Unit 02 Atomic Structure Just How Small is





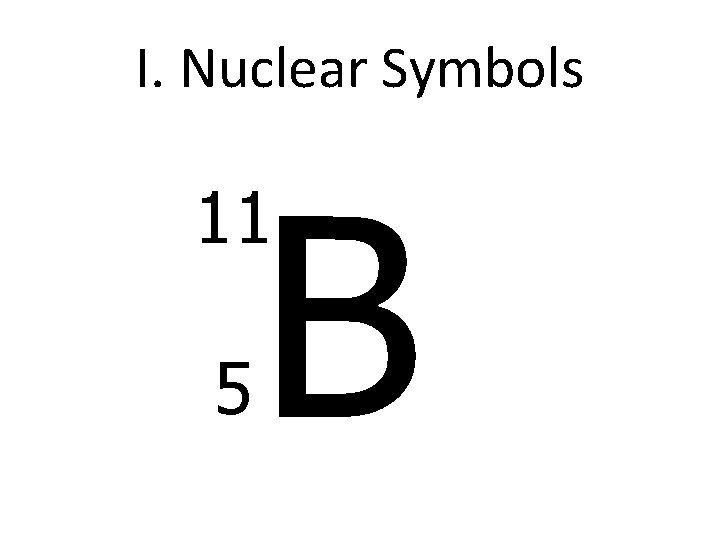


- Slides: 41

Unit 02 Atomic Structure

Just How Small is an Atom? You don’t need to write. • A speck 0. 1 mm in diameter (about half the size of a period at the end of the sentence) requires one million atoms. • It would require a million atoms, edge to edge, to match the thickness of a page of paper.

Can you see an atom? • Technically, you cannot "see" anything smaller than the shortest wavelength of light that you can see it with. • But there are ways to "visualize" it, like Atomic Force Microscopy. But these are all just measurements converted to computer images, and are not in any real sense "seeing" the atom. • You can't see atoms in any normal sense of using an optical microscope. • You don't get an optical image, but it does allow you to map out an image of the atoms of a molecule. To do this you use a metallic tip which interacts with the atoms you want to image. As you move the tip over the atoms, you pass a current, called a tunneling current, between the tip and the atom. This current is extremely sensitive to the distance between the atom and the tip.

- REMEMBER FROM: Elements, Mixtures, and Compounds - Element - a pure substance made up of one type of atom. - organized on periodic table - each element has a unique number of protons…its atomic number protons

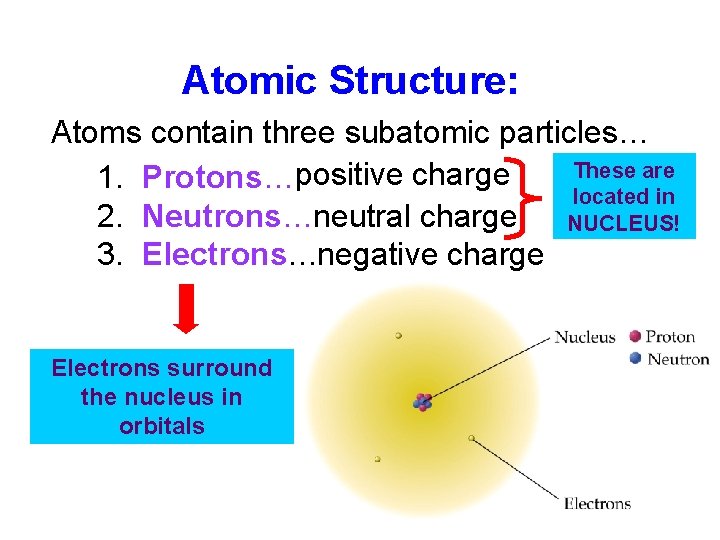
Atomic Structure: Atoms contain three subatomic particles… These are 1. Protons…positive charge located in 2. Neutrons…neutral charge NUCLEUS! 3. Electrons…negative charge Electrons surround the nucleus in orbitals

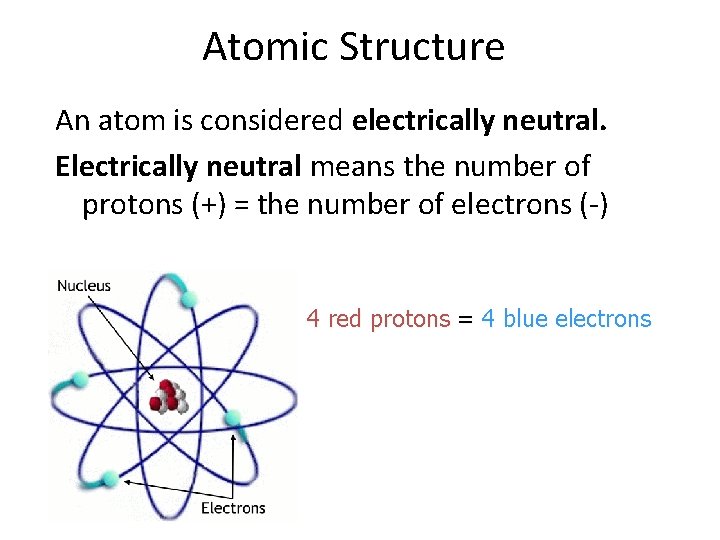
Atomic Structure An atom is considered electrically neutral. Electrically neutral means the number of protons (+) = the number of electrons (-) 4 red protons = 4 blue electrons

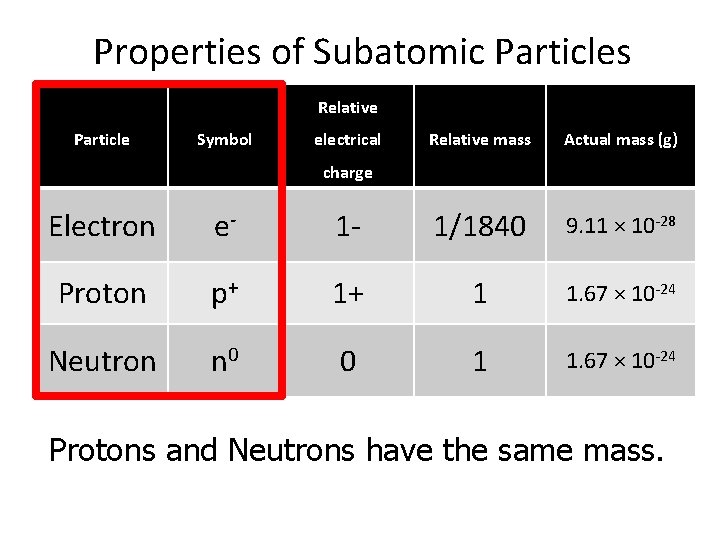
Properties of Subatomic Particles Relative Particle Symbol electrical Relative mass Actual mass (g) charge Electron e- 1 - 1/1840 9. 11 × 10 -28 Proton p+ 1+ 1 1. 67 × 10 -24 Neutron n 0 0 1 1. 67 × 10 -24 Protons and Neutrons have the same mass.

