Please note these are the actual videorecorded proceedings















- Slides: 45

Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content.

Module 10: Acute Myeloid Leukemia Drs Stone and Pollyea


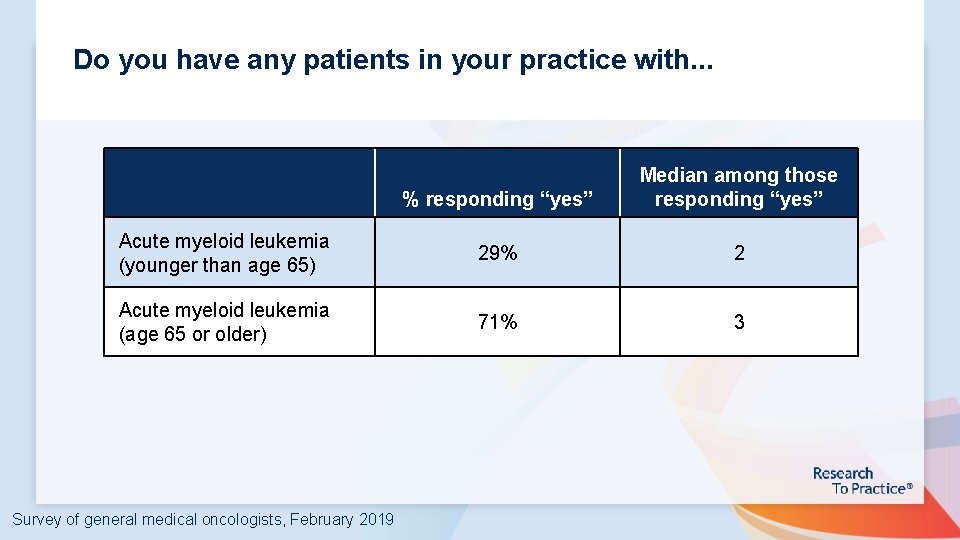
Do you have any patients in your practice with. . . % responding “yes” Median among those responding “yes” Acute myeloid leukemia (younger than age 65) 29% 2 Acute myeloid leukemia (age 65 or older) 71% 3 Survey of general medical oncologists, February 2019

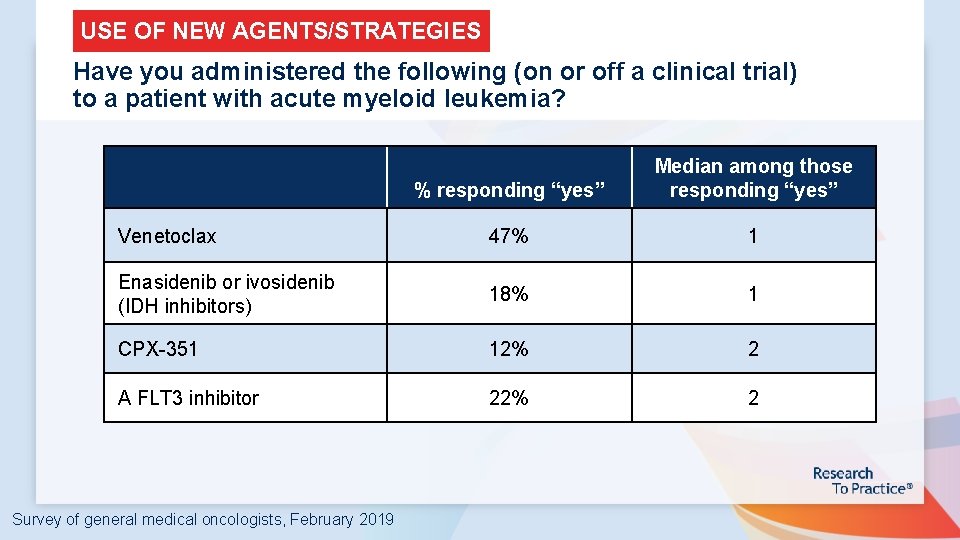
USE OF NEW AGENTS/STRATEGIES Have you administered the following (on or off a clinical trial) to a patient with acute myeloid leukemia? % responding “yes” Median among those responding “yes” Venetoclax 47% 1 Enasidenib or ivosidenib (IDH inhibitors) 18% 1 CPX-351 12% 2 A FLT 3 inhibitor 22% 2 Survey of general medical oncologists, February 2019

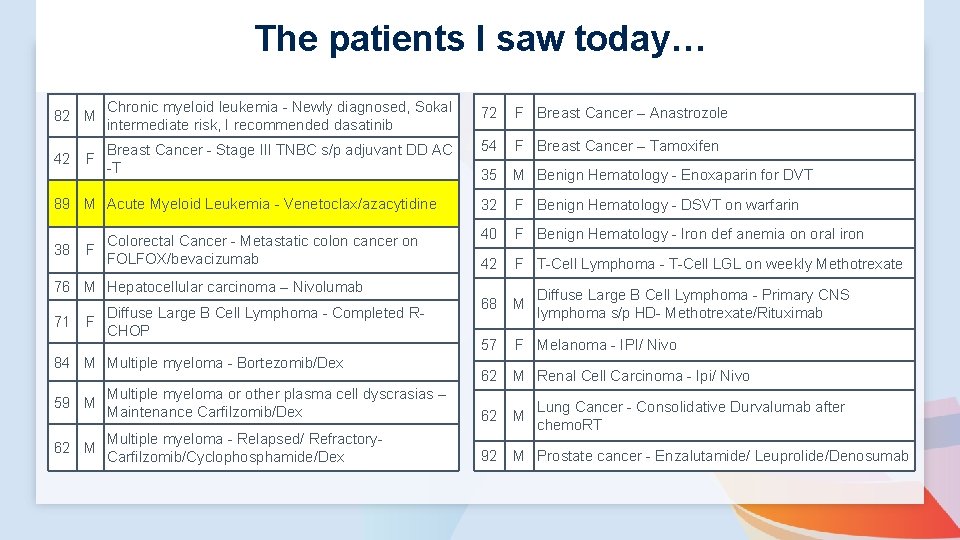
The patients I saw today… 82 M Chronic myeloid leukemia - Newly diagnosed, Sokal intermediate risk, I recommended dasatinib 72 F Breast Cancer – Anastrozole 42 F Breast Cancer - Stage III TNBC s/p adjuvant DD AC -T 54 F Breast Cancer – Tamoxifen 35 M Benign Hematology - Enoxaparin for DVT 89 M Acute Myeloid Leukemia - Venetoclax/azacytidine 32 F Benign Hematology - DSVT on warfarin Colorectal Cancer - Metastatic colon cancer on 38 F FOLFOX/bevacizumab 40 F Benign Hematology - Iron def anemia on oral iron 42 F T-Cell Lymphoma - T-Cell LGL on weekly Methotrexate 68 M 57 F Melanoma - IPI/ Nivo 62 M Renal Cell Carcinoma - Ipi/ Nivo 76 M Hepatocellular carcinoma – Nivolumab 71 F Diffuse Large B Cell Lymphoma - Completed RCHOP 84 M Multiple myeloma - Bortezomib/Dex Diffuse Large B Cell Lymphoma - Primary CNS lymphoma s/p HD- Methotrexate/Rituximab 59 M Multiple myeloma or other plasma cell dyscrasias – Maintenance Carfilzomib/Dex 62 M Multiple myeloma - Relapsed/ Refractory- Carfilzomib/Cyclophosphamide/Dex 92 M Prostate cancer - Enzalutamide/ Leuprolide/Denosumab Lung Cancer - Consolidative Durvalumab after chemo. RT

Current Testing Algorithms; Treatment for Patients with a Genomic Alteration Richard M Stone, MD Chief of Staff Director, Translational Research, Leukemia Division, Medical Oncology Dana-Farber Cancer Institute Professor of Medicine Harvard Medical School Boston, MA

Disclosures- Richard M Stone, MD • Consulting relationships past three years: – Abb. Vie*; Actinium, Agios*; Amgen; Argenix (DSMB); Arog*; Astellas; Astra. Zeneca; Celgene (includes DSMB and steering committee); Fujifilm, Janssen; Juno; Macrogen-ics; Novartis*; Ono; Orsenix; Pfizer; Roche; Stemline, Sumitomo; Takeda (DSMB) – * denotes support to my institution for clinical trials on which I was local PI • Securities, employment, promotional activities, intellectual property, gifts, grants – None

