Please note these are the actual videorecorded proceedings



- Slides: 15

Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content.

CAR T-Cell Therapy for B-Cell Lymphomas Sattva S Neelapu, MD Professor and Deputy Chair ad interim Department of Lymphoma and Myeloma The University of Texas MD Anderson Cancer Center Houston, TX

Disclosures Advisory Committee and Consulting Agreements Celgene Corporation, Gilead Sciences Inc, Kite Pharma Inc, Merck, Novartis, Pfizer Inc, Unum Therapeutics Contracted Research Bristol-Myers Squibb Company, Cellectis, Gilead Sciences Inc, Karus Therapeutics, Kite Pharma Inc, Merck, Poseida Therapeutics Inc

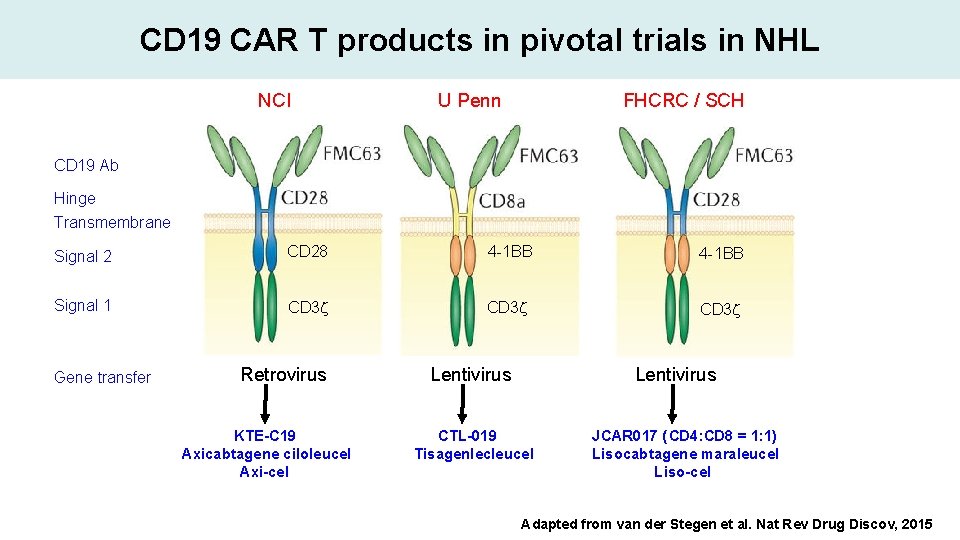
CD 19 CAR T products in pivotal trials in NHL NCI U Penn FHCRC / SCH CD 19 Ab Hinge Transmembrane Signal 2 CD 28 4 -1 BB Signal 1 CD 3 z Gene transfer Retrovirus KTE-C 19 Axicabtagene ciloleucel Axi-cel Lentivirus CTL-019 Tisagenlecleucel JCAR 017 (CD 4: CD 8 = 1: 1) Lisocabtagene maraleucel Liso-cel Adapted from van der Stegen et al. Nat Rev Drug Discov, 2015

