Warmup What is the atomic of Cesium How








































- Slides: 63

Warm-up • What is the atomic # of Cesium? How many protons, electrons, and neutrons does one atom of Cesium have? What group is it in?

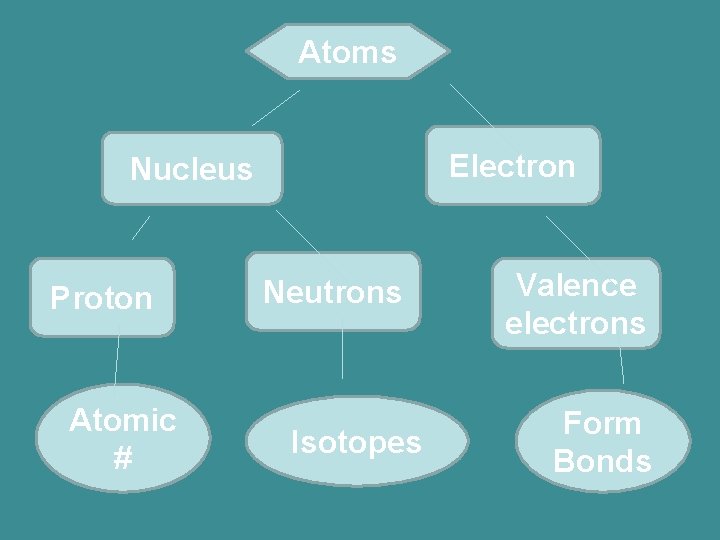
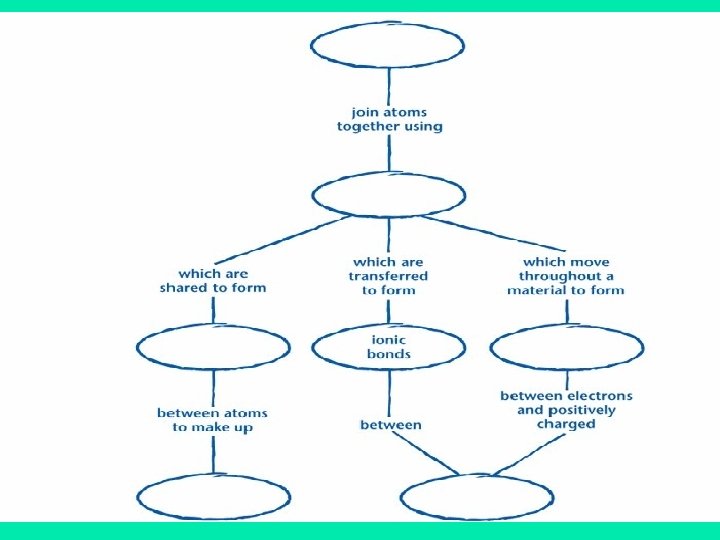
Warm- up Create a concept map with the following terms: Atom, Nucleus, Proton, Electrons, Isotope, neutrons, Atomic #, valence electrons, forms bonds.

Atoms Electron Nucleus Proton Atomic # Neutrons Isotopes Valence electrons Form Bonds

Warm-up • Describe the difference between a covalent and an ionic compound. • Draw an electron dot configuration for … Cl 2 and NH 3

Chemical reactions and bonding • Objectives: • Examine chemical reactions through the behavior of valence electrons. • Explain what happens to chemical bonds during a chemical reaction. • What is a charged atom and how does an atom become charged? • How do atoms combine to form compounds?

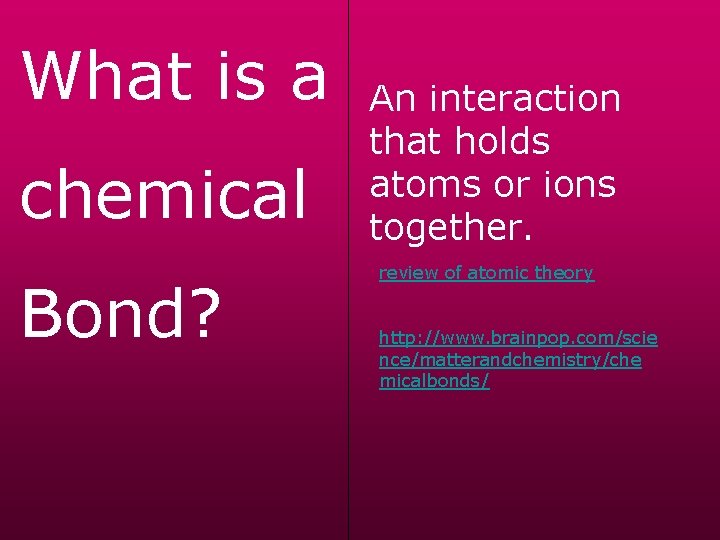
What is a chemical Bond? An interaction that holds atoms or ions together. review of atomic theory http: //www. brainpop. com/scie nce/matterandchemistry/che micalbonds/

What is chemical Bonding? The combining of atoms to form compounds.