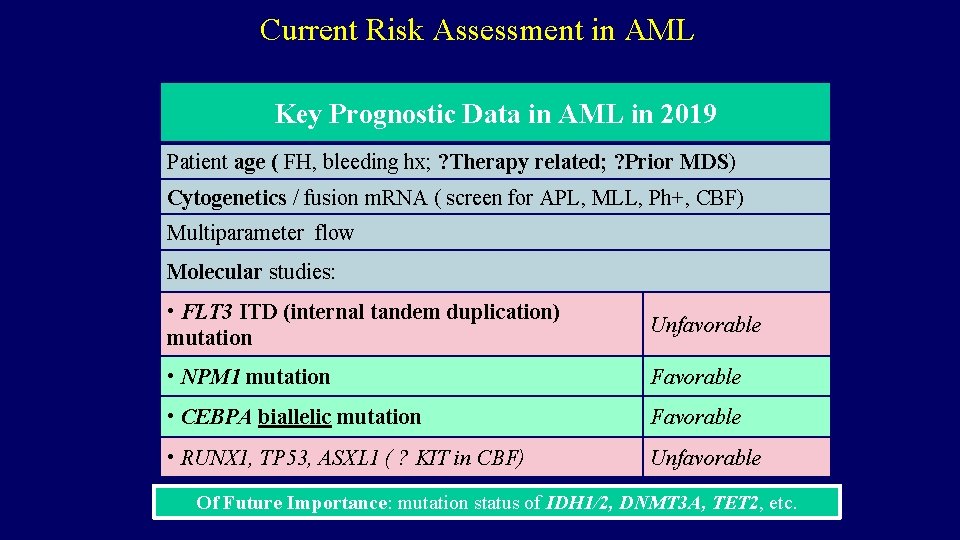
Current Risk Assessment in AML Key Prognostic Data in AML in 2019 Patient age ( FH, bleeding hx; ? Therapy related; ? Prior MDS) Cytogenetics / fusion m. RNA ( screen for APL, MLL, Ph+, CBF) Multiparameter flow Molecular studies: • FLT 3 ITD (internal tandem duplication) mutation Unfavorable • NPM 1 mutation Favorable • CEBPA biallelic mutation Favorable • RUNX 1, TP 53, ASXL 1 ( ? KIT in CBF) Unfavorable Of Future Importance: mutation status of IDH 1/2, DNMT 3 A, TET 2, etc.

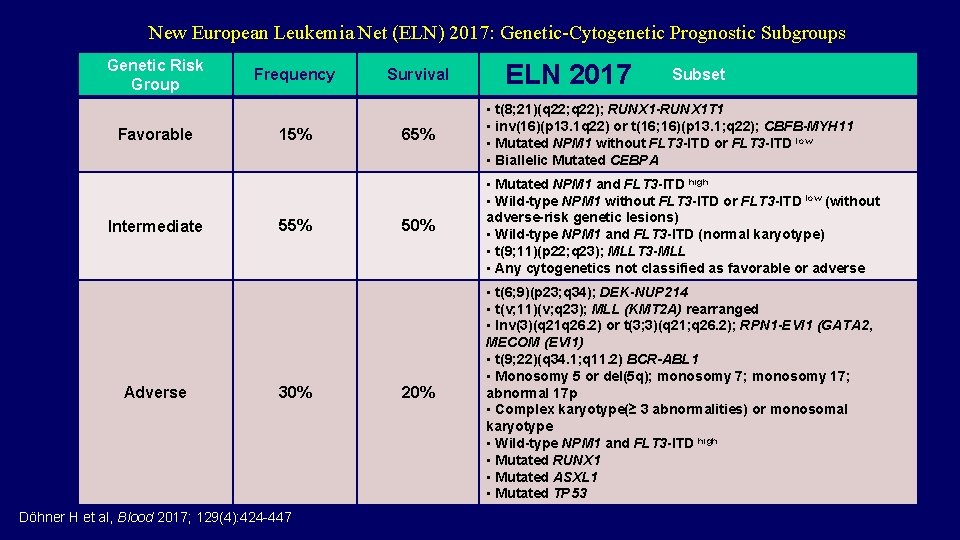
New European Leukemia Net (ELN) 2017: Genetic-Cytogenetic Prognostic Subgroups Genetic Risk Group Favorable Intermediate Adverse Frequency 15% 55% 30% Döhner H et al, Blood 2017; 129(4): 424 -447 Survival ELN 2017 Subset 65% • t(8; 21)(q 22; q 22); RUNX 1 -RUNX 1 T 1 • inv(16)(p 13. 1 q 22) or t(16; 16)(p 13. 1; q 22); CBFB-MYH 11 • Mutated NPM 1 without FLT 3 -ITD or FLT 3 -ITD low • Biallelic Mutated CEBPA 50% • Mutated NPM 1 and FLT 3 -ITD high • Wild-type NPM 1 without FLT 3 -ITD or FLT 3 -ITD low (without adverse-risk genetic lesions) • Wild-type NPM 1 and FLT 3 -ITD (normal karyotype) • t(9; 11)(p 22; q 23); MLLT 3 -MLL • Any cytogenetics not classified as favorable or adverse 20% • t(6; 9)(p 23; q 34); DEK-NUP 214 • t(v; 11)(v; q 23); MLL (KMT 2 A) rearranged • Inv(3)(q 21 q 26. 2) or t(3; 3)(q 21; q 26. 2); RPN 1 -EVI 1 (GATA 2, MECOM (EVI 1) • t(9; 22)(q 34. 1; q 11. 2) BCR-ABL 1 • Monosomy 5 or del(5 q); monosomy 7; monosomy 17; abnormal 17 p • Complex karyotype(≥ 3 abnormalities) or monosomal karyotype • Wild-type NPM 1 and FLT 3 -ITD high • Mutated RUNX 1 • Mutated ASXL 1 • Mutated TP 53

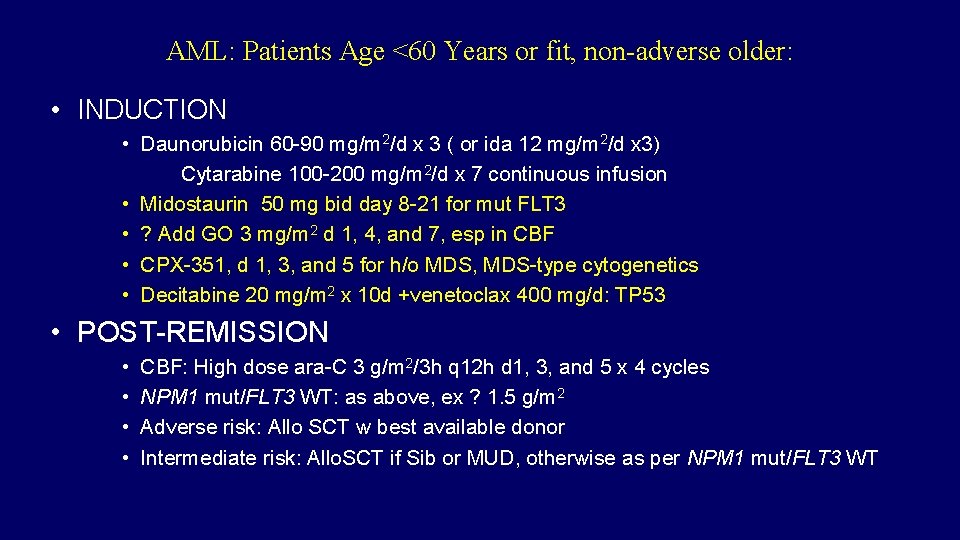
AML: Patients Age <60 Years or fit, non-adverse older: • INDUCTION • Daunorubicin 60 -90 mg/m 2/d x 3 ( or ida 12 mg/m 2/d x 3) Cytarabine 100 -200 mg/m 2/d x 7 continuous infusion • Midostaurin 50 mg bid day 8 -21 for mut FLT 3 • ? Add GO 3 mg/m 2 d 1, 4, and 7, esp in CBF • CPX-351, d 1, 3, and 5 for h/o MDS, MDS-type cytogenetics • Decitabine 20 mg/m 2 x 10 d +venetoclax 400 mg/d: TP 53 • POST-REMISSION • • CBF: High dose ara-C 3 g/m 2/3 h q 12 h d 1, 3, and 5 x 4 cycles NPM 1 mut/FLT 3 WT: as above, ex ? 1. 5 g/m 2 Adverse risk: Allo SCT w best available donor Intermediate risk: Allo. SCT if Sib or MUD, otherwise as per NPM 1 mut/FLT 3 WT

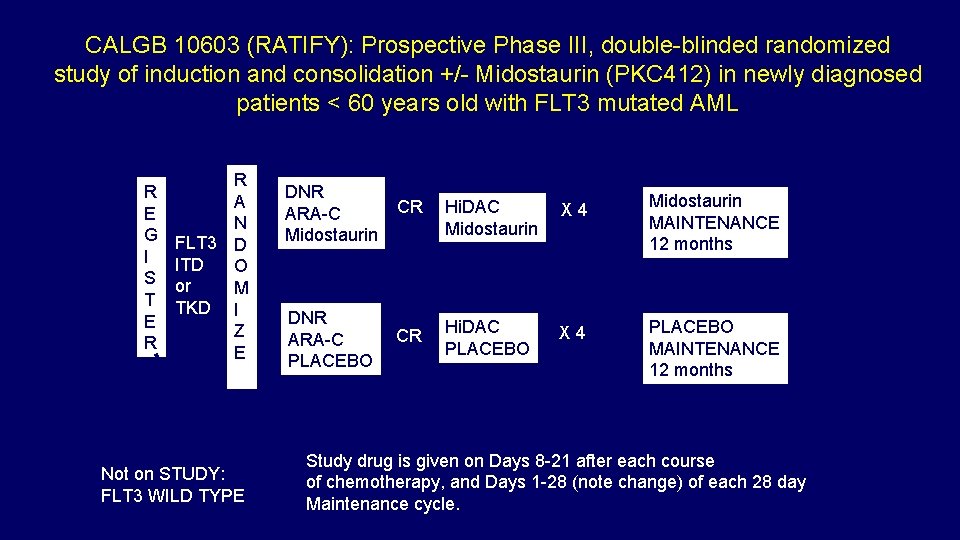
CALGB 10603 (RATIFY): Prospective Phase III, double-blinded randomized study of induction and consolidation +/- Midostaurin (PKC 412) in newly diagnosed patients < 60 years old with FLT 3 mutated AML R E G I S T E R R A N FLT 3 D ITD O or M TKD I Z E Not on STUDY: FLT 3 WILD TYPE DNR ARA-C Midostaurin CR Hi. DAC Midostaurin X 4 Midostaurin MAINTENANCE 12 months DNR ARA-C PLACEBO CR Hi. DAC PLACEBO X 4 PLACEBO MAINTENANCE 12 months Study drug is given on Days 8 -21 after each course of chemotherapy, and Days 1 -28 (note change) of each 28 day Maintenance cycle.