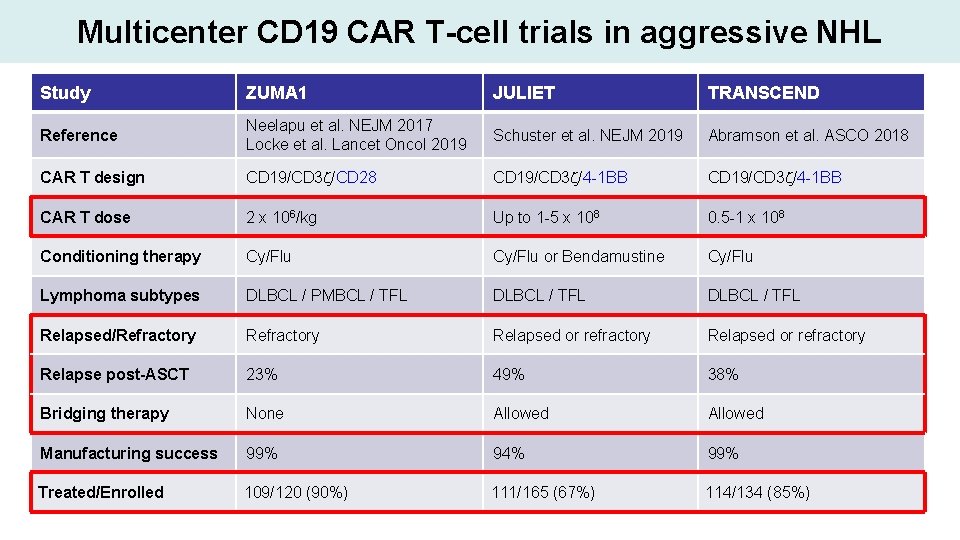
Multicenter CD 19 CAR T-cell trials in aggressive NHL Study ZUMA 1 JULIET TRANSCEND Reference Neelapu et al. NEJM 2017 Locke et al. Lancet Oncol 2019 Schuster et al. NEJM 2019 Abramson et al. ASCO 2018 CAR T design CD 19/CD 3 z/CD 28 CD 19/CD 3 z/4 -1 BB CAR T dose 2 x 106/kg Up to 1 -5 x 108 0. 5 -1 x 108 Conditioning therapy Cy/Flu or Bendamustine Cy/Flu Lymphoma subtypes DLBCL / PMBCL / TFL DLBCL / TFL Relapsed/Refractory Relapsed or refractory Relapse post-ASCT 23% 49% 38% Bridging therapy None Allowed Manufacturing success 99% 94% 99% Treated/Enrolled 109/120 (90%) 111/165 (67%) 114/134 (85%)

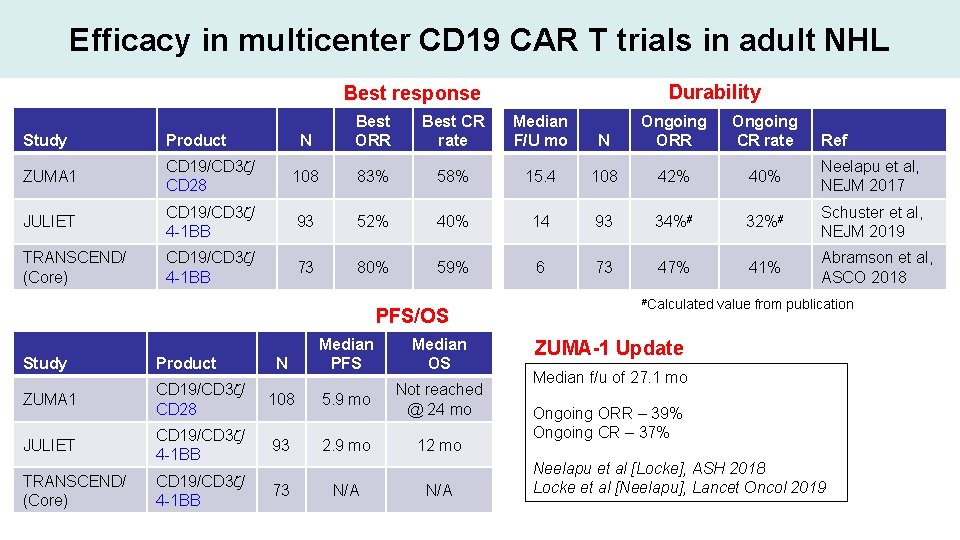
Efficacy in multicenter CD 19 CAR T trials in adult NHL Durability Best response N Best ORR Best CR rate Median F/U mo N Ongoing ORR Ongoing CR rate Study Product ZUMA 1 CD 19/CD 3 z/ CD 28 108 83% 58% 15. 4 108 42% 40% Neelapu et al, NEJM 2017 JULIET CD 19/CD 3 z/ 4 -1 BB 93 52% 40% 14 93 34%# 32%# Schuster et al, NEJM 2019 TRANSCEND/ (Core) CD 19/CD 3 z/ 4 -1 BB 73 80% 59% 6 73 47% 41% Abramson et al, ASCO 2018 PFS/OS N Median PFS Median OS Study Product ZUMA 1 CD 19/CD 3 z/ CD 28 108 5. 9 mo Not reached @ 24 mo JULIET CD 19/CD 3 z/ 4 -1 BB 93 2. 9 mo 12 mo TRANSCEND/ (Core) CD 19/CD 3 z/ 4 -1 BB 73 N/A #Calculated Ref value from publication ZUMA-1 Update Median f/u of 27. 1 mo Ongoing ORR – 39% Ongoing CR – 37% Neelapu et al [Locke], ASH 2018 Locke et al [Neelapu], Lancet Oncol 2019

