Please note these are the actual videorecorded proceedings

















- Slides: 72

Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content.

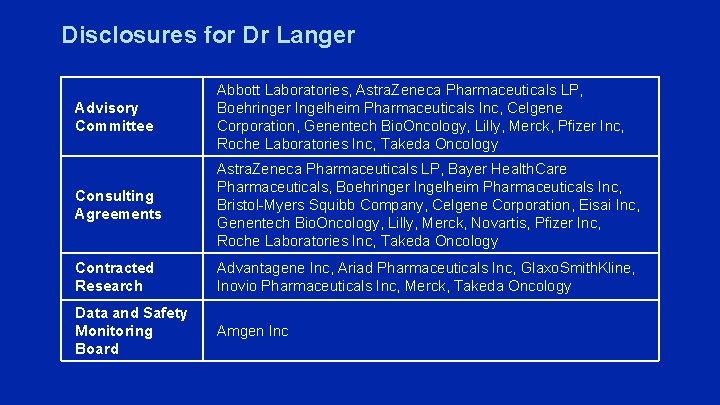
Disclosures for Dr Langer Advisory Committee Abbott Laboratories, Astra. Zeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals Inc, Celgene Corporation, Genentech Bio. Oncology, Lilly, Merck, Pfizer Inc, Roche Laboratories Inc, Takeda Oncology Consulting Agreements Astra. Zeneca Pharmaceuticals LP, Bayer Health. Care Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals Inc, Bristol-Myers Squibb Company, Celgene Corporation, Eisai Inc, Genentech Bio. Oncology, Lilly, Merck, Novartis, Pfizer Inc, Roche Laboratories Inc, Takeda Oncology Contracted Research Advantagene Inc, Ariad Pharmaceuticals Inc, Glaxo. Smith. Kline, Inovio Pharmaceuticals Inc, Merck, Takeda Oncology Data and Safety Monitoring Board Amgen Inc

Disclosures for Dr Hanna Contracted Research Bristol-Myers Squibb Company, Merck

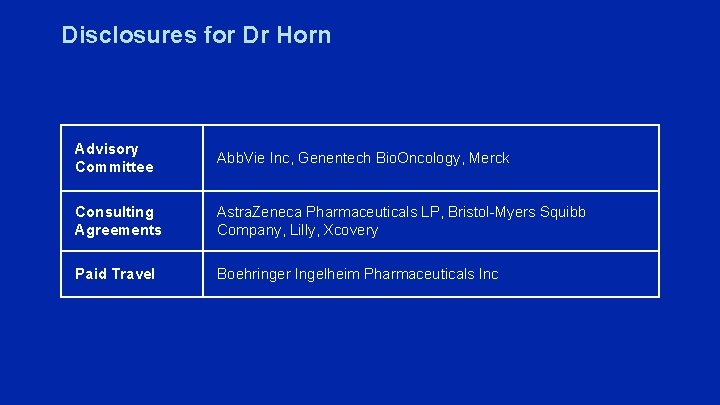
Disclosures for Dr Horn Advisory Committee Abb. Vie Inc, Genentech Bio. Oncology, Merck Consulting Agreements Astra. Zeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Lilly, Xcovery Paid Travel Boehringer Ingelheim Pharmaceuticals Inc

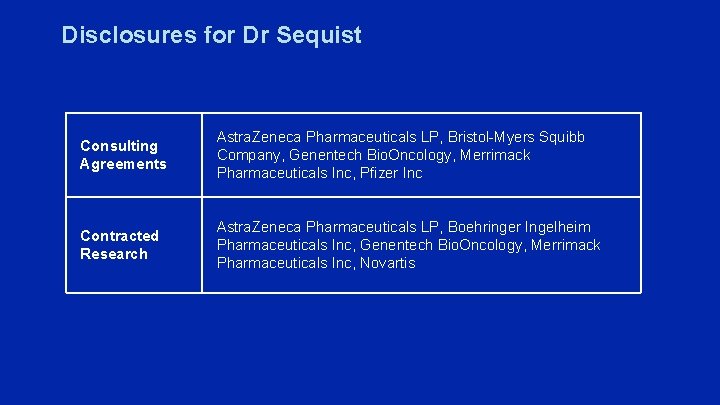
Disclosures for Dr Sequist Consulting Agreements Astra. Zeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Genentech Bio. Oncology, Merrimack Pharmaceuticals Inc, Pfizer Inc Contracted Research Astra. Zeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals Inc, Genentech Bio. Oncology, Merrimack Pharmaceuticals Inc, Novartis

Agenda Introduction – The New Taxonomy of Metastatic Non-Small Cell Lung Cancer (NSCLC) Module 1 – Emerging Research Data with and Potential Clinical Role of Immune Checkpoint Inhibitors in the Management of Locally Advanced NSCLC Module 2 – Integration of Anti-PD-1/PD-L 1 Antibodies into Current Treatment Algorithms for Patients with Metastatic NSCLC Module 3 – Ongoing Evaluation of Existing and Emerging Immunotherapeutic Approaches, Including Combination Strategies Module 4 – Identification and Management of Immune-Mediated and Other Toxicities Associated with Checkpoint Inhibitors; Relative Contraindications for Patients with Existing Autoimmune Disease

In preparation for this meeting, we conducted a survey of 7 lung cancer clinical investigators: Indiana University Vanderbilt University of Pennsylvania Massachusetts General Hospital Cancer Center Yale Cancer Institute Memorial Sloan Kettering Cancer Center Sarah Cannon Research Institute

Agenda Introduction – The New Taxonomy of Metastatic Non-Small Cell Lung Cancer (NSCLC) Module 1 – Emerging Research Data with and Potential Clinical Role of Immune Checkpoint Inhibitors in the Management of Locally Advanced NSCLC Module 2 – Integration of Anti-PD-1/PD-L 1 Antibodies into Current Treatment Algorithms for Patients with Metastatic NSCLC Module 3 – Ongoing Evaluation of Existing and Emerging Immunotherapeutic Approaches, Including Combination Strategies Module 4 – Identification and Management of Immune-Mediated and Other Toxicities Associated with Checkpoint Inhibitors; Relative Contraindications for Patients with Existing Autoimmune Disease

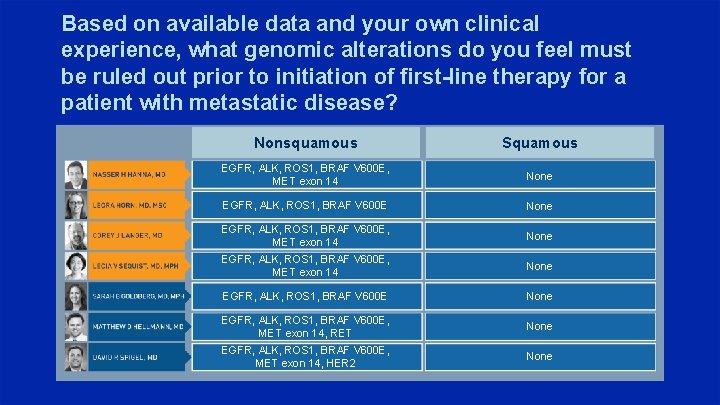
Based on available data and your own clinical experience, what genomic alterations do you feel must be ruled out prior to initiation of first-line therapy for a patient with metastatic disease? Nonsquamous Squamous EGFR, ALK, ROS 1, BRAF V 600 E, MET exon 14 None EGFR, ALK, ROS 1, BRAF V 600 E None EGFR, ALK, ROS 1, BRAF V 600 E, MET exon 14, RET None EGFR, ALK, ROS 1, BRAF V 600 E, MET exon 14, HER 2 None

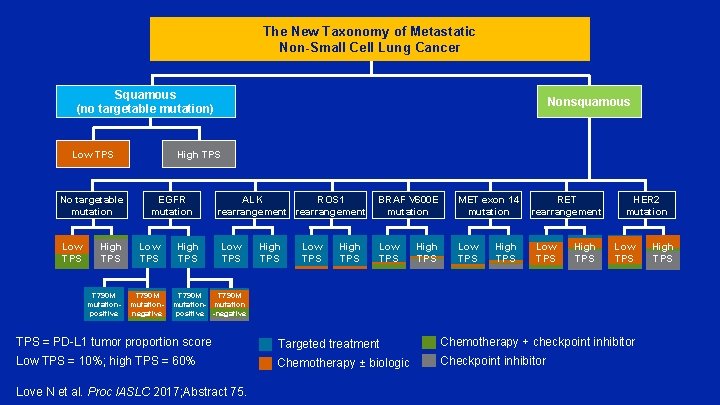
The New Taxonomy of Metastatic Non-Small Cell Lung Cancer Squamous (no targetable mutation) Low TPS No targetable mutation Low TPS High TPS T 790 M mutationpositive Nonsquamous High TPS EGFR mutation Low TPS T 790 M mutationnegative High TPS ALK ROS 1 rearrangement Low TPS High TPS BRAF V 600 E mutation MET exon 14 RET mutation rearrangement Low TPS High TPS HER 2 mutation Low TPS T 790 M mutation- mutation positive -negative TPS = PD-L 1 tumor proportion score Targeted treatment Chemotherapy + checkpoint inhibitor Low TPS = 10%; high TPS = 60% Chemotherapy ± biologic Checkpoint inhibitor Love N et al. Proc IASLC 2017; Abstract 75. High TPS

Osimertinib vs Standard of Care (So. C) EGFR-TKI as First-Line Therapy in Patients (pts) with EGFRm Advanced NSCLC: FLAURA Ramalingam S et al. Proc ESMO 2017; Abstract LBA 2_PR.

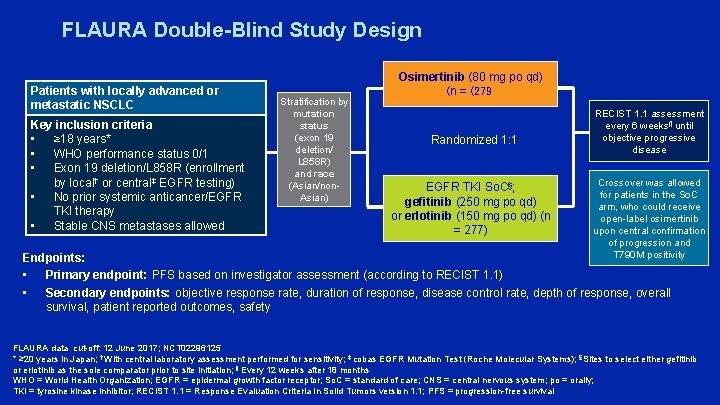
FLAURA Double-Blind Study Design Patients with locally advanced or metastatic NSCLC Key inclusion criteria • ≥ 18 years* • WHO performance status 0/1 • Exon 19 deletion/L 858 R (enrollment by local† or central‡ EGFR testing) • No prior systemic anticancer/EGFR TKI therapy • Stable CNS metastases allowed Stratification by mutation status (exon 19 deletion/ L 858 R) and race (Asian/non. Asian) Osimertinib (80 mg po qd) (n = (279 Randomized 1: 1 EGFR TKI So. C§; gefitinib (250 mg po qd) or erlotinib (150 mg po qd) (n = 277) RECIST 1. 1 assessment every 6 weeks¶ until objective progressive disease Crossover was allowed for patients in the So. C arm, who could receive open-label osimertinib upon central confirmation of progression and T 790 M positivity Endpoints: • Primary endpoint: PFS based on investigator assessment (according to RECIST 1. 1) • Secondary endpoints: objective response rate, duration of response, disease control rate, depth of response, overall survival, patient reported outcomes, safety FLAURA data cut-off: 12 June 2017; NCT 02296125 * ≥ 20 years in Japan; † With central laboratory assessment performed for sensitivity; ‡ cobas EGFR Mutation Test (Roche Molecular Systems); §Sites to select either gefitinib or erlotinib as the sole comparator prior to site initiation; ¶ Every 12 weeks after 18 months WHO = World Health Organization; EGFR = epidermal growth factor receptor; So. C = standard of care; CNS = central nervous system; po = orally; TKI = tyrosine kinase inhibitor; RECIST 1. 1 = Response Evaluation Criteria In Solid Tumors version 1. 1; PFS = progression-free survival