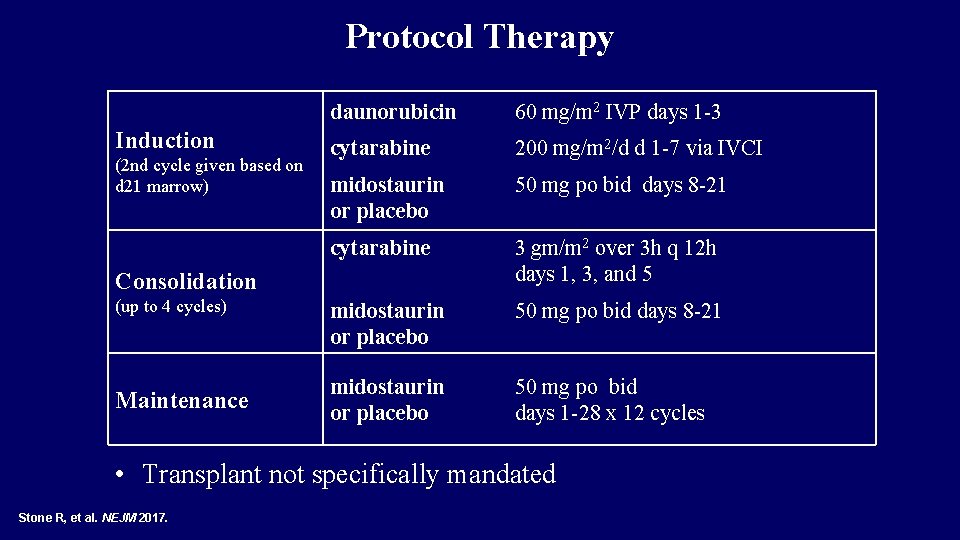
Protocol Therapy Induction (2 nd cycle given based on d 21 marrow) daunorubicin 60 mg/m 2 IVP days 1 -3 cytarabine 200 mg/m 2/d d 1 -7 via IVCI midostaurin or placebo 50 mg po bid days 8 -21 cytarabine 3 gm/m 2 over 3 h q 12 h days 1, 3, and 5 midostaurin or placebo 50 mg po bid days 8 -21 midostaurin or placebo 50 mg po bid days 1 -28 x 12 cycles Consolidation (up to 4 cycles) Maintenance • Transplant not specifically mandated Stone R, et al. NEJM 2017.

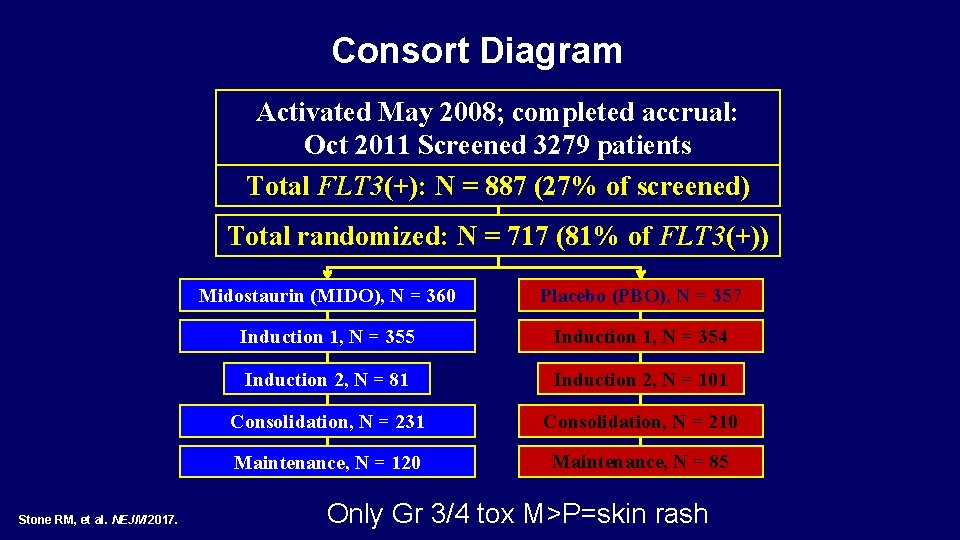
Consort Diagram Activated May 2008; completed accrual: Oct 2011 Screened 3279 patients Total FLT 3(+): N = 887 (27% of screened) Total randomized: N = 717 (81% of FLT 3(+)) Stone RM, et al. NEJM 2017. Midostaurin (MIDO), N = 360 Placebo (PBO), N = 357 Induction 1, N = 355 Induction 1, N = 354 Induction 2, N = 81 Induction 2, N = 101 Consolidation, N = 231 Consolidation, N = 210 Maintenance, N = 120 Maintenance, N = 85 Only Gr 3/4 tox M>P=skin rash

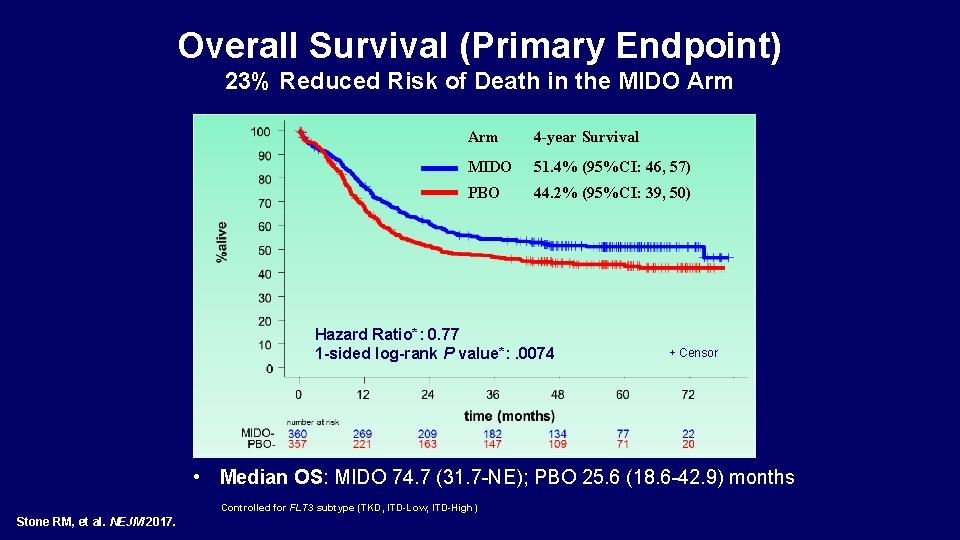
Overall Survival (Primary Endpoint) 23% Reduced Risk of Death in the MIDO Arm 4 -year Survival MIDO 51. 4% (95%CI: 46, 57) PBO 44. 2% (95%CI: 39, 50) Hazard Ratio*: 0. 77 1 -sided log-rank P value*: . 0074 + Censor • Median OS: MIDO 74. 7 (31. 7 -NE); PBO 25. 6 (18. 6 -42. 9) months NE, not estimable *Controlled for FLT 3 subtype (TKD, ITD-Low, ITD-High) Stone RM, et al. NEJM 2017.

