jokes Q What is the show cesium and
















- Slides: 17

jokes • Q: What is the show cesium and iodine love watching together? • A: CSI • Q: Anyone know any jokes about sodium? • A: Na • Q: Did you hear how the date with oxygen and potassium went? • A: It went OK

Ions C. 3 -S. C. 1 -I will be able to find the charge/oxidation number for multiple elements.

What is an Ion? • An Ion is a charged atom. • Every element on the periodic table wants to have a full outer shell.

How can an Atom Become Charged? • THE ONLY WAY AN ATOM CAN BECOME CHARGED IS IF IT GAINS OR LOSES ELECTRONS!! • Remember Protons and Neutron are found tightly packed in the nucleus. • Also if you change the number of protons an atom has you completely change the element itself.

Metals • They are found on the left side of the periodic table therefore they need to lose electrons to become stable • Positive Ions are called cations

Non-Metals • Non-metals are found on the right side of the periodic table therefore they need to gain electrons to become stable. • Negative ions are called anions

Finding the charge • Find the charge for the following Ions Na Cl Al Ne

Ionic Compounds C. 3 -S. C. 3 -I can draw Lewis diagrams to show ionic bonding

Ionic Compounds • Bonds which occur between a metal and a non-metal. Opposites Attract!!!

Drawing ionic compounds • We can use Lewis diagrams to show ionic bonds form. The objective is to balance the number of electrons the metal gives up with the number of electrons the non-metal gains. • Ex. Sodium and chlorine • Ex. Magnesium and nitrogen

Naming and Finding the formula for ionic compounds. C. 2 -S. C. 2 -I will be able to name and find the formula for ionic compounds.

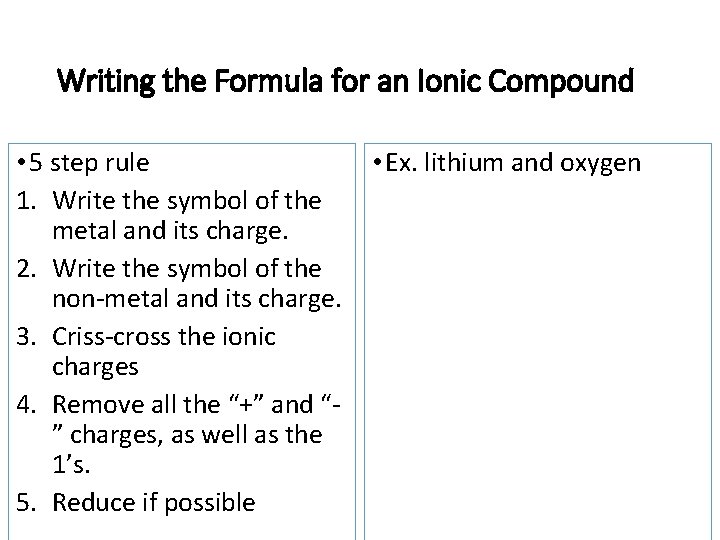
Review: Drawing ionic compounds • lithium and oxygen Formula It is much easier to find the formula for ionic compounds. Lets check it out….

Writing the Formula for an Ionic Compound • 5 step rule 1. Write the symbol of the metal and its charge. 2. Write the symbol of the non-metal and its charge. 3. Criss-cross the ionic charges 4. Remove all the “+” and “” charges, as well as the 1’s. 5. Reduce if possible • Ex. lithium and oxygen

Lets try a couple… • 1. Barium and Fluorine • 2. Aluminum and Phosphorus • 3. Sodium and Arsenic

Naming Ionic Compounds • 3 step rule • Ex. Li. Cl 1. Write the full name of the metal first 2. Write the prefix of the non-metal. 3. Add the ending “ide”

Lets try a couple… • 1. Al 2 O 3 • 2. Ba. S • 3. K 2 O • 4. Ca 2 C

Bonding with classmates…
 What show do cesium and iodine love to watch
What show do cesium and iodine love to watch Cesium viewshed
Cesium viewshed Cesium fountain atomic clock
Cesium fountain atomic clock Iron(iii) chloride (aq) + cesium phosphate (aq)
Iron(iii) chloride (aq) + cesium phosphate (aq) How many protons does cesium have
How many protons does cesium have Predict whether cesium forms cs or cs2 ions
Predict whether cesium forms cs or cs2 ions Cesium java
Cesium java Romeo and juliet knock knock jokes
Romeo and juliet knock knock jokes Romeo and juliet puns
Romeo and juliet puns Romeo and juliet jokes
Romeo and juliet jokes How does paris react to juliet's death
How does paris react to juliet's death Riddles and jokes
Riddles and jokes Are you dirty minded quiz
Are you dirty minded quiz Punz pronouns
Punz pronouns Phrasal verbs jokes
Phrasal verbs jokes Joke indicators
Joke indicators Ego jokes
Ego jokes Black comedy examples
Black comedy examples