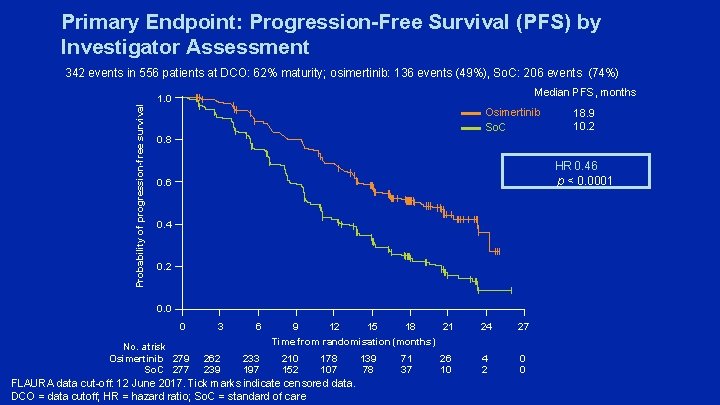
Primary Endpoint: Progression-Free Survival (PFS) by Investigator Assessment Probability of progression-free survival 342 events in 556 patients at DCO: 62% maturity; osimertinib: 136 events (49%), So. C: 206 events (74%) Median PFS, months 1. 0 Osimertinib So. C 18. 9 10. 2 0. 8 HR 0. 46 p < 0. 0001 0. 6 0. 4 0. 2 0. 0 0 No. at risk Osimertinib 279 So. C 277 3 6 262 239 233 197 21 9 12 15 18 Time from randomisation (months) 210 152 178 107 FLAURA data cut-off: 12 June 2017. Tick marks indicate censored data. DCO = data cutoff; HR = hazard ratio; So. C = standard of care 139 78 71 37 26 10 24 27 4 2 0 0

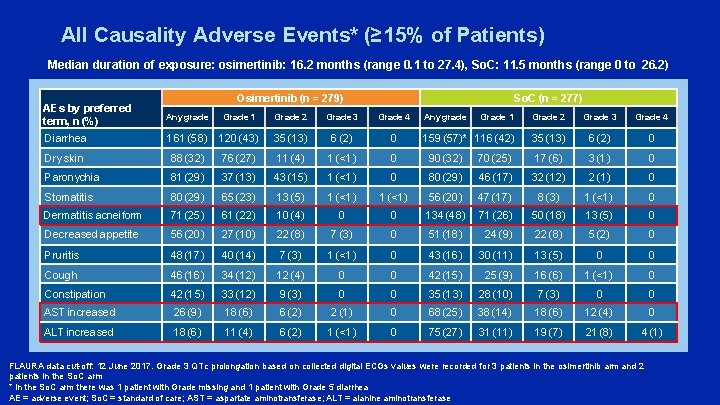
All Causality Adverse Events* (≥ 15% of Patients) Median duration of exposure: osimertinib: 16. 2 months (range 0. 1 to 27. 4), So. C: 11. 5 months (range 0 to 26. 2) Osimertinib (n = 279) So. C (n = 277) AEs by preferred term, n (%) Any grade Grade 1 Grade 2 Grade 3 Grade 4 Diarrhea 161 (58) 120 (43) 35 (13) 6 (2) 0 Dry skin 88 (32) 76 (27) 11 (4) 1 (<1) 0 90 (32) Paronychia 81 (29) 37 (13) 43 (15) 1 (<1) 0 Stomatitis 80 (29) 65 (23) 13 (5) 1 (<1) Dermatitis acneiform 71 (25) 61 (22) 10 (4) Decreased appetite 56 (20) 27 (10) Pruritis 48 (17) Cough Any grade Grade 1 Grade 2 Grade 3 Grade 4 159 (57)* 116 (42) 35 (13) 6 (2) 0 70 (25) 17 (6) 3 (1) 0 80 (29) 46 (17) 32 (12) 2 (1) 0 1 (<1) 56 (20) 47 (17) 8 (3) 1 (<1) 0 0 0 134 (48) 71 (26) 50 (18) 13 (5) 0 22 (8) 7 (3) 0 51 (18) 24 (9) 22 (8) 5 (2) 0 40 (14) 7 (3) 1 (<1) 0 43 (16) 30 (11) 13 (5) 0 0 46 (16) 34 (12) 12 (4) 0 0 42 (15) 25 (9) 16 (6) 1 (<1) 0 Constipation 42 (15) 33 (12) 9 (3) 0 0 35 (13) 28 (10) 7 (3) 0 0 AST increased 26 (9) 18 (6) 6 (2) 2 (1) 0 68 (25) 38 (14) 18 (6) 12 (4) 0 ALT increased 18 (6) 11 (4) 6 (2) 1 (<1) 0 75 (27) 31 (11) 19 (7) 21 (8) 4 (1) FLAURA data cut-off: 12 June 2017. Grade 3 QTc prolongation based on collected digital ECGs values were recorded for 3 patients in the osimertinib arm and 2 patients in the So. C arm * In the So. C arm there was 1 patient with Grade missing and 1 patient with Grade 5 diarrhea AE = adverse event; So. C = standard of care; AST = aspartate aminotransferase; ALT = alanine aminotransferase

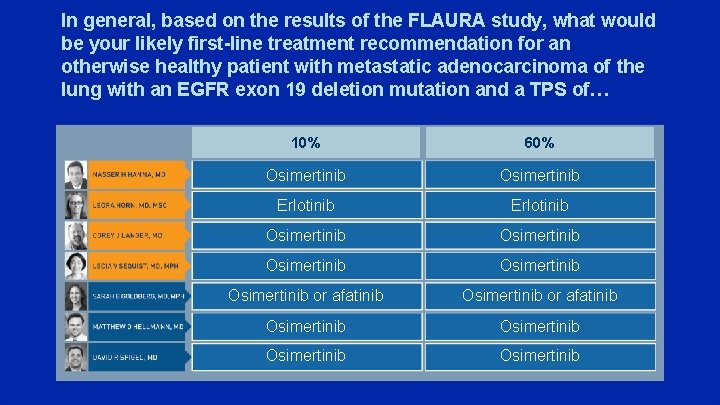
In general, based on the results of the FLAURA study, what would be your likely first-line treatment recommendation for an otherwise healthy patient with metastatic adenocarcinoma of the lung with an EGFR exon 19 deletion mutation and a TPS of… 10% 60% Osimertinib Erlotinib Osimertinib Osimertinib or afatinib Osimertinib

Agenda Introduction – The New Taxonomy of Metastatic Non-Small Cell Lung Cancer (NSCLC) Module 1 – Emerging Research Data with and Potential Clinical Role of Immune Checkpoint Inhibitors in the Management of Locally Advanced NSCLC Module 2 – Integration of Anti-PD-1/PD-L 1 Antibodies into Current Treatment Algorithms for Patients with Metastatic NSCLC Module 3 – Ongoing Evaluation of Existing and Emerging Immunotherapeutic Approaches, Including Combination Strategies Module 4 – Identification and Management of Immune-Mediated and Other Toxicities Associated with Checkpoint Inhibitors; Relative Contraindications for Patients with Existing Autoimmune Disease

PACIFIC: A Double-Blind, Placebo-Controlled Phase III Study of Durvalumab after Chemoradiation Therapy (CRT) in Patients with Stage III, Locally Advanced, Unresectable NSCLC Paz-Ares L et al. Proc ESMO 2017; Abstract LBA 1.

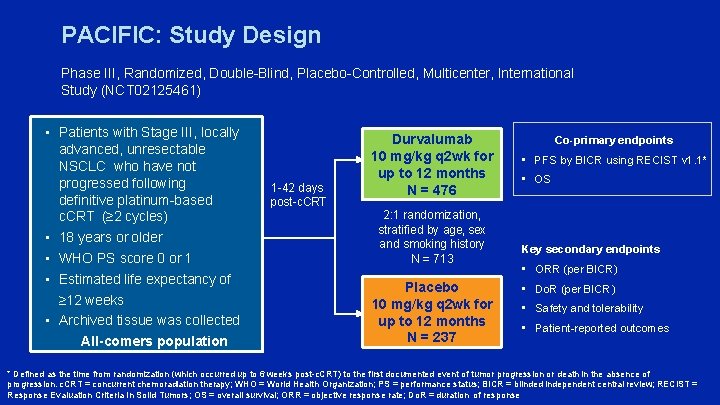
PACIFIC: Study Design Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicenter, International Study (NCT 02125461) • Patients with Stage III, locally advanced, unresectable NSCLC who have not progressed following definitive platinum-based c. CRT (≥ 2 cycles) • 18 years or older • WHO PS score 0 or 1 • Estimated life expectancy of ≥ 12 weeks • Archived tissue was collected All-comers population 1 -42 days post-c. CRT Durvalumab 10 mg/kg q 2 wk for up to 12 months N = 476 2: 1 randomization, stratified by age, sex and smoking history N = 713 Placebo 10 mg/kg q 2 wk for up to 12 months N = 237 Co-primary endpoints • PFS by BICR using RECIST v 1. 1* • OS Key secondary endpoints • ORR (per BICR) • Do. R (per BICR) • Safety and tolerability • Patient-reported outcomes * Defined as the time from randomization (which occurred up to 6 weeks post-c. CRT) to the first documented event of tumor progression or death in the absence of progression. c. CRT = concurrent chemoradiation therapy; WHO = World Health Organization; PS = performance status; BICR = blinded independent central review; RECIST = Response Evaluation Criteria In Solid Tumors; OS = overall survival; ORR = objective response rate; Do. R = duration of response

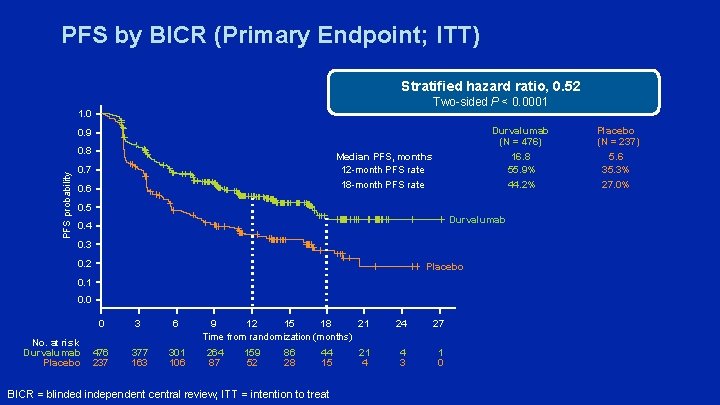
PFS by BICR (Primary Endpoint; ITT) Stratified hazard ratio, 0. 52 Two-sided P < 0. 0001 1. 0 Durvalumab (N = 476) 16. 8 55. 9% 44. 2% 0. 9 PFS probability 0. 8 Median PFS, months 12 -month PFS rate 18 -month PFS rate 0. 7 0. 6 0. 5 Durvalumab 0. 4 0. 3 0. 2 Placebo 0. 1 0. 0 No. at risk Durvalumab Placebo 0 3 6 476 237 377 163 301 106 21 9 12 15 18 Time from randomization (months) 264 87 159 52 86 28 44 15 BICR = blinded independent central review; ITT = intention to treat 21 4 24 27 4 3 1 0 Placebo (N = 237) 5. 6 35. 3% 27. 0%

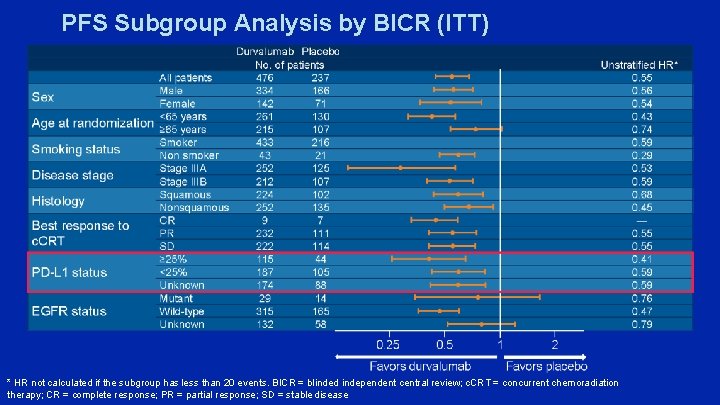
PFS Subgroup Analysis by BICR (ITT) * HR not calculated if the subgroup has less than 20 events. BICR = blinded independent central review; c. CRT = concurrent chemoradiation therapy; CR = complete response; PR = partial response; SD = stable disease

