Limiting Reactants Limiting Reactants Def The reactant that





















- Slides: 24
Limiting Reactants

Limiting Reactants • Def. The reactant that is completely consumed in a chemical reaction and limits the amount of product that can be formed

Limiting Reactants • The limiting reactant limits the reaction and, thus, determines how much of the product forms. • The left-over reactants are called excess reactants.

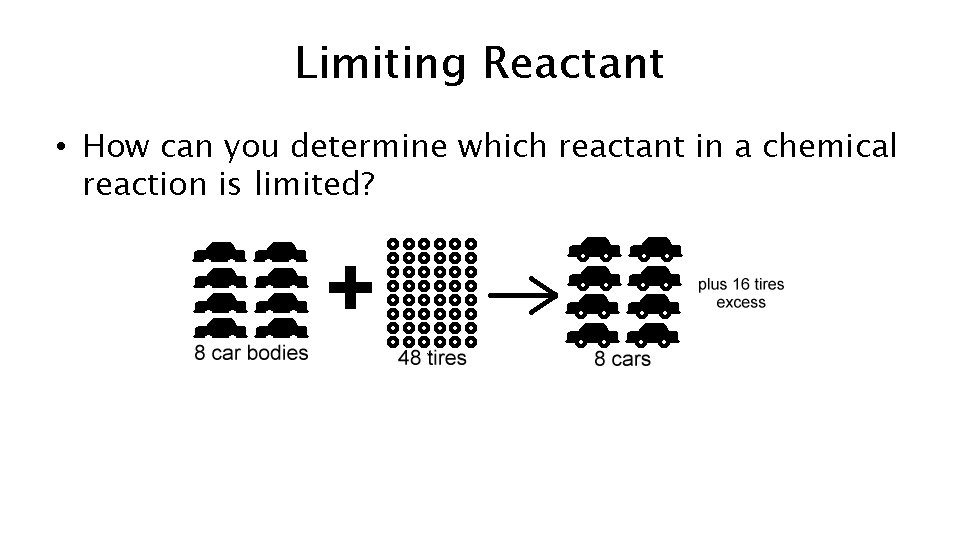
Limiting Reactant • How can you determine which reactant in a chemical reaction is limited?

How to find Limiting Reactant • 1 st- Write out the balance chemical equation. • 2 nd- Find the number of moles of a reactant from each product given. rd • 3 -The reactant with the smallest number of grams is the limiting reactant. The one with the higher amount is the excess reactant.

Warm up • What is the limiting reactant and the excess reactant when 35. 00 grams of Magnesium and 15. 00 g of Nitrogen are used to produce Magnesium Nitride.

• One way to produce hydrogen sulfide gas is by the reaction of iron (II) sulfide with hydrochloric acid (HCl). If 10. 2 grams of HCl is added to 13. 2 g Fe. S, what is the limiting reactant and excess reactant.

Determining how much Product can be formed st • 1 Take the number of moles of Limiting reactant and convert to the grams of product.

Determining the Limiting Reactant • In the reaction below, 40. 0 g of sodium hydroxide (Na. OH) reacts with 60. 0 g of sulfuric acid (H 2 SO 4). How much of Sodium Sulfate can be made?

Determine how much excess remains • 1 st take the amount, usually grams, of the limiting reactant that you were giving initially and find how many grams of the excess reactant it could make in grams • 2 nd Subtract the beginning amount of the excess reactant by the amount that you found in step number one. This amount will be how much excess remains

Practice 1) One way to produce hydrogen sulfide gas is by the reaction of Iron(III) sulfide with hydrochloric acid. Fe. S + 2 HCl Fe. Cl 2 + H 2 S If 10. 2 g HCl is added to 13. 2 g Fe. S, how many grams of H 2 S can be formed? What is the mass of the excess reactant remaining?

Practice • Suppose that a solution containing 3. 50 grams of Na 3 PO 4 is mixed with a solution containing 6. 40 grams of Ba(NO 3)2. Na 3 PO 4 + Ba(NO 3)2 Ba 3(PO 4)2 + Na. NO 3 a)What is the limiting reactant and what is the excess reactant b)How many grams of Ba 3(PO 4)2 can be formed? c)How much of the excess reactant remains

Warm Up • A 2. 00 g sample of ammonia is mixed with 4. 00 g of oxygen in the equation below. What is the limiting reactant, how much water can be produced and how much excess reactant remains after the reaction has stopped? • 4 NH 3(g) + 5 O 2(g) 4 NO(g) + 6 H 2 O(g)

Yield of Chemical Reaction • Theoretical Yield- the calculated quantity of product in a reaction • Actual Yield- The measured mass formed in the reaction • Note: Actual yield of chemical reactions are often less than theoretical yield

Percent Yield • Is the ratio of the actual yield to theoretical yield times 100%. • Percent Yield = actual yield x 100 % theoretical yield

Example • If the actual yield of Mg 3 N 2 is 47. 87 g and theoretical yield is 48. 85 g what is the percent yield?

Example • Calculate theoretical yield of Zn. S, in grams, that can be made from 0. 488 g Zn and 0. 503 g S 8. 8 Zn + S 8 8 Zn. S If the actual yield is 0. 606 g Zn. S, what is the percent yield?

Practice • Calculate theoretical yield of C 2 H 5 Cl if 112 g of C 2 H 5 OH is reacted with 34. 7 g of PCl 3 based on the reaction below. If 23. 7 g of C 2 H 5 Cl is produced, what is the percent yield? 3 C 2 H 5 OH + PCl 3 3 C 2 H 5 Cl + H 3 PO 3

Example • What mass of acetic acid is needed to prepare 252 g ethyl acetate if the expected percent yield is 85%?

Solution Concentration and Solution Stoichiometry • Solution : Homogeneous mixture of two or more substances • Majority Component of the solution – Solvent • Minority component - solute • If water acts as a solvent – Aqueous solution

Solutions • Dilute solution : More solvent+ less solute • Concentrated Solution: Less solvent+ more solute • The solution concentration can be expressed by the term molarity(M)

Molarity • amount of solute in moles • Molarity = • volume of solution in L

Problems • How you make 1 L of 1 M solution of Na. Cl? • Calculate the molarity of a solution made by adding 45. 4 g of Na. NO 3 to a flask and dissolving it with water to create a total volume of 2. 50 L? • What mass of KBr in grams do you need to make 250 ml of a 1. 5 M KBr solution?

Problems • How many grams of Sucrose(C 12 H 22 O 11 ) • are in 1. 55 L of 0. 758 M sucrose solution?