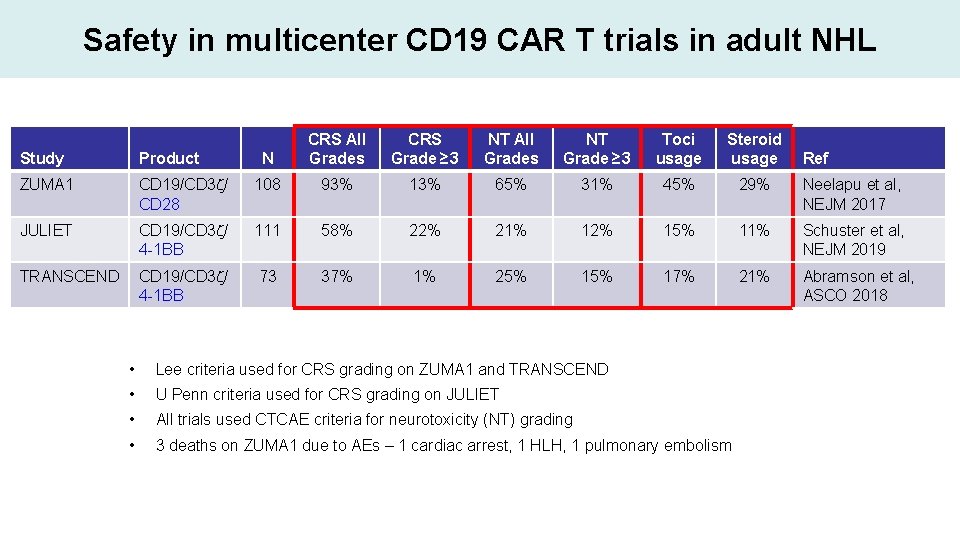
Safety in multicenter CD 19 CAR T trials in adult NHL N CRS All Grades CRS Grade ≥ 3 NT All Grades NT Grade ≥ 3 Toci usage Steroid usage Study Product ZUMA 1 CD 19/CD 3 z/ CD 28 108 93% 13% 65% 31% 45% 29% Neelapu et al, NEJM 2017 JULIET CD 19/CD 3 z/ 4 -1 BB 111 58% 22% 21% 12% 15% 11% Schuster et al, NEJM 2019 TRANSCEND CD 19/CD 3 z/ 4 -1 BB 73 37% 1% 25% 17% 21% Abramson et al, ASCO 2018 • Lee criteria used for CRS grading on ZUMA 1 and TRANSCEND • U Penn criteria used for CRS grading on JULIET • All trials used CTCAE criteria for neurotoxicity (NT) grading • 3 deaths on ZUMA 1 due to AEs – 1 cardiac arrest, 1 HLH, 1 pulmonary embolism Ref

Case 1 • 34 yo F diagnosed with stage II DLBCL • Achieved a CR after R-CHOP x 6. • Relapsed 6 months later and was treated with R-ICE x 2 CR • Autologous stem cell transplant • Relapsed 6 mo after ASCT • Received axi-cel anti-CD 19 CAR T cell therapy

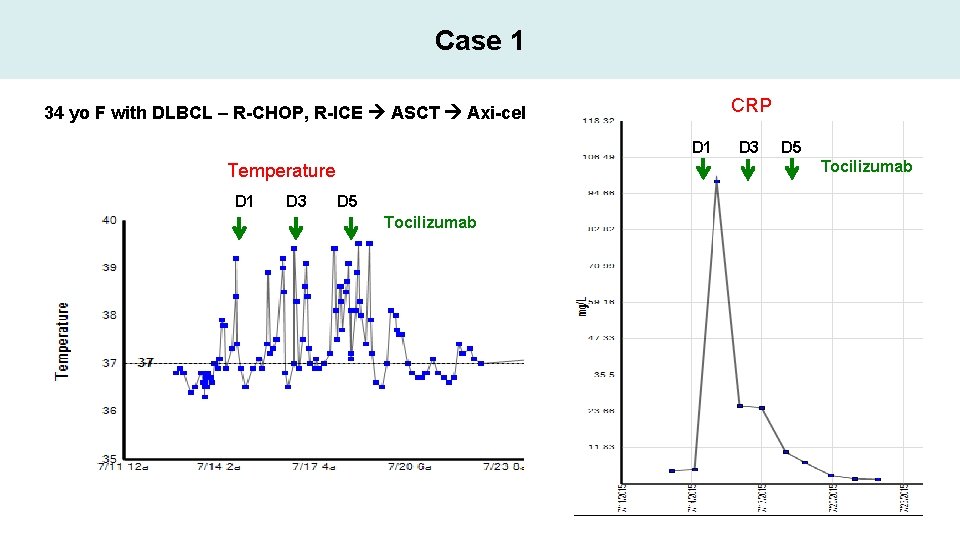
Case 1 CRP 34 yo F with DLBCL – R-CHOP, R-ICE ASCT Axi-cel D 1 D 3 D 5 Tocilizumab Temperature D 1 D 3 D 5 Tocilizumab

Case 1: Impaired handwriting is a sensitive sign of neurotoxicity Day 4 9 am MMSE score 29/30 Day 5 01: 30 PM Toci 8 mg/kg 27/30 Day 5 03: 30 PM 27/30 Day 6 9 am 29/30

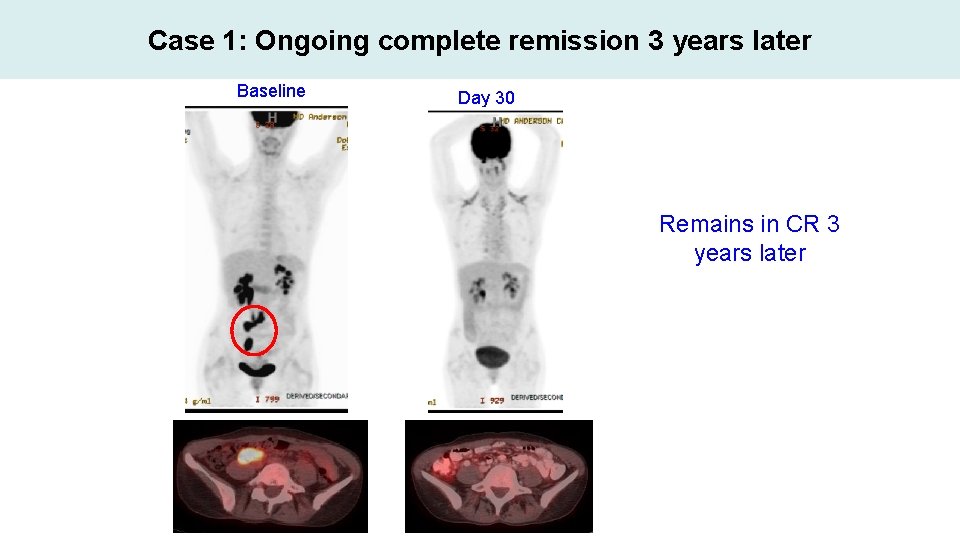
Case 1: Ongoing complete remission 3 years later Baseline Day 30 Remains in CR 3 years later

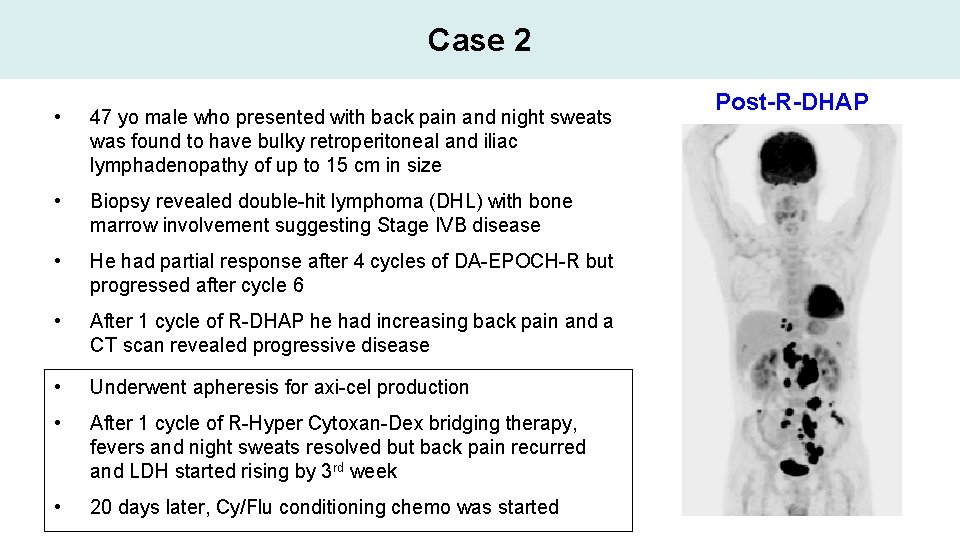
Case 2 • 47 yo male who presented with back pain and night sweats was found to have bulky retroperitoneal and iliac lymphadenopathy of up to 15 cm in size • Biopsy revealed double-hit lymphoma (DHL) with bone marrow involvement suggesting Stage IVB disease • He had partial response after 4 cycles of DA-EPOCH-R but progressed after cycle 6 • After 1 cycle of R-DHAP he had increasing back pain and a CT scan revealed progressive disease • Underwent apheresis for axi-cel production • After 1 cycle of R-Hyper Cytoxan-Dex bridging therapy, fevers and night sweats resolved but back pain recurred and LDH started rising by 3 rd week • 20 days later, Cy/Flu conditioning chemo was started Post-R-DHAP

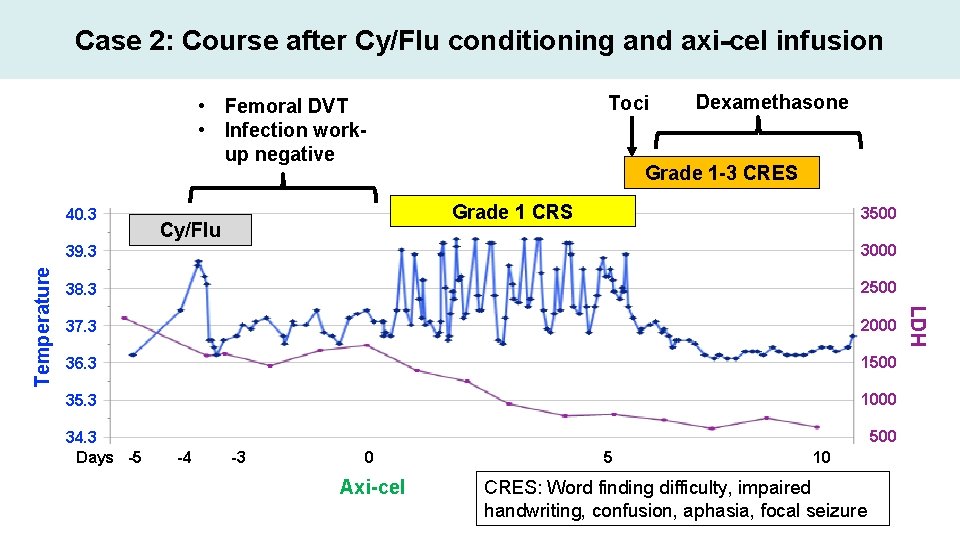
Case 2: Course after Cy/Flu conditioning and axi-cel infusion Toci • Femoral DVT • Infection workup negative Grade 1 -3 CRES Grade 1 CRS Cy/Flu 3500 39. 3 3000 38. 3 2500 37. 3 2000 36. 3 1500 35. 3 1000 34. 3 Days -5 500 -4 -3 0 Axi-cel 5 10 CRES: Word finding difficulty, impaired handwriting, confusion, aphasia, focal seizure LDH Temperature 40. 3 Dexamethasone

Case 2: CRS and CRES management • • CRS – – Fever managed with acetaminophen, ibuprofen, cooling blanket – – – IV hydration Blood and urine cultures, chest x-ray to rule out infection, blood cultures q 48 hrs for fever Broad spectrum antibiotics with vancomycin and cefepime Filgrastim for neutropenia CRES – – – Tocilizumab for grade 1 CRES with concurrent grade 1 CRS EEG, CT head, MRI brain Neurology consult Lorazepam for focal seizure; Levetiracetam increased to 1, 500 mg q 12 hrs Aspiration precautions Dexamethasone at 10 mg IV q 6 hrs for grade 3 CRES with taper after grade 1 CRES

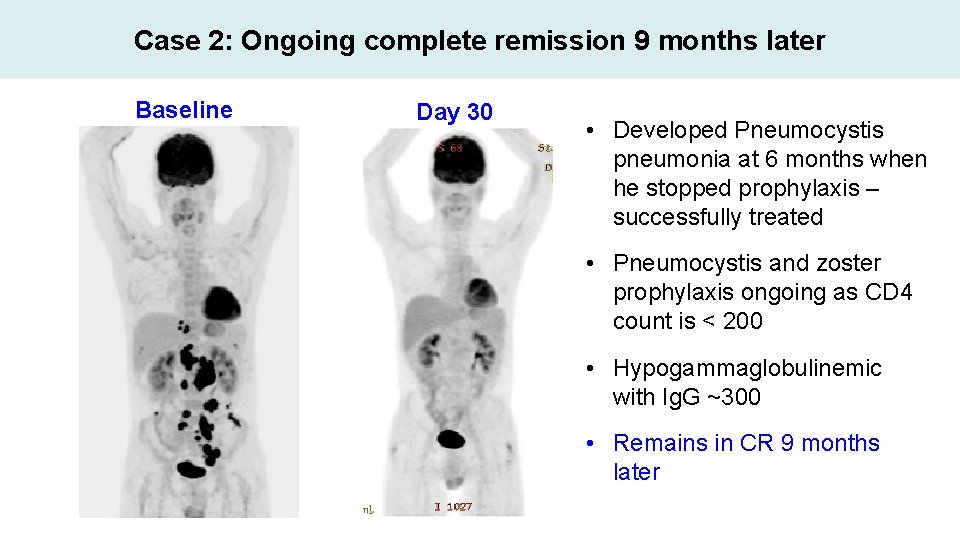
Case 2: Ongoing complete remission 9 months later Baseline Day 30 • Developed Pneumocystis pneumonia at 6 months when he stopped prophylaxis – successfully treated • Pneumocystis and zoster prophylaxis ongoing as CD 4 count is < 200 • Hypogammaglobulinemic with Ig. G ~300 • Remains in CR 9 months later