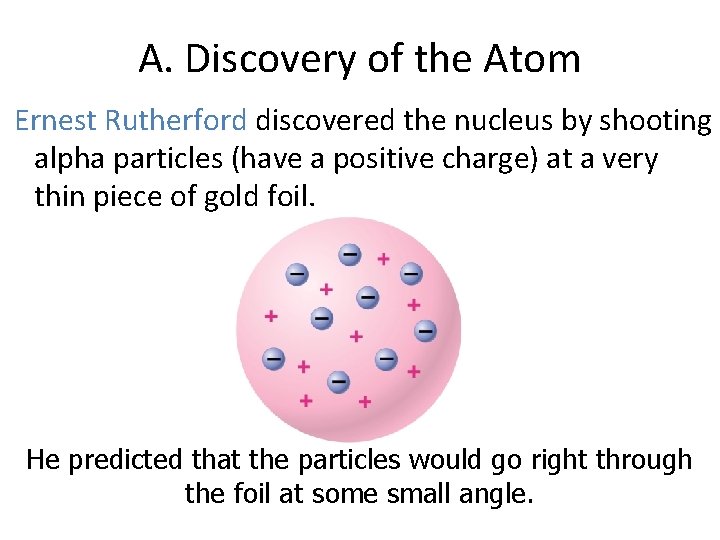
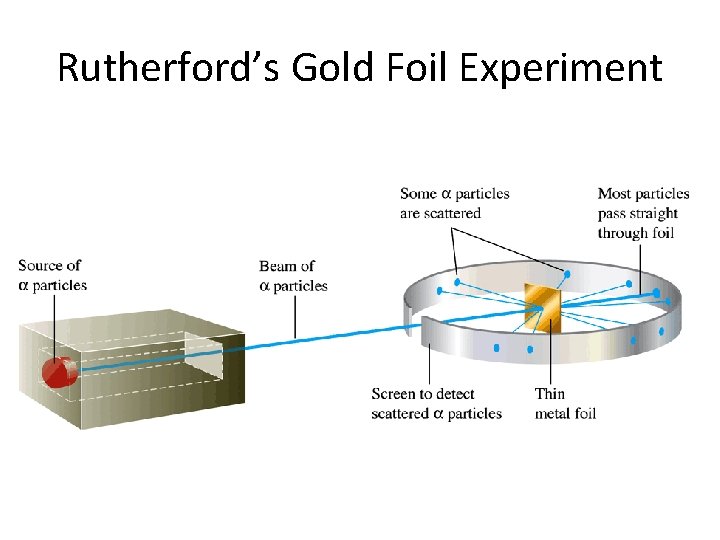
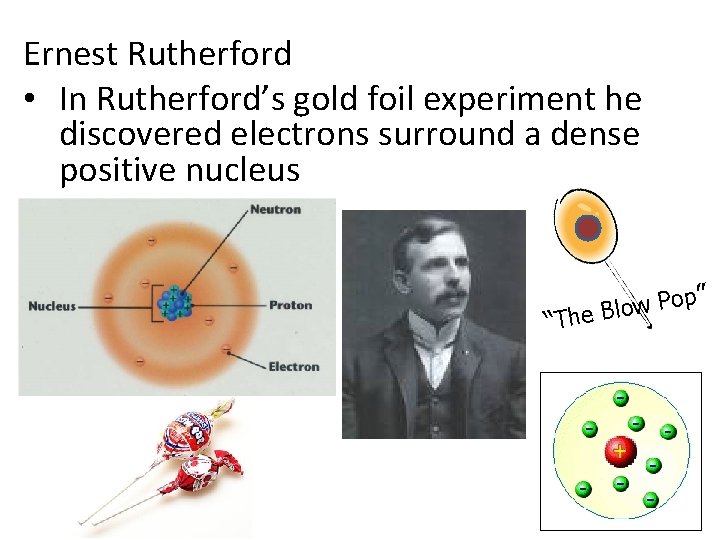
A. Discovery of the Atom Ernest Rutherford discovered the nucleus by shooting alpha particles (have a positive charge) at a very thin piece of gold foil. He predicted that the particles would go right through the foil at some small angle.

Rutherford’s Gold Foil Experiment

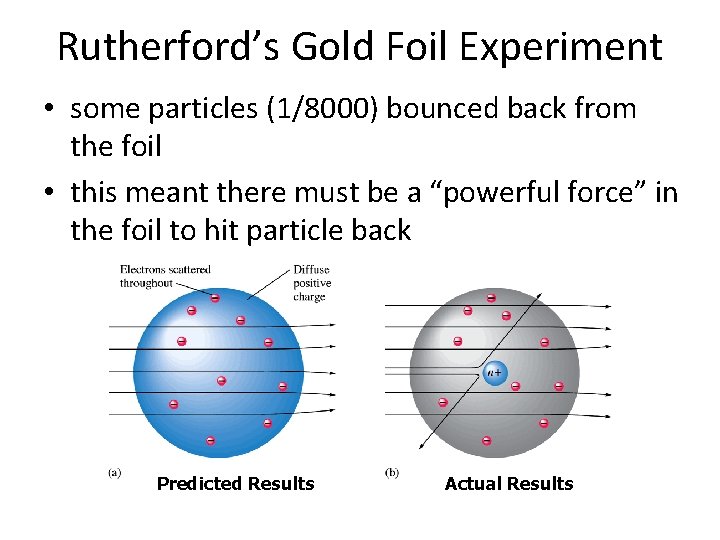
Rutherford’s Gold Foil Experiment • some particles (1/8000) bounced back from the foil • this meant there must be a “powerful force” in the foil to hit particle back Predicted Results Actual Results

Discovery of the Atom Purpose: The students will find the shape of different items and relate this to the early scientist that made discoveries about the shape and size of the atom. Procedure: 1. Title the left side of your spiral Discovery of the Atom. 2. For each item you will write the letters then draw your predicted shape of the item. 3. Then you will write 1 sentence describing why they think your prediction is the shape of the item. A: Item in brown bag – Use your hands to feel the shape of the item. B: Item in clay – Using the toothpicks provided find the shape of the object enclosed in the modeling clay. C: Black box – Maneuver the black box with a marble inside to discover the shape of the object enclosed.

B. Models of the Atom

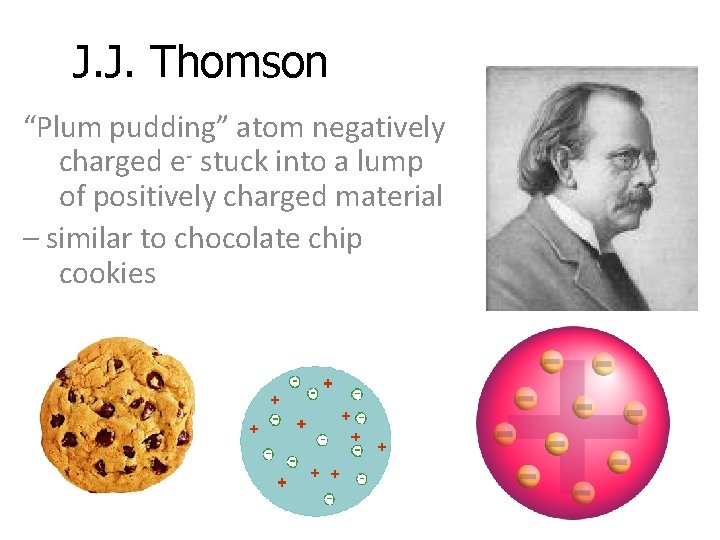
J. J. Thomson “Plum pudding” atom negatively charged e- stuck into a lump of positively charged material – similar to chocolate chip cookies

Ernest Rutherford • In Rutherford’s gold foil experiment he discovered electrons surround a dense positive nucleus ” p o P w lo “The B

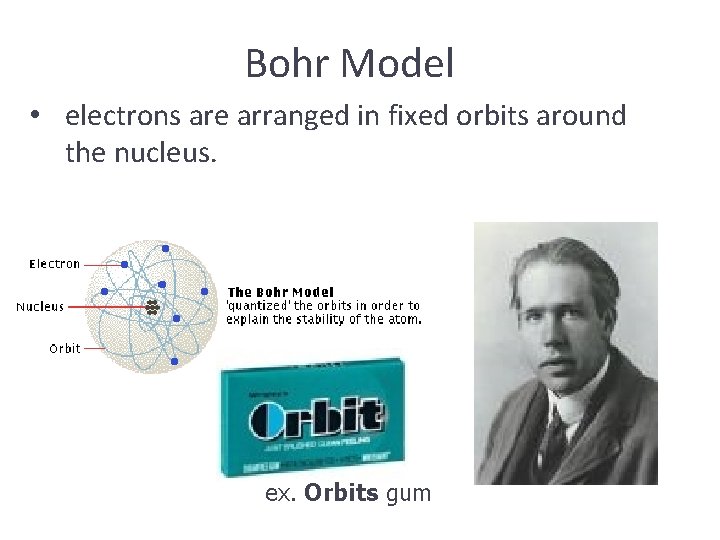
Bohr Model • electrons are arranged in fixed orbits around the nucleus. ex. Orbits gum

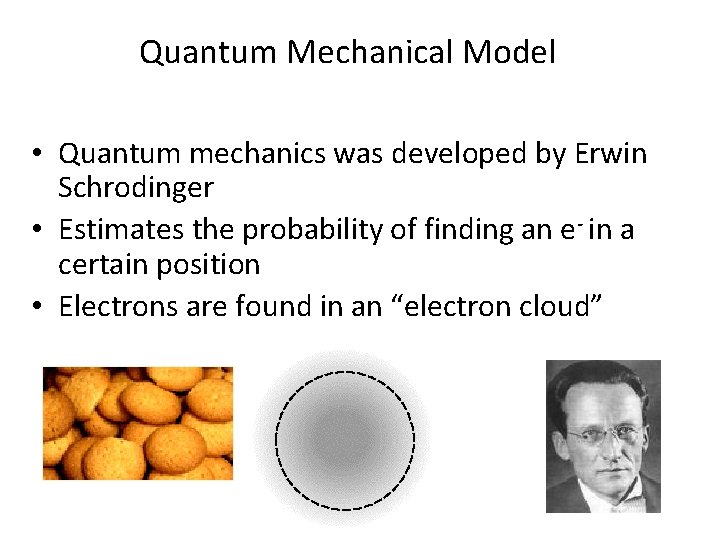
Quantum Mechanical Model • Quantum mechanics was developed by Erwin Schrodinger • Estimates the probability of finding an e- in a certain position • Electrons are found in an “electron cloud”
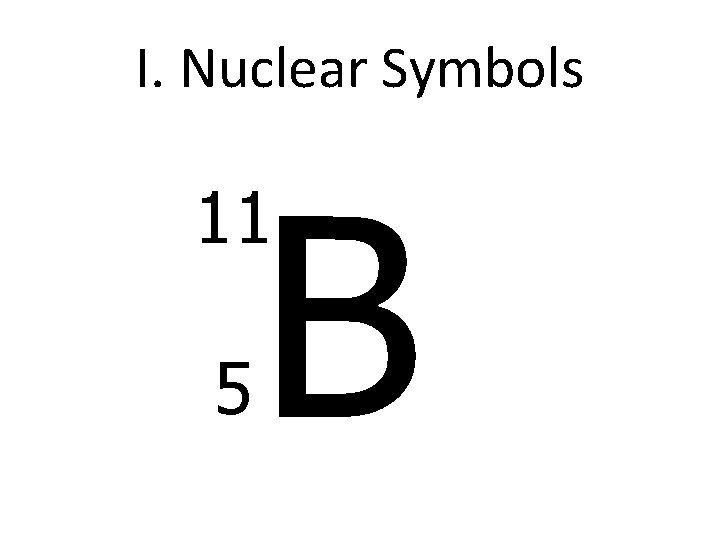
I. Nuclear Symbols B 11 5

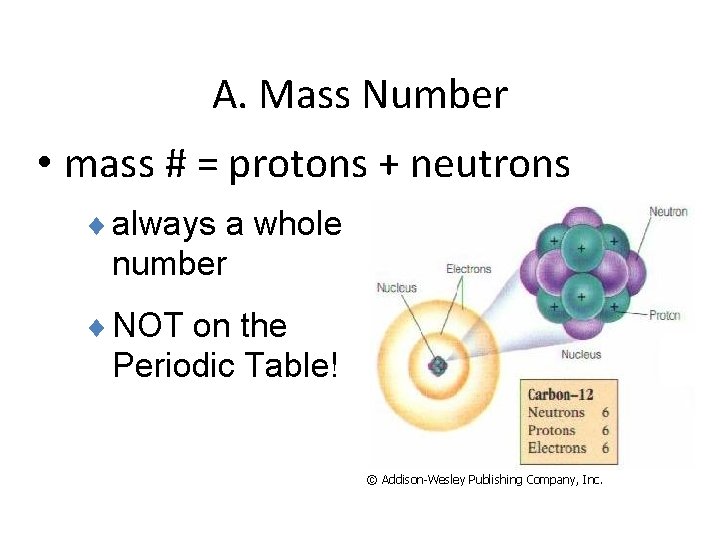
A. Mass Number • mass # = protons + neutrons ¨ always a whole number ¨ NOT on the Periodic Table! © Addison-Wesley Publishing Company, Inc.

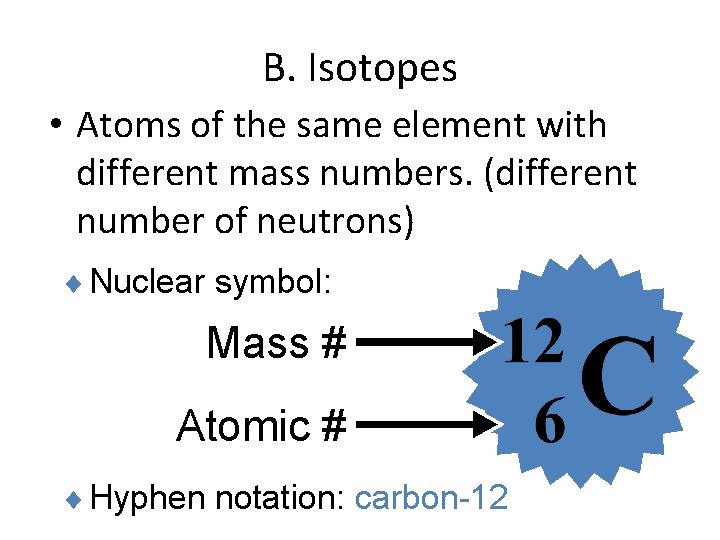
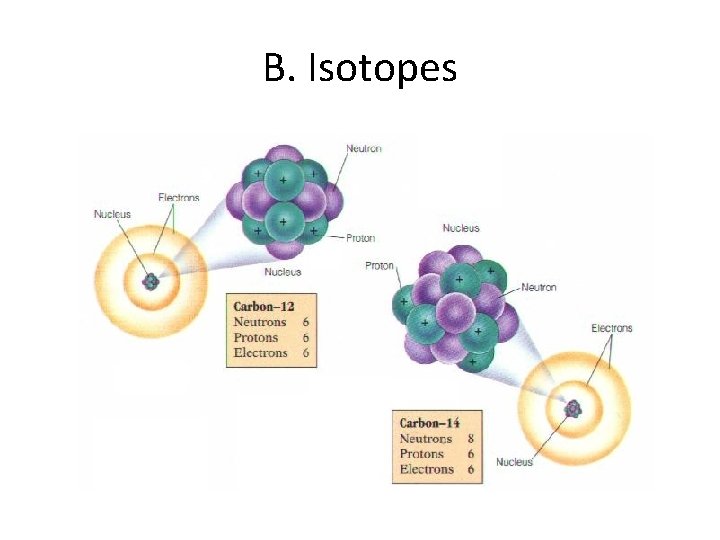
B. Isotopes • Atoms of the same element with different mass numbers. (different number of neutrons) ¨ Nuclear symbol: Mass # Atomic # ¨ Hyphen notation: carbon-12

B. Isotopes

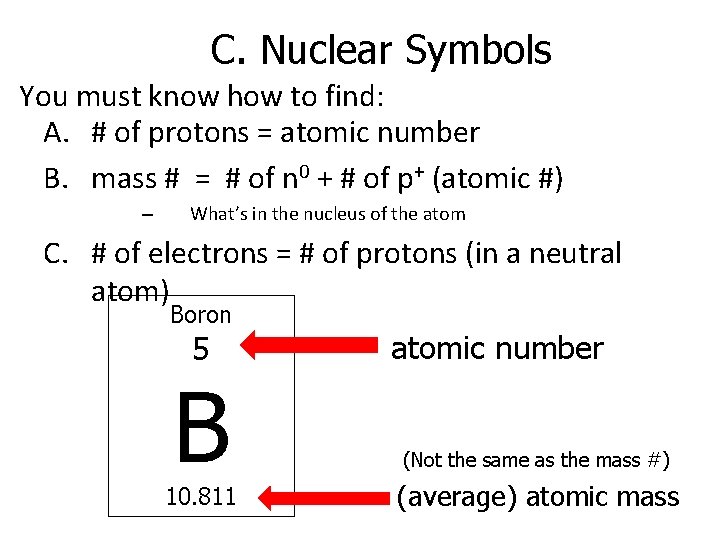
C. Nuclear Symbols You must know how to find: A. # of protons = atomic number B. mass # = # of n 0 + # of p+ (atomic #) – What’s in the nucleus of the atom C. # of electrons = # of protons (in a neutral atom) Boron 5 B 10. 811 atomic number (Not the same as the mass #) (average) atomic mass

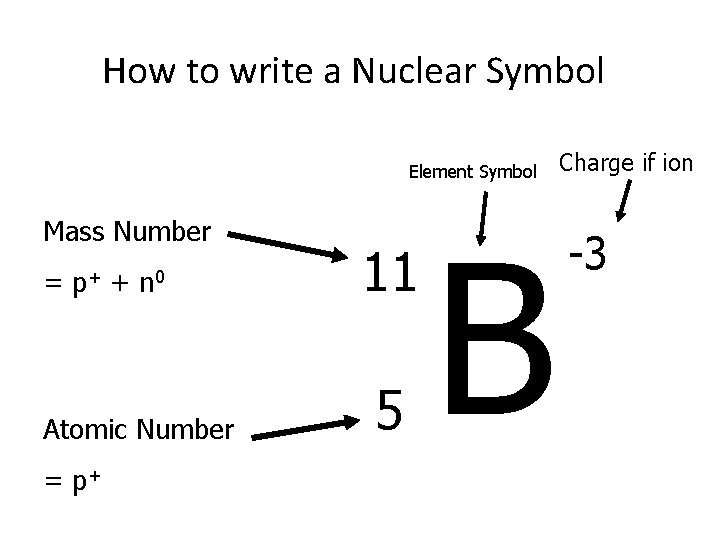
How to write a Nuclear Symbol Element Symbol Mass Number = p+ + n 0 Atomic Number = p+ 11 5 B Charge if ion -3

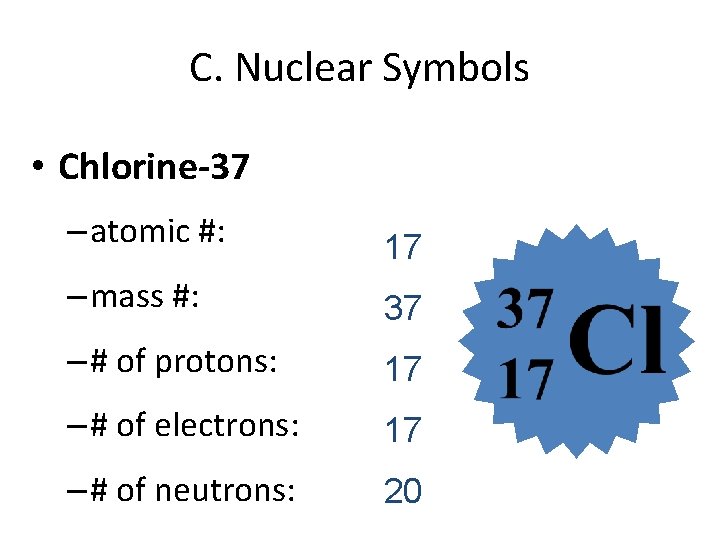
C. Nuclear Symbols • Chlorine-37 – atomic #: 17 – mass #: 37 – # of protons: 17 – # of electrons: 17 – # of neutrons: 20

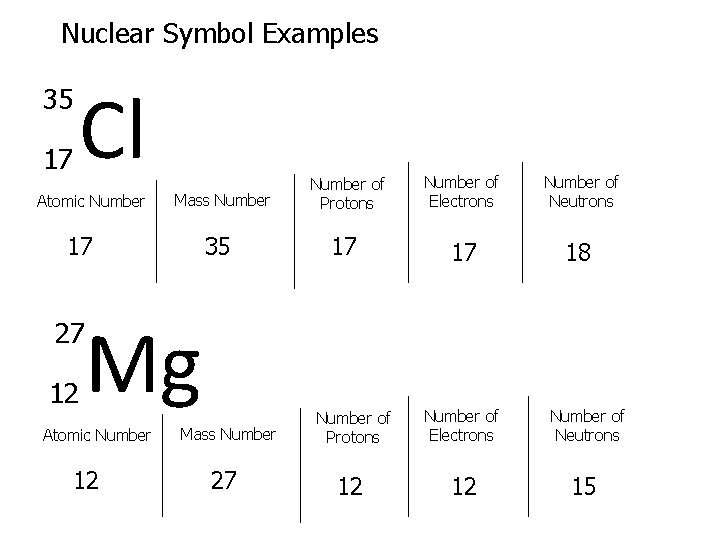
Nuclear Symbol Examples 35 17 Cl Atomic Number Mass Number 17 35 Mg Number of Protons Number of Electrons Number of Neutrons 17 17 18 27 12 Atomic Number 12 Mass Number 27 Number of Protons Number of Electrons Number of Neutrons 12 12 15

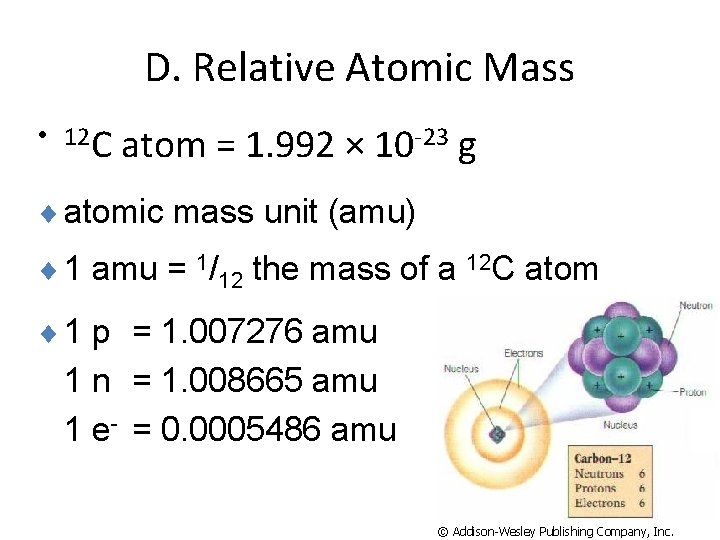
D. Relative Atomic Mass • 12 C atom = 1. 992 × 10 -23 g ¨ atomic mass unit (amu) ¨ 1 amu = 1/12 the mass of a 12 C atom ¨ 1 p = 1. 007276 amu 1 n = 1. 008665 amu 1 e- = 0. 0005486 amu © Addison-Wesley Publishing Company, Inc.

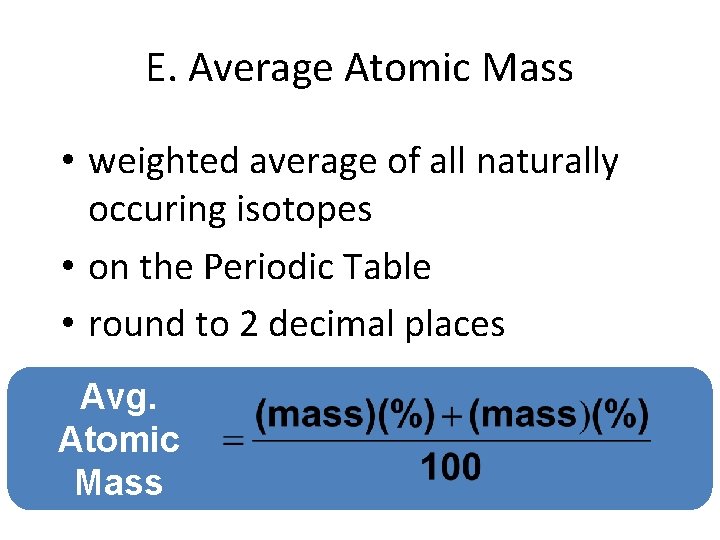
E. Average Atomic Mass • weighted average of all naturally occuring isotopes • on the Periodic Table • round to 2 decimal places Avg. Atomic Mass

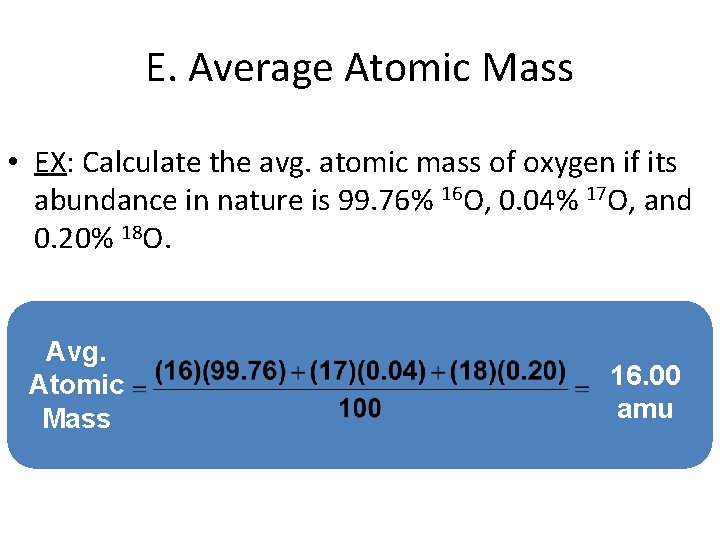
E. Average Atomic Mass • EX: Calculate the avg. atomic mass of oxygen if its abundance in nature is 99. 76% 16 O, 0. 04% 17 O, and 0. 20% 18 O. Avg. Atomic Mass 16. 00 amu

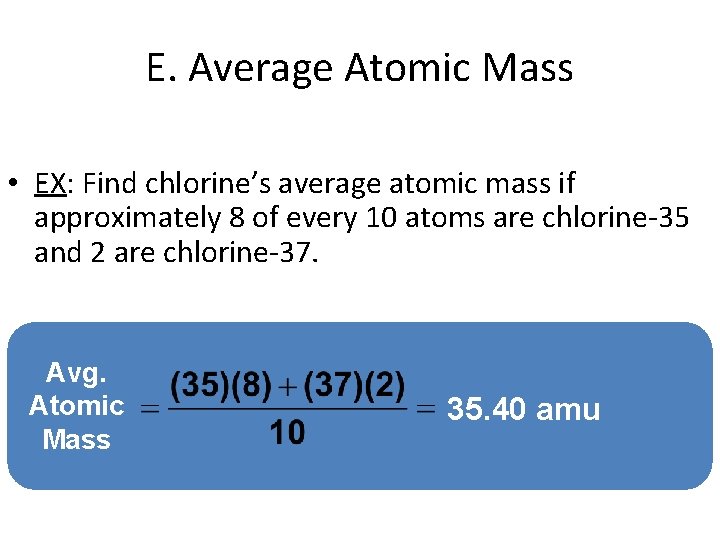
E. Average Atomic Mass • EX: Find chlorine’s average atomic mass if approximately 8 of every 10 atoms are chlorine-35 and 2 are chlorine-37. Avg. Atomic Mass 35. 40 amu

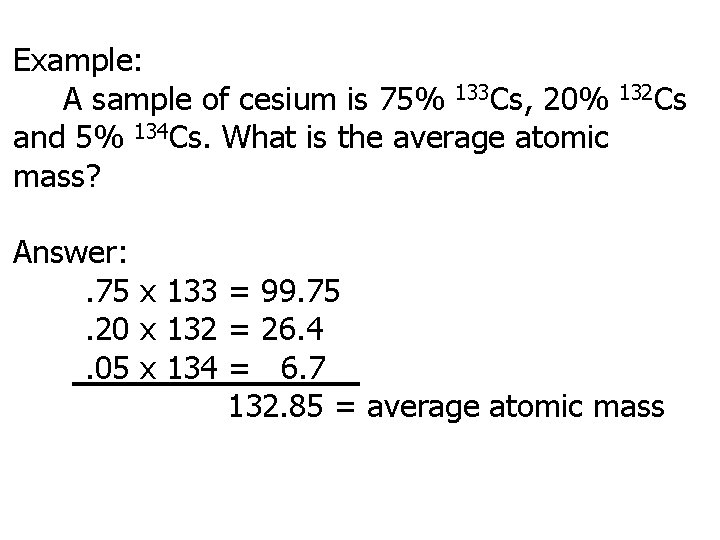
Example: A sample of cesium is 75% 133 Cs, 20% and 5% 134 Cs. What is the average atomic mass? 132 Cs Answer: . 75 x 133 = 99. 75. 20 x 132 = 26. 4. 05 x 134 = 6. 7 132. 85 = average atomic mass

II. The Periodic Table Periodic Law – properties of elements can be predicted by their position on the periodic table

A. History of the Periodic Table • Dmitri Mendeleev (1871) – Developed the first periodic table – It was arranged by atomic mass because atomic number had not been discovered – He was able to predict properties of elements

A. History of the Periodic Table • Henry Moseley (1913) - developed the modern periodic table - arranged in order of increasing atomic number

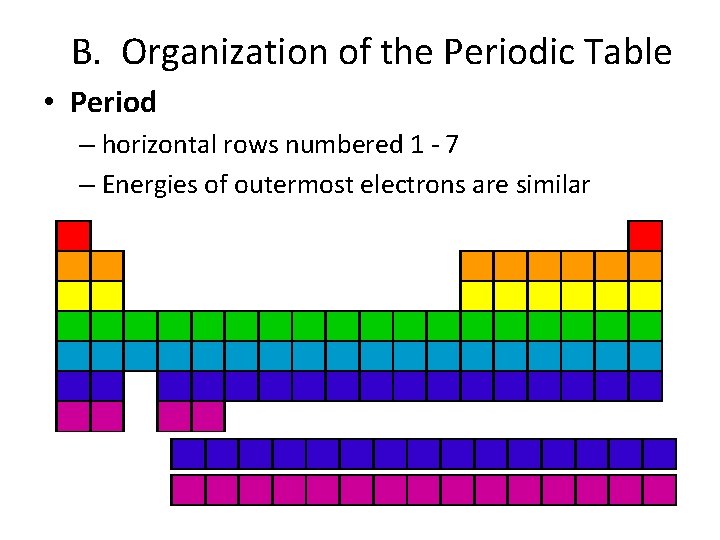
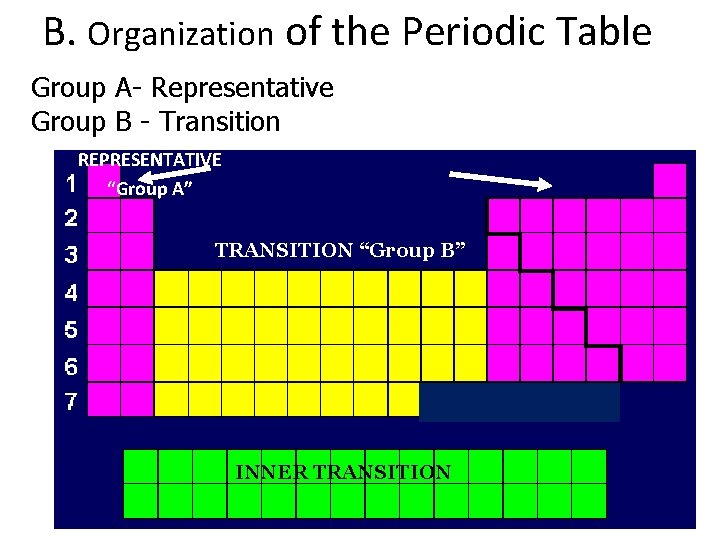
B. Organization of the Periodic Table • Period – horizontal rows numbered 1 - 7 – Energies of outermost electrons are similar

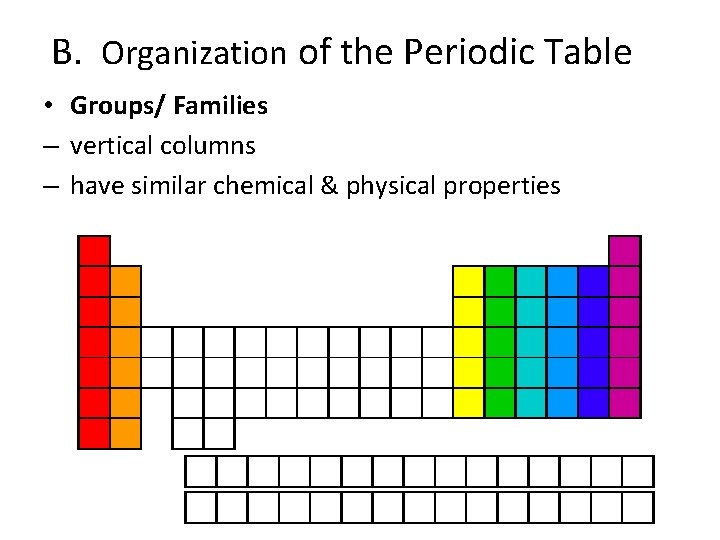
B. Organization of the Periodic Table • Groups/ Families – vertical columns – have similar chemical & physical properties

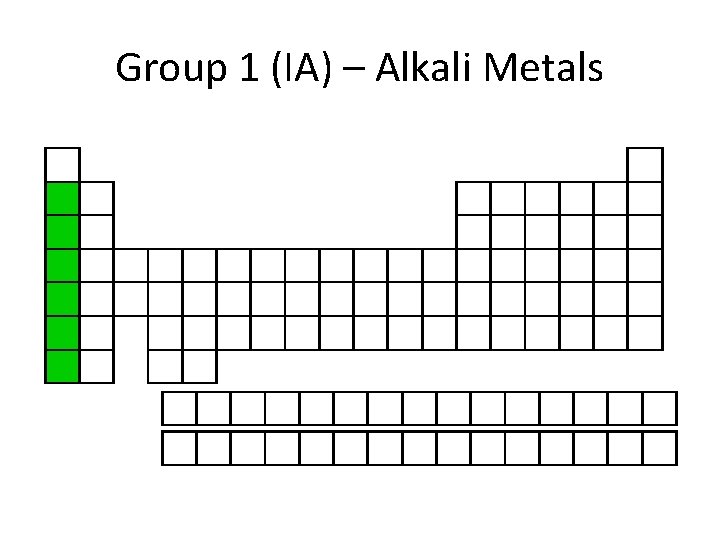
Group 1 (IA) – Alkali Metals

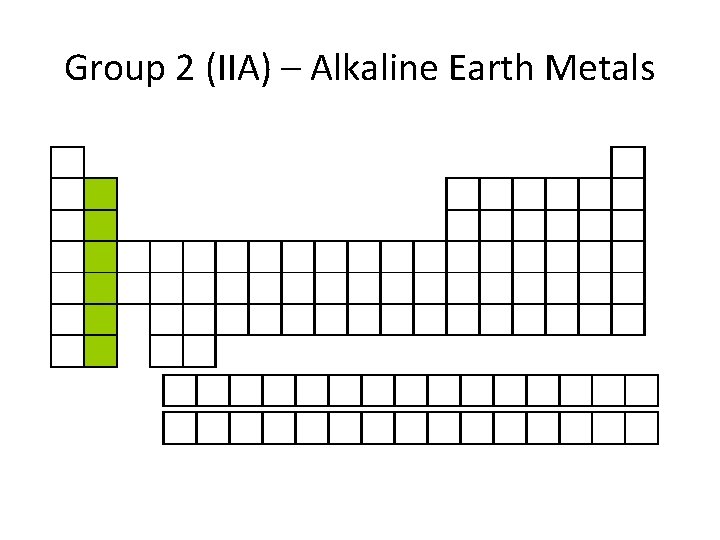
Group 2 (IIA) – Alkaline Earth Metals

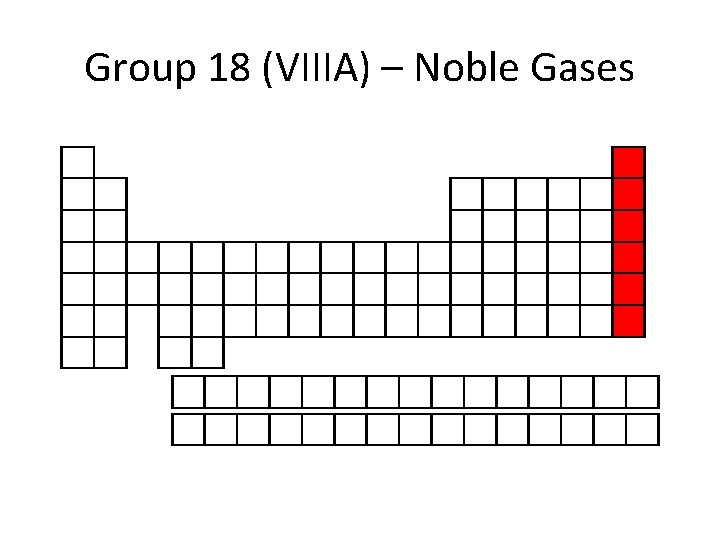
Group 18 (VIIIA) – Noble Gases

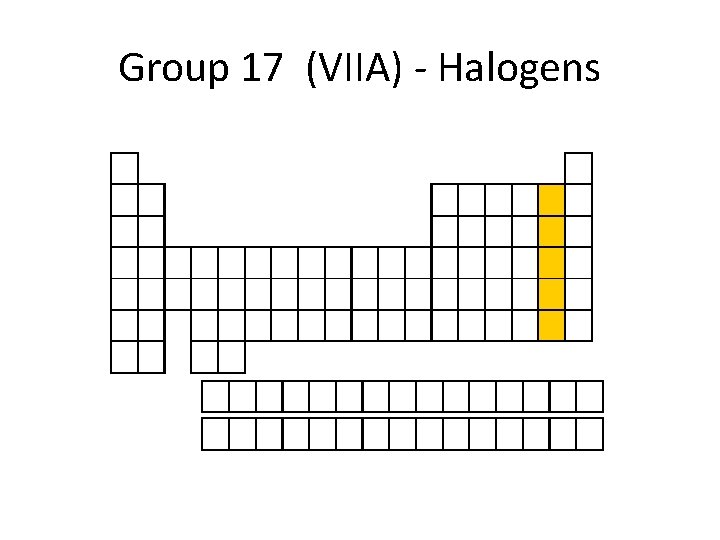
Group 17 (VIIA) - Halogens

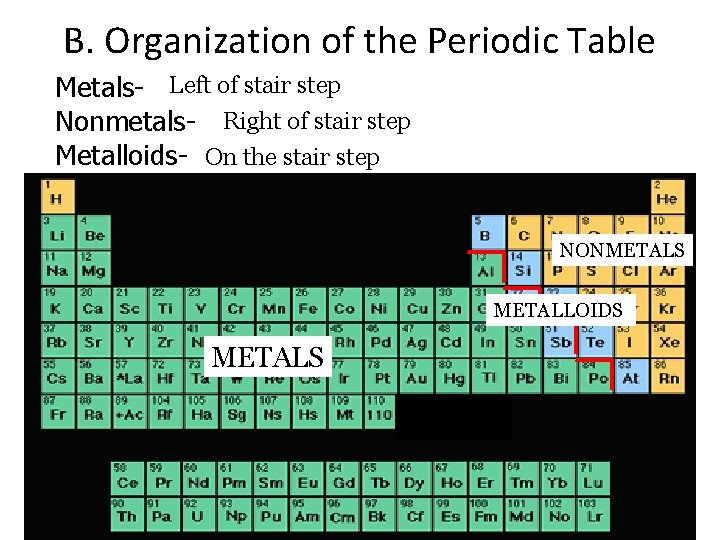
B. Organization of the Periodic Table Metals- Left of stair step Nonmetals- Right of stair step Metalloids- On the stair step NONMETALS METALLOIDS METALS

B. Organization of the Periodic Table Group A- Representative Group B - Transition REPRESENTATIVE “Group A” TRANSITION “Group B” INNER TRANSITION

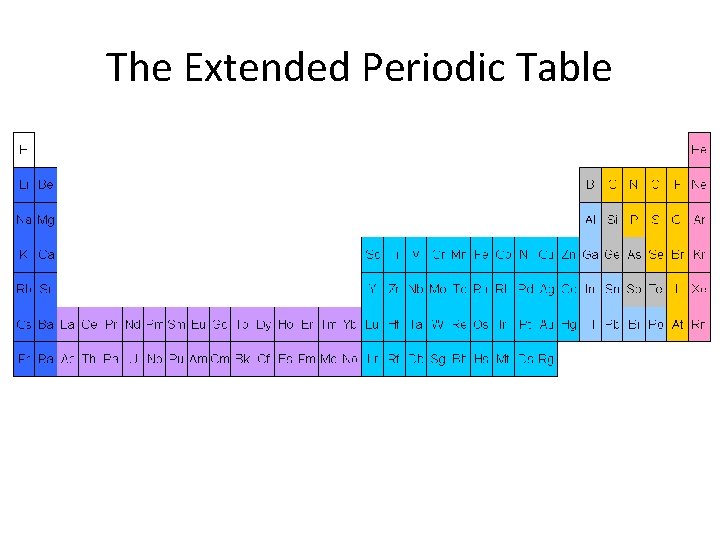
The Extended Periodic Table