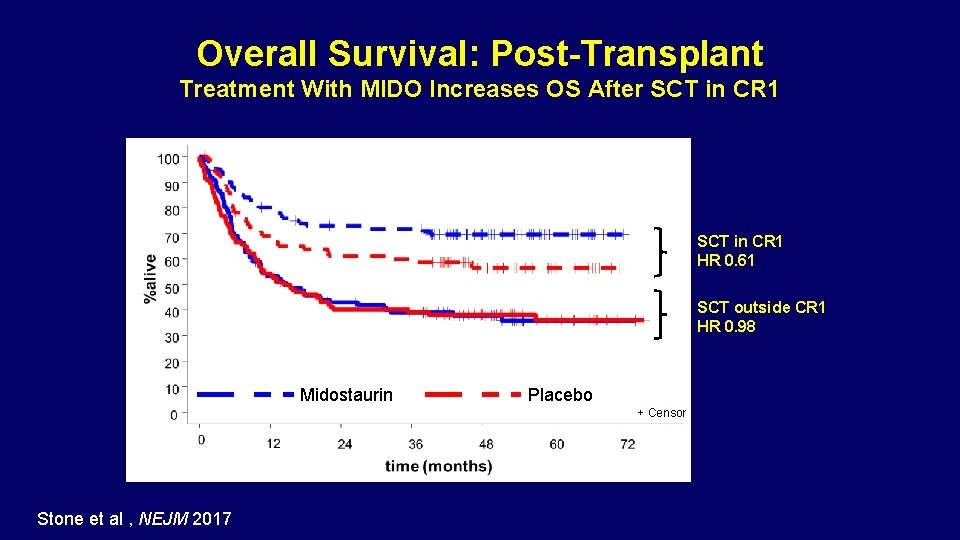
Overall Survival: Post-Transplant Treatment With MIDO Increases OS After SCT in CR 1 HR 0. 61 SCT outside CR 1 HR 0. 98 Midostaurin Placebo + Censor Stone RM, et al. Blood. 2015; 126: Abstract 6. Stone et al , NEJM 2017

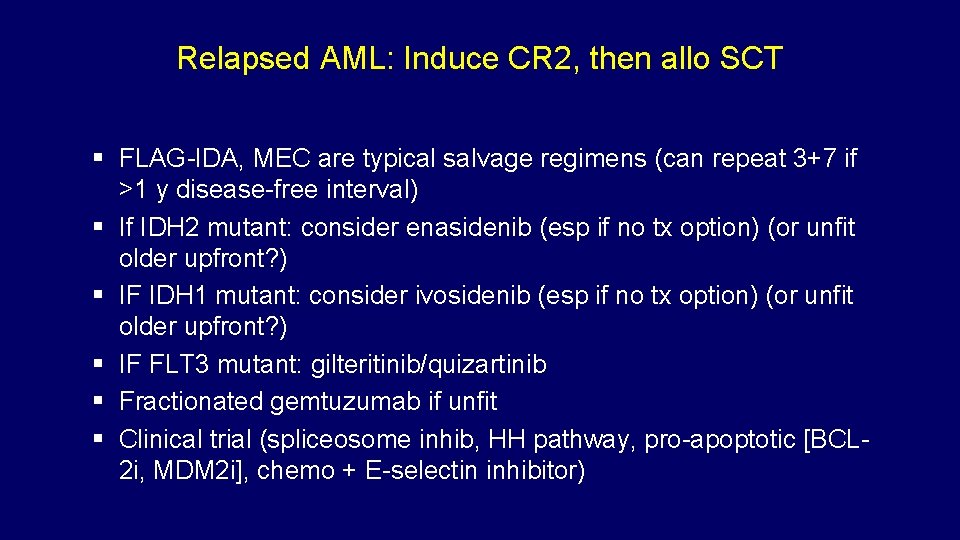
Relapsed AML: Induce CR 2, then allo SCT FLAG-IDA, MEC are typical salvage regimens (can repeat 3+7 if >1 y disease-free interval) If IDH 2 mutant: consider enasidenib (esp if no tx option) (or unfit older upfront? ) IF IDH 1 mutant: consider ivosidenib (esp if no tx option) (or unfit older upfront? ) IF FLT 3 mutant: gilteritinib/quizartinib Fractionated gemtuzumab if unfit Clinical trial (spliceosome inhib, HH pathway, pro-apoptotic [BCL 2 i, MDM 2 i], chemo + E-selectin inhibitor) Smith, BD, et al. Blood 2004 Knapper, S, et al. Blood 2006

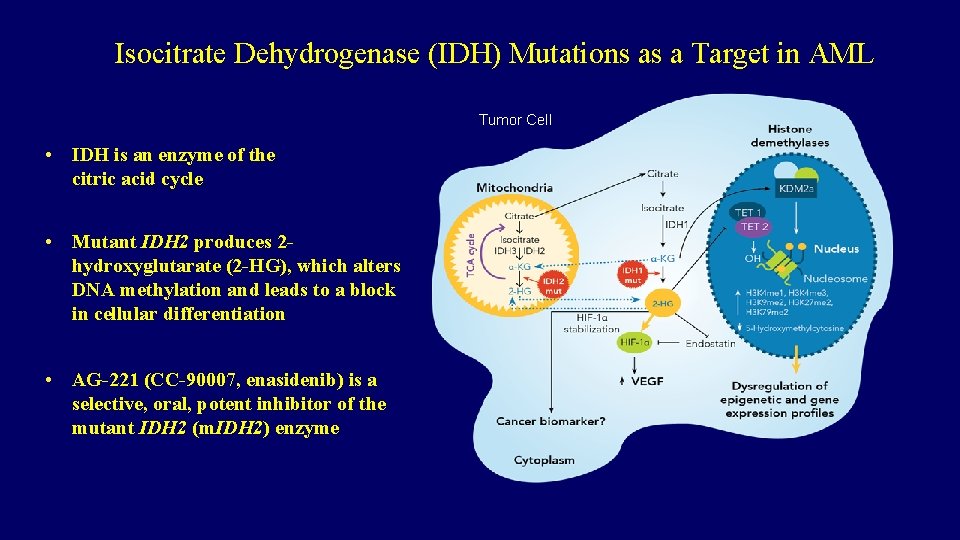
Isocitrate Dehydrogenase (IDH) Mutations as a Target in AML Tumor Cell • IDH is an enzyme of the citric acid cycle • Mutant IDH 2 produces 2 hydroxyglutarate (2 -HG), which alters DNA methylation and leads to a block in cellular differentiation • AG-221 (CC-90007, enasidenib) is a selective, oral, potent inhibitor of the mutant IDH 2 (m. IDH 2) enzyme

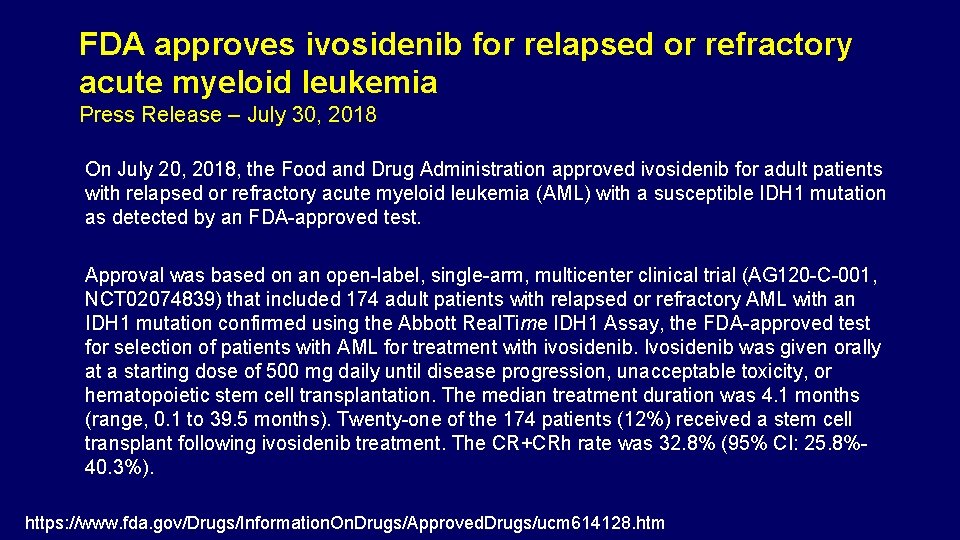
FDA approves ivosidenib for relapsed or refractory acute myeloid leukemia Press Release – July 30, 2018 On July 20, 2018, the Food and Drug Administration approved ivosidenib for adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible IDH 1 mutation as detected by an FDA-approved test. Approval was based on an open-label, single-arm, multicenter clinical trial (AG 120 -C-001, NCT 02074839) that included 174 adult patients with relapsed or refractory AML with an IDH 1 mutation confirmed using the Abbott Real. Time IDH 1 Assay, the FDA-approved test for selection of patients with AML for treatment with ivosidenib. Ivosidenib was given orally at a starting dose of 500 mg daily until disease progression, unacceptable toxicity, or hematopoietic stem cell transplantation. The median treatment duration was 4. 1 months (range, 0. 1 to 39. 5 months). Twenty-one of the 174 patients (12%) received a stem cell transplant following ivosidenib treatment. The CR+CRh rate was 32. 8% (95% CI: 25. 8%40. 3%). https: //www. fda. gov/Drugs/Information. On. Drugs/Approved. Drugs/ucm 614128. htm

