The Structure of The Atom Atom An atom





- Slides: 13

The Structure of The Atom

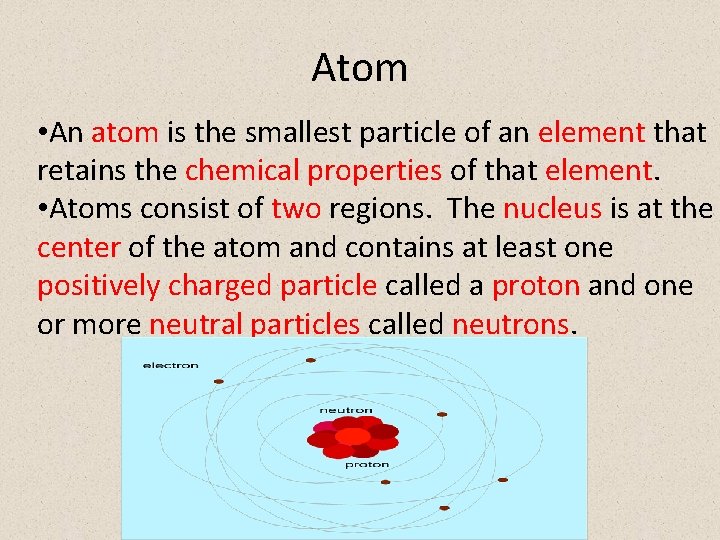
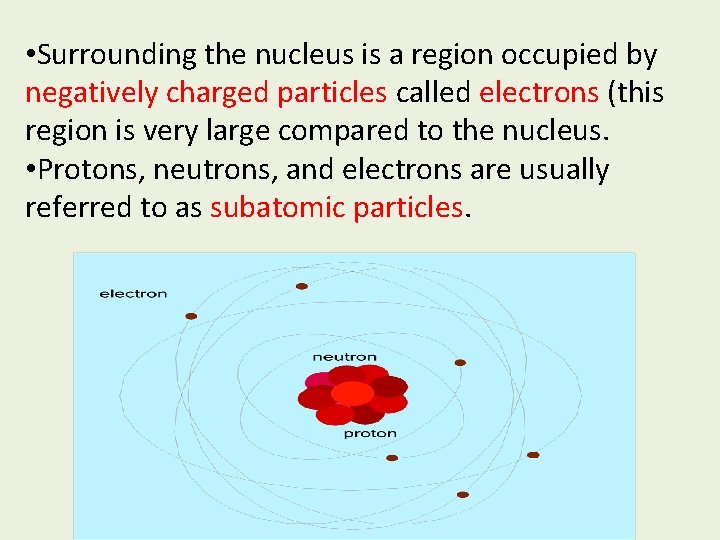
Atom • An atom is the smallest particle of an element that retains the chemical properties of that element. • Atoms consist of two regions. The nucleus is at the center of the atom and contains at least one positively charged particle called a proton and one or more neutral particles called neutrons.

• Surrounding the nucleus is a region occupied by negatively charged particles called electrons (this region is very large compared to the nucleus. • Protons, neutrons, and electrons are usually referred to as subatomic particles.

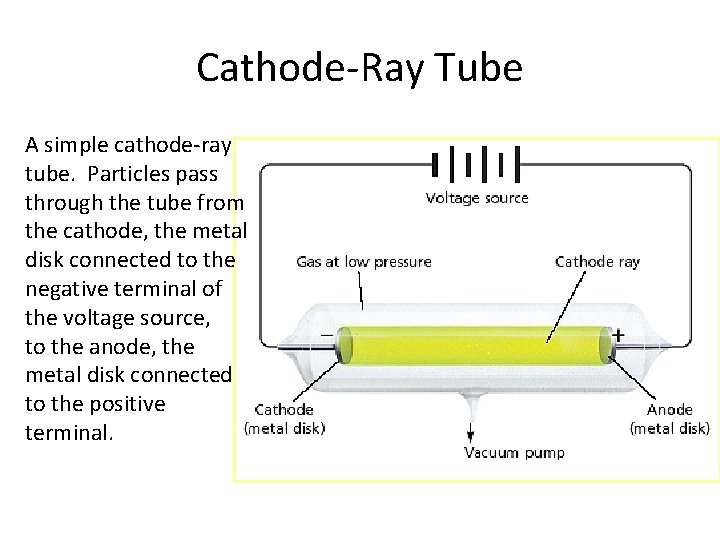
Discovery of the Electron • The discovery of the electron occurred in the late 1800 s when scientists were investigating the relationship between electricity and matter. The experiments were carried out in cathode-ray tubes.

Cathode-Ray Tube A simple cathode-ray tube. Particles pass through the tube from the cathode, the metal disk connected to the negative terminal of the voltage source, to the anode, the metal disk connected to the positive terminal.

• The experiments carried out by Joseph John Thomson in 1897, showed the particles that composed the cathode ray were negatively charged. • Thomson also thought that atoms must contain some positive charge. • Thomson suggested that an atom consisted of a cloud of positive charge with the negative electrons embedded in it. This model is often called the “plum-pudding” model. • Watch video clip.

• In 1909 experiments conducted by Robert A. Millikan showed that atoms were electrically neutral and must contain a positive charge to balance the negative electrons. They also showed that atoms must contain other particles that account for most of their mass. • Watch video clip.

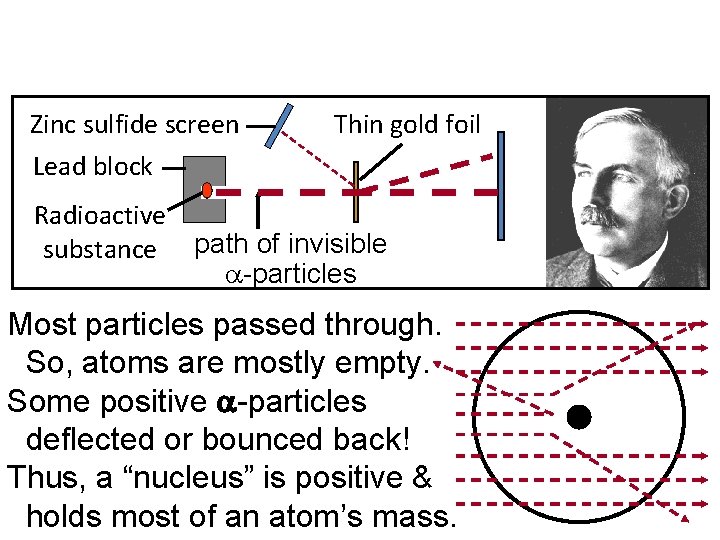
Discovery of the Atomic Nucleus • In 1911 Ernest Rutherford, Hans Geiger, and Ernest Marsden bombarded a thin piece of gold foil with fast-moving alpha particles. Some particles were redirected back toward the source. • Rutherford concluded that this was caused by a densely packed bundle of matter with a positive charge (nucleus).

Zinc sulfide screen Lead block Radioactive substance Thin gold foil path of invisible -particles Most particles passed through. So, atoms are mostly empty. Some positive -particles deflected or bounced back! Thus, a “nucleus” is positive & holds most of an atom’s mass.

Composition of the Atomic Nucleus • The nuclei by atoms of different elements differ in the number of protons they contain (the number of protons in an atom’s nucleus determine the atom’s identity.

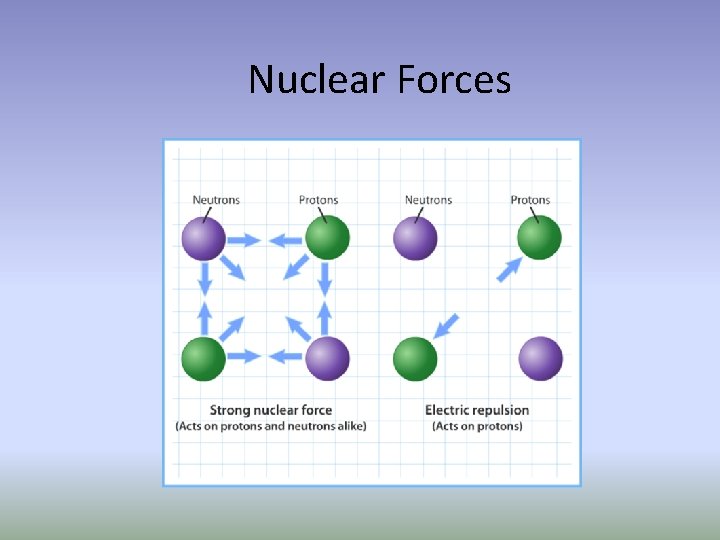
If protons are positively charged how does the nucleus stay together? Nuclear Forces!! When two protons are extremely close to each other, there is a strong attraction between them. A similar attraction exists when neutrons are very close to each other or when protons and neutrons are very close together. These short-range proton-neutron, proton-proton, and neutron-neutron forces hold the nuclear particles together and are referred to as nuclear forces.

Nuclear Forces

The Sizes of Atoms • The sizes of atoms are expressed in picometers ( 1 pm = 1 x 10 -12 m). • To get an idea of how small a picometer is, consider that 1 cm is the same fractional part of 103 km (about 600 miles) as 100 pm is of 1 cm. • Atomic radii range from about 40 to 270 pm.