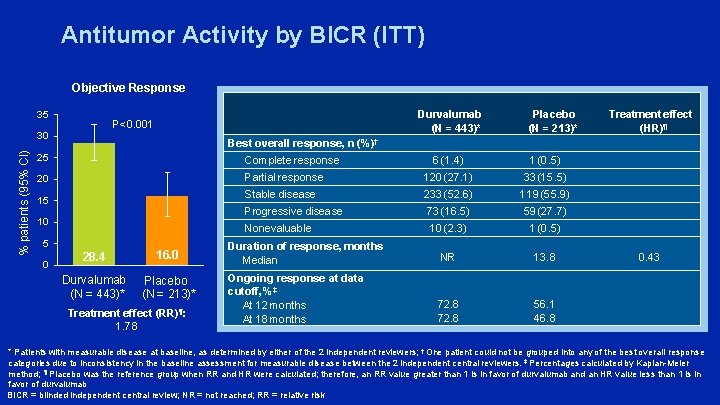
Antitumor Activity by BICR (ITT) Objective Response 35 30 % patients (95% CI) Durvalumab (N = 443)* P<0. 001 Complete response 15 10 5 0 Treatment effect (HR)¶ Best overall response, n (%)† 25 20 Placebo (N = 213)* 28. 4 16. 0 Durvalumab Placebo (N = 443)* (N = 213)* Treatment effect (RR)¶: 1. 78 6 (1. 4) 1 (0. 5) Partial response 120 (27. 1) 33 (15. 5) Stable disease 233 (52. 6) 119 (55. 9) Progressive disease 73 (16. 5) 59 (27. 7) Nonevaluable 10 (2. 3) 1 (0. 5) Duration of response, months Median NR 13. 8 Ongoing response at data cutoff, %‡ At 12 months At 18 months 72. 8 56. 1 46. 8 0. 43 * Patients with measurable disease at baseline, as determined by either of the 2 independent reviewers; † One patient could not be grouped into any of the best overall response categories due to inconsistency in the baseline assessment for measurable disease between the 2 independent central reviewers. ‡ Percentages calculated by Kaplan-Meier method; ¶ Placebo was the reference group when RR and HR were calculated; therefore, an RR value greater than 1 is in favor of durvalumab and an HR value less than 1 is in favor of durvalumab BICR = blinded independent central review; NR = not reached; RR = relative risk

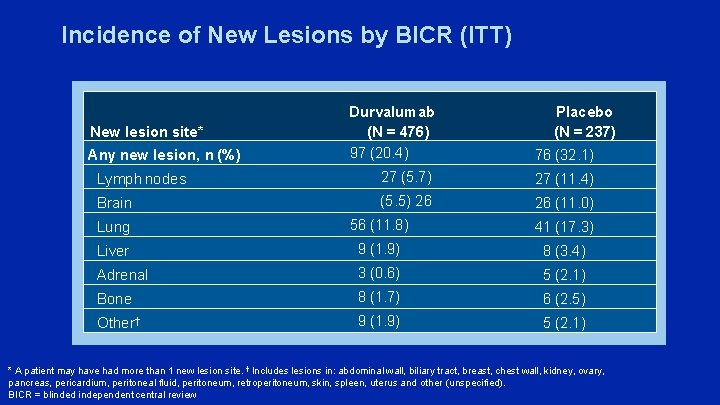
Incidence of New Lesions by BICR (ITT) Durvalumab (N = 476) 97 (20. 4) 76 (32. 1) Lymph nodes 27 (5. 7) 27 (11. 4) Brain (5. 5) 26 26 (11. 0) Lung 56 (11. 8) 41 (17. 3) Liver 9 (1. 9) 8 (3. 4) Adrenal 3 (0. 6) 5 (2. 1) Bone 8 (1. 7) 6 (2. 5) Other† 9 (1. 9) 5 (2. 1) New lesion site* Any new lesion, n (%) Placebo (N = 237) * A patient may have had more than 1 new lesion site. † Includes lesions in: abdominal wall, biliary tract, breast, chest wall, kidney, ovary, pancreas, pericardium, peritoneal fluid, peritoneum, retroperitoneum, skin, spleen, uterus and other (unspecified). BICR = blinded independent central review

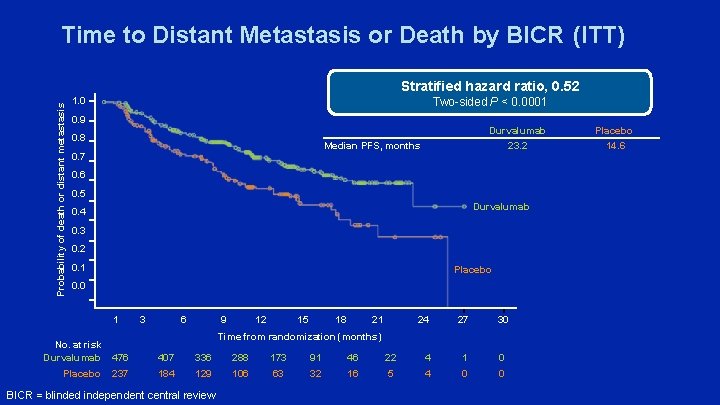
Time to Distant Metastasis or Death by BICR (ITT) Probability of death or distant metastasis Stratified hazard ratio, 0. 52 Two-sided P < 0. 0001 1. 0 0. 9 0. 8 Durvalumab 23. 2 Median PFS, months 0. 7 0. 6 0. 5 Durvalumab 0. 4 0. 3 0. 2 0. 1 Placebo 0. 0 1 3 6 9 12 15 18 21 24 27 30 Time from randomization (months) No. at risk Durvalumab 476 407 336 288 173 91 46 22 4 1 0 Placebo 237 184 129 106 63 32 16 5 4 0 0 BICR = blinded independent central review Placebo 14. 6

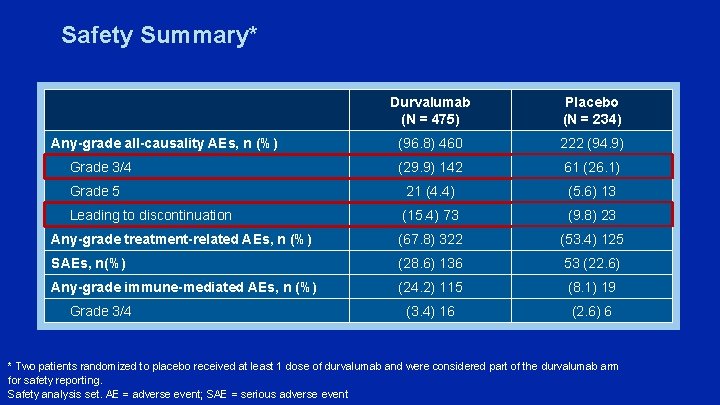
Safety Summary* Durvalumab (N = 475) Placebo (N = 234) (96. 8) 460 222 (94. 9) (29. 9) 142 61 (26. 1) Grade 5 21 (4. 4) (5. 6) 13 Leading to discontinuation (15. 4) 73 (9. 8) 23 Any-grade treatment-related AEs, n (%) (67. 8) 322 (53. 4) 125 SAEs, n(%) (28. 6) 136 53 (22. 6) Any-grade immune-mediated AEs, n (%) (24. 2) 115 (8. 1) 19 (3. 4) 16 (2. 6) 6 Any-grade all-causality AEs, n (%) Grade 3/4 * Two patients randomized to placebo received at least 1 dose of durvalumab and were considered part of the durvalumab arm for safety reporting. Safety analysis set. AE = adverse event; SAE = serious adverse event

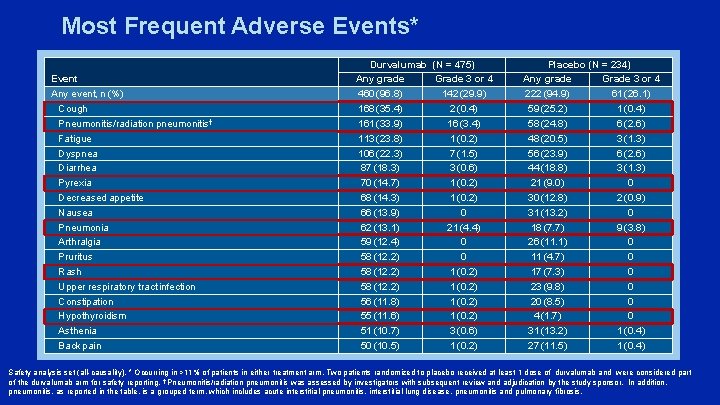
Most Frequent Adverse Events* Event Any event, n (%) Cough Pneumonitis/radiation pneumonitis† Fatigue Dyspnea Diarrhea Pyrexia Decreased appetite Nausea Pneumonia Arthralgia Pruritus Rash Upper respiratory tract infection Constipation Hypothyroidism Asthenia Back pain Durvalumab (N = 475) Any grade Grade 3 or 4 460 (96. 8) 142 (29. 9) 168 (35. 4) 2 (0. 4) 161 (33. 9) 16 (3. 4) 113 (23. 8) 1 (0. 2) 106 (22. 3) 7 (1. 5) 87 (18. 3) 3 (0. 6) 70 (14. 7) 1 (0. 2) 68 (14. 3) 1 (0. 2) 66 (13. 9) 0 62 (13. 1) 21 (4. 4) 59 (12. 4) 0 58 (12. 2) 1 (0. 2) 56 (11. 8) 1 (0. 2) 55 (11. 6) 1 (0. 2) 51 (10. 7) 3 (0. 6) 50 (10. 5) 1 (0. 2) Placebo (N = 234) Any grade Grade 3 or 4 222 (94. 9) 61 (26. 1) 59 (25. 2) 1 (0. 4) 58 (24. 8) 6 (2. 6) 48 (20. 5) 3 (1. 3) 56 (23. 9) 6 (2. 6) 44 (18. 8) 3 (1. 3) 21 (9. 0) 0 30 (12. 8) 2 (0. 9) 31 (13. 2) 0 18 (7. 7) 9 (3. 8) 26 (11. 1) 0 11 (4. 7) 0 17 (7. 3) 0 23 (9. 8) 0 20 (8. 5) 0 4 (1. 7) 0 31 (13. 2) 1 (0. 4) 27 (11. 5) 1 (0. 4) Safety analysis set (all-causality). * Occurring in >11% of patients in either treatment arm. Two patients randomized to placebo received at least 1 dose of durvalumab and were considered part of the durvalumab arm for safety reporting. † Pneumonitis/radiation pneumonitis was assessed by investigators with subsequent review and adjudication by the study sponsor. In addition, pneumonitis, as reported in the table, is a grouped term, which includes acute interstitial pneumonitis, interstitial lung disease, pneumonitis and pulmonary fibrosis.

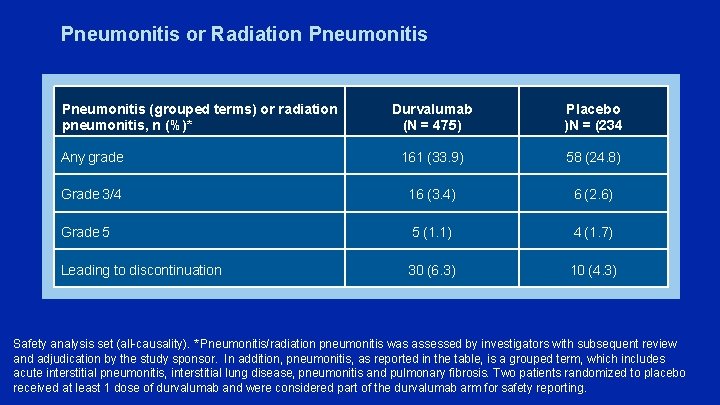
Pneumonitis or Radiation Pneumonitis (grouped terms) or radiation pneumonitis, n (%)* Durvalumab (N = 475) Placebo )N = (234 Any grade 161 (33. 9) 58 (24. 8) Grade 3/4 16 (3. 4) 6 (2. 6) Grade 5 5 (1. 1) 4 (1. 7) Leading to discontinuation 30 (6. 3) 10 (4. 3) Safety analysis set (all-causality). * Pneumonitis/radiation pneumonitis was assessed by investigators with subsequent review and adjudication by the study sponsor. In addition, pneumonitis, as reported in the table, is a grouped term, which includes acute interstitial pneumonitis, interstitial lung disease, pneumonitis and pulmonary fibrosis. Two patients randomized to placebo received at least 1 dose of durvalumab and were considered part of the durvalumab arm for safety reporting.