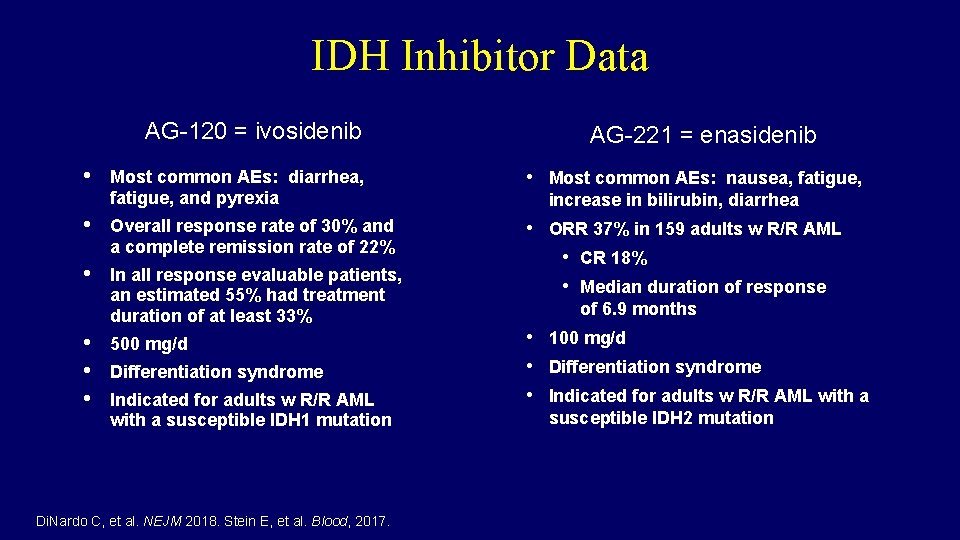
IDH Inhibitor Data AG-120 = ivosidenib • AG-221 = enasidenib Most common AEs: diarrhea, fatigue, and pyrexia • Most common AEs: nausea, fatigue, • Overall response rate of 30% and a complete remission rate of 22% • In all response evaluable patients, an estimated 55% had treatment duration of at least 33% • ORR 37% in 159 adults w R/R AML • CR 18% • Median duration of response • • • 500 mg/d Differentiation syndrome Indicated for adults w R/R AML with a susceptible IDH 1 mutation Di. Nardo C, et al. NEJM 2018. Stein E, et al. Blood, 2017. increase in bilirubin, diarrhea of 6. 9 months • 100 mg/d • Differentiation syndrome • Indicated for adults w R/R AML with a susceptible IDH 2 mutation

ASH 2018: IDH Inhibitor Data Sets • Stein EM et al. Ivosidenib or Enasidenib Combined with Induction and Consolidation Chemotherapy in Patients with Newly Diagnosed AML with an IDH 1 or IDH 2 Mutation Is Safe, Effective, and Leads to MRD-Negative Complete Remissions. Proc ASH 2018; Abstract 560. • Di. Nardo CD et al. Ivosidenib (AG-120) Induced Durable Remissions and Transfusion Independence in Patients with IDH 1 -Mutant Relapsed or Refractory Myelodysplastic Syndrome: Results from a Phase 1 Dose Escalation and Expansion Study. Proc ASH 2018; Abstract 1812. • Stein EM et al. Enasidenib Is Highly Active in Previously Untreated IDH 2 Mutant AML: Early Results from the Beat AML Master Trial. Proc ASH 2018; Abstract 287.

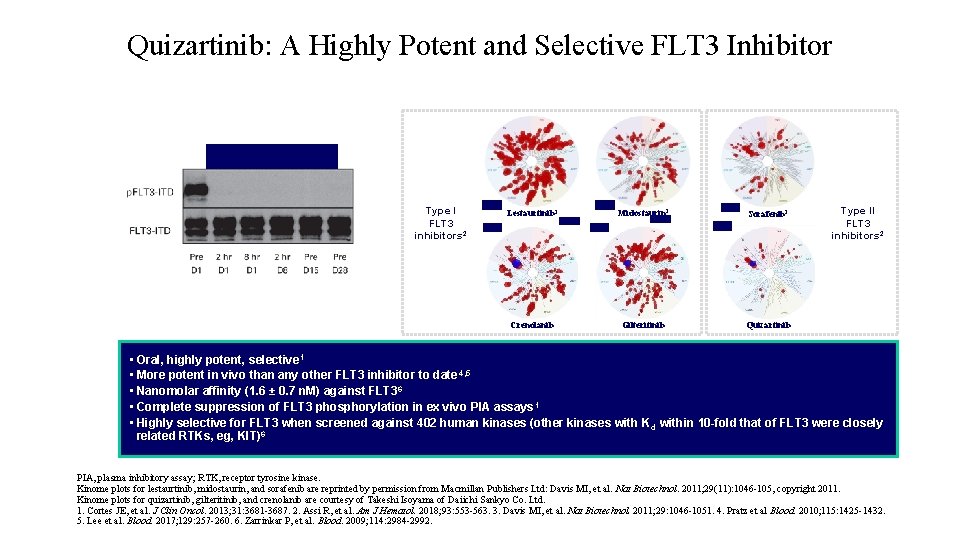
Quizartinib: A Highly Potent and Selective FLT 3 Inhibitor Quizartinib 60 mg 1 Type I FLT 3 inhibitors 2 Lestaurtinib 3 Midostaurin 3 Sorafenib 3 Crenolanib Gilteritinib Quizartinib Type II FLT 3 inhibitors 2 Cortes JE, et al. J Clin Oncol. 2013; 31(29): 3681 -3687. Reprinted with permission. © 2013 American Society of Clinical Oncology. All rights reserved. • Oral, highly potent, selective 1 • More potent in vivo than any other FLT 3 inhibitor to date 4, 5 • Nanomolar affinity (1. 6 ± 0. 7 n. M) against FLT 3 6 • Complete suppression of FLT 3 phosphorylation in ex vivo PIA assays 1 • Highly selective for FLT 3 when screened against 402 human kinases (other kinases with K d within 10 -fold that of FLT 3 were closely related RTKs, eg, KIT)6 PIA, plasma inhibitory assay; RTK, receptor tyrosine kinase. Kinome plots for lestaurtinib, midostaurin, and sorafenib are reprinted by permission from Macmillan Publishers Ltd: Davis MI, et al. Nat Biotechnol. 2011; 29(11): 1046 -105, copyright 2011. Kinome plots for quizartinib, gilteritinib, and crenolanib are courtesy of Takeshi Isoyama of Daiichi Sankyo Co. Ltd. 1. Cortes JE, et al. J Clin Oncol. 2013; 31: 3681 -3687. 2. Assi R, et al. Am J Hematol. 2018; 93: 553 -563. 3. Davis MI, et al. Nat Biotechnol. 2011; 29: 1046 -1051. 4. Pratz et al Blood. 2010; 115: 1425 -1432. 5. Lee et al. Blood. 2017; 129: 257 -260. 6. Zarrinkar P, et al. Blood. 2009; 114: 2984 -2992.

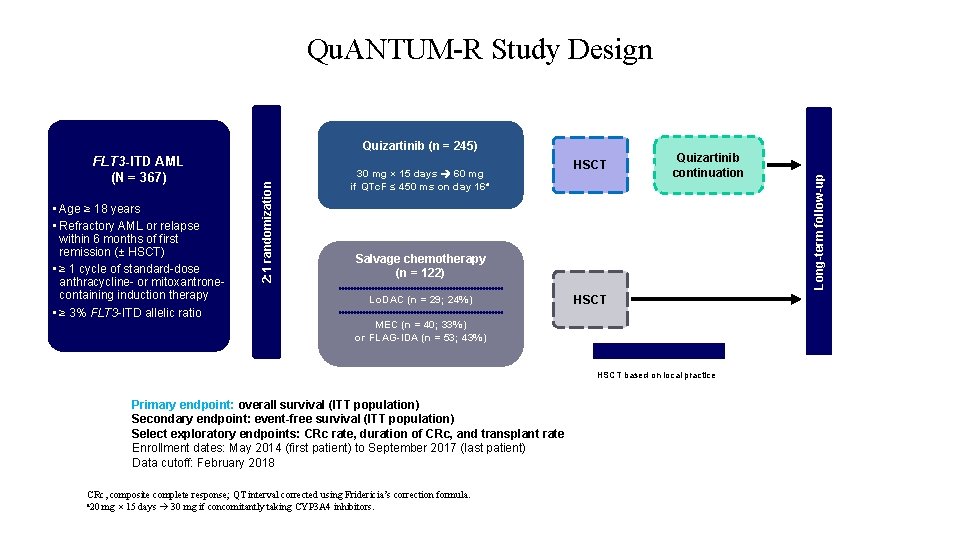
FLT 3 -ITD AML (N = 367) • Age ≥ 18 years • Refractory AML or relapse within 6 months of first remission (± HSCT) • ≥ 1 cycle of standard-dose anthracycline- or mitoxantronecontaining induction therapy • ≥ 3% FLT 3 -ITD allelic ratio 2: 1 randomization Quizartinib (n = 245) 30 mg × 15 days 60 mg if QTc. F ≤ 450 ms on day 16 a HSCT Quizartinib continuation Salvage chemotherapy (n = 122) Lo. DAC (n = 29; 24%) HSCT MEC (n = 40; 33%) or FLAG-IDA (n = 53; 43%) Optional treatments HSCT based on local practice Primary endpoint: overall survival (ITT population) Secondary endpoint: event-free survival (ITT population) Select exploratory endpoints: CRc rate, duration of CRc, and transplant rate Enrollment dates: May 2014 (first patient) to September 2017 (last patient) Data cutoff: February 2018 CRc, composite complete response; QT interval corrected using Fridericia’s correction formula. a 20 mg × 15 days 30 mg if concomitantly taking CYP 3 A 4 inhibitors. Long-term follow-up Qu. ANTUM-R Study Design