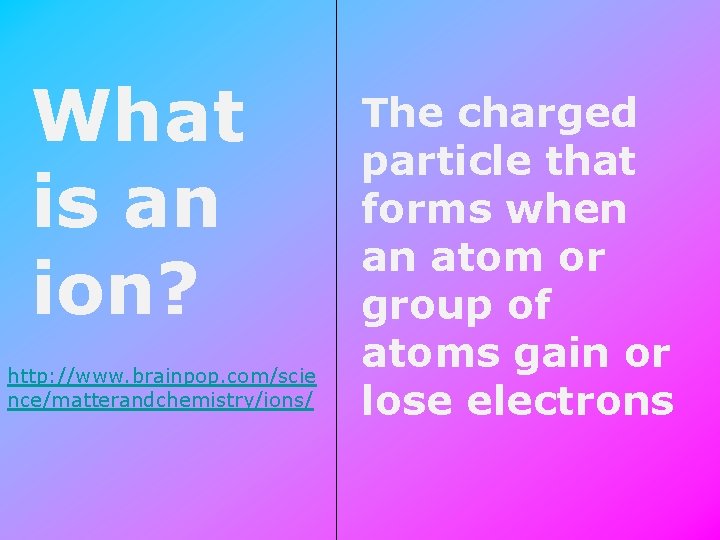
What is an ion? http: //www. brainpop. com/scie nce/matterandchemistry/ions/ The charged particle that forms when an atom or group of atoms gain or lose electrons

Reactivity in group 1 - the loss of 1 valence electron Myth Busters- methane gas (CH 4) Alkali metal reactions with water

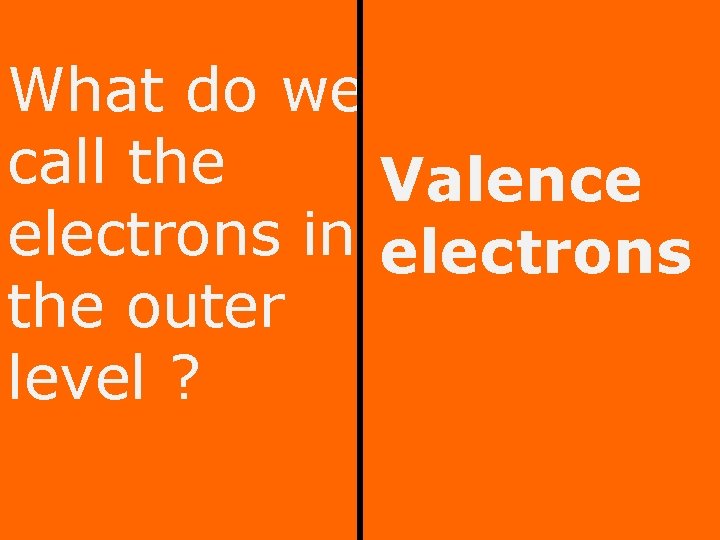
What do we call the Valence electrons in electrons the outer level ?

Why are valence electrons important? • They determine whether a bond will form between elements. In most atoms 8 is considered a full outer level, but hydrogen and helium only need two to be stable.

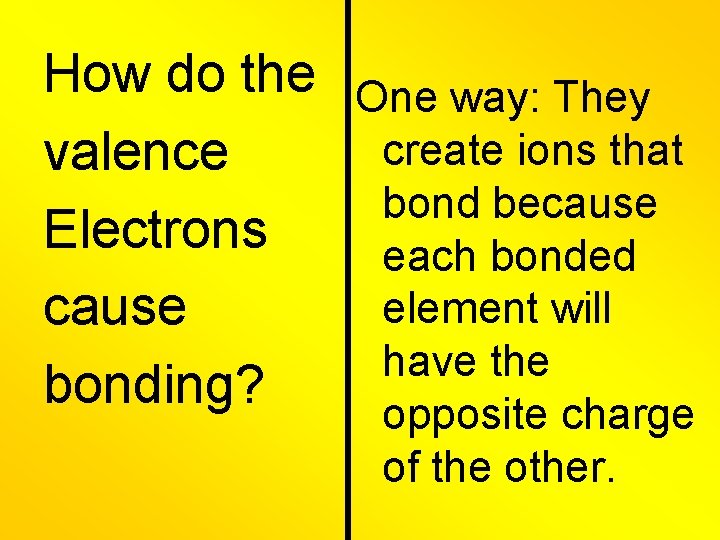
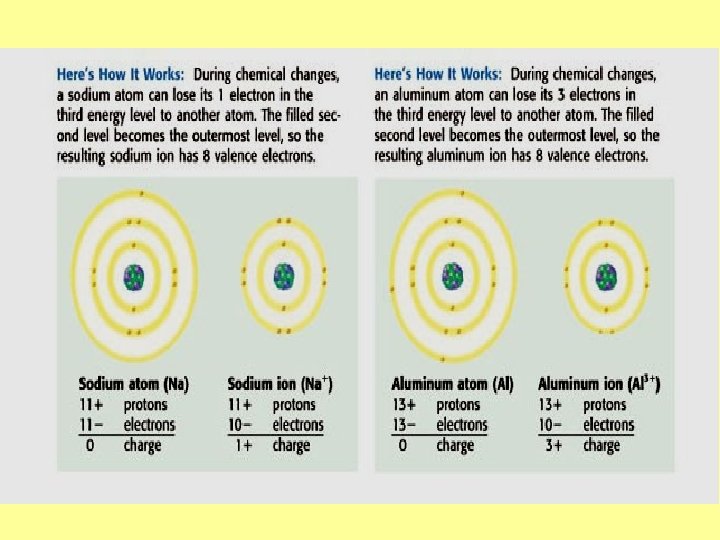
How do the One way: They create ions that valence bond because Electrons each bonded element will cause have the bonding? opposite charge of the other.

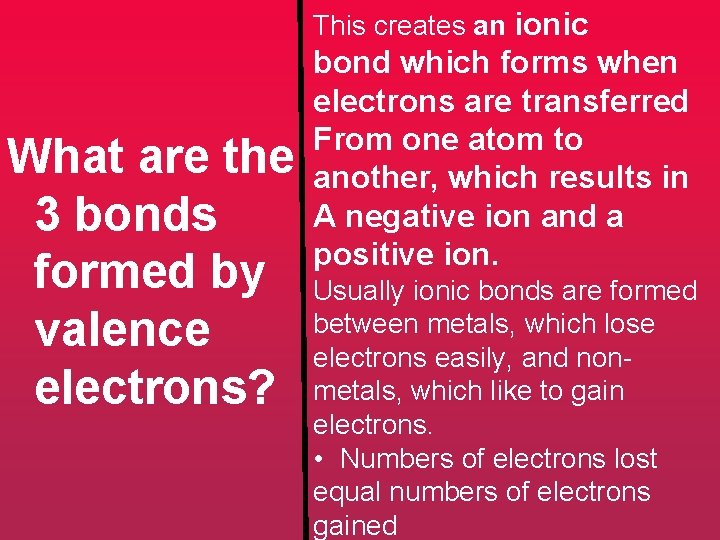
This creates an ionic What are the 3 bonds formed by valence electrons? bond which forms when electrons are transferred From one atom to another, which results in A negative ion and a positive ion. Usually ionic bonds are formed between metals, which lose electrons easily, and nonmetals, which like to gain electrons. • Numbers of electrons lost equal numbers of electrons gained

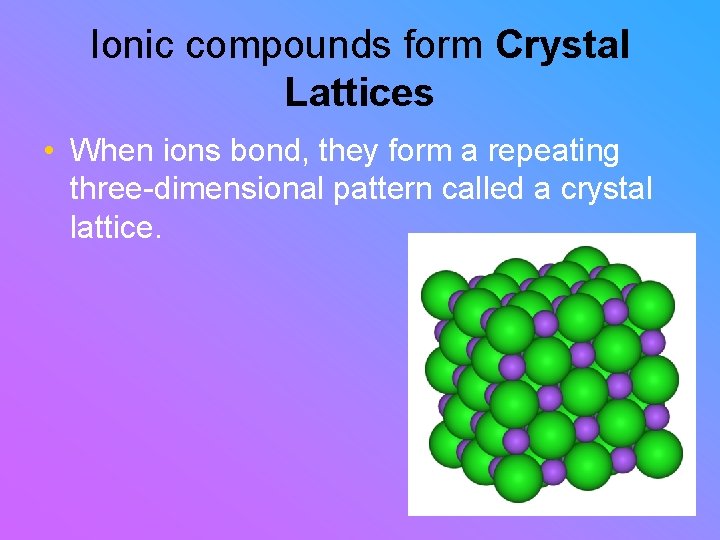
Ionic compounds form Crystal Lattices • When ions bond, they form a repeating three-dimensional pattern called a crystal lattice.

Naming Ionic Compounds • When metals and non-metals form ionic compounds, the metal becomes a positively charged ion and the non-metal becomes a negatively charged ion. Names of ions with negative charges end in “ide” such as oxide, chloride, sulfide, fluoride

Ionic Compounds and Their Properties • Brittleness Ionic compounds tend to be brittle solids at room temperature. So, they usually break apart when hit. This property is due to the arrangement of ions in a repeating three-dimensional pattern called a crystal lattice. • High Melting Points Because of the strong ionic bonds that hold ions together, ionic compounds have high melting points. • Solubility and Electrical Conductivity Many ionic compounds are highly soluble. The solution that forms when an ionic compound dissolves in water can conduct an electric current.