Summary • Durvalumab demonstrated a statistically significant and robust improvement in PFS versus placebo (HR 0. 52; P < 0. 0001; median improvement of >11 months) at a planned interim analysis. • PFS improvement with durvalumab was observed across all prespecified subgroups. • Durvalumab demonstrated a clinically meaningful benefit in ORR (28. 4% vs 16. 0%; p < 0. 001), with durable responses versus placebo (median Do. R not reached vs 13. 8 months). • Patients receiving durvalumab had a lower incidence of new lesions, including new brain metastases, compared to patients receiving placebo. • The safety profile of durvalumab was consistent with that of other immunotherapies and with its known safety profile as monotherapy in patients with more advanced disease; 1 no new safety signals were identified. • The study remains blinded to OS. 1. Antonia SJ et al. Poster presented at the 41 st European Society for Medical Oncology Annual Meeting, Copenhagen, October 7 -11, 2016. ORR = overall response rate; Do. R = duration of response

Conclusion • Durvalumab is a promising new therapeutic option in patients with Stage III, locally advanced, unresectable NSCLC who have completed c. CRT = concurrent chemoradiation therapy

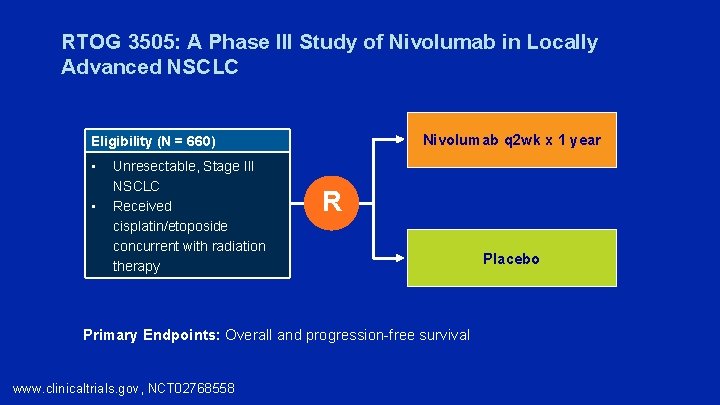
RTOG 3505: A Phase III Study of Nivolumab in Locally Advanced NSCLC Nivolumab q 2 wk x 1 year Eligibility (N = 660) • • Unresectable, Stage III NSCLC Received cisplatin/etoposide concurrent with radiation therapy R Primary Endpoints: Overall and progression-free survival www. clinicaltrials. gov, NCT 02768558 Placebo

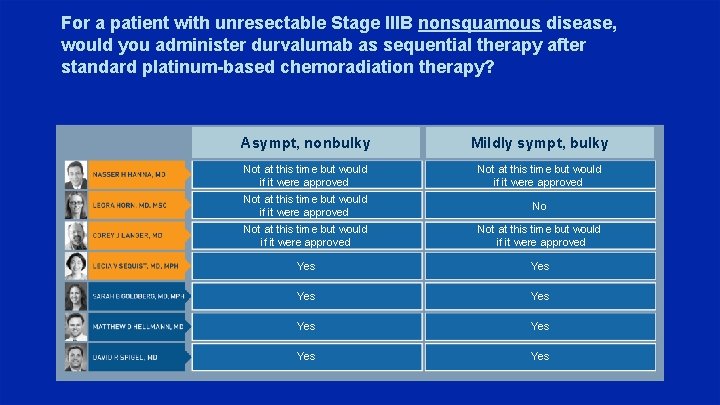
For a patient with unresectable Stage IIIB nonsquamous disease, would you administer durvalumab as sequential therapy after standard platinum-based chemoradiation therapy? Asympt, nonbulky Mildly sympt, bulky Not at this time but would if it were approved No Not at this time but would if it were approved Yes Yes

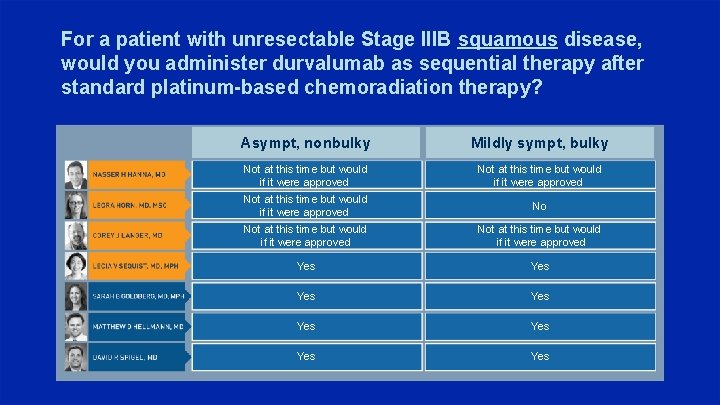
For a patient with unresectable Stage IIIB squamous disease, would you administer durvalumab as sequential therapy after standard platinum-based chemoradiation therapy? Asympt, nonbulky Mildly sympt, bulky Not at this time but would if it were approved No Not at this time but would if it were approved Yes Yes

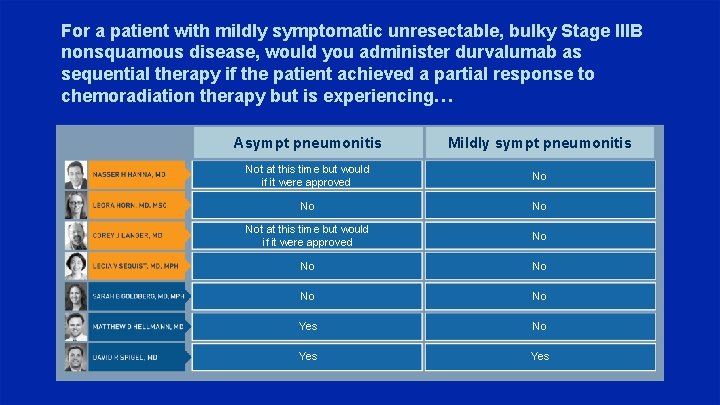
For a patient with mildly symptomatic unresectable, bulky Stage IIIB nonsquamous disease, would you administer durvalumab as sequential therapy if the patient achieved a partial response to chemoradiation therapy but is experiencing… Asympt pneumonitis Mildly sympt pneumonitis Not at this time but would if it were approved No Not at this time but would if it were approved No No No Yes Yes

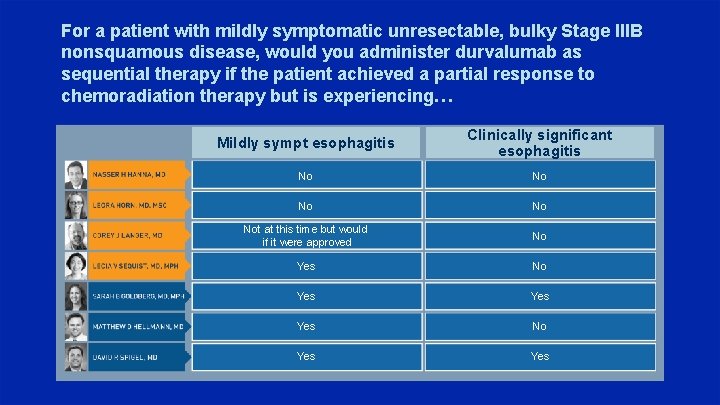
For a patient with mildly symptomatic unresectable, bulky Stage IIIB nonsquamous disease, would you administer durvalumab as sequential therapy if the patient achieved a partial response to chemoradiation therapy but is experiencing… Mildly sympt esophagitis Clinically significant esophagitis No No Not at this time but would if it were approved No Yes Yes No Yes

Agenda Introduction – The New Taxonomy of Metastatic Non-Small Cell Lung Cancer (NSCLC) Module 1 – Emerging Research Data with and Potential Clinical Role of Immune Checkpoint Inhibitors in the Management of Locally Advanced NSCLC Module 2 – Integration of Anti-PD-1/PD-L 1 Antibodies into Current Treatment Algorithms for Patients with Metastatic NSCLC Module 3 – Ongoing Evaluation of Existing and Emerging Immunotherapeutic Approaches, Including Combination Strategies Module 4 – Identification and Management of Immune-Mediated and Other Toxicities Associated with Checkpoint Inhibitors; Relative Contraindications for Patients with Existing Autoimmune Disease

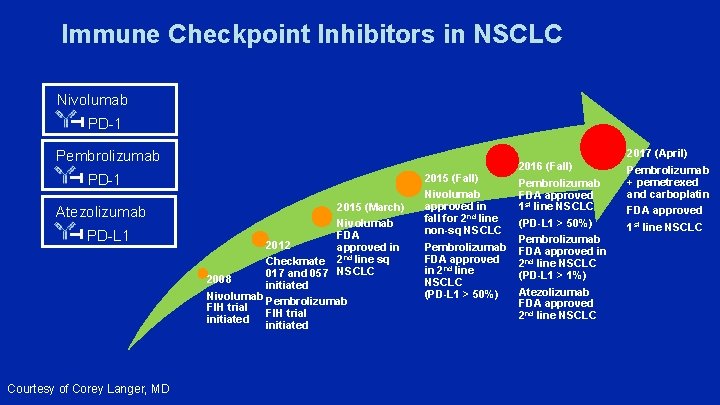
Immune Checkpoint Inhibitors in NSCLC Nivolumab PD-1 Pembrolizumab PD-1 Atezolizumab PD-L 1 Courtesy of Corey Langer, MD 2015 (March) Nivolumab FDA 2012 approved in Checkmate 2 nd line sq 017 and 057 NSCLC 2008 initiated Nivolumab Pembrolizumab FIH trial initiated 2015 (Fall) Nivolumab approved in fall for 2 nd line non-sq NSCLC Pembrolizumab FDA approved in 2 nd line NSCLC (PD-L 1 > 50%) 2016 (Fall) Pembrolizumab FDA approved 1 st line NSCLC (PD-L 1 > 50%) Pembrolizumab FDA approved in 2 nd line NSCLC (PD-L 1 > 1%) Atezolizumab FDA approved 2 nd line NSCLC 2017 (April) Pembrolizumab + pemetrexed and carboplatin FDA approved 1 st line NSCLC

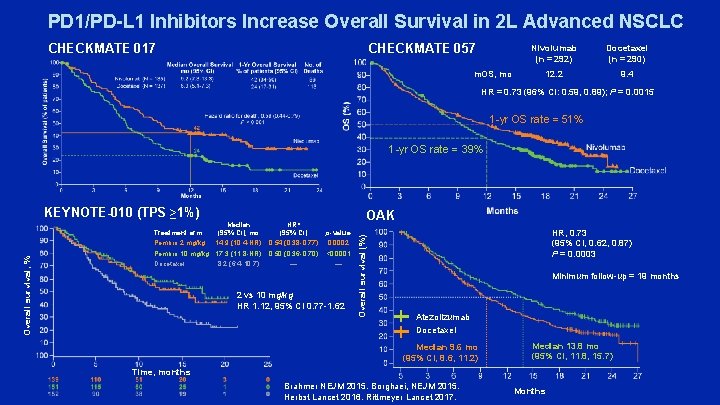
PD 1/PD-L 1 Inhibitors Increase Overall Survival in 2 L Advanced NSCLC CHECKMATE 057 CHECKMATE 017 m. OS, mo Nivolumab (n = 292) Docetaxel (n = 290) 12. 2 9. 4 HR = 0. 73 (96% CI: 0. 59, 0. 89); P = 0. 0015 1 -yr OS rate = 51% 1 -yr OS rate = 39% KEYNOTE-010 (TPS ≥ 1%) Median (95% CI), mo 14. 9 (10. 4 -NR) Pembro 10 mg/kg 17. 3 (11. 8 -NR) Docetaxel 8. 2 (6. 4 -10. 7) HR* (95% CI) 0. 54 (0. 38 -0. 77) OAK p-value 0. 0002 0. 50 (0. 36 -0. 70) <0. 0001 — — 2 vs 10 mg/kg HR 1. 12, 95% CI 0. 77 -1. 62 Overall survival (%) Overall survival, % Treatment arm Pembro 2 mg/kg HR, 0. 73 (95% CI, 0. 62, 0. 87) P = 0. 0003 Minimum follow-up = 19 months Atezolizumab Docetaxel Median 9. 6 mo (95% CI, 8. 6, 11. 2) Median 13. 8 mo (95% CI, 11. 8, 15. 7) Time, months Brahmer NEJM 2015. Borghaei, NEJM 2015. Herbst Lancet 2016. Rittmeyer Lancet 2017. Months

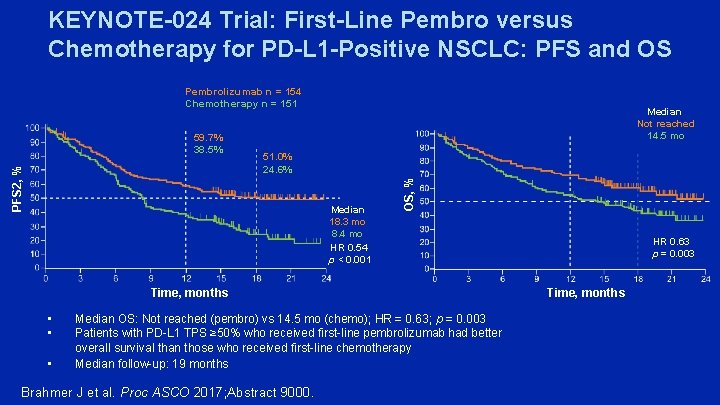
KEYNOTE-024 Trial: First-Line Pembro versus Chemotherapy for PD-L 1 -Positive NSCLC: PFS and OS Pembrolizumab n = 154 Chemotherapy n = 151 51. 0% 24. 6% Median 18. 3 mo 8. 4 mo HR 0. 54 p < 0. 001 OS, % PFS 2, % 59. 7% 38. 5% Median Not reached 14. 5 mo Time, months • • • Median OS: Not reached (pembro) vs 14. 5 mo (chemo); HR = 0. 63; p = 0. 003 Patients with PD-L 1 TPS ≥ 50% who received first-line pembrolizumab had better overall survival than those who received first-line chemotherapy Median follow-up: 19 months Brahmer J et al. Proc ASCO 2017; Abstract 9000. HR 0. 63 p = 0. 003 Time, months

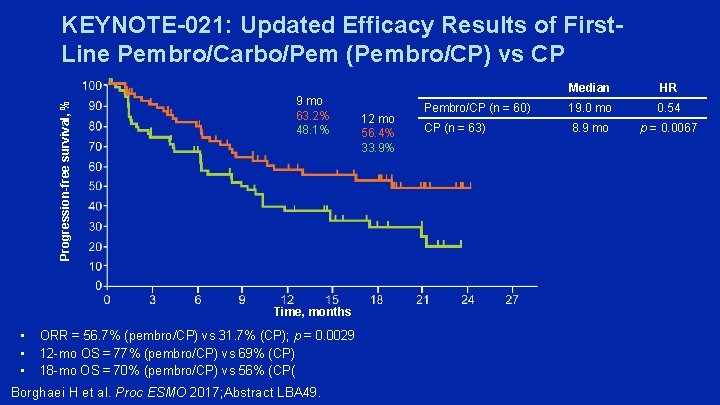
Progression-free survival, % KEYNOTE-021: Updated Efficacy Results of First. Line Pembro/Carbo/Pem (Pembro/CP) vs CP 9 mo 63. 2% 48. 1% Time, months • • • ORR = 56. 7% (pembro/CP) vs 31. 7% (CP); p = 0. 0029 12 -mo OS = 77% (pembro/CP) vs 69% (CP) 18 -mo OS = 70% (pembro/CP) vs 56% (CP( Borghaei H et al. Proc ESMO 2017; Abstract LBA 49. 12 mo 56. 4% 33. 9% Median HR Pembro/CP (n = 60) 19. 0 mo 0. 54 CP (n = 63) 8. 9 mo p = 0. 0067

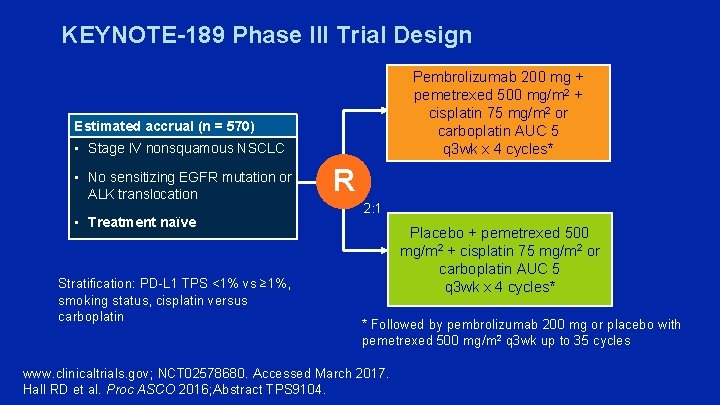
KEYNOTE-189 Phase III Trial Design Pembrolizumab 200 mg + pemetrexed 500 mg/m 2 + cisplatin 75 mg/m 2 or carboplatin AUC 5 q 3 wk x 4 cycles* Estimated accrual (n = 570) • Stage IV nonsquamous NSCLC • No sensitizing EGFR mutation or ALK translocation • Treatment naïve Stratification: PD-L 1 TPS <1% vs ≥ 1%, smoking status, cisplatin versus carboplatin R 2: 1 Placebo + pemetrexed 500 mg/m 2 + cisplatin 75 mg/m 2 or carboplatin AUC 5 q 3 wk x 4 cycles* * Followed by pembrolizumab 200 mg or placebo with pemetrexed 500 mg/m 2 q 3 wk up to 35 cycles www. clinicaltrials. gov; NCT 02578680. Accessed March 2017. Hall RD et al. Proc ASCO 2016; Abstract TPS 9104.

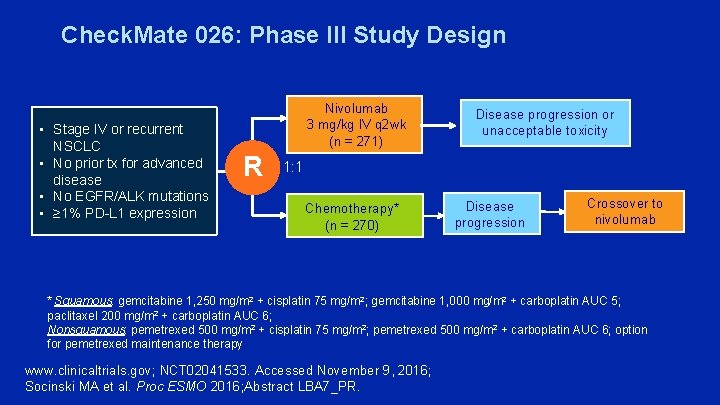
Check. Mate 026: Phase III Study Design • Stage IV or recurrent NSCLC • No prior tx for advanced disease • No EGFR/ALK mutations • ≥ 1% PD-L 1 expression Nivolumab 3 mg/kg IV q 2 wk (n = 271) R Disease progression or unacceptable toxicity 1: 1 Chemotherapy* (n = 270) Disease progression Crossover to nivolumab * Squamous: gemcitabine 1, 250 mg/m 2 + cisplatin 75 mg/m 2; gemcitabine 1, 000 mg/m 2 + carboplatin AUC 5; paclitaxel 200 mg/m 2 + carboplatin AUC 6; Nonsquamous: pemetrexed 500 mg/m 2 + cisplatin 75 mg/m 2; pemetrexed 500 mg/m 2 + carboplatin AUC 6; option for pemetrexed maintenance therapy www. clinicaltrials. gov; NCT 02041533. Accessed November 9, 2016; Socinski MA et al. Proc ESMO 2016; Abstract LBA 7_PR.

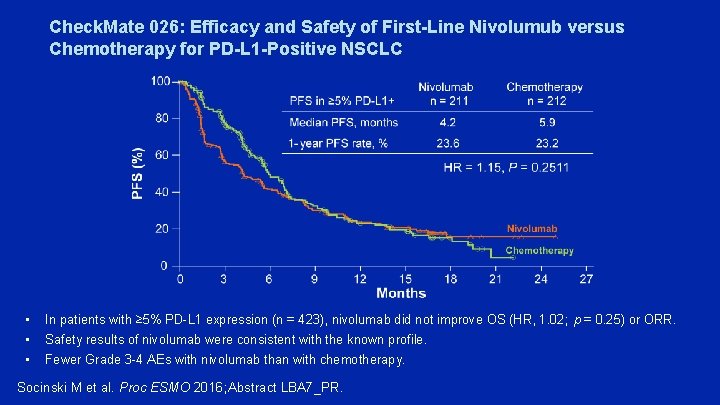
Check. Mate 026: Efficacy and Safety of First-Line Nivolumub versus Chemotherapy for PD-L 1 -Positive NSCLC • • • In patients with ≥ 5% PD-L 1 expression (n = 423), nivolumab did not improve OS (HR, 1. 02; p = 0. 25) or ORR. Safety results of nivolumab were consistent with the known profile. Fewer Grade 3 -4 AEs with nivolumab than with chemotherapy. Socinski M et al. Proc ESMO 2016; Abstract LBA 7_PR.

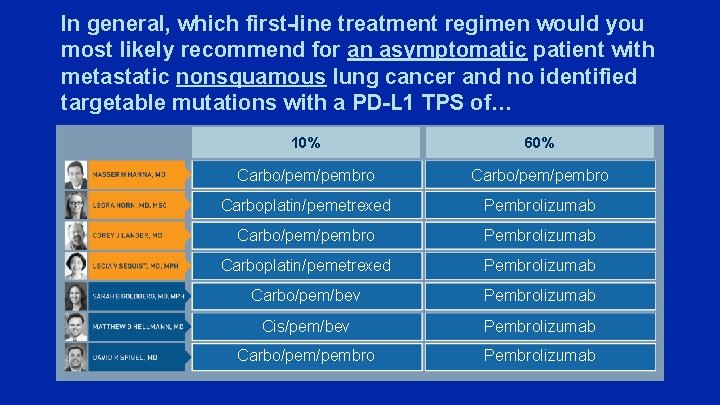
In general, which first-line treatment regimen would you most likely recommend for an asymptomatic patient with metastatic nonsquamous lung cancer and no identified targetable mutations with a PD-L 1 TPS of… 10% 60% Carbo/pem/pembro Carboplatin/pemetrexed Pembrolizumab Carbo/pembro Pembrolizumab Carboplatin/pemetrexed Pembrolizumab Carbo/pem/bev Pembrolizumab Cis/pem/bev Pembrolizumab Carbo/pembro Pembrolizumab

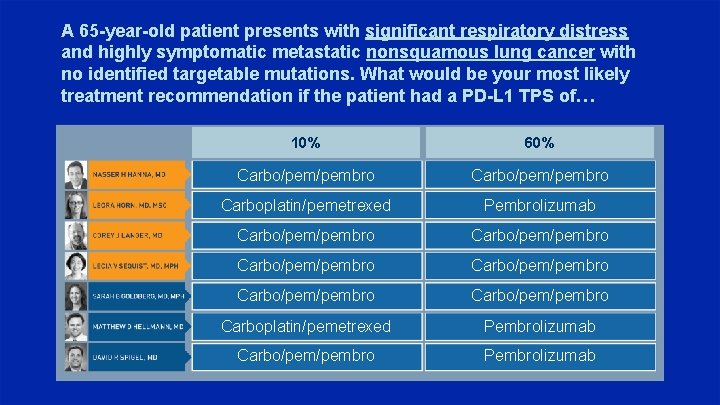
A 65 -year-old patient presents with significant respiratory distress and highly symptomatic metastatic nonsquamous lung cancer with no identified targetable mutations. What would be your most likely treatment recommendation if the patient had a PD-L 1 TPS of… 10% 60% Carbo/pem/pembro Carboplatin/pemetrexed Pembrolizumab Carbo/pem/pembro Carbo/pem/pembro Carboplatin/pemetrexed Pembrolizumab Carbo/pembro Pembrolizumab

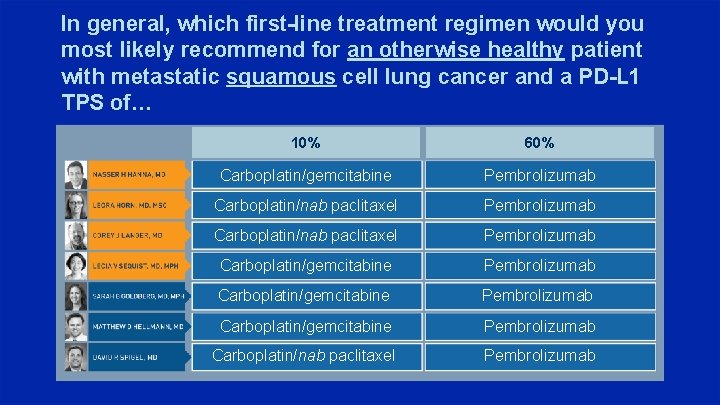
In general, which first-line treatment regimen would you most likely recommend for an otherwise healthy patient with metastatic squamous cell lung cancer and a PD-L 1 TPS of… 10% 60% Carboplatin/gemcitabine Pembrolizumab Carboplatin/nab paclitaxel Pembrolizumab Carboplatin/gemcitabine Pembrolizumab Carboplatin/nab paclitaxel Pembrolizumab

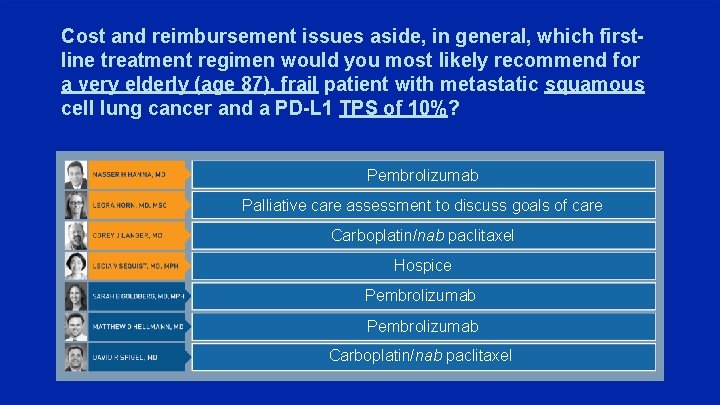
Cost and reimbursement issues aside, in general, which firstline treatment regimen would you most likely recommend for a very elderly (age 87), frail patient with metastatic squamous cell lung cancer and a PD-L 1 TPS of 10%? Pembrolizumab Palliative care assessment to discuss goals of care Carboplatin/nab paclitaxel Hospice Pembrolizumab Carboplatin/nab paclitaxel

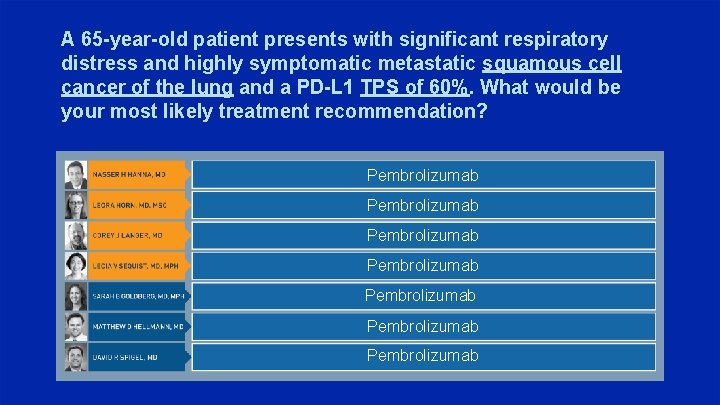
A 65 -year-old patient presents with significant respiratory distress and highly symptomatic metastatic squamous cell cancer of the lung and a PD-L 1 TPS of 60%. What would be your most likely treatment recommendation? Pembrolizumab Pembrolizumab

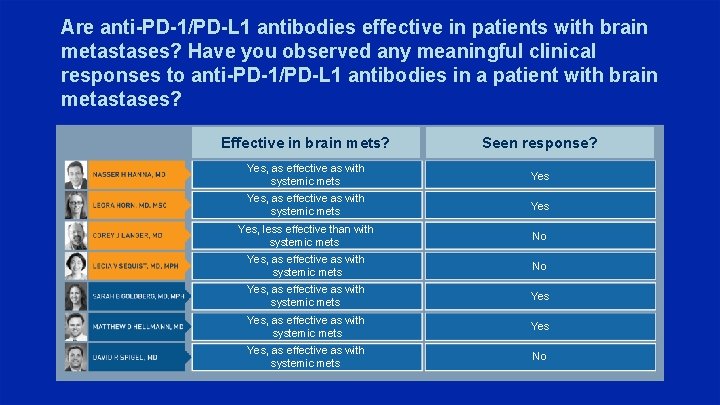
Are anti-PD-1/PD-L 1 antibodies effective in patients with brain metastases? Have you observed any meaningful clinical responses to anti-PD-1/PD-L 1 antibodies in a patient with brain metastases? Effective in brain mets? Seen response? Yes, as effective as with systemic mets Yes Yes, less effective than with systemic mets No Yes, as effective as with systemic mets Yes Yes, as effective as with systemic mets No

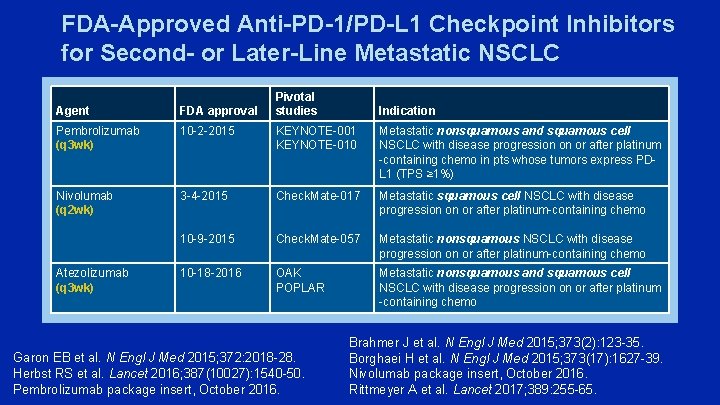
FDA-Approved Anti-PD-1/PD-L 1 Checkpoint Inhibitors for Second- or Later-Line Metastatic NSCLC Pivotal studies Agent FDA approval Pembrolizumab (q 3 wk) 10 -2 -2015 KEYNOTE-001 KEYNOTE-010 Metastatic nonsquamous and squamous cell NSCLC with disease progression on or after platinum -containing chemo in pts whose tumors express PDL 1 (TPS ≥ 1%) Nivolumab (q 2 wk) 3 -4 -2015 Check. Mate-017 Metastatic squamous cell NSCLC with disease progression on or after platinum-containing chemo 10 -9 -2015 Check. Mate-057 Metastatic nonsquamous NSCLC with disease progression on or after platinum-containing chemo 10 -18 -2016 OAK POPLAR Metastatic nonsquamous and squamous cell NSCLC with disease progression on or after platinum -containing chemo Atezolizumab (q 3 wk) Garon EB et al. N Engl J Med 2015; 372: 2018 -28. Herbst RS et al. Lancet 2016; 387(10027): 1540 -50. Pembrolizumab package insert, October 2016. Indication Brahmer J et al. N Engl J Med 2015; 373(2): 123 -35. Borghaei H et al. N Engl J Med 2015; 373(17): 1627 -39. Nivolumab package insert, October 2016. Rittmeyer A et al. Lancet 2017; 389: 255 -65.

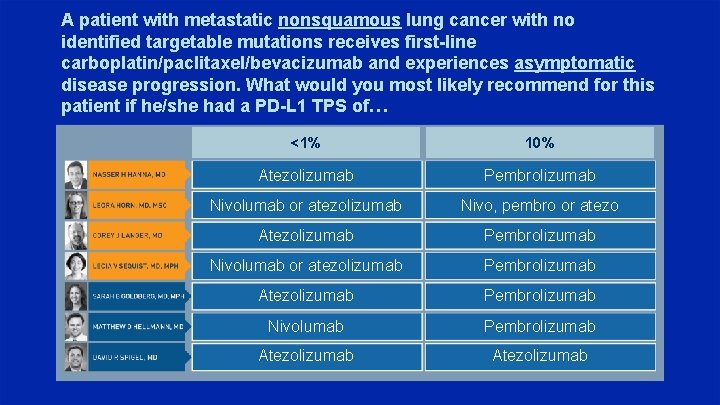
A patient with metastatic nonsquamous lung cancer with no identified targetable mutations receives first-line carboplatin/paclitaxel/bevacizumab and experiences asymptomatic disease progression. What would you most likely recommend for this patient if he/she had a PD-L 1 TPS of… <1% 10% Atezolizumab Pembrolizumab Nivolumab or atezolizumab Nivo, pembro or atezo Atezolizumab Pembrolizumab Nivolumab or atezolizumab Pembrolizumab Atezolizumab Pembrolizumab Nivolumab Pembrolizumab Atezolizumab

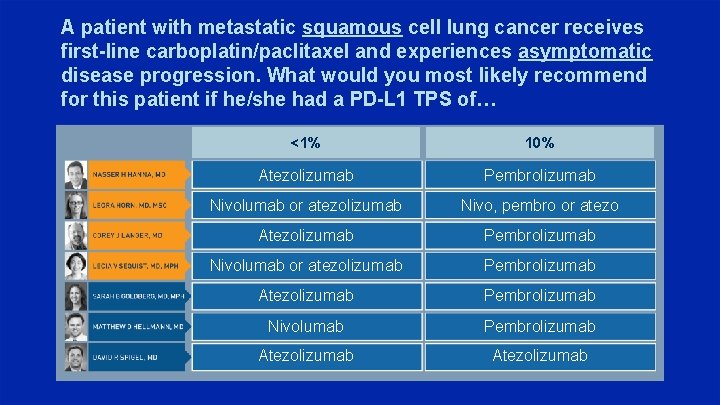
A patient with metastatic squamous cell lung cancer receives first-line carboplatin/paclitaxel and experiences asymptomatic disease progression. What would you most likely recommend for this patient if he/she had a PD-L 1 TPS of… <1% 10% Atezolizumab Pembrolizumab Nivolumab or atezolizumab Nivo, pembro or atezo Atezolizumab Pembrolizumab Nivolumab or atezolizumab Pembrolizumab Atezolizumab Pembrolizumab Nivolumab Pembrolizumab Atezolizumab

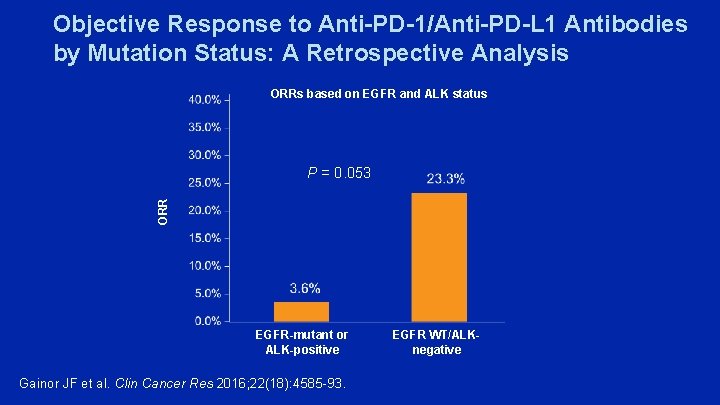
Objective Response to Anti-PD-1/Anti-PD-L 1 Antibodies by Mutation Status: A Retrospective Analysis ORRs based on EGFR and ALK status ORR P = 0. 053 EGFR-mutant or ALK-positive Gainor JF et al. Clin Cancer Res 2016; 22(18): 4585 -93. EGFR WT/ALKnegative

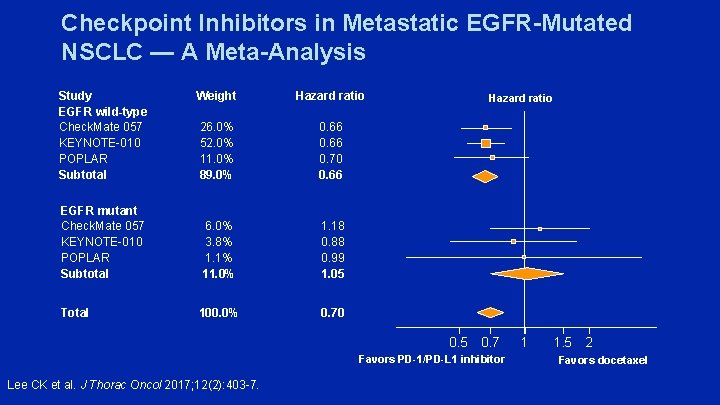
Checkpoint Inhibitors in Metastatic EGFR-Mutated NSCLC — A Meta-Analysis Study EGFR wild-type Check. Mate 057 KEYNOTE-010 POPLAR Subtotal Weight Hazard ratio 26. 0% 52. 0% 11. 0% 89. 0% 0. 66 0. 70 0. 66 EGFR mutant Check. Mate 057 KEYNOTE-010 POPLAR Subtotal 6. 0% 3. 8% 1. 1% 11. 0% 1. 18 0. 88 0. 99 1. 05 Total 100. 0% 0. 70 Hazard ratio 0. 5 0. 7 Favors PD-1/PD-L 1 inhibitor Lee CK et al. J Thorac Oncol 2017; 12(2): 403 -7. 1 1. 5 2 Favors docetaxel

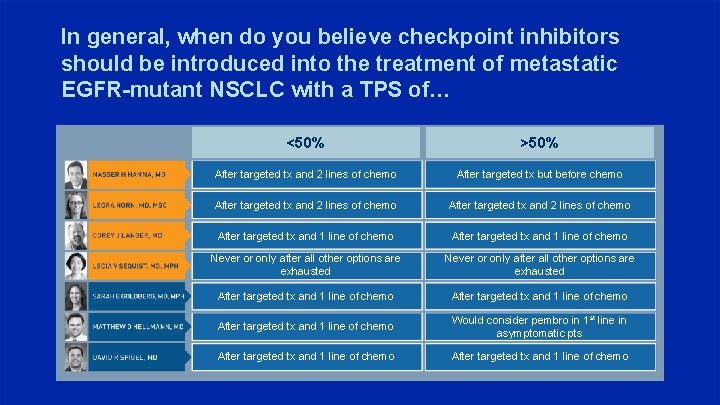
In general, when do you believe checkpoint inhibitors should be introduced into the treatment of metastatic EGFR-mutant NSCLC with a TPS of… <50% >50% After targeted tx and 2 lines of chemo After targeted tx but before chemo After targeted tx and 2 lines of chemo After targeted tx and 1 line of chemo Never or only after all other options are exhausted After targeted tx and 1 line of chemo Would consider pembro in 1 st line in asymptomatic pts After targeted tx and 1 line of chemo

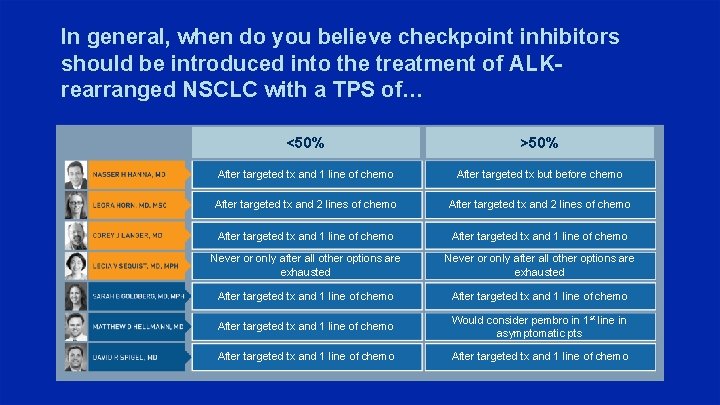
In general, when do you believe checkpoint inhibitors should be introduced into the treatment of ALKrearranged NSCLC with a TPS of… <50% >50% After targeted tx and 1 line of chemo After targeted tx but before chemo After targeted tx and 2 lines of chemo After targeted tx and 1 line of chemo Never or only after all other options are exhausted After targeted tx and 1 line of chemo Would consider pembro in 1 st line in asymptomatic pts After targeted tx and 1 line of chemo

Agenda Introduction – The New Taxonomy of Metastatic Non-Small Cell Lung Cancer (NSCLC) Module 1 – Emerging Research Data with and Potential Clinical Role of Immune Checkpoint Inhibitors in the Management of Locally Advanced NSCLC Module 2 – Integration of Anti-PD-1/PD-L 1 Antibodies into Current Treatment Algorithms for Patients with Metastatic NSCLC Module 3 – Ongoing Evaluation of Existing and Emerging Immunotherapeutic Approaches, Including Combination Strategies Module 4 – Identification and Management of Immune-Mediated and Other Toxicities Associated with Checkpoint Inhibitors; Relative Contraindications for Patients with Existing Autoimmune Disease

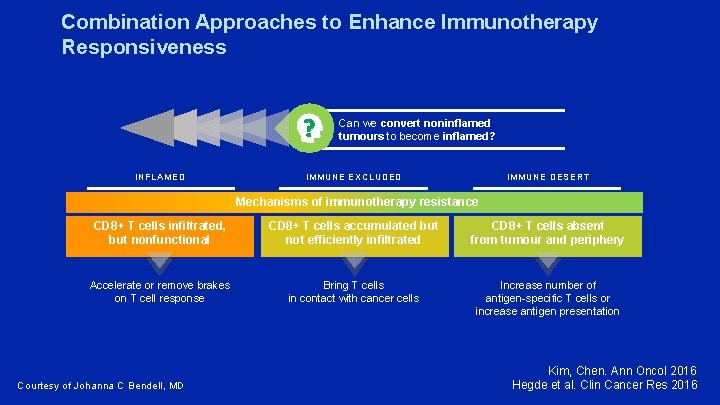
Combination Approaches to Enhance Immunotherapy Responsiveness Can we convert noninflamed tumours to become inflamed? INFLAMED IMMUNE EXCLUDED IMMUNE DESERT Mechanisms of immunotherapy resistance CD 8+ T cells infiltrated, but nonfunctional CD 8+ T cells accumulated but not efficiently infiltrated CD 8+ T cells absent from tumour and periphery Accelerate or remove brakes on T cell response Bring T cells in contact with cancer cells Increase number of antigen-specific T cells or increase antigen presentation Courtesy of Johanna C Bendell, MD Kim, Chen. Ann Oncol 2016 Hegde et al. Clin Cancer Res 2016

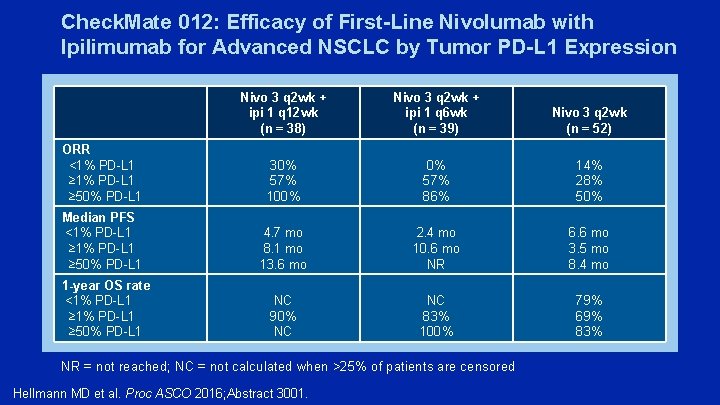
Check. Mate 012: Efficacy of First-Line Nivolumab with Ipilimumab for Advanced NSCLC by Tumor PD-L 1 Expression Nivo 3 q 2 wk + ipi 1 q 12 wk (n = 38) Nivo 3 q 2 wk + ipi 1 q 6 wk (n = 39) Nivo 3 q 2 wk (n = 52) ORR <1% PD-L 1 ≥ 50% PD-L 1 30% 57% 100% 0% 57% 86% 14% 28% 50% Median PFS <1% PD-L 1 ≥ 50% PD-L 1 4. 7 mo 8. 1 mo 13. 6 mo 2. 4 mo 10. 6 mo NR 6. 6 mo 3. 5 mo 8. 4 mo 1 -year OS rate <1% PD-L 1 ≥ 50% PD-L 1 NC 90% NC NC 83% 100% 79% 69% 83% NR = not reached; NC = not calculated when >25% of patients are censored Hellmann MD et al. Proc ASCO 2016; Abstract 3001.

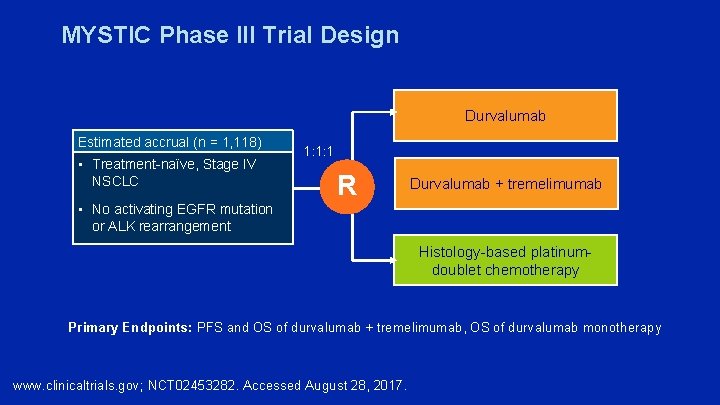
MYSTIC Phase III Trial Design Durvalumab Estimated accrual (n = 1, 118) • Treatment-naïve, Stage IV NSCLC 1: 1: 1 R Durvalumab + tremelimumab • No activating EGFR mutation or ALK rearrangement Histology-based platinumdoublet chemotherapy Primary Endpoints: PFS and OS of durvalumab + tremelimumab, OS of durvalumab monotherapy www. clinicaltrials. gov; NCT 02453282. Accessed August 28, 2017.

Phase III MYSTIC Trial Does Not Meet Its Primary Endpoint of PFS Press Release — July 27, 2017 The combination of durvalumab and tremelimumab did not meet the primary endpoint of improving PFS compared to standard of care (So. C) in patients whose tumors express PD-L 1 on 25% or more of their cancer cells (as determined by the VENTANA PD-L 1 [SP 263] assay). As a secondary endpoint, although not formally tested, durvalumab monotherapy would not have met a prespecified threshold of PFS benefit over So. C in this disease setting. The trial will continue to assess two additional primary endpoints of overall survival (OS) for durvalumab monotherapy and OS for the durvalumab plus tremelimumab combination. Final OS data from both primary endpoints are expected during the first half of 2018. https: //www. astrazeneca. com/media-centre/press-releases/2017/astrazeneca-reports-initial-resultsfrom-the-ongoing-mystic-trial-in-stage-iv-lung-cancer-27072017. html

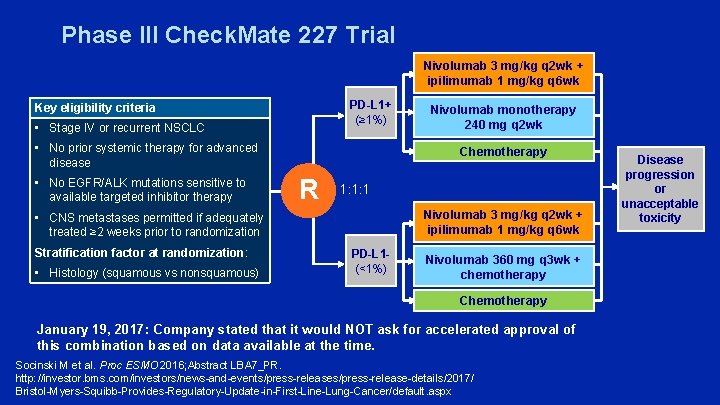
Phase III Check. Mate 227 Trial Nivolumab 3 mg/kg q 2 wk + ipilimumab 1 mg/kg q 6 wk PD-L 1+ (≥ 1%) Key eligibility criteria • Stage IV or recurrent NSCLC • No prior systemic therapy for advanced disease • No EGFR/ALK mutations sensitive to available targeted inhibitor therapy Chemotherapy R 1: 1: 1 Nivolumab 3 mg/kg q 2 wk + ipilimumab 1 mg/kg q 6 wk • CNS metastases permitted if adequately treated ≥ 2 weeks prior to randomization Stratification factor at randomization: • Histology (squamous vs nonsquamous) Nivolumab monotherapy 240 mg q 2 wk PD-L 1(<1%) Nivolumab 360 mg q 3 wk + chemotherapy Chemotherapy January 19, 2017: Company stated that it would NOT ask for accelerated approval of this combination based on data available at the time. Socinski M et al. Proc ESMO 2016; Abstract LBA 7_PR. http: //investor. bms. com/investors/news-and-events/press-release-details/2017/ Bristol-Myers-Squibb-Provides-Regulatory-Update-in-First-Line-Lung-Cancer/default. aspx Disease progression or unacceptable toxicity

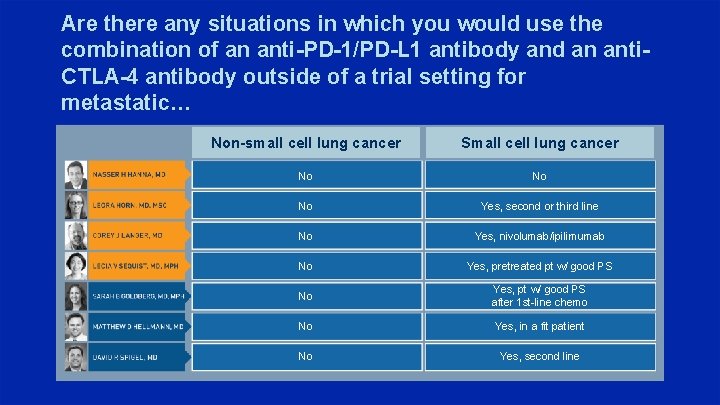
Are there any situations in which you would use the combination of an anti-PD-1/PD-L 1 antibody and an anti. CTLA-4 antibody outside of a trial setting for metastatic… Non-small cell lung cancer Small cell lung cancer No No No Yes, second or third line No Yes, nivolumab/ipilimumab No Yes, pretreated pt w/ good PS No Yes, pt w/ good PS after 1 st-line chemo No Yes, in a fit patient No Yes, second line

Have you or would you administer targeted therapy (eg, an EGFR TKI or ALK inhibitor) in combination with a PD -1/PD-L 1 antibody to a patient with metastatic NSCLC? I haven’t and would not I have as part of clinical trials and have seen significant toxicity I haven’t and would not

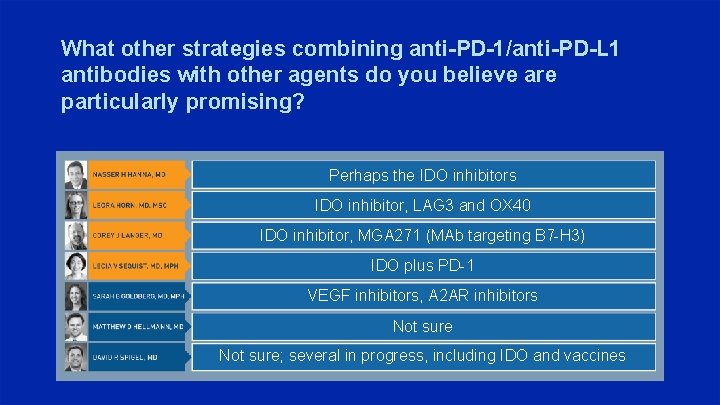
What other strategies combining anti-PD-1/anti-PD-L 1 antibodies with other agents do you believe are particularly promising? Perhaps the IDO inhibitors IDO inhibitor, LAG 3 and OX 40 IDO inhibitor, MGA 271 (MAb targeting B 7 -H 3) IDO plus PD-1 VEGF inhibitors, A 2 AR inhibitors Not sure; several in progress, including IDO and vaccines

Agenda Introduction – The New Taxonomy of Metastatic Non-Small Cell Lung Cancer (NSCLC) Module 1 – Emerging Research Data with and Potential Clinical Role of Immune Checkpoint Inhibitors in the Management of Locally Advanced NSCLC Module 2 – Integration of Anti-PD-1/PD-L 1 Antibodies into Current Treatment Algorithms for Patients with Metastatic NSCLC Module 3 – Ongoing Evaluation of Existing and Emerging Immunotherapeutic Approaches, Including Combination Strategies Module 4 – Identification and Management of Immune-Mediated and Other Toxicities Associated with Checkpoint Inhibitors; Relative Contraindications for Patients with Existing Autoimmune Disease

How many patients have you had in your practice in whom anti-PD-1/anti-PD-L 1 therapy was stopped because of toxicity, protocol requirements, et cetera, who experienced sustained responses (ie, more than 6 months) after treatment was discontinued? About 10% Too many to count 3 -4 2 -3 Several Dozens 25+

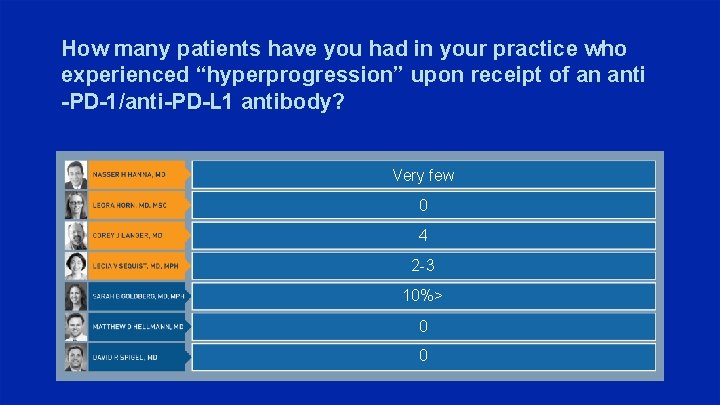
How many patients have you had in your practice who experienced “hyperprogression” upon receipt of an anti -PD-1/anti-PD-L 1 antibody? Very few 0 4 2 -3 10%> 0 0

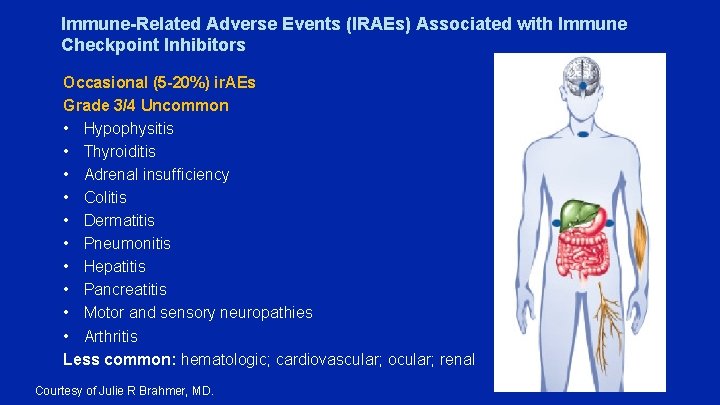
Immune-Related Adverse Events (IRAEs) Associated with Immune Checkpoint Inhibitors Occasional (5 -20%) ir. AEs Grade 3/4 Uncommon • Hypophysitis • Thyroiditis • Adrenal insufficiency • Colitis • Dermatitis • Pneumonitis • Hepatitis • Pancreatitis • Motor and sensory neuropathies • Arthritis Less common: hematologic; cardiovascular; ocular; renal Courtesy of Julie R Brahmer, MD.

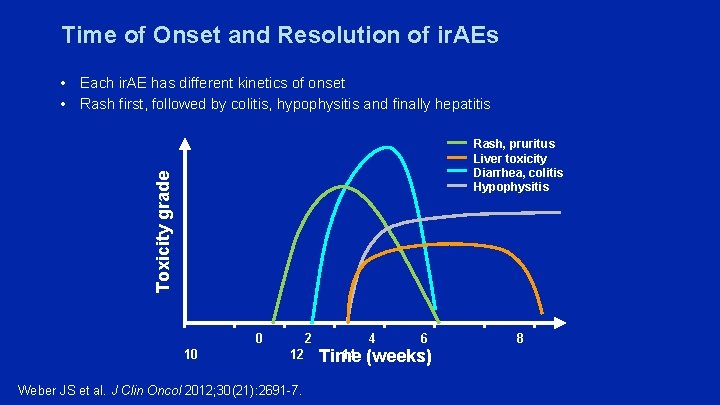
Time of Onset and Resolution of ir. AEs • Each ir. AE has different kinetics of onset • Rash first, followed by colitis, hypophysitis and finally hepatitis Toxicity grade Rash, pruritus Liver toxicity Diarrhea, colitis Hypophysitis 10 0 2 4 6 12 14 Time (weeks) Weber JS et al. J Clin Oncol 2012; 30(21): 2691 -7. 8

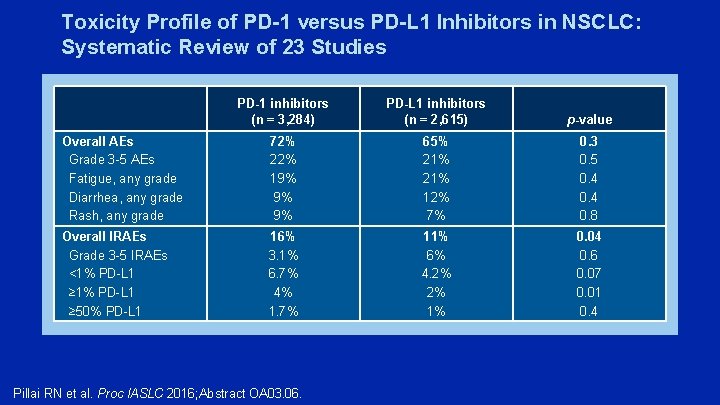
Toxicity Profile of PD-1 versus PD-L 1 Inhibitors in NSCLC: Systematic Review of 23 Studies PD-1 inhibitors (n = 3, 284) PD-L 1 inhibitors (n = 2, 615) p-value Overall AEs Grade 3 -5 AEs Fatigue, any grade Diarrhea, any grade Rash, any grade 72% 22% 19% 9% 9% 65% 21% 12% 7% 0. 3 0. 5 0. 4 0. 8 Overall IRAEs Grade 3 -5 IRAEs <1% PD-L 1 ≥ 50% PD-L 1 16% 3. 1% 6. 7% 4% 1. 7% 11% 6% 4. 2% 2% 1% 0. 04 0. 6 0. 07 0. 01 0. 4 Pillai RN et al. Proc IASLC 2016; Abstract OA 03. 06.

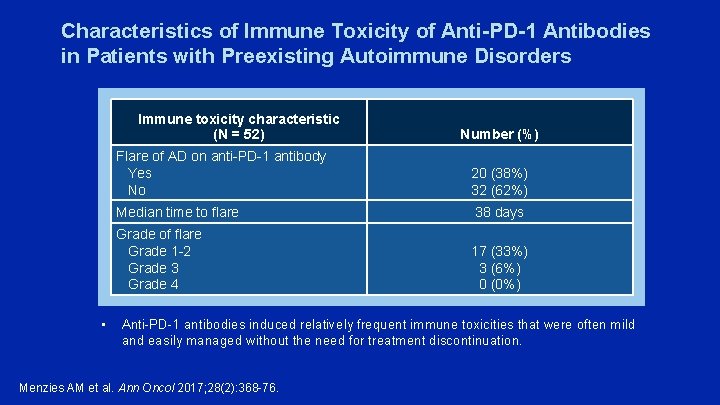
Anti-PD-1 Therapy in Patients with Advanced Melanoma and Preexisting Autoimmune Disorders (AD) • 119 patients from 13 academic tertiary referral centers received anti-PD-1 antibodies • In patients with preexisting AD (N = 52), the response rate was 33% • 20 (38%) patients had a flare of AD requiring immunosuppression, including – 7/13 with rheumatoid arthritis – 3/3 with polymyalgia rheumatica – 2/2 with Sjogren’s syndrome – 2/2 with immune thrombocytopaenic purpura – 3/8 with psoriasis • No patients with gastrointestinal (N = 6) or neurological disorders (N = 5) flared • Only 2 (4%) patients permanently discontinued treatment due to flare, but 15 (29%) developed other ir. AEs and 4 (8%) permanently discontinued treatment Menzies AM et al. Ann Oncol 2017; 28(2): 368 -76.

Characteristics of Immune Toxicity of Anti-PD-1 Antibodies in Patients with Preexisting Autoimmune Disorders Immune toxicity characteristic (N = 52) • Number (%) Flare of AD on anti-PD-1 antibody Yes No 20 (38%) 32 (62%) Median time to flare 38 days Grade of flare Grade 1 -2 Grade 3 Grade 4 17 (33%) 3 (6%) 0 (0%) Anti-PD-1 antibodies induced relatively frequent immune toxicities that were often mild and easily managed without the need for treatment discontinuation. Menzies AM et al. Ann Oncol 2017; 28(2): 368 -76.

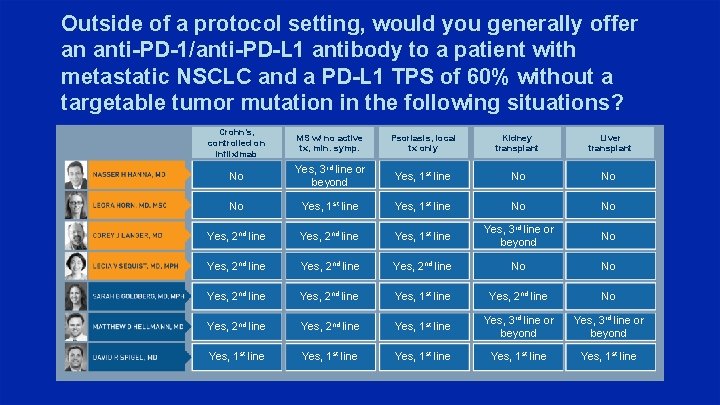
Outside of a protocol setting, would you generally offer an anti-PD-1/anti-PD-L 1 antibody to a patient with metastatic NSCLC and a PD-L 1 TPS of 60% without a targetable tumor mutation in the following situations? Crohn’s, controlled on infliximab MS w/ no active tx, min. symp. Psoriasis, local tx only Kidney transplant Liver transplant No Yes, 3 rd line or beyond Yes, 1 st line No No No Yes, 1 st line No No Yes, 2 nd line Yes, 1 st line Yes, 3 rd line or beyond No Yes, 2 nd line No No Yes, 2 nd line Yes, 1 st line Yes, 2 nd line No Yes, 2 nd line Yes, 1 st line Yes, 3 rd line or beyond Yes, 1 st line Yes, 1 st line