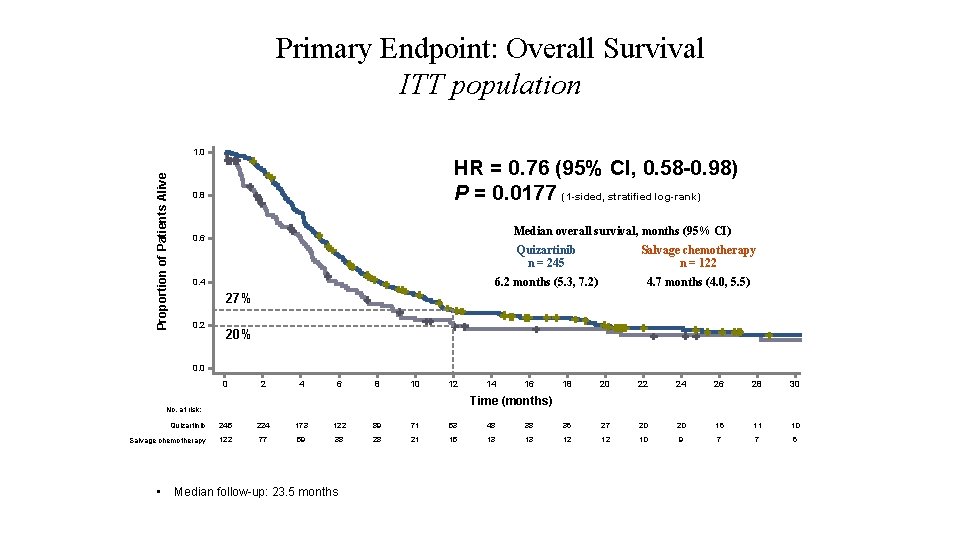
Primary Endpoint: Overall Survival ITT population Proportion of Patients Alive 1. 0 HR = 0. 76 (95% CI, 0. 58 -0. 98) P = 0. 0177 (1 -sided, stratified log-rank) 0. 8 Median overall survival, months (95% CI) 0. 6 0. 4 Quizartinib n = 245 Salvage chemotherapy n = 122 6. 2 months (5. 3, 7. 2) 4. 7 months (4. 0, 5. 5) 27% 0. 2 20% 0. 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 Time (months) No. at risk: Quizartinib 245 224 173 122 89 71 53 48 38 36 27 20 20 16 11 10 Salvage chemotherapy 122 77 59 38 28 21 15 13 13 12 12 10 9 7 7 6 • Median follow-up: 23. 5 months

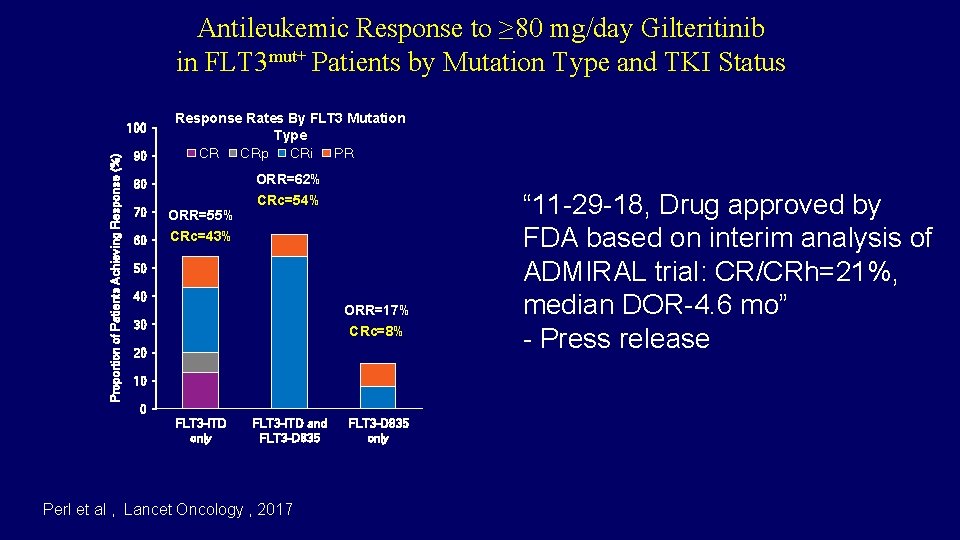
Antileukemic Response to ≥ 80 mg/day Gilteritinib in FLT 3 mut+ Patients by Mutation Type and TKI Status 90 Response Rates By FLT 3 Mutation Type CR CRp CRi PR 80 ORR=62% Proportion of Patients Achieving Response (%) 100 70 ORR=55% 60 CRc=43% CRc=54% 50 40 ORR=17% 30 CRc=8% 20 10 0 FLT 3 -ITD only FLT 3 -ITD and FLT 3 -D 835 Perl et al , Lancet Oncology , 2017 FLT 3 -D 835 only “ 11 -29 -18, Drug approved by FDA based on interim analysis of ORR=42% ADMIRAL trial: CR/CRh=21%, median DOR-4. 6 mo” - Press release

Module 10: Acute Myeloid Leukemia Drs Stone and Pollyea

Recently Approved and Emerging Therapeutic Approaches Daniel A. Pollyea, MD Associate Professor of Medicine University of Colorado School of Medicine

Disclosures Advisory Committee Abb. Vie Inc, Astellas Pharma Global Development Inc, Celgene Corporation, Celyad, Daiichi Sankyo Inc, Gilead Sciences Inc Contracted Research Abb. Vie Inc Data and Safety Monitoring Board/Committee Glyco. Mimetics Inc, Tolero Pharmaceuticals

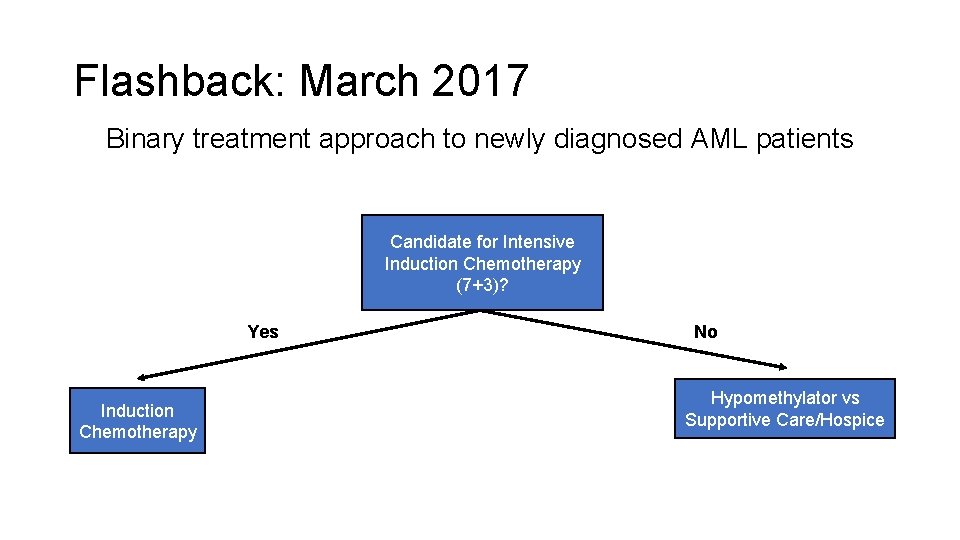
Flashback: March 2017 Binary treatment approach to newly diagnosed AML patients Candidate for Intensive Induction Chemotherapy (7+3)? Yes Induction Chemotherapy No Hypomethylator vs Supportive Care/Hospice

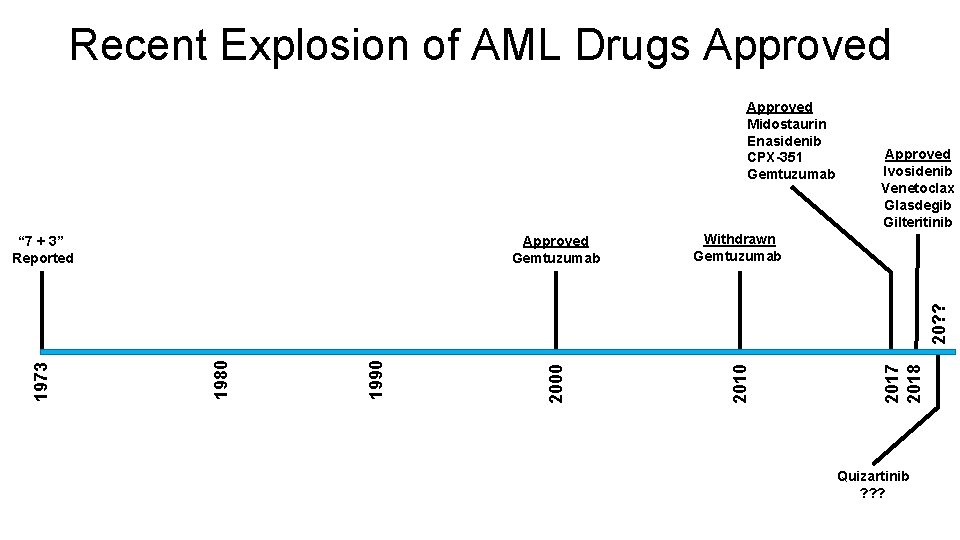
Recent Explosion of AML Drugs Approved Withdrawn Gemtuzumab Approved Ivosidenib Venetoclax Glasdegib Gilteritinib 2017 2018 1990 1980 1973 20? ? Approved Gemtuzumab 2010 “ 7 + 3” Reported 2000 Approved Midostaurin Enasidenib CPX-351 Gemtuzumab Quizartinib ? ? ?