Lewis structure & Animation of bonds • http: //glencoe. mcgrawhill. com/sites/0078741858/student_view 0/ unit 2/chapter 5/interactive_tables. html • animation of covalent and ionic bonds



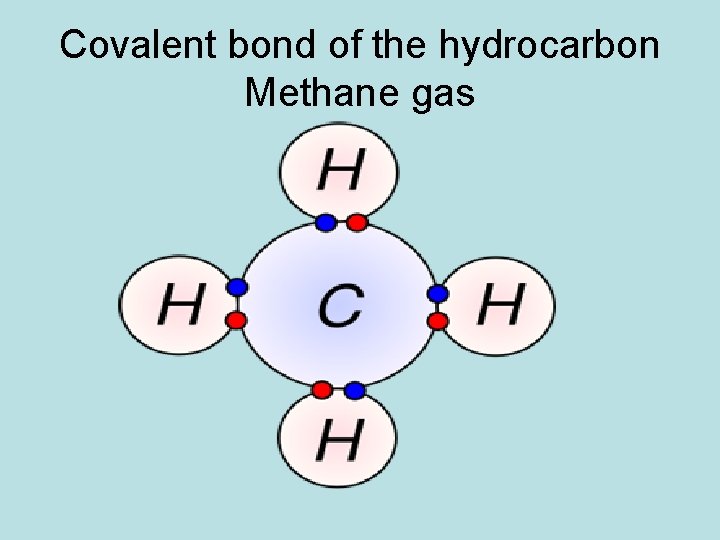
What is a covalent bond? 2 nd way valence electrons form bonds: A covalent bond forms when two or more atoms share one or more pairs of electrons.

Covalent Bonds and Molecules • Substances containing covalent bonds consist of individual particles called molecules. A molecule usually consists of two or more atoms joined in a definite ratio. A water molecule is shown on the slide after next. • These bonds generally form between nonmetals, where it takes too much energy to transfer electrons so the atoms just share electrons.


Covalent Compounds and Their Properties • Low Solubility Many covalent compounds are not soluble in water, which means that they do not dissolve well in water. • Low Melting Points Less heat is needed to separate the molecules of covalent compounds, so these compounds have much lower melting and boiling points than ionic compounds do. • Electrical Conductivity Although most covalent compounds don’t dissolve in water, some do. Most of the covalent compounds that dissolve in water form solutions that do not conduct electricity well.


Covalent bond of the hydrocarbon Methane gas

• Third way valence electrons form bonds: A metallic bond is • What Is a a bond formed by Metallic the attraction Bond? between positively Animation charged metal ions more metallic bond and the electrons animation in the metal.

How do electrons move throughout Metals with a metallic bond? Bonding in metals is a result of the metal atoms being so close to one another that their outermost energy levels overlap. This overlapping allows valence electrons to move throughout the metal.

Properties of Metals • Conducting Electric Current Metallic bonding allows metals to conduct electric current. For example, The moving valence electrons can carry electric current through copper wire to light a lamp • Reshaping Metals Because the electrons swim freely around the metal ions, the atoms in metals can be rearranged. As a result, metals can be reshaped. The properties of ductility (the ability to be drawn into wires) and malleability (the ability to be hammered into sheets) describe a metal’s ability to be reshaped. • Bending Without Breaking Metal objects can be bent without being broken.



Chemical reaction Notes

Warm-up: Review • What are three types of chemical bonds? • Describe what is happening to the atoms in each of these bonds. (remember it is all about the electrons)

• Ionic, covalent, and metallic • In an ionic bond electrons are gained and lost (In table salt-sodium gives to chlorine so that chlorine can have a full set), In covalent bonds electrons are shared (water- 2 Hydrogen and one oxygen share so that they all have a full set), metallic contain positive ions that are attracted to the electrons of neighboring atoms allowing them to overlap (valence electrons can move from atom to atom, which allows electrical conductivity). • animation of ionic and covalent bonds

Objectives • Describe how chemical reactions produce new substances that have different chemical and physical properties. • Identify four signs that indicate that a chemical reaction might be taking place. • Explain what happens to chemical bonds during a chemical reaction.

• What is a chemical reaction? • The process by which one or more substances change to produce one or more different / new substances.

-change of color What are some signs of a Chemical reaction? (Remember chapter one of our first book? ) -production of odor -formation of a gas -bubbles/foaming/ fizzing -production of light, heat, electricity, sound -also something new called a precipitate, This is combining solutions to create a solid. So in other words liquid + liquid = solid

Is this a chemical reaction? ? ? • http: //freeschool. wordpress. com/2008/01/1 1/fun-chemical-reaction-experiment-briggs -rauscher-reaction/

Precipitates

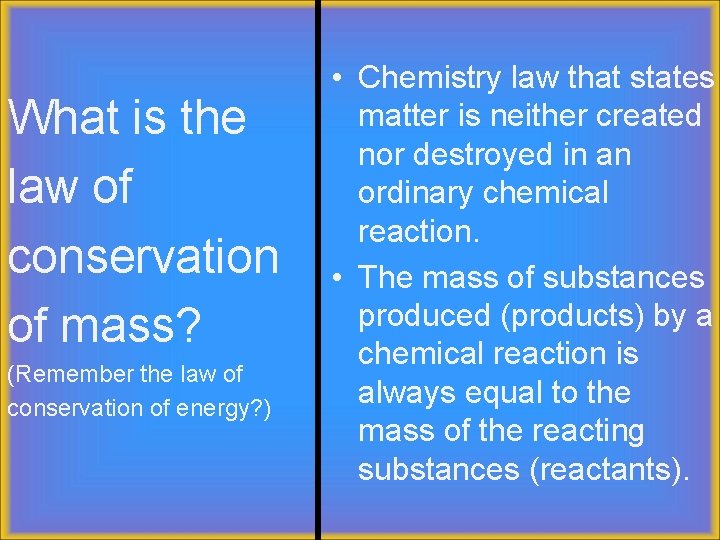
What is the law of conservation of mass? (Remember the law of conservation of energy? ) • Chemistry law that states matter is neither created nor destroyed in an ordinary chemical reaction. • The mass of substances produced (products) by a chemical reaction is always equal to the mass of the reacting substances (reactants).

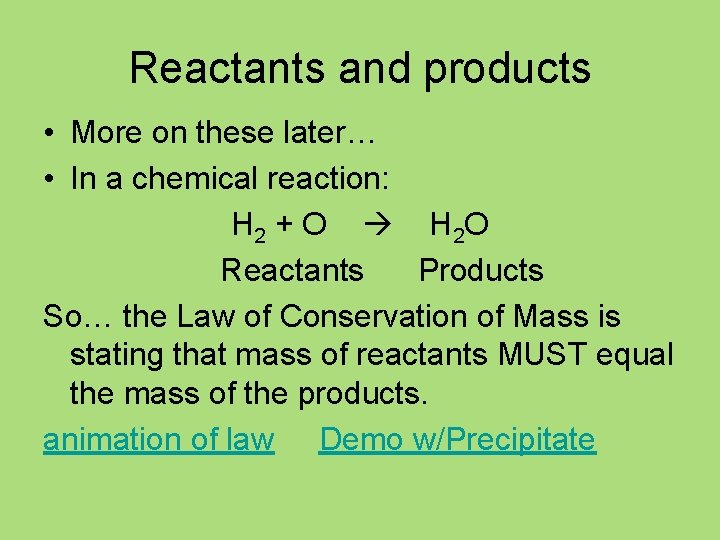
Reactants and products • More on these later… • In a chemical reaction: H 2 + O H 2 O Reactants Products So… the Law of Conservation of Mass is stating that mass of reactants MUST equal the mass of the products. animation of law Demo w/Precipitate

Lab Jobs • Job #1: Lab manager/discussion leader and get final mass of bottle, balloon, vinegar and baking soda. • Job #2: Goggle and Vinegar getter, balloon holder • Job #3: Baking soda getter, baking soda “masser” and pourer (into balloon). • Job # 4: Reader, recorder, and get initial mass of bottle, balloon, vinegar and baking soda.

One surefire way to tell that a chemical reaction has taken place: If the material that comes out of the reaction has totally different Properties than the substances that went into the reaction. This happens because the bonds that created the original substances Have been broken and new bonds have formed in new ways To create something different. New bonds=new properties=new substance

• What are diatomic molecules? Molecules made up of two atoms of the same element. O 2 H 2

Warm-up Describe the law of conservation of matter and how it was proven or disproven in the results of your lab with the balloon.

Objectives for Ch. 2 section 2 • Interpret and write simple chemical formulas. • Write and balance simple chemical equations. • Explain how a balanced equation shows the law of conservation of mass.

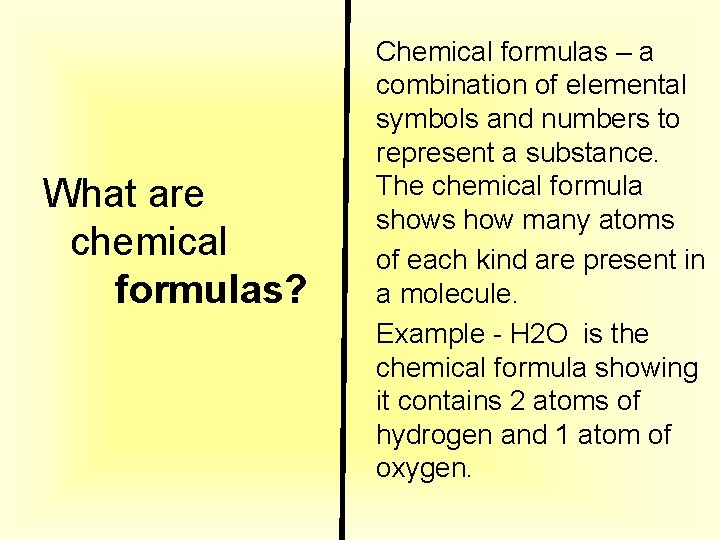
What are chemical formulas? Chemical formulas – a combination of elemental symbols and numbers to represent a substance. The chemical formula shows how many atoms of each kind are present in a molecule. Example - H 2 O is the chemical formula showing it contains 2 atoms of hydrogen and 1 atom of oxygen.

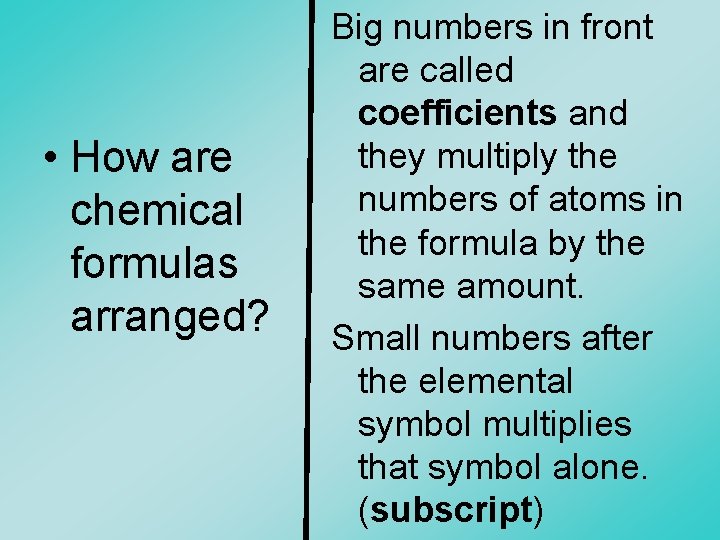
• How are chemical formulas arranged? Big numbers in front are called coefficients and they multiply the numbers of atoms in the formula by the same amount. Small numbers after the elemental symbol multiplies that symbol alone. (subscript)

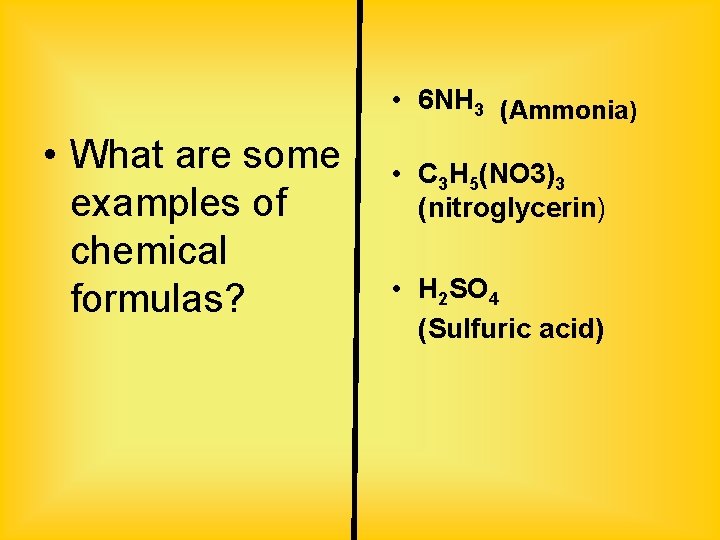
• 6 NH 3 (Ammonia) • What are some examples of chemical formulas? • C 3 H 5(NO 3)3 (nitroglycerin) • H 2 SO 4 (Sulfuric acid)

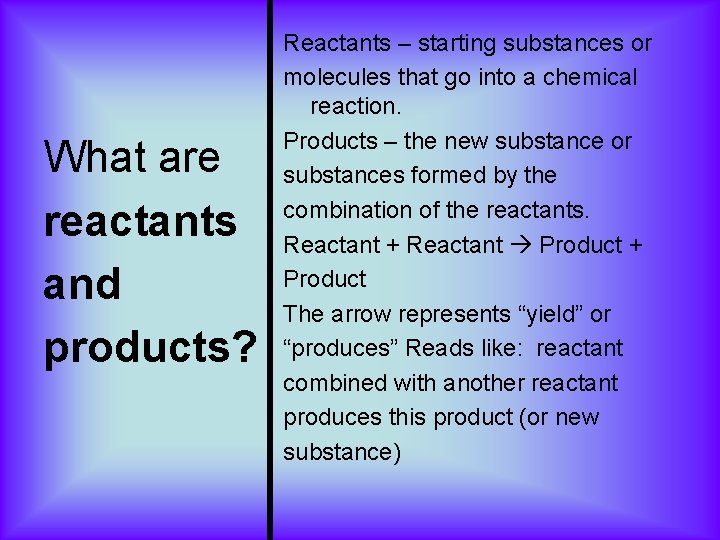
What are reactants and products? Reactants – starting substances or molecules that go into a chemical reaction. Products – the new substance or substances formed by the combination of the reactants. Reactant + Reactant Product + Product The arrow represents “yield” or “produces” Reads like: reactant combined with another reactant produces this product (or new substance)

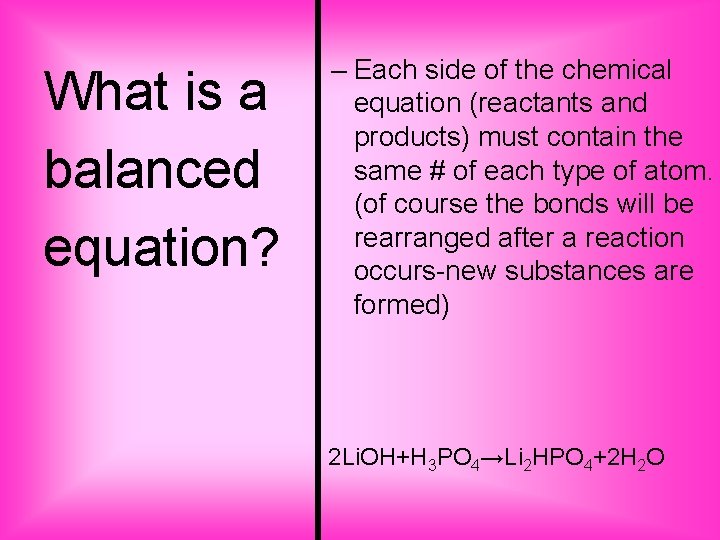
What is a balanced equation? – Each side of the chemical equation (reactants and products) must contain the same # of each type of atom. (of course the bonds will be rearranged after a reaction occurs-new substances are formed) 2 Li. OH+H 3 PO 4→Li 2 HPO 4+2 H 2 O


What are the prefixes used to determine the # of atoms in a molecule/ compound? 1 mono 2 di 3 tri-, tris 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona 10 deca-

Warm-up http: //quizlet. com/test/227 305/

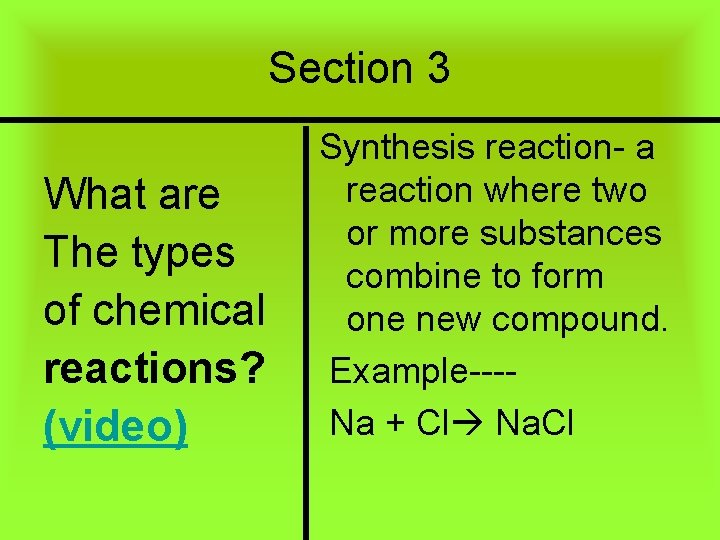
Section 3 What are The types of chemical reactions? (video) Synthesis reaction- a reaction where two or more substances combine to form one new compound. Example---Na + Cl Na. Cl

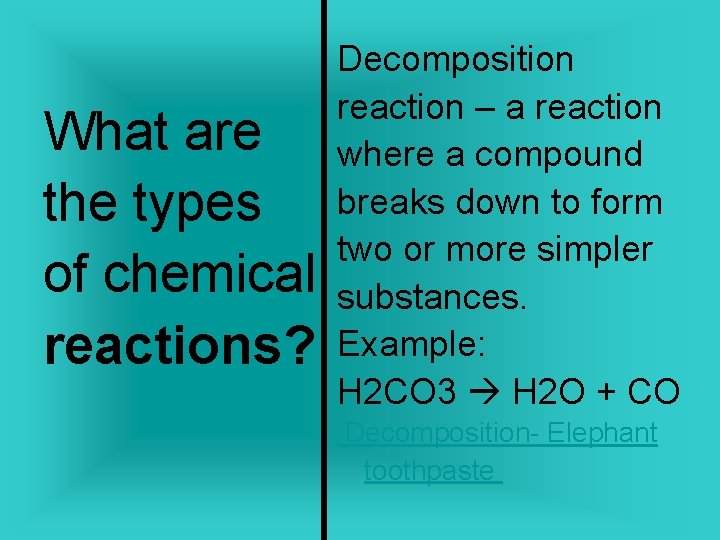
What are the types of chemical reactions? Decomposition reaction – a reaction where a compound breaks down to form two or more simpler substances. Example: H 2 CO 3 H 2 O + CO Decomposition- Elephant toothpaste

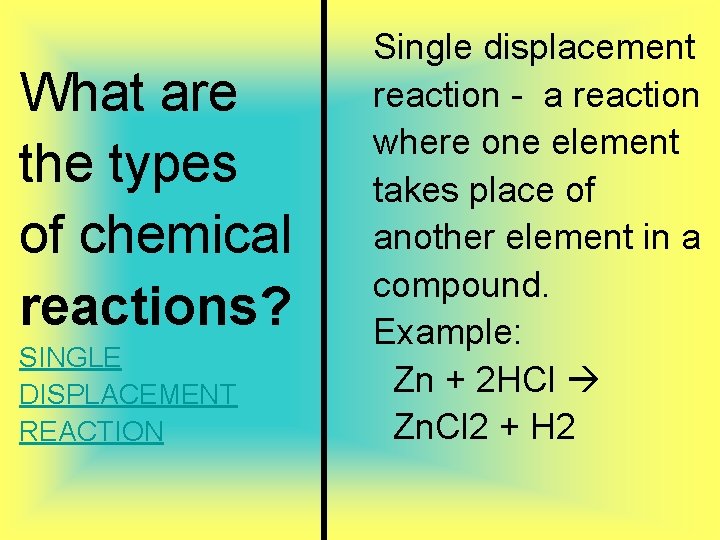
What are the types of chemical reactions? SINGLE DISPLACEMENT REACTION Single displacement reaction - a reaction where one element takes place of another element in a compound. Example: Zn + 2 HCl Zn. Cl 2 + H 2

What are the types of chemical reactions? DOUBLE DISPLACEMENT Double displacement reaction – a reaction where a gas, a solid precipitate, or a molecular compound forms from the exchange of ions between two compounds. Example: Na. Cl + Ag. F Na. F + Ag. Cl

Double Displacement http: //study. com/academy/ lesson/doubledisplacement-reactiondefinition-examples. html

Warm-up • Just for fun you mix up a solution of salt (sodium chloride or Na. Cl) and water and record how many days in the sun it takes to evaporate the water and leave only salt in the container. Is this a chemical reaction? Use the terms chemical/physical properties and precipitate to describe why this is or is not a chemical reaction.

Answer: • This is not a chemical reaction it is a physical change. The physical property observed is a change of state. In a chemical reaction a solid can precipitate out of 2 liquids. We saw this happen in the lab with single displacements. The copper precipitated out of the copper chloride solution when Iron was added to create a reaction. In the warm-up situation water evaporated leaving the sodium chloride.

REVIEW All reactions

What are the two Types of energy transfer in chemical reactions? Endothermic Video Endo #2 Exothermic video Exo #2

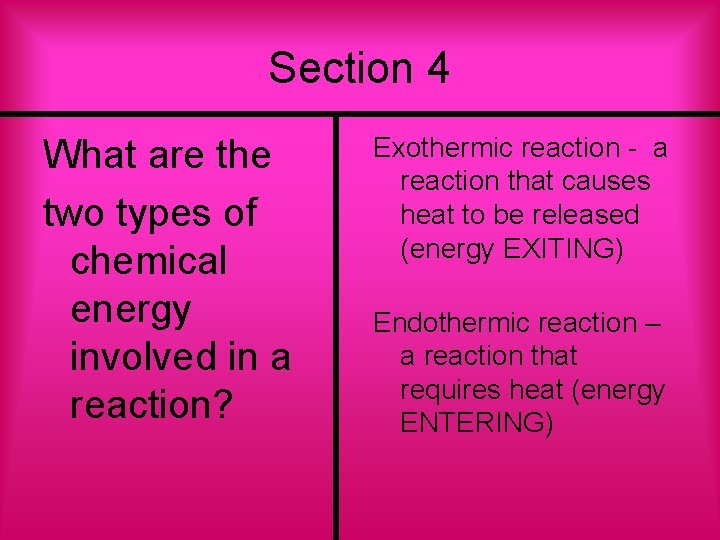
Section 4 What are the two types of chemical energy involved in a reaction? Exothermic reaction - a reaction that causes heat to be released (energy EXITING) Endothermic reaction – a reaction that requires heat (energy ENTERING)
 Cesium fountain atomic clock
Cesium fountain atomic clock Cesium java
Cesium java Iron(iii) chloride (aq) + cesium phosphate (aq)
Iron(iii) chloride (aq) + cesium phosphate (aq) How many protons does cesium have
How many protons does cesium have Cesium viewshed
Cesium viewshed Predict whether cesium forms cs or cs2 ions
Predict whether cesium forms cs or cs2 ions What show do cesium and iodine love to watch
What show do cesium and iodine love to watch Persuasive essay quotes
Persuasive essay quotes Multiplication property of exponents
Multiplication property of exponents Stratified warmup
Stratified warmup Tinman calculator
Tinman calculator Java warmup
Java warmup Warmup ratio
Warmup ratio Surface area warm up
Surface area warm up Warmup 65
Warmup 65 Warmup 65
Warmup 65 Rhyme glow
Rhyme glow Define:warmup
Define:warmup Warmup end
Warmup end Gmass warmup
Gmass warmup Mass of oxygen
Mass of oxygen Relative atomic mass of beryllium
Relative atomic mass of beryllium Difference between atomic number and atomic mass
Difference between atomic number and atomic mass Atomic size trend
Atomic size trend Atomic number vs atomic radius
Atomic number vs atomic radius Ionic radius trends
Ionic radius trends Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu điện thế nghỉ
điện thế nghỉ Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Frameset trong html5
Frameset trong html5 Gấu đi như thế nào
Gấu đi như thế nào Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Bảng số nguyên tố
Bảng số nguyên tố Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Hệ hô hấp
Hệ hô hấp Tư thế ngồi viết
Tư thế ngồi viết đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Tư thế worms-breton
Tư thế worms-breton Bổ thể
Bổ thể ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết Thẻ vin
Thẻ vin Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Diễn thế sinh thái là
Diễn thế sinh thái là Ví dụ về giọng cùng tên
Ví dụ về giọng cùng tên Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Hát lên người ơi alleluia
Hát lên người ơi alleluia Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Hổ sinh sản vào mùa nào
Hổ sinh sản vào mùa nào đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công của trọng lực
Công của trọng lực Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lời thề hippocrates
Lời thề hippocrates