Venetoclax • Specific inhibitor of BCL-2 • FDA approved November 2018 with azacitidine, decitabine or LDAC for newly diagnosed AML patients who are: • 75 years or older OR • Have comorbidities that preclude the use of intensive induction chemotherapy

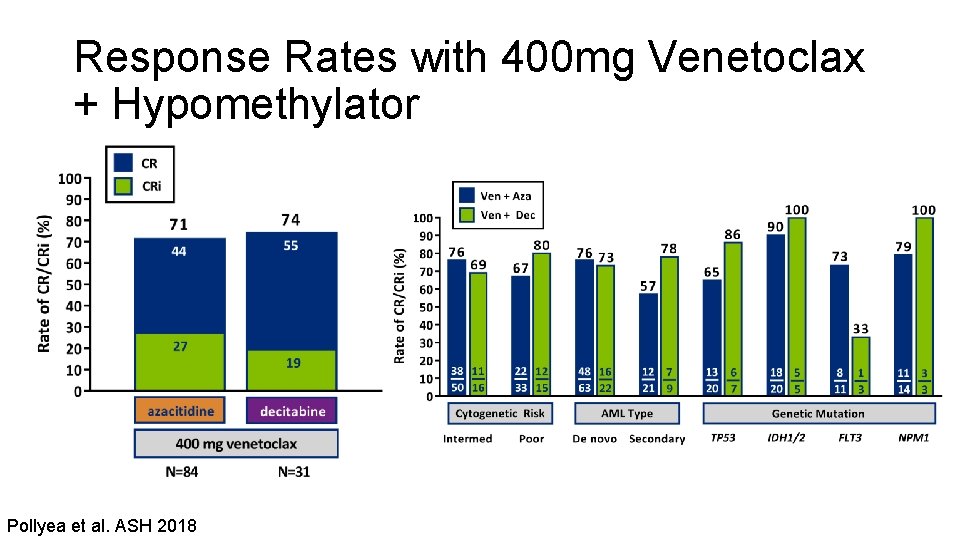
Response Rates with 400 mg Venetoclax + Hypomethylator Pollyea et al. ASH 2018

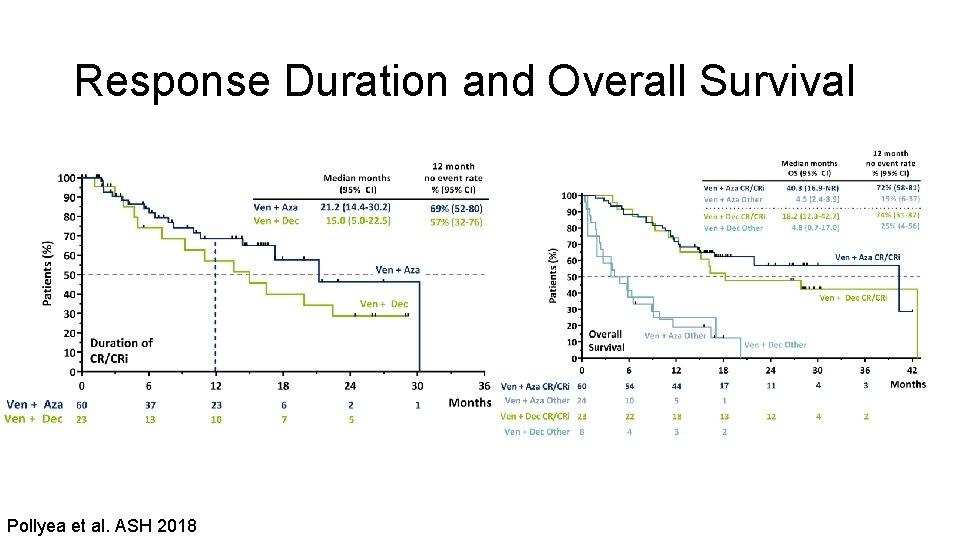
Response Duration and Overall Survival Pollyea et al. ASH 2018

Toxicity of Special Interest: TLS • Mitigation techniques included pre-emptive hospitalization, rapid intra-patient dose escalation, fluids and monitoring • No TLS was observed in hypomethylator backbone escalation/expansion patients (N=145) • 1 “laboratory TLS” case in LDAC backbone escalation/expansion patients (N=82) Does this mean we don’t have to worry about TLS or that we need to aggressively mitigate?

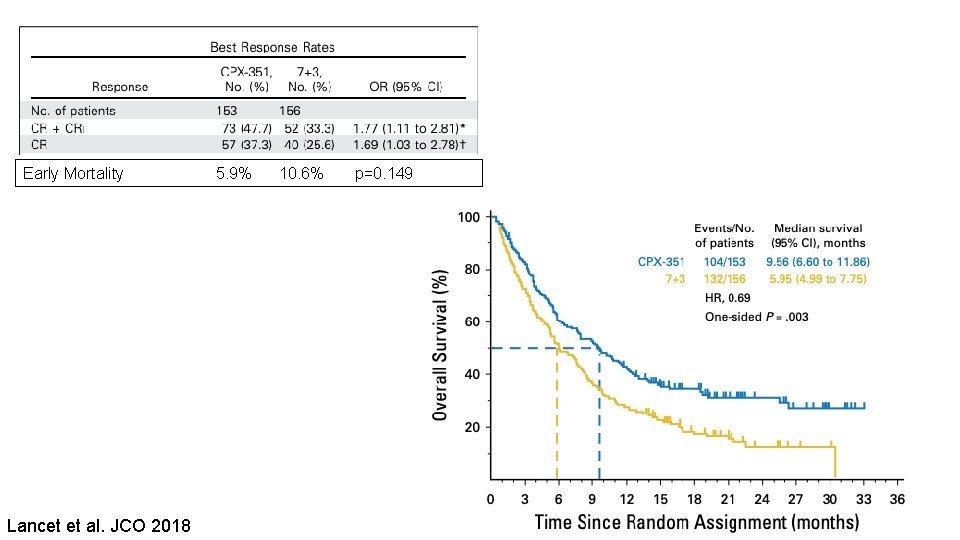
Liposomal Daunorubicin + Cytarabine (CPX-351) • Approved for newly diagnosed t-AML or AML with MDS-related changes • Superior response rate • Survival benefit, with reduced toxicity, for this population when compared with standard 7+3

Early Mortality 5. 9% 10. 6% p=0. 149 Lancet et al. JCO 2018

Other Ongoing Studies with CPX-351 • With venetoclax • With gemtuzumab • With enasidenib

Glasdegib • Inhibitor of Smoothened, key component of the Hedgehog signaling pathway • Activated in cancer/leukemia cells, particularly stem cell populations • Now FDA approved with LDAC for newly diagnosed AML patients 75 and older or those who cannot tolerate intensive chemotherapy

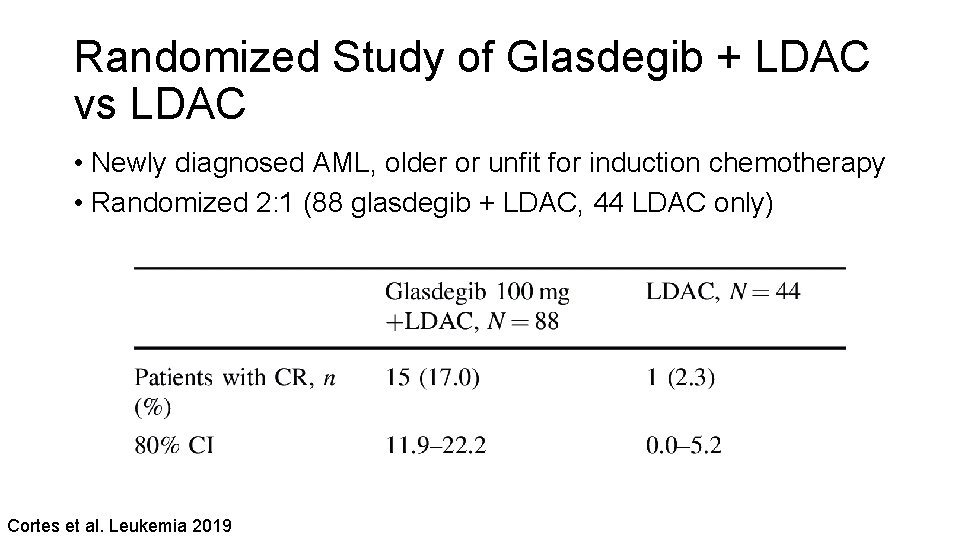
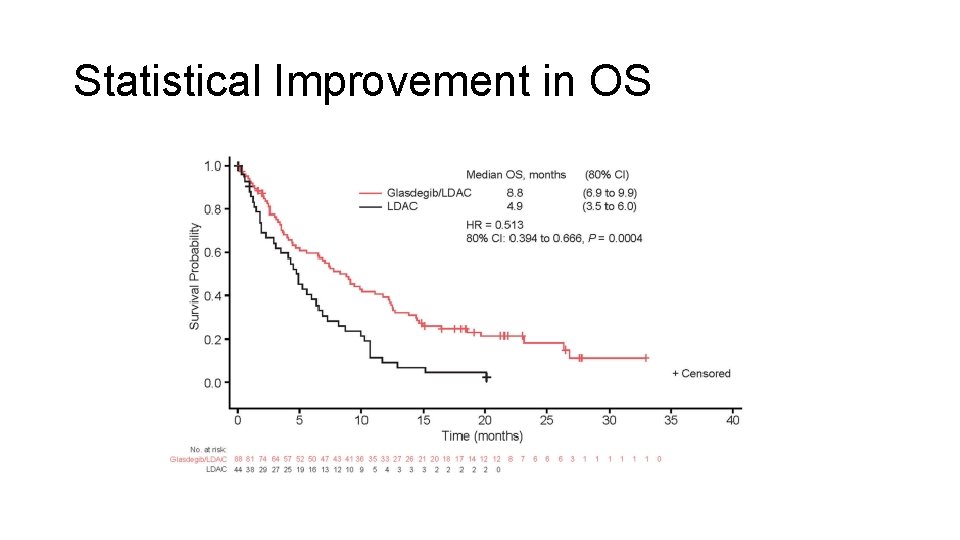
Randomized Study of Glasdegib + LDAC vs LDAC • Newly diagnosed AML, older or unfit for induction chemotherapy • Randomized 2: 1 (88 glasdegib + LDAC, 44 LDAC only) Cortes et al. Leukemia 2019

Statistical Improvement in OS

What is the Clinical Role for Glasdegib? • When to use it vs otherapies?

Module 10: Acute Myeloid Leukemia Boards Mini-Review: Dr Stone Topic/issue: Management of hyperleukocytosis, including leukapheresis

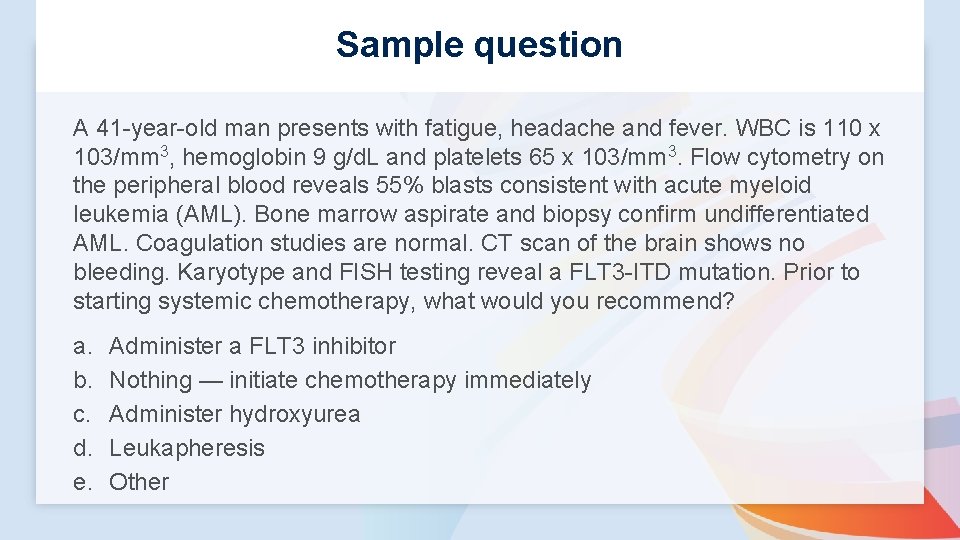
Sample question A 41 -year-old man presents with fatigue, headache and fever. WBC is 110 x 103/mm 3, hemoglobin 9 g/d. L and platelets 65 x 103/mm 3. Flow cytometry on the peripheral blood reveals 55% blasts consistent with acute myeloid leukemia (AML). Bone marrow aspirate and biopsy confirm undifferentiated AML. Coagulation studies are normal. CT scan of the brain shows no bleeding. Karyotype and FISH testing reveal a FLT 3 -ITD mutation. Prior to starting systemic chemotherapy, what would you recommend? a. b. c. d. e. Administer a FLT 3 inhibitor Nothing — initiate chemotherapy immediately Administer hydroxyurea Leukapheresis Other

Module 10: Acute Myeloid Leukemia Boards Mini-Review: Dr Pollyea Topic/issue: Prevention of infections (antimicrobials, vaccines, antifungals) and variability in approach based on disease stage and treatment; impact of drug-drug interactions

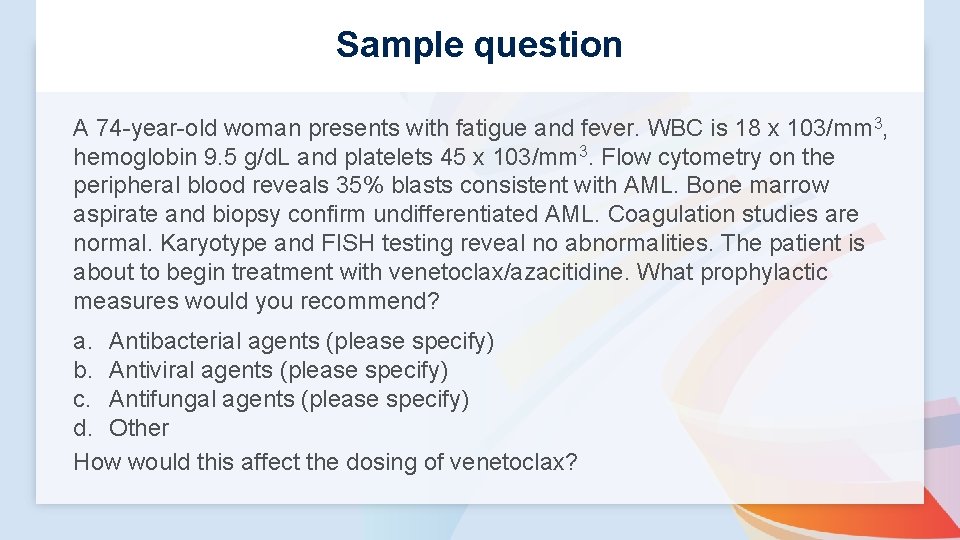
Sample question A 74 -year-old woman presents with fatigue and fever. WBC is 18 x 103/mm 3, hemoglobin 9. 5 g/d. L and platelets 45 x 103/mm 3. Flow cytometry on the peripheral blood reveals 35% blasts consistent with AML. Bone marrow aspirate and biopsy confirm undifferentiated AML. Coagulation studies are normal. Karyotype and FISH testing reveal no abnormalities. The patient is about to begin treatment with venetoclax/azacitidine. What prophylactic measures would you recommend? a. Antibacterial agents (please specify) b. Antiviral agents (please specify) c. Antifungal agents (please specify) d. Other How would this affect the dosing of venetoclax?
 Antigentest åre
Antigentest åre To stop proceedings temporarily; move to another place
To stop proceedings temporarily; move to another place Divorce laws in india
Divorce laws in india Flow chart of court proceedings in the philippines
Flow chart of court proceedings in the philippines Meeting proceedings
Meeting proceedings Definition of sanctifying grace
Definition of sanctifying grace Actual yield
Actual yield Raymond carver will you please be quiet please
Raymond carver will you please be quiet please Please note that comma
Please note that comma Signal word
Signal word Linear method of note taking
Linear method of note taking Debit memo
Debit memo Difference between note making and note taking
Difference between note making and note taking Simple discount rate
Simple discount rate Financial documents order
Financial documents order Difference between note making and note taking
Difference between note making and note taking Debit note example
Debit note example Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Hệ hô hấp
Hệ hô hấp Lp html
Lp html Bảng số nguyên tố lớn hơn 1000
Bảng số nguyên tố lớn hơn 1000 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Tư thế worm breton
Tư thế worm breton ưu thế lai là gì
ưu thế lai là gì Thẻ vin
Thẻ vin Tư thế ngồi viết
Tư thế ngồi viết Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết V cc cc
V cc cc Thể thơ truyền thống
Thể thơ truyền thống Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con đại từ thay thế
đại từ thay thế Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính thế năng
Công thức tính thế năng 101012 bằng
101012 bằng Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể