Please note these are the actual videorecorded proceedings
















- Slides: 71

Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content.

Oncology Grand Rounds Emerging Strategies in Non-Small Cell Lung Cancer Nurse and Physician Investigators Discuss New Agents, Novel Therapies and Actual Cases from Practice Friday, April 12, 2019 12: 15 PM – 1: 45 PM Faculty Kelly EH Goodwin, MSN, RN, ANP-BC Judy Pagtama, RN, MSN, FNP-C Anne S Tsao, MD Joel W Neal, MD, Ph. D Moderator Neil Love, MD

Oncology Grand Rounds Series

Disclosures for Moderator Neil Love, MD Dr Love is president and CEO of Research To Practice receives funds in the form of educational grants to develop CME activities from the following commercial interests: Abb. Vie Inc, Acerta Pharma — A member of the Astra. Zeneca Group, Adaptive Biotechnologies, Agendia Inc, Agios Pharmaceuticals Inc, Amgen Inc, Ariad Pharmaceuticals Inc, Array Bio. Pharma Inc, Astellas Pharma Global Development Inc, Astra. Zeneca Pharmaceuticals LP, Bayer Health. Care Pharmaceuticals, Biodesix Inc, bio. Theranostics Inc, Boehringer Ingelheim Pharmaceuticals Inc, Boston Biomedical Inc, Bristol-Myers Squibb Company, Celgene Corporation, Clovis Oncology, Daiichi Sankyo Inc, Dendreon Pharmaceuticals Inc, Eisai Inc, Exelixis Inc, Foundation Medicine, Genentech, Genmab, Genomic Health Inc, Gilead Sciences Inc, Guardant Health, Halozyme Inc, Immuno. Gen Inc, Incyte Corporation, Infinity Pharmaceuticals Inc, Ipsen Biopharmaceuticals Inc, Janssen Biotech Inc, administered by Janssen Scientific Affairs LLC, Jazz Pharmaceuticals Inc, Kite Pharma Inc, Lexicon Pharmaceuticals Inc, Lilly, Loxo Oncology, Merck, Merrimack Pharmaceuticals Inc, Myriad Genetic Laboratories Inc, Natera Inc, Novartis, Oncopeptides, Pfizer Inc, Pharmacyclics LLC, an Abb. Vie Company, Prometheus Laboratories Inc, Puma Biotechnology Inc, Regeneron Pharmaceuticals Inc, Sandoz Inc, a Novartis Division, Sanofi Genzyme, Seattle Genetics, Sirtex Medical Ltd, Spectrum Pharmaceuticals Inc, Taiho Oncology Inc, Takeda Oncology, Tesaro, Teva Oncology, Tokai Pharmaceuticals Inc and Tolero Pharmaceuticals.

Kelly EH Goodwin, MSN, RN, ANP-BC Thoracic Cancer Center, Massachusetts General Hospital Boston, Massachusetts

Disclosures for Ms Goodwin No relevant conflicts of interest to disclose

Joel W Neal, MD, Ph. D Assistant Professor of Medicine Division of Oncology Stanford Cancer Institute Stanford University Stanford, California

Disclosures for Dr Neal Advisory Committee and Consulting Agreements Ariad Pharmaceuticals Inc, Astra. Zeneca Pharmaceuticals LP, Exelixis Inc, Genentech, Jounce Therapeutics Inc, Lilly, Loxo Oncology, Roche Laboratories Inc, Takeda Oncology Contracted Research Ariad Pharmaceuticals Inc, Boehringer Ingelheim Pharmaceuticals Inc, Exelixis Inc, Genentech, Merck, Nektar, Novartis, Roche Laboratories Inc, Takeda Oncology

Judy Pagtama, RN, MSN, FNP-C Nurse Practitioner Stanford Cancer Institute Stanford, California

Disclosures for Ms Pagtama No relevant conflicts of interest to disclose

Anne S Tsao, MD Professor Director, Mesothelioma Program Director, Thoracic Chemo-Radiation Program Department of Thoracic/Head and Neck Medical Oncology The University of Texas MD Anderson Cancer Center Houston, Texas

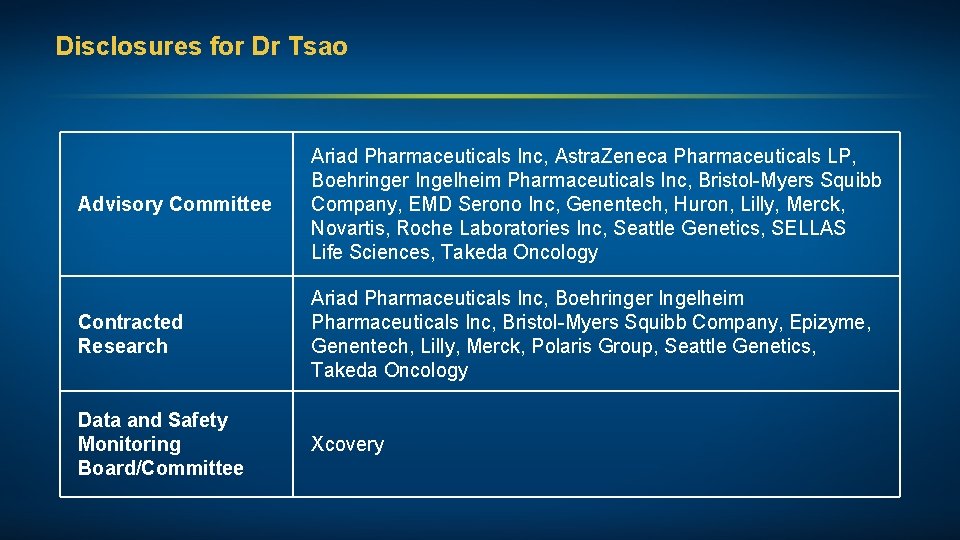
Disclosures for Dr Tsao Advisory Committee Ariad Pharmaceuticals Inc, Astra. Zeneca Pharmaceuticals LP, Boehringer Ingelheim Pharmaceuticals Inc, Bristol-Myers Squibb Company, EMD Serono Inc, Genentech, Huron, Lilly, Merck, Novartis, Roche Laboratories Inc, Seattle Genetics, SELLAS Life Sciences, Takeda Oncology Contracted Research Ariad Pharmaceuticals Inc, Boehringer Ingelheim Pharmaceuticals Inc, Bristol-Myers Squibb Company, Epizyme, Genentech, Lilly, Merck, Polaris Group, Seattle Genetics, Takeda Oncology Data and Safety Monitoring Board/Committee Xcovery

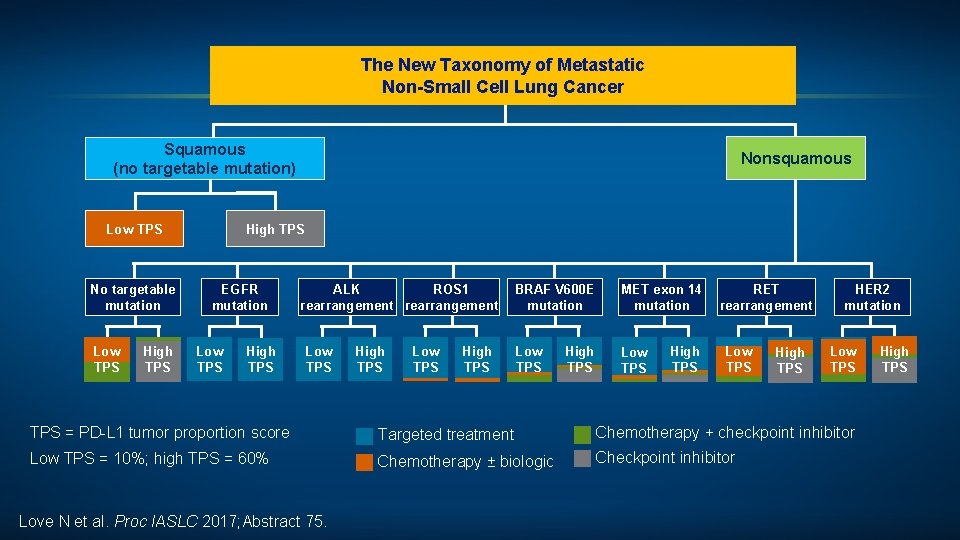
The New Taxonomy of Metastatic Non-Small Cell Lung Cancer Squamous (no targetable mutation) Low TPS No targetable mutation Low TPS High TPS Nonsquamous High TPS EGFR mutation Low TPS High TPS ALK ROS 1 rearrangement Low TPS High TPS BRAF V 600 E mutation MET exon 14 RET mutation rearrangement Low TPS High TPS HER 2 mutation Low TPS = PD-L 1 tumor proportion score Targeted treatment Chemotherapy + checkpoint inhibitor Low TPS = 10%; high TPS = 60% Chemotherapy ± biologic Checkpoint inhibitor Love N et al. Proc IASLC 2017; Abstract 75. High TPS

Emerging Strategies in NSCLC: Agenda EGFR-Mutated Metastatic NSCLC Immune Checkpoint Inhibitor Therapy for Metastatic NSCLC Immunotherapy as Consolidation After Chemoradiation of Locally Advanced NSCLC

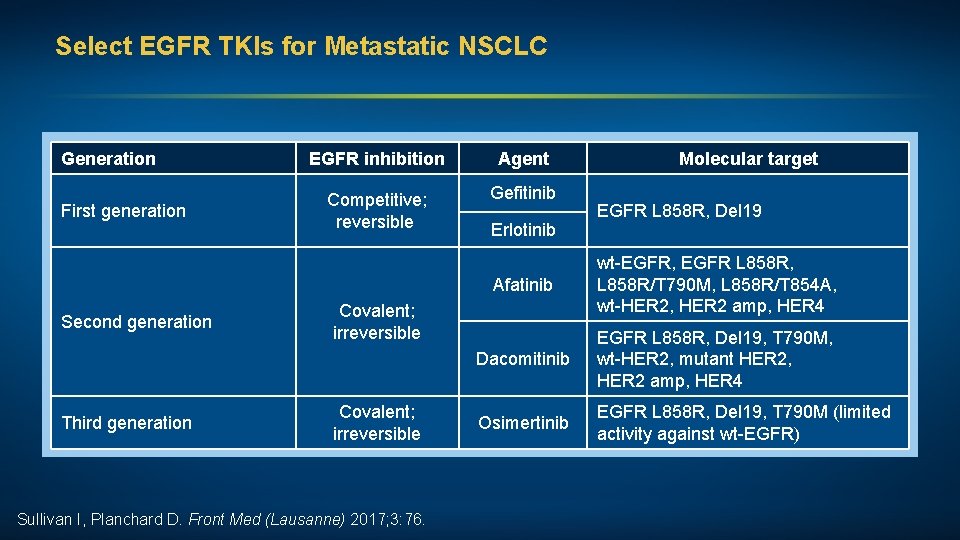
Select EGFR TKIs for Metastatic NSCLC Generation First generation Second generation Third generation EGFR inhibition Agent Competitive; reversible Gefitinib Erlotinib Sullivan I, Planchard D. Front Med (Lausanne) 2017; 3: 76. EGFR L 858 R, Del 19 Afatinib wt-EGFR, EGFR L 858 R, L 858 R/T 790 M, L 858 R/T 854 A, wt-HER 2, HER 2 amp, HER 4 Dacomitinib EGFR L 858 R, Del 19, T 790 M, wt-HER 2, mutant HER 2, HER 2 amp, HER 4 Osimertinib EGFR L 858 R, Del 19, T 790 M (limited activity against wt-EGFR) Covalent; irreversible Molecular target

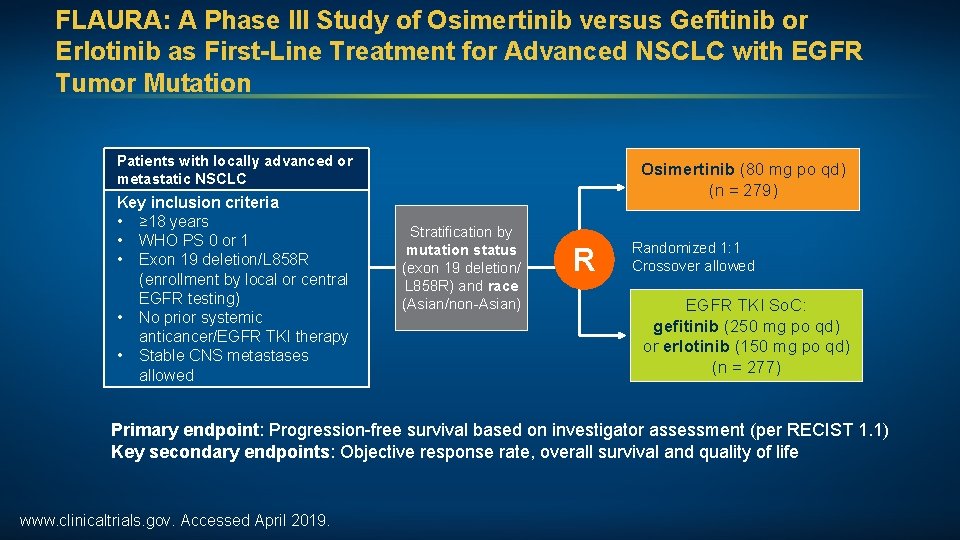
FLAURA: A Phase III Study of Osimertinib versus Gefitinib or Erlotinib as First-Line Treatment for Advanced NSCLC with EGFR Tumor Mutation Patients with locally advanced or metastatic NSCLC Key inclusion criteria • ≥ 18 years • WHO PS 0 or 1 • Exon 19 deletion/L 858 R (enrollment by local or central EGFR testing) • No prior systemic anticancer/EGFR TKI therapy • Stable CNS metastases allowed Osimertinib (80 mg po qd) (n = 279) Stratification by mutation status (exon 19 deletion/ L 858 R) and race (Asian/non-Asian) R Randomized 1: 1 Crossover allowed EGFR TKI So. C: gefitinib (250 mg po qd) or erlotinib (150 mg po qd) (n = 277) Primary endpoint: Progression-free survival based on investigator assessment (per RECIST 1. 1) Key secondary endpoints: Objective response rate, overall survival and quality of life www. clinicaltrials. gov. Accessed April 2019.

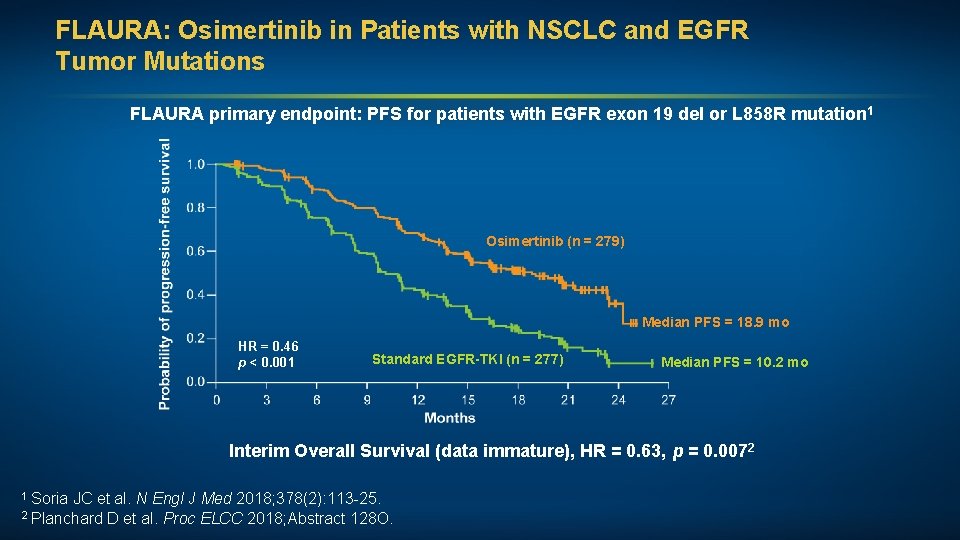
FLAURA: Osimertinib in Patients with NSCLC and EGFR Tumor Mutations FLAURA primary endpoint: PFS for patients with EGFR exon 19 del or L 858 R mutation 1 Osimertinib (n = 279) Median PFS = 18. 9 mo HR = 0. 46 p < 0. 001 Standard EGFR-TKI (n = 277) Median PFS = 10. 2 mo Interim Overall Survival (data immature), HR = 0. 63, p = 0. 0072 1 Soria JC et al. N Engl J Med 2018; 378(2): 113 -25. ELCC 2018; Abstract 128 O. 2 Planchard D et al. Proc

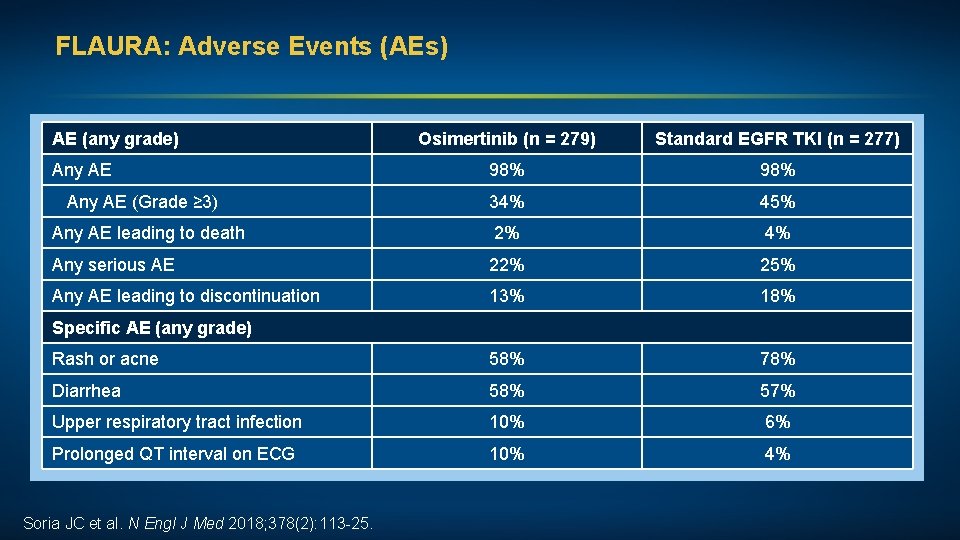
FLAURA: Adverse Events (AEs) AE (any grade) Osimertinib (n = 279) Standard EGFR TKI (n = 277) Any AE 98% Any AE (Grade ≥ 3) 34% 45% Any AE leading to death 2% 4% Any serious AE 22% 25% Any AE leading to discontinuation 13% 18% Rash or acne 58% 78% Diarrhea 58% 57% Upper respiratory tract infection 10% 6% Prolonged QT interval on ECG 10% 4% Specific AE (any grade) Soria JC et al. N Engl J Med 2018; 378(2): 113 -25.

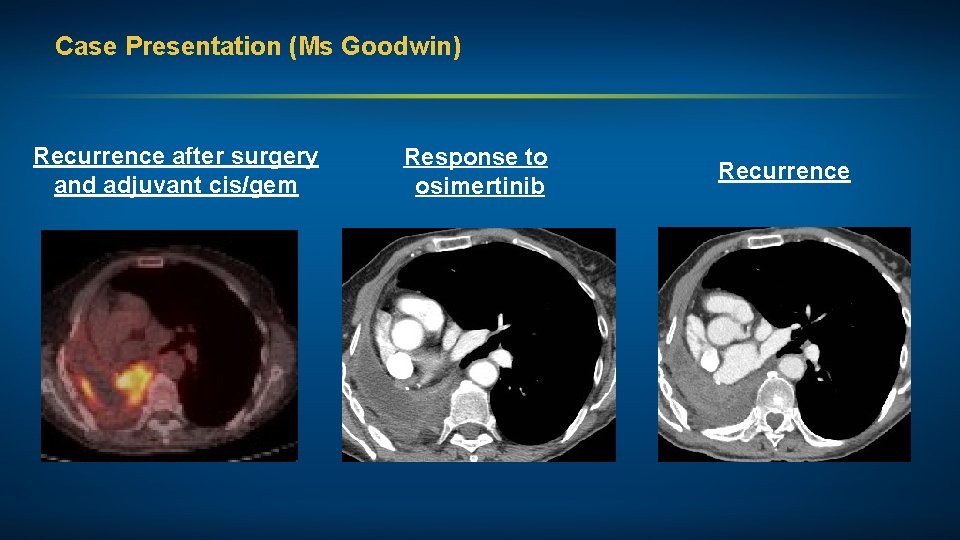
Case Presentation (Ms Goodwin) A woman in her early 70 s, a nonsmoker, with Stage IV adenosquamous NSCLC (EGFR 19 deletion, EGFR amplification and subsequent MET amplification) Treatments received • R pneumonectomy + adjuvant cisplatin/gemcitabine • Resection of left frontal mass and CNS radiation therapy (RT) • Osimertinib • Continued osimertinib + crizotinib Other considerations • Prior history of depression • Very involved daughter shields mother from bad news; pt defers to daughter

Case Presentation (Ms Goodwin) Recurrence after surgery and adjuvant cis/gem Response to osimertinib Recurrence

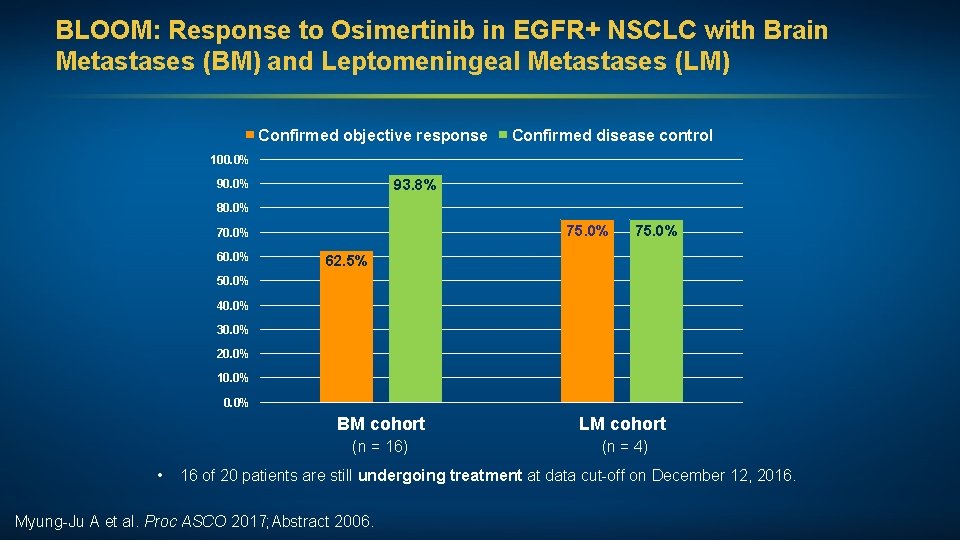
BLOOM: Response to Osimertinib in EGFR+ NSCLC with Brain Metastases (BM) and Leptomeningeal Metastases (LM) Confirmed objective response Confirmed disease control 100. 0% 93. 8% 90. 0% 80. 0% 75. 0% 70. 0% 60. 0% 75. 0% 62. 5% 50. 0% 40. 0% 30. 0% 20. 0% 10. 0% • BM cohort LM cohort (n = 16) (n = 4) 16 of 20 patients are still undergoing treatment at data cut-off on December 12, 2016. Myung-Ju A et al. Proc ASCO 2017; Abstract 2006.

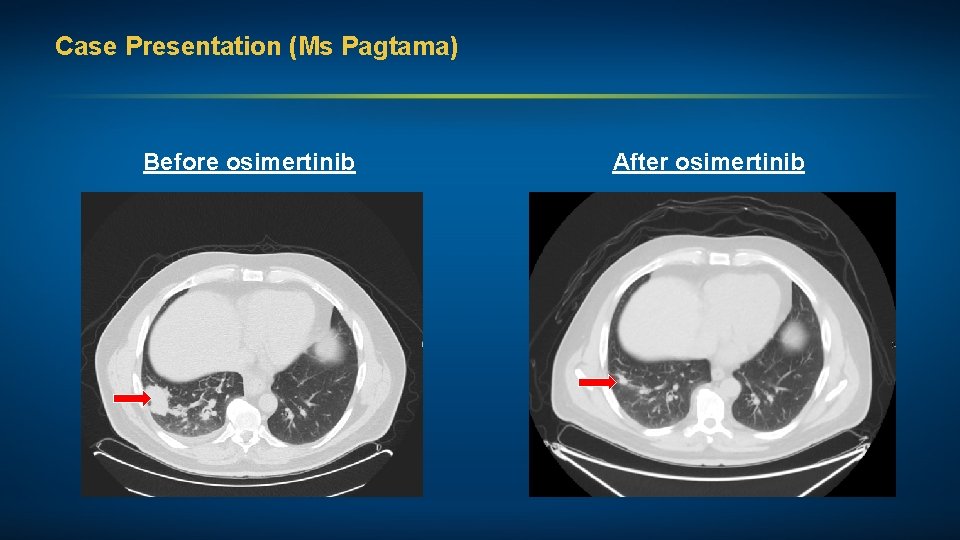
Case Presentation (Ms Pagtama) A man in his late 50 s with Stage IV adenocarcinoma of the lung (EGFR exon 19 deletion, PD-L 1 0%) • Right pleural effusion, single brain metastasis Treatments received • Osimertinib Other considerations • Works full time • Married, with 3 children • Spiritual, Zen meditation • Osimertinib-related mild rash, dry skin and alternating diarrhea and constipation

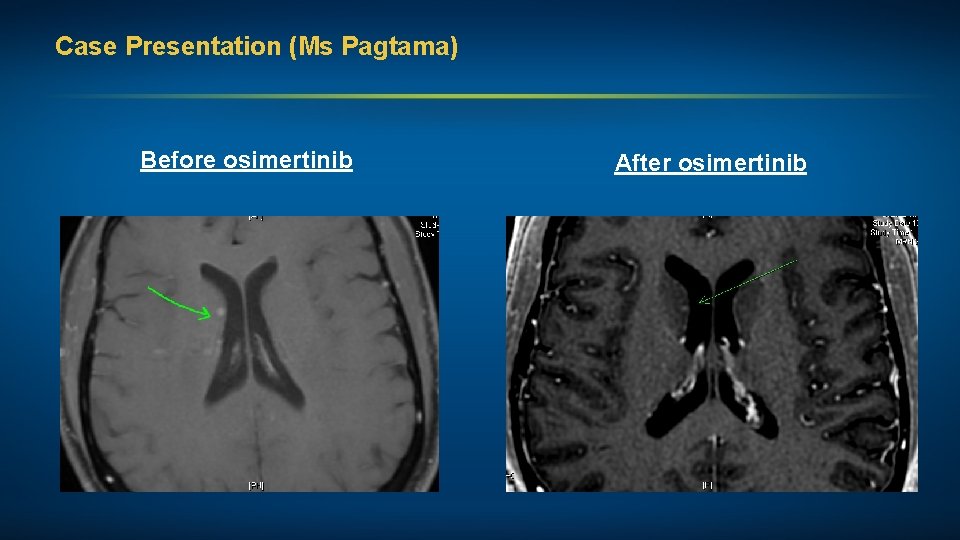
Case Presentation (Ms Pagtama) Before osimertinib After osimertinib

Case Presentation (Ms Pagtama) Before osimertinib After osimertinib

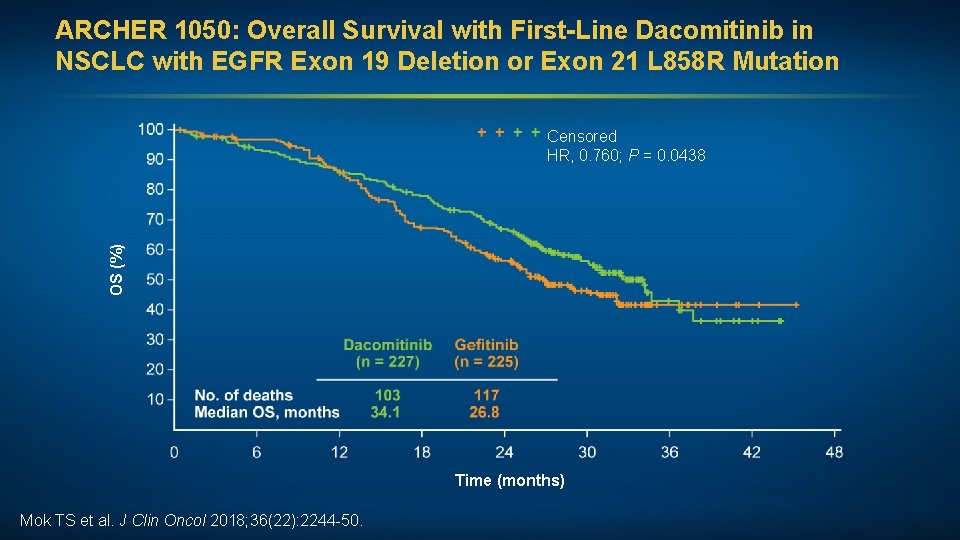
ARCHER 1050: Overall Survival with First-Line Dacomitinib in NSCLC with EGFR Exon 19 Deletion or Exon 21 L 858 R Mutation OS (%) Censored HR, 0. 760; P = 0. 0438 Time (months) Mok TS et al. J Clin Oncol 2018; 36(22): 2244 -50.

FDA Approves Dacomitinib for First-Line NSCLC with EGFR Exon 19 Deletion or Exon 21 L 858 R Mutation Press Release — September 27, 2018 “On Sept. 27, 2018, the Food and Drug Administration approved dacomitinib tablets for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L 858 R substitution mutations as detected by an FDA-approved test. Approval was based on a randomized, multicenter, open-label, active controlled trial (ARCHER 1050; NCT 01774721) comparing the safety and efficacy of dacomitinib to gefitinib in 452 patients with unresectable, metastatic NSCLC. ” https: //www. fda. gov/Drugs/Information. On. Drugs/Approved. Drugs/ucm 621967. htm

Emerging Strategies in NSCLC: Agenda EGFR-Mutated Metastatic NSCLC Immune Checkpoint Inhibitor Therapy for Metastatic NSCLC Immunotherapy as Consolidation After Chemoradiation of Locally Advanced NSCLC

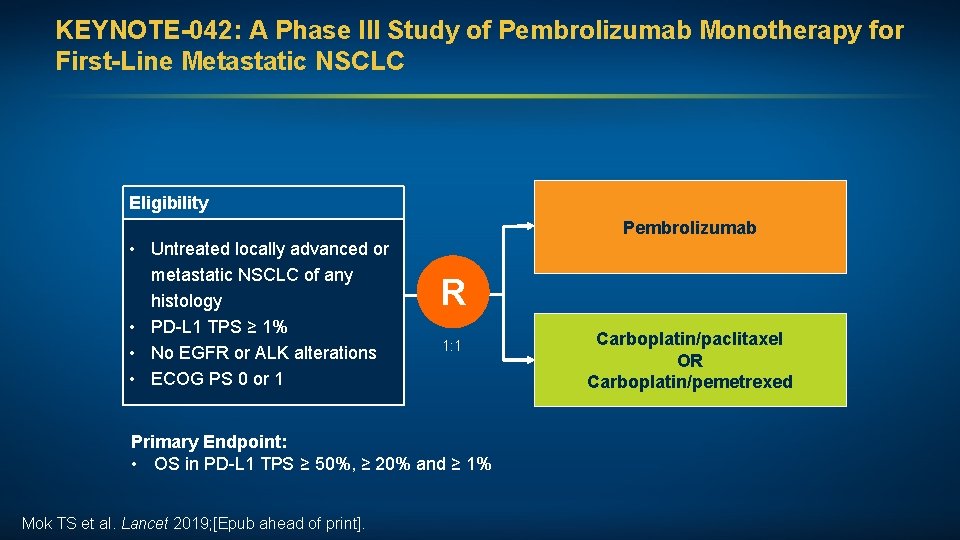
KEYNOTE-042: A Phase III Study of Pembrolizumab Monotherapy for First-Line Metastatic NSCLC Eligibility Pembrolizumab • Untreated locally advanced or metastatic NSCLC of any histology • PD-L 1 TPS ≥ 1% • No EGFR or ALK alterations • ECOG PS 0 or 1 R 1: 1 Primary Endpoint: • OS in PD-L 1 TPS ≥ 50%, ≥ 20% and ≥ 1% Mok TS et al. Lancet 2019; [Epub ahead of print]. Carboplatin/paclitaxel OR Carboplatin/pemetrexed

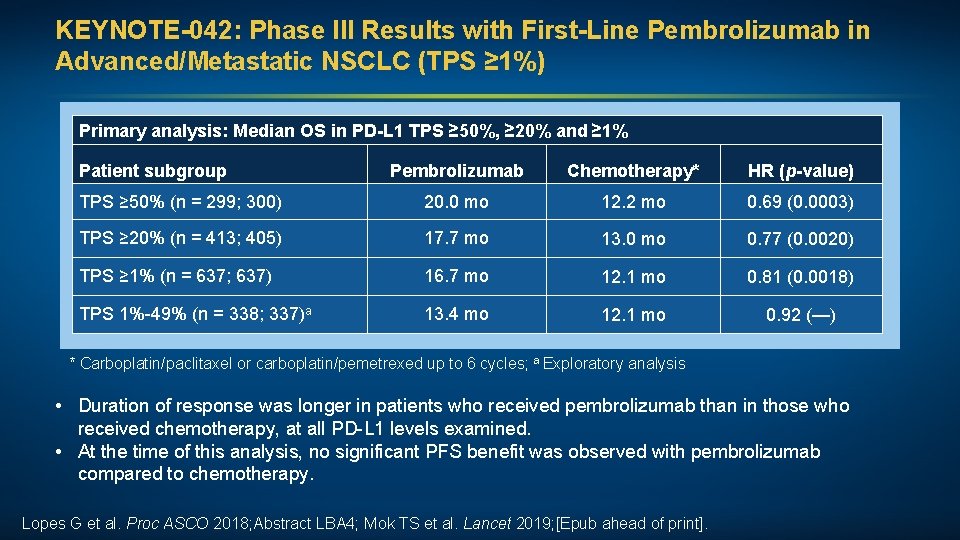
KEYNOTE-042: Phase III Results with First-Line Pembrolizumab in Advanced/Metastatic NSCLC (TPS ≥ 1%) Primary analysis: Median OS in PD-L 1 TPS ≥ 50%, ≥ 20% and ≥ 1% Patient subgroup Pembrolizumab Chemotherapy* HR (p-value) TPS ≥ 50% (n = 299; 300) 20. 0 mo 12. 2 mo 0. 69 (0. 0003) TPS ≥ 20% (n = 413; 405) 17. 7 mo 13. 0 mo 0. 77 (0. 0020) TPS ≥ 1% (n = 637; 637) 16. 7 mo 12. 1 mo 0. 81 (0. 0018) TPS 1%-49% (n = 338; 337)a 13. 4 mo 12. 1 mo 0. 92 (—) * Carboplatin/paclitaxel or carboplatin/pemetrexed up to 6 cycles; a Exploratory analysis • Duration of response was longer in patients who received pembrolizumab than in those who received chemotherapy, at all PD-L 1 levels examined. • At the time of this analysis, no significant PFS benefit was observed with pembrolizumab compared to chemotherapy. Lopes G et al. Proc ASCO 2018; Abstract LBA 4; Mok TS et al. Lancet 2019; [Epub ahead of print].

FDA expands pembrolizumab indication for first-line treatment of NSCLC (TPS ≥ 1%) Press Release — April 11, 2019 “On April 11, 2019, the Food and Drug Administration approved pembrolizumab for the first-line treatment of patients with stage III non-small cell lung cancer (NSCLC) who are not candidates for surgical resection or definitive chemoradiation or metastatic NSCLC. Patients’ tumors must have no EGFR or ALK genomic aberrations and express PD-L 1 (Tumor Proportion Score [TPS] ≥ 1%) determined by an FDA-approved test. Pembrolizumab was previously approved as a single agent for the first-line treatment of patients with metastatic NSCLC whose tumors express PD-L 1 TPS ≥ 50%. Approval was based on KEYNOTE‑ 042 (NCT 02220894), a randomized, multicenter, open-label, active-controlled trial conducted in 1274 patients with stage III or IV NSCLC who had not received prior systemic treatment for metastatic NSCLC and whose tumors expressed PD-L 1 (TPS ≥ 1%). ” https: //www. fda. gov/Drugs/Information. On. Drugs/Approved. Drugs/ucm 635857. htm

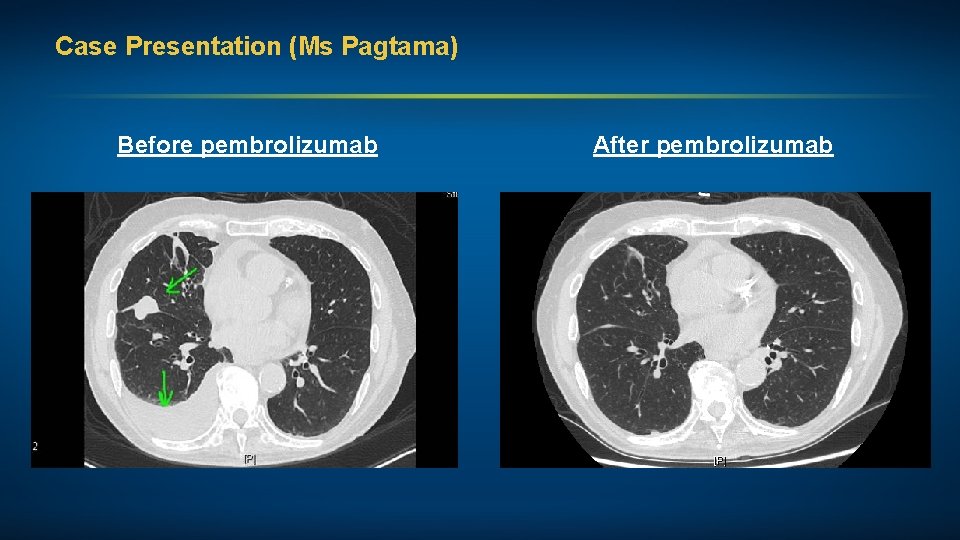
Case Presentation (Ms Pagtama) A man in his mid-90 s with Stage IV pan-wild-type adenocarcinoma of the lung (PD-L 1 60%) Treatments received • Pembrolizumab 200 mg (fixed dose) due to poor renal function Other considerations • Retired physicist • Married, wife with dementia • Continues to drive, remains independent

Case Presentation (Ms Pagtama) Before pembrolizumab After pembrolizumab

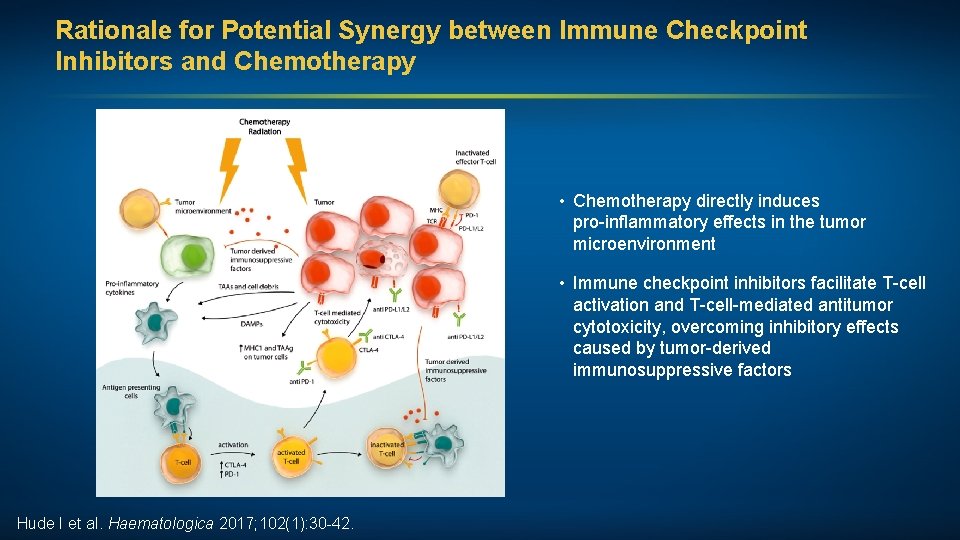
Rationale for Potential Synergy between Immune Checkpoint Inhibitors and Chemotherapy • Chemotherapy directly induces pro-inflammatory effects in the tumor microenvironment • Immune checkpoint inhibitors facilitate T-cell activation and T-cell-mediated antitumor cytotoxicity, overcoming inhibitory effects caused by tumor-derived immunosuppressive factors Hude I et al. Haematologica 2017; 102(1): 30 -42.

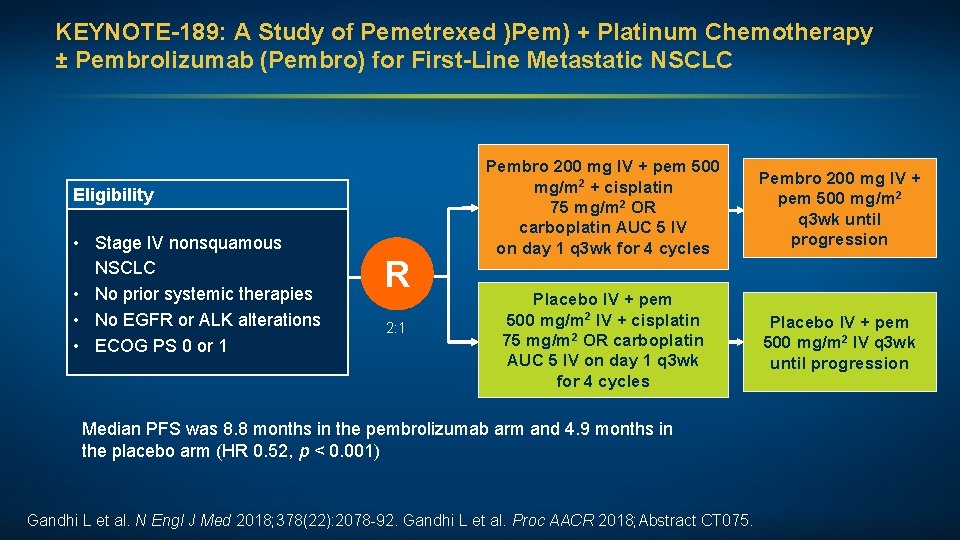
KEYNOTE-189: A Study of Pemetrexed )Pem) + Platinum Chemotherapy ± Pembrolizumab (Pembro) for First-Line Metastatic NSCLC Eligibility • Stage IV nonsquamous NSCLC • No prior systemic therapies • No EGFR or ALK alterations • ECOG PS 0 or 1 R 2: 1 Pembro 200 mg IV + pem 500 mg/m 2 + cisplatin 75 mg/m 2 OR carboplatin AUC 5 IV on day 1 q 3 wk for 4 cycles Pembro 200 mg IV + pem 500 mg/m 2 q 3 wk until progression Placebo IV + pem 500 mg/m 2 IV + cisplatin 75 mg/m 2 OR carboplatin AUC 5 IV on day 1 q 3 wk for 4 cycles Placebo IV + pem 500 mg/m 2 IV q 3 wk until progression Median PFS was 8. 8 months in the pembrolizumab arm and 4. 9 months in the placebo arm (HR 0. 52, p < 0. 001) Gandhi L et al. N Engl J Med 2018; 378(22): 2078 -92. Gandhi L et al. Proc AACR 2018; Abstract CT 075.

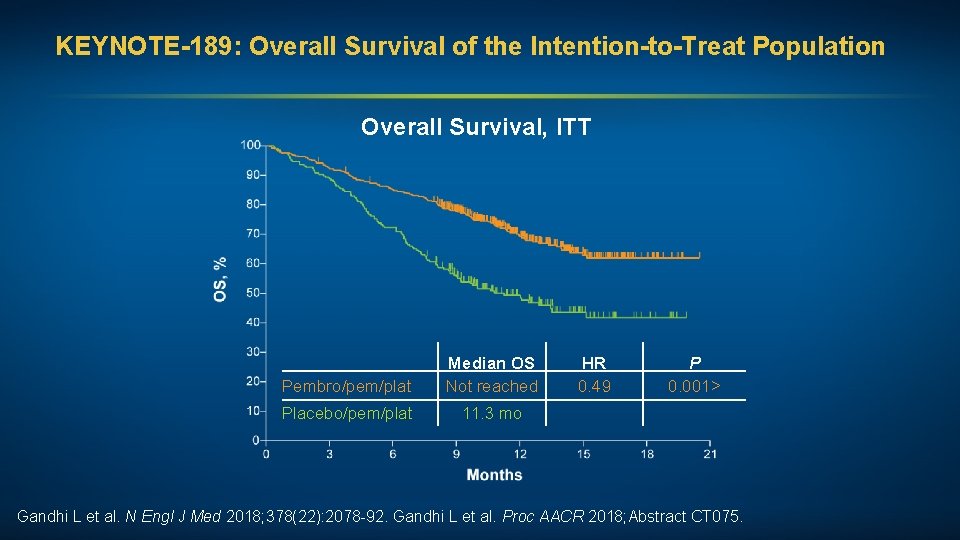
KEYNOTE-189: Overall Survival of the Intention-to-Treat Population Overall Survival, ITT Pembro/pem/plat Median OS Not reached Placebo/pem/plat 11. 3 mo HR 0. 49 P 0. 001> Gandhi L et al. N Engl J Med 2018; 378(22): 2078 -92. Gandhi L et al. Proc AACR 2018; Abstract CT 075.

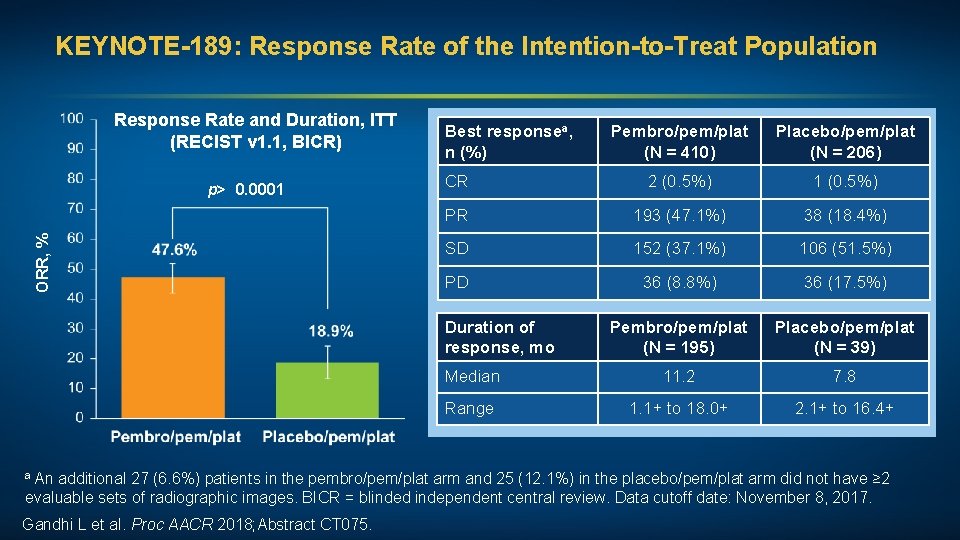
KEYNOTE-189: Response Rate of the Intention-to-Treat Population Response Rate and Duration, ITT (RECIST v 1. 1, BICR) ORR, % p> 0. 0001 Best responsea, n (%) Pembro/pem/plat (N = 410) Placebo/pem/plat (N = 206) CR 2 (0. 5%) 1 (0. 5%) PR 193 (47. 1%) 38 (18. 4%) SD 152 (37. 1%) 106 (51. 5%) PD 36 (8. 8%) 36 (17. 5%) Pembro/pem/plat (N = 195) Placebo/pem/plat (N = 39) Median 11. 2 7. 8 Range 1. 1+ to 18. 0+ 2. 1+ to 16. 4+ Duration of response, mo a An additional 27 (6. 6%) patients in the pembro/pem/plat arm and 25 (12. 1%) in the placebo/pem/plat arm did not have ≥ 2 evaluable sets of radiographic images. BICR = blinded independent central review. Data cutoff date: November 8, 2017. Gandhi L et al. Proc AACR 2018; Abstract CT 075.

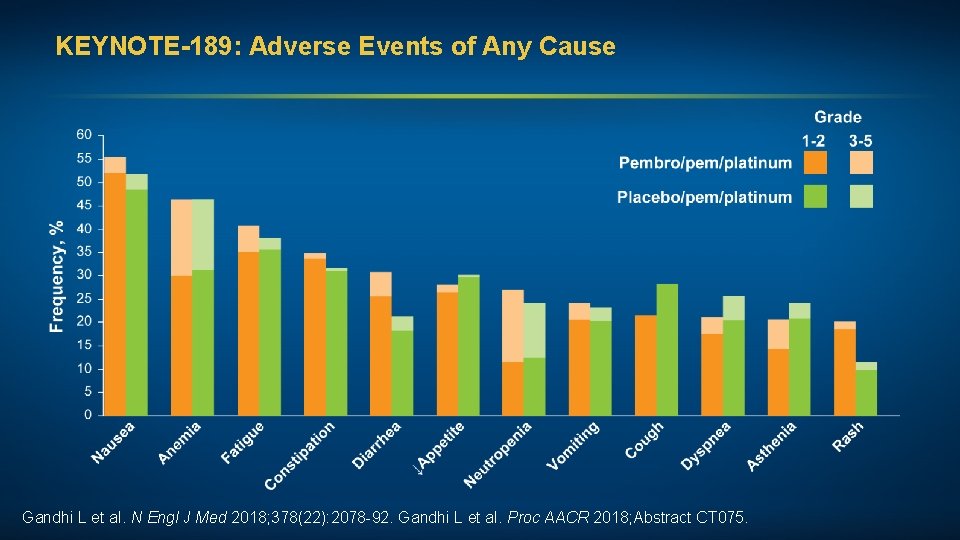
KEYNOTE-189: Adverse Events of Any Cause Gandhi L et al. N Engl J Med 2018; 378(22): 2078 -92. Gandhi L et al. Proc AACR 2018; Abstract CT 075.

FDA Approval of Pembrolizumab in Combination with Chemo for First-Line Treatment of Metastatic Nonsquamous NSCLC August 20, 2018 “On August 20, 2018, the Food and Drug Administration approved pembrolizumab in combination with pemetrexed and platinum as first-line treatment of patients with metastatic, non-squamous non-small cell lung cancer (NSq. NSCLC), with no EGFR or ALK genomic tumor aberrations. This action is based on the results of KEYNOTE-189 (NCT 02578680), a randomized, multicenter, double-blind, active controlled study enrolling 616 patients receiving first-line treatment for metastatic NSq. NSCLC. Patients were randomized (2: 1) to receive pembrolizumab (or placebo) in combination with pemetrexed, and investigator’s choice of either cisplatin or carboplatin every 3 weeks for 4 cycles followed by pembrolizumab (or placebo) and pemetrexed. Treatment with pembrolizumab continued until disease progression, unacceptable toxicity, or a maximum of 24 months. ” https: //www. fda. gov/Drugs/Information. On. Drugs/Approved. Drugs/ucm 617471. htm

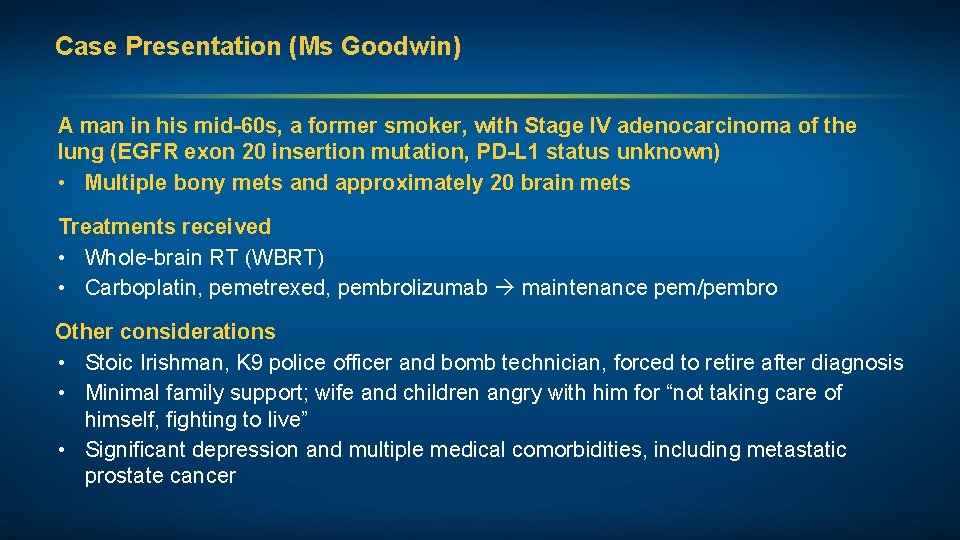
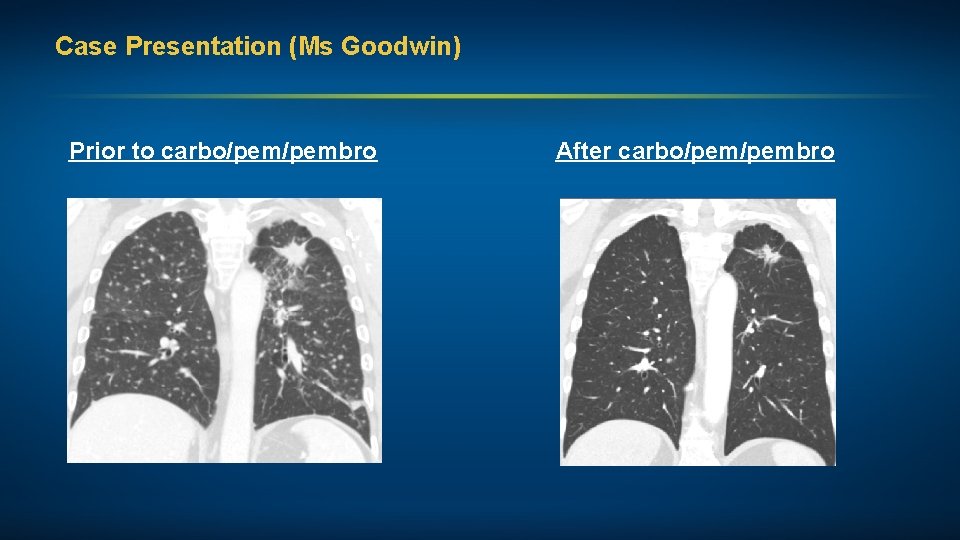
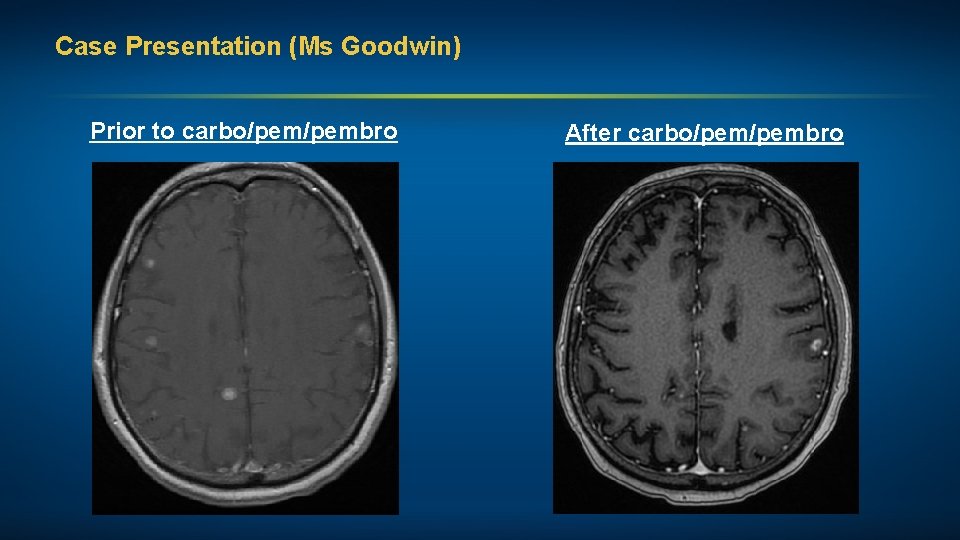
Case Presentation (Ms Goodwin) A man in his mid-60 s, a former smoker, with Stage IV adenocarcinoma of the lung (EGFR exon 20 insertion mutation, PD-L 1 status unknown) • Multiple bony mets and approximately 20 brain mets Treatments received • Whole-brain RT (WBRT) • Carboplatin, pemetrexed, pembrolizumab maintenance pem/pembro Other considerations • Stoic Irishman, K 9 police officer and bomb technician, forced to retire after diagnosis • Minimal family support; wife and children angry with him for “not taking care of himself, fighting to live” • Significant depression and multiple medical comorbidities, including metastatic prostate cancer

Case Presentation (Ms Goodwin) Prior to carbo/pembro After carbo/pembro

Case Presentation (Ms Goodwin) Prior to carbo/pembro After carbo/pembro

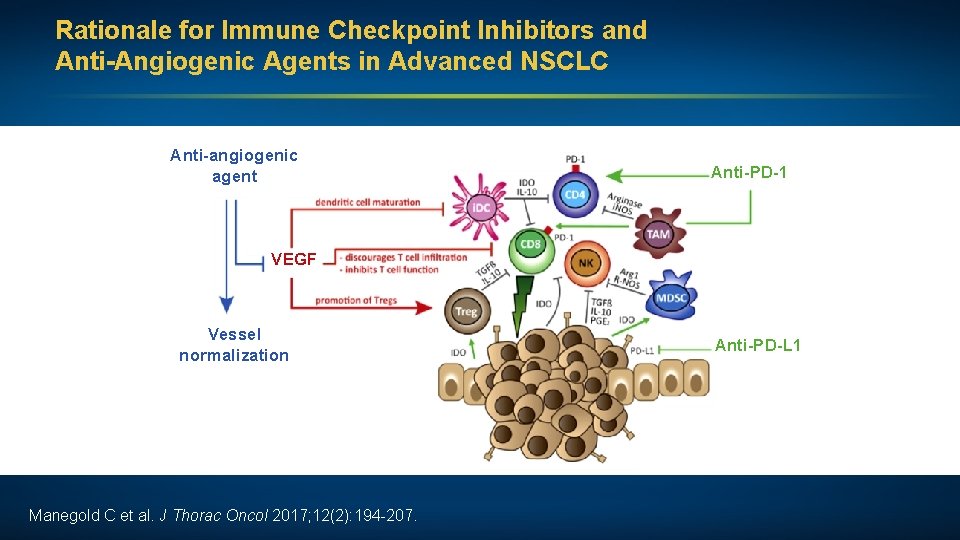
Rationale for Immune Checkpoint Inhibitors and Anti-Angiogenic Agents in Advanced NSCLC Anti-angiogenic agent Anti-PD-1 VEGF Vessel normalization Manegold C et al. J Thorac Oncol 2017; 12(2): 194 -207. Anti-PD-L 1

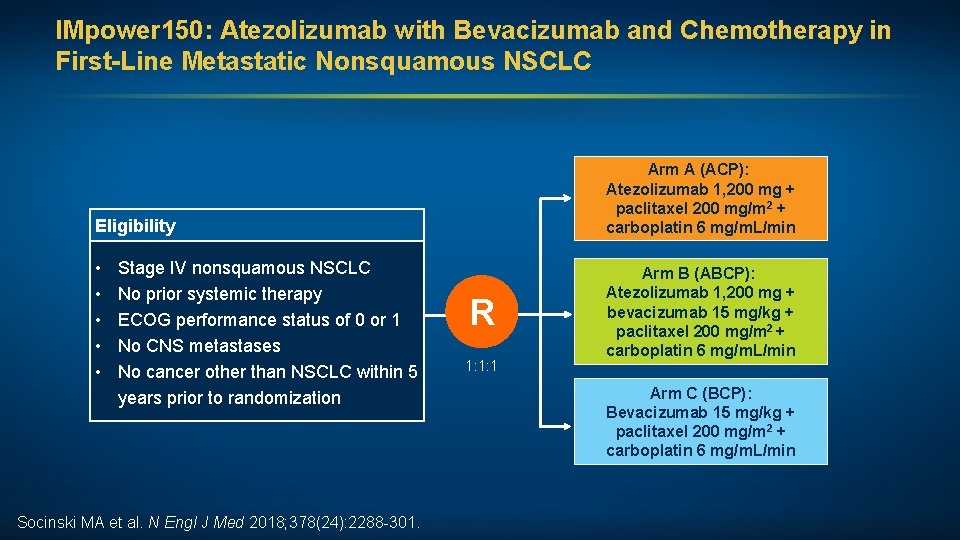
IMpower 150: Atezolizumab with Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC Arm A (ACP): Atezolizumab 1, 200 mg + paclitaxel 200 mg/m 2 + carboplatin 6 mg/m. L/min Eligibility • • • Stage IV nonsquamous NSCLC No prior systemic therapy ECOG performance status of 0 or 1 No CNS metastases No cancer other than NSCLC within 5 years prior to randomization Socinski MA et al. N Engl J Med 2018; 378(24): 2288 -301. R 1: 1: 1 Arm B (ABCP): Atezolizumab 1, 200 mg + bevacizumab 15 mg/kg + paclitaxel 200 mg/m 2 + carboplatin 6 mg/m. L/min Arm C (BCP): Bevacizumab 15 mg/kg + paclitaxel 200 mg/m 2 + carboplatin 6 mg/m. L/min

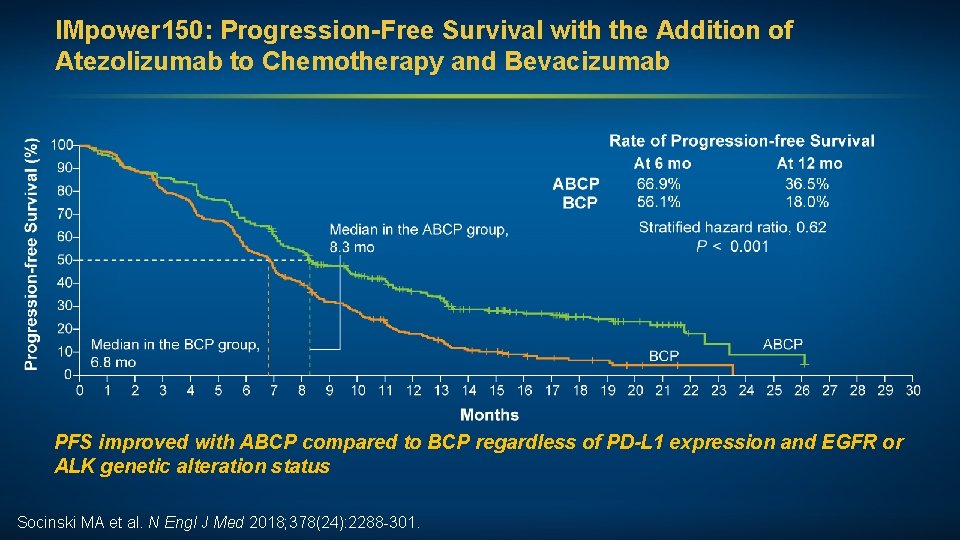
IMpower 150: Progression-Free Survival with the Addition of Atezolizumab to Chemotherapy and Bevacizumab PFS improved with ABCP compared to BCP regardless of PD-L 1 expression and EGFR or ALK genetic alteration status Socinski MA et al. N Engl J Med 2018; 378(24): 2288 -301.

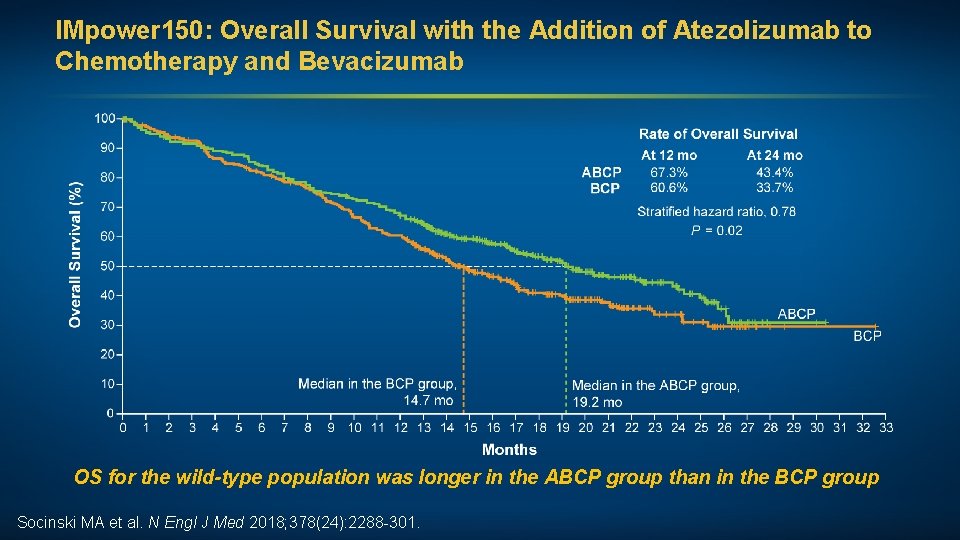
IMpower 150: Overall Survival with the Addition of Atezolizumab to Chemotherapy and Bevacizumab OS for the wild-type population was longer in the ABCP group than in the BCP group Socinski MA et al. N Engl J Med 2018; 378(24): 2288 -301.

FDA Approval of Atezolizumab in Combination with Chemo and Bevacizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC December 6, 2018 “On December 6, 2018, the Food and Drug Administration approved atezolizumab, in combination with bevacizumab, paclitaxel, and carboplatin for the first-line treatment of patients with metastatic non-squamous, non-small cell lung cancer (NSq NSCLC) with no EGFR or ALK genomic tumor aberrations. Approval was based on the IMpower 150 trial (NCT 02366143), an open-label, randomized (1: 1: 1), three-arm trial enrolling 1202 patients receiving first-line treatment for metastatic NSq NSCLC. Eighty-seven percent (1045 patients) were identified as not having EGFR or ALK tumor mutations. Following completion of 4 or 6 cycles of carboplatin and paclitaxel, patients continued to receive bevacizumab in the 4 -drug arm and the control arm and continued to receive atezolizumab in the two experimental arms until disease progression or unacceptable toxicity. ” https: //www. fda. gov/Drugs/Information. On. Drugs/Approved. Drugs/ucm 627874. htm

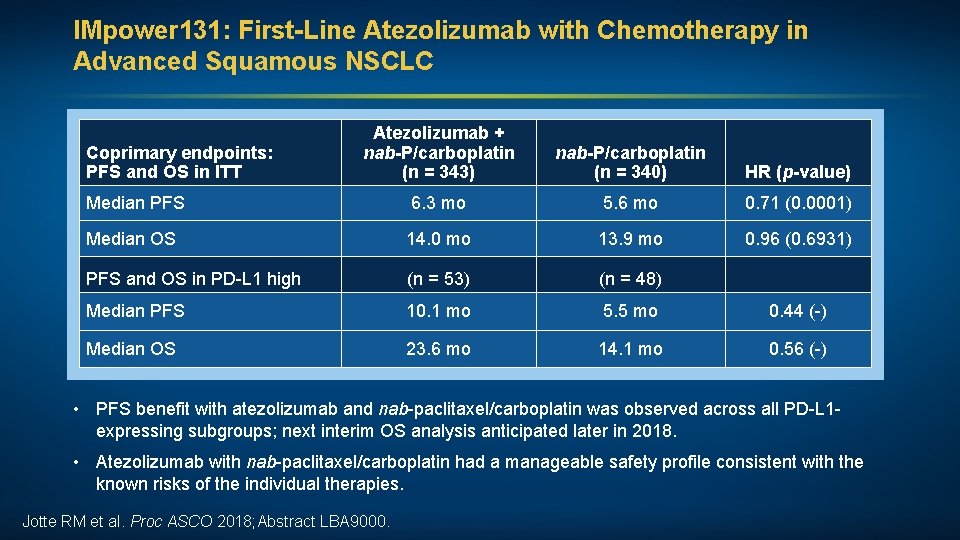
IMpower 131: First-Line Atezolizumab with Chemotherapy in Advanced Squamous NSCLC Atezolizumab + nab-P/carboplatin (n = 343) nab-P/carboplatin (n = 340) HR (p-value) Median PFS 6. 3 mo 5. 6 mo 0. 71 (0. 0001) Median OS 14. 0 mo 13. 9 mo 0. 96 (0. 6931) PFS and OS in PD-L 1 high (n = 53) (n = 48) Median PFS 10. 1 mo 5. 5 mo 0. 44 (-) Median OS 23. 6 mo 14. 1 mo 0. 56 (-) Coprimary endpoints: PFS and OS in ITT • PFS benefit with atezolizumab and nab-paclitaxel/carboplatin was observed across all PD-L 1 expressing subgroups; next interim OS analysis anticipated later in 2018. • Atezolizumab with nab-paclitaxel/carboplatin had a manageable safety profile consistent with the known risks of the individual therapies. Jotte RM et al. Proc ASCO 2018; Abstract LBA 9000.

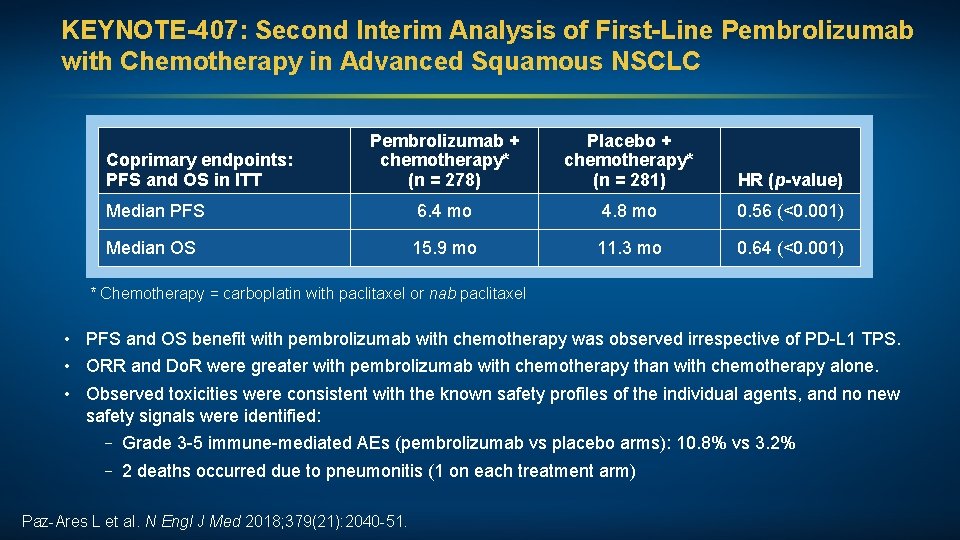
KEYNOTE-407: Second Interim Analysis of First-Line Pembrolizumab with Chemotherapy in Advanced Squamous NSCLC Pembrolizumab + chemotherapy* (n = 278) Placebo + chemotherapy* (n = 281) HR (p-value) Median PFS 6. 4 mo 4. 8 mo 0. 56 (<0. 001) Median OS 15. 9 mo 11. 3 mo 0. 64 (<0. 001) Coprimary endpoints: PFS and OS in ITT * Chemotherapy = carboplatin with paclitaxel or nab paclitaxel • PFS and OS benefit with pembrolizumab with chemotherapy was observed irrespective of PD-L 1 TPS. • ORR and Do. R were greater with pembrolizumab with chemotherapy than with chemotherapy alone. • Observed toxicities were consistent with the known safety profiles of the individual agents, and no new safety signals were identified: – Grade 3 -5 immune-mediated AEs (pembrolizumab vs placebo arms): 10. 8% vs 3. 2% – 2 deaths occurred due to pneumonitis (1 on each treatment arm) Paz-Ares L et al. N Engl J Med 2018; 379(21): 2040 -51.

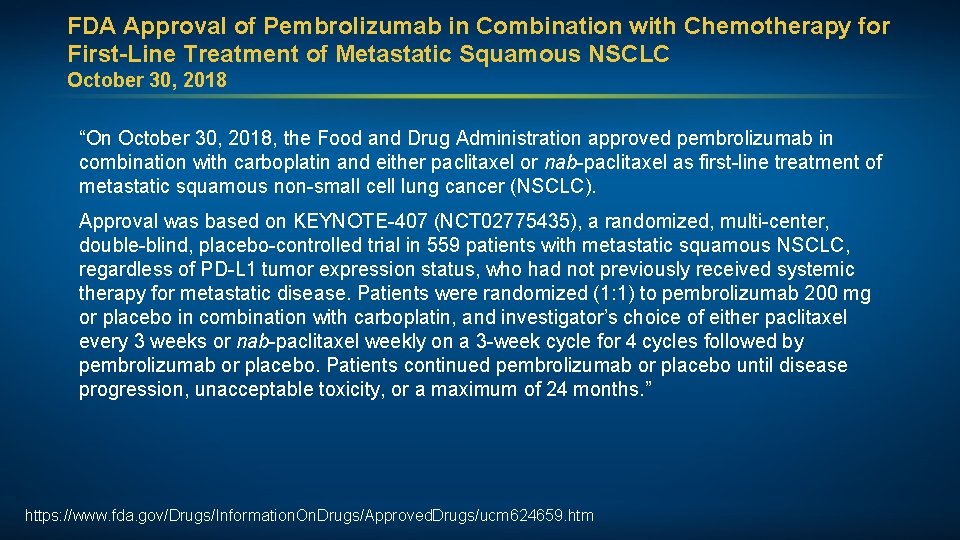
FDA Approval of Pembrolizumab in Combination with Chemotherapy for First-Line Treatment of Metastatic Squamous NSCLC October 30, 2018 “On October 30, 2018, the Food and Drug Administration approved pembrolizumab in combination with carboplatin and either paclitaxel or nab-paclitaxel as first-line treatment of metastatic squamous non-small cell lung cancer (NSCLC). Approval was based on KEYNOTE-407 (NCT 02775435), a randomized, multi-center, double-blind, placebo-controlled trial in 559 patients with metastatic squamous NSCLC, regardless of PD-L 1 tumor expression status, who had not previously received systemic therapy for metastatic disease. Patients were randomized (1: 1) to pembrolizumab 200 mg or placebo in combination with carboplatin, and investigator’s choice of either paclitaxel every 3 weeks or nab-paclitaxel weekly on a 3 -week cycle for 4 cycles followed by pembrolizumab or placebo. Patients continued pembrolizumab or placebo until disease progression, unacceptable toxicity, or a maximum of 24 months. ” https: //www. fda. gov/Drugs/Information. On. Drugs/Approved. Drugs/ucm 624659. htm

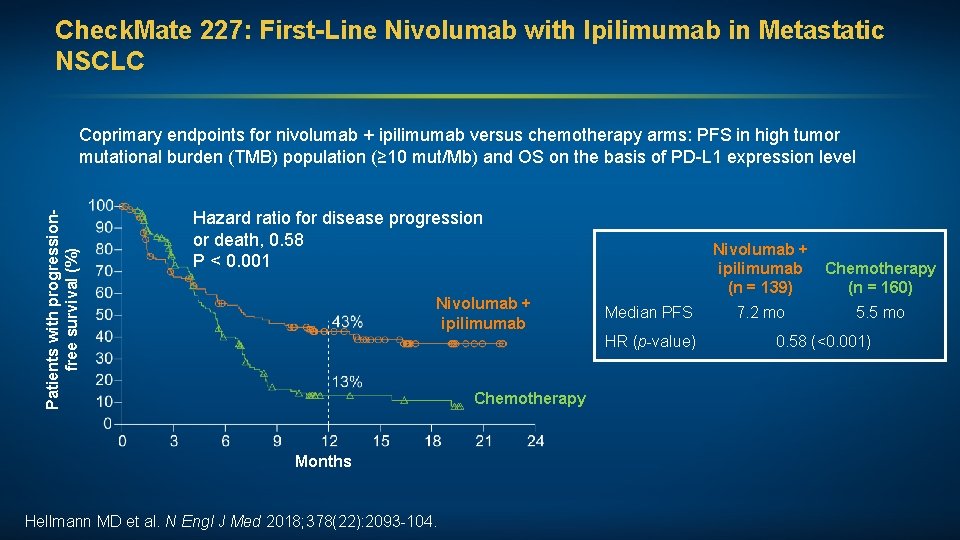
Check. Mate 227: First-Line Nivolumab with Ipilimumab in Metastatic NSCLC Patients with progressionfree survival (%) Coprimary endpoints for nivolumab + ipilimumab versus chemotherapy arms: PFS in high tumor mutational burden (TMB) population (≥ 10 mut/Mb) and OS on the basis of PD-L 1 expression level Hazard ratio for disease progression or death, 0. 58 P < 0. 001 Nivolumab + ipilimumab Median PFS HR (p-value) Chemotherapy Months Hellmann MD et al. N Nivolumab + ipilimumab Chemotherapy (n = 160) (n = 139) Engl J Med 2018; 378(22): 2093 -104. 7. 2 mo 5. 5 mo 0. 58 (<0. 001)

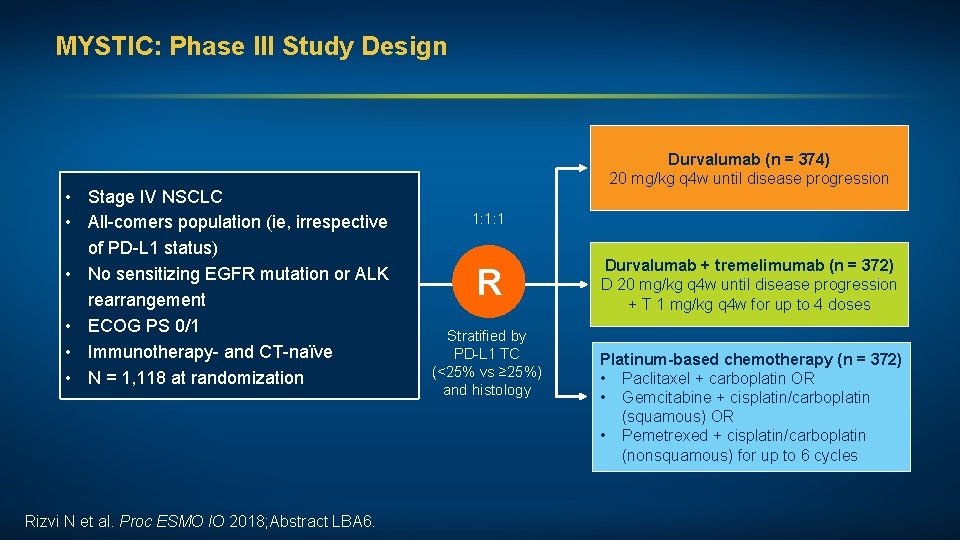
MYSTIC: Phase III Study Design • Stage IV NSCLC • All-comers population (ie, irrespective of PD-L 1 status) • No sensitizing EGFR mutation or ALK rearrangement • ECOG PS 0/1 • Immunotherapy- and CT-naïve • N = 1, 118 at randomization Rizvi N et al. Proc ESMO IO 2018; Abstract LBA 6. Durvalumab (n = 374) 20 mg/kg q 4 w until disease progression 1: 1: 1 R Stratified by PD-L 1 TC (<25% vs ≥ 25%) and histology Durvalumab + tremelimumab (n = 372) D 20 mg/kg q 4 w until disease progression + T 1 mg/kg q 4 w for up to 4 doses Platinum-based chemotherapy (n = 372) • Paclitaxel + carboplatin OR • Gemcitabine + cisplatin/carboplatin (squamous) OR • Pemetrexed + cisplatin/carboplatin (nonsquamous) for up to 6 cycles

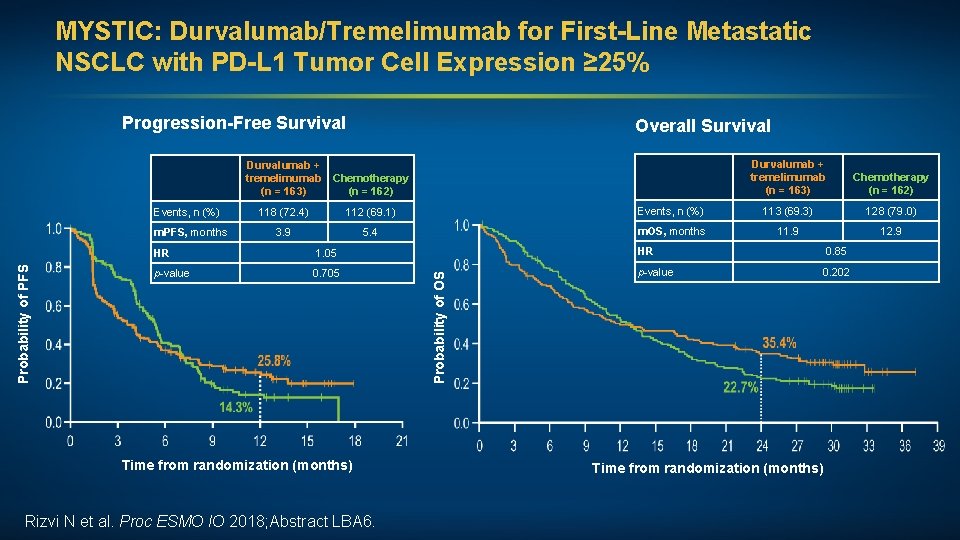
MYSTIC: Durvalumab/Tremelimumab for First-Line Metastatic NSCLC with PD-L 1 Tumor Cell Expression ≥ 25% Progression-Free Survival Overall Survival Durvalumab + tremelimumab Chemotherapy (n = 163) (n = 162) Events, n (%) 112 (69. 1) Events, n (%) 113 (69. 3) 128 (79. 0) 3. 9 5. 4 m. OS, months 11. 9 12. 9 HR 1. 05 HR 0. 85 p-value 0. 705 p-value 0. 202 Time from randomization (months) Rizvi N et al. Proc Chemotherapy (n = 162) 118 (72. 4) ESMO IO 2018; Abstract LBA 6. Probability of OS Probability of PFS m. PFS, months Durvalumab + tremelimumab (n = 163) Time from randomization (months)

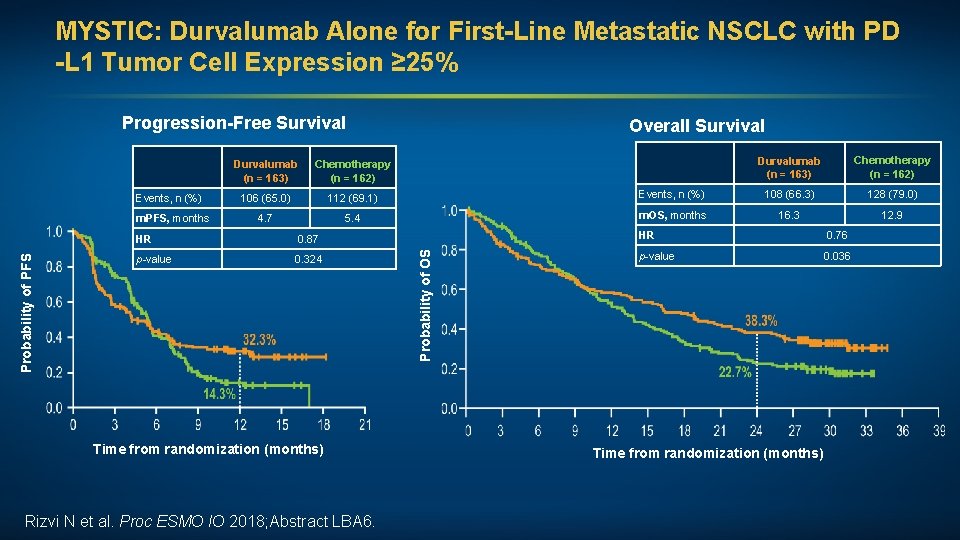
MYSTIC: Durvalumab Alone for First-Line Metastatic NSCLC with PD -L 1 Tumor Cell Expression ≥ 25% Progression-Free Survival Probability of PFS m. PFS, months Chemotherapy (n = 162) 106 (65. 0) 112 (69. 1) 4. 7 5. 4 HR 0. 87 p-value 0. 324 Time from randomization (months) Rizvi N et al. Proc ESMO IO 2018; Abstract LBA 6. Probability of OS Events, n (%) Durvalumab (n = 163) Overall Survival Durvalumab (n = 163) Chemotherapy (n = 162) Events, n (%) 108 (66. 3) 128 (79. 0) m. OS, months 16. 3 12. 9 HR 0. 76 p-value 0. 036 Time from randomization (months)

J Clin Oncol 2018; 36(17): 1714 -68.

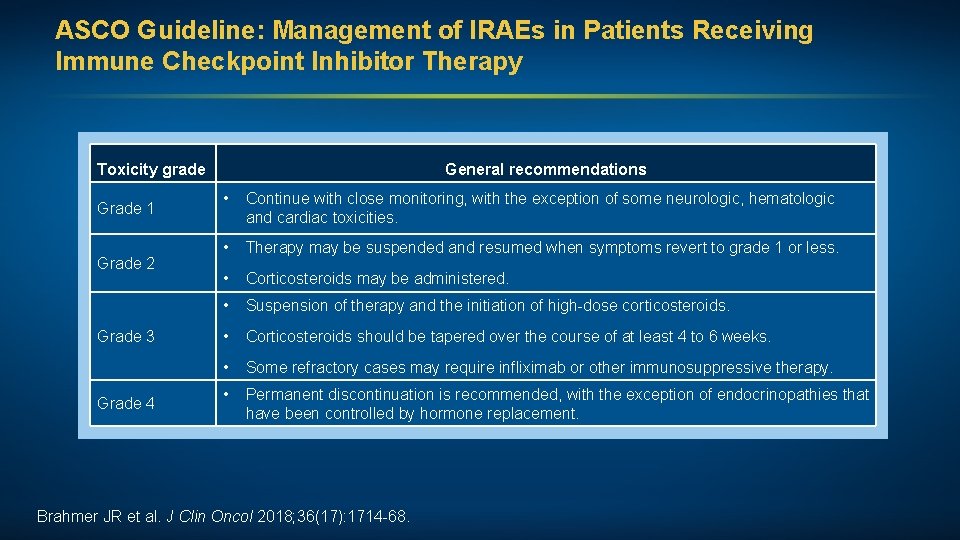
ASCO Guideline: Management of IRAEs in Patients Receiving Immune Checkpoint Inhibitor Therapy Toxicity grade Grade 1 Grade 2 Grade 3 Grade 4 General recommendations • Continue with close monitoring, with the exception of some neurologic, hematologic and cardiac toxicities. • Therapy may be suspended and resumed when symptoms revert to grade 1 or less. • Corticosteroids may be administered. • Suspension of therapy and the initiation of high-dose corticosteroids. • Corticosteroids should be tapered over the course of at least 4 to 6 weeks. • Some refractory cases may require infliximab or other immunosuppressive therapy. • Permanent discontinuation is recommended, with the exception of endocrinopathies that have been controlled by hormone replacement. Brahmer JR et al. J Clin Oncol 2018; 36(17): 1714 -68.

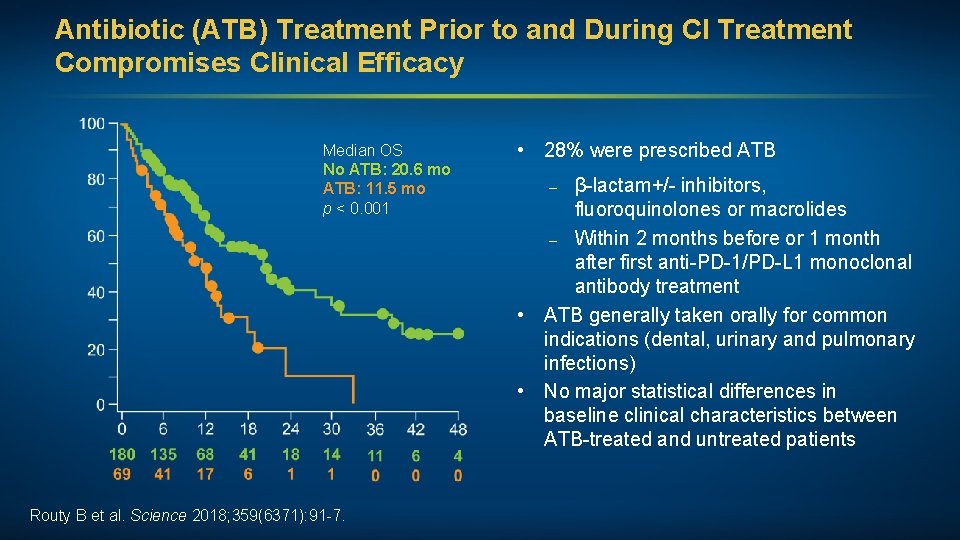
Antibiotic (ATB) Treatment Prior to and During CI Treatment Compromises Clinical Efficacy Median OS No ATB: 20. 6 mo ATB: 11. 5 mo p < 0. 001 Routy B et al. Science 2018; 359(6371): 91 -7. • 28% were prescribed ATB – β-lactam+/- inhibitors, fluoroquinolones or macrolides – Within 2 months before or 1 month after first anti-PD-1/PD-L 1 monoclonal antibody treatment • ATB generally taken orally for common indications (dental, urinary and pulmonary infections) • No major statistical differences in baseline clinical characteristics between ATB-treated and untreated patients

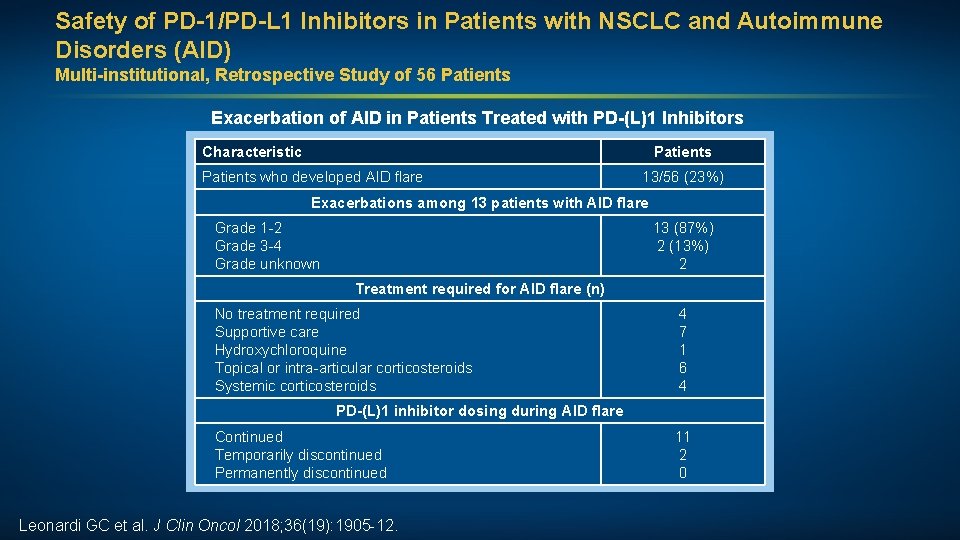
Safety of PD-1/PD-L 1 Inhibitors in Patients with NSCLC and Autoimmune Disorders (AID) Multi-institutional, Retrospective Study of 56 Patients Exacerbation of AID in Patients Treated with PD-(L)1 Inhibitors Characteristic Patients who developed AID flare 13/56 (23%) Exacerbations among 13 patients with AID flare Grade 1 -2 Grade 3 -4 Grade unknown 13 (87%) 2 (13%) 2 Treatment required for AID flare (n) No treatment required Supportive care Hydroxychloroquine Topical or intra-articular corticosteroids Systemic corticosteroids 4 7 1 6 4 PD-(L)1 inhibitor dosing during AID flare Continued Temporarily discontinued Permanently discontinued Leonardi GC et al. J Clin Oncol 2018; 36(19): 1905 -12. 11 2 0

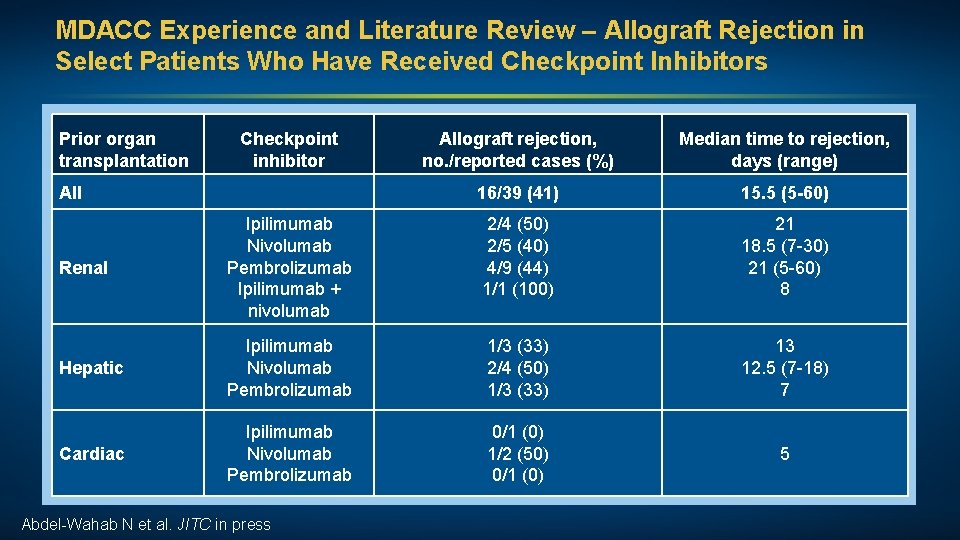
MDACC Experience and Literature Review – Allograft Rejection in Select Patients Who Have Received Checkpoint Inhibitors Prior organ transplantation Checkpoint inhibitor Allograft rejection, no. /reported cases (%) Median time to rejection, days (range) 16/39 (41) 15. 5 (5 -60) 2/4 (50) 2/5 (40) 4/9 (44) 1/1 (100) 21 18. 5 (7 -30) 21 (5 -60) 8 Hepatic Ipilimumab Nivolumab Pembrolizumab 1/3 (33) 2/4 (50) 1/3 (33) 13 12. 5 (7 -18) 7 Cardiac Ipilimumab Nivolumab Pembrolizumab 0/1 (0) 1/2 (50) 0/1 (0) 5 All Renal Ipilimumab Nivolumab Pembrolizumab Ipilimumab + nivolumab Abdel-Wahab N et al. JITC in press

Emerging Strategies in NSCLC: Agenda EGFR-Mutated Metastatic NSCLC Immune Checkpoint Inhibitor Therapy for Metastatic NSCLC Immunotherapy as Consolidation After Chemoradiation of Locally Advanced NSCLC

Case Presentation (Ms Pagtama) A man in his early 70 s, a former smoker, with locally recurrent adenocarcinoma of the lung in a subcarinal lymph node (PD-L 1 80%) Treatments received • Induction carboplatin and pemetrexed with concurrent chemoradiation • Durvalumab Other considerations • Retired and married with 4 adult children • Former heavy drinker, who quit after initial lung cancer diagnosis • Anxiety and depression since cancer recurrence • Worsening joint pain and difficulty with fatigue

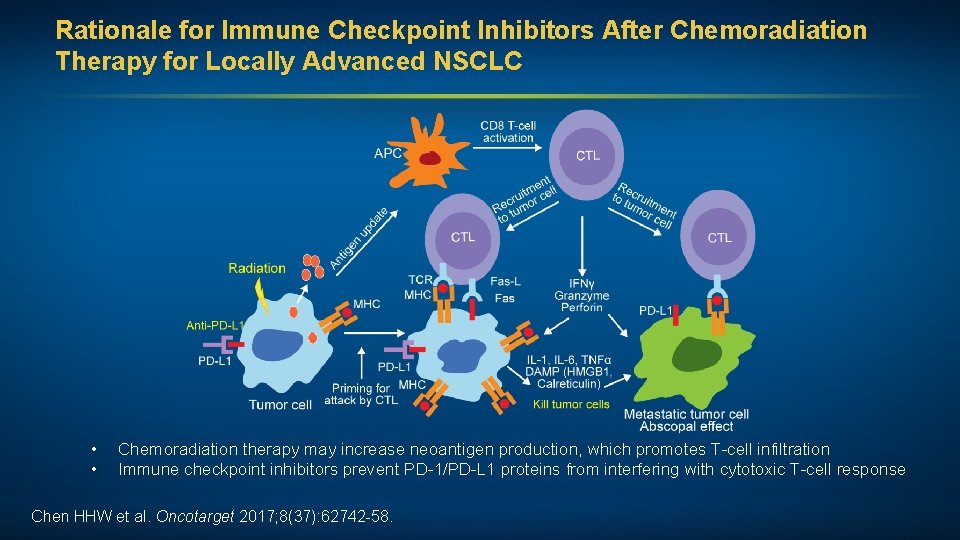
Rationale for Immune Checkpoint Inhibitors After Chemoradiation Therapy for Locally Advanced NSCLC • • Chemoradiation therapy may increase neoantigen production, which promotes T-cell infiltration Immune checkpoint inhibitors prevent PD-1/PD-L 1 proteins from interfering with cytotoxic T-cell response Chen HHW et al. Oncotarget 2017; 8(37): 62742 -58.

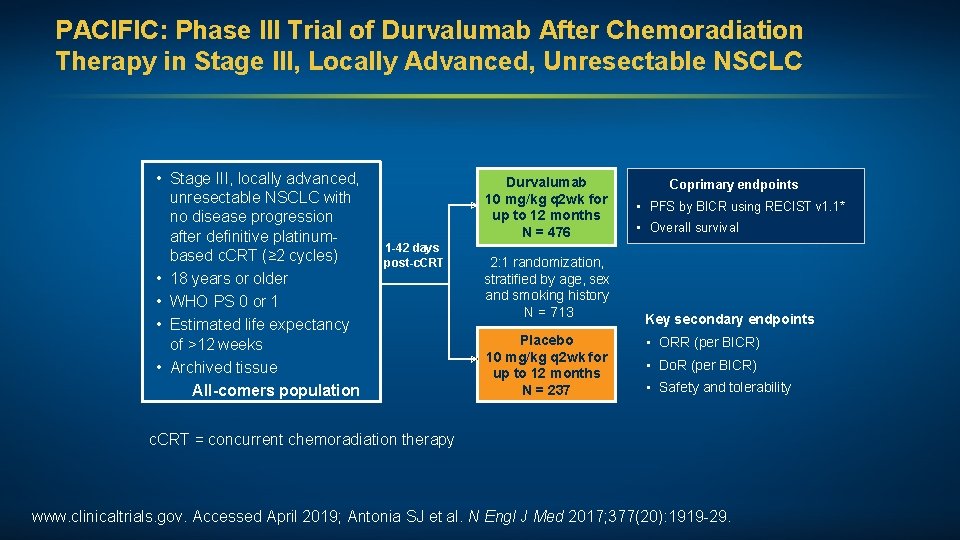
PACIFIC: Phase III Trial of Durvalumab After Chemoradiation Therapy in Stage III, Locally Advanced, Unresectable NSCLC • Stage III, locally advanced, unresectable NSCLC with no disease progression after definitive platinumbased c. CRT (≥ 2 cycles) • 18 years or older • WHO PS 0 or 1 • Estimated life expectancy of >12 weeks • Archived tissue All-comers population Durvalumab 10 mg/kg q 2 wk for up to 12 months N = 476 1 -42 days post-c. CRT 2: 1 randomization, stratified by age, sex and smoking history N = 713 Placebo 10 mg/kg q 2 wk for up to 12 months N = 237 Coprimary endpoints • PFS by BICR using RECIST v 1. 1* • Overall survival Key secondary endpoints • ORR (per BICR) • Do. R (per BICR) • Safety and tolerability c. CRT = concurrent chemoradiation therapy www. clinicaltrials. gov. Accessed April 2019; Antonia SJ et al. N Engl J Med 2017; 377(20): 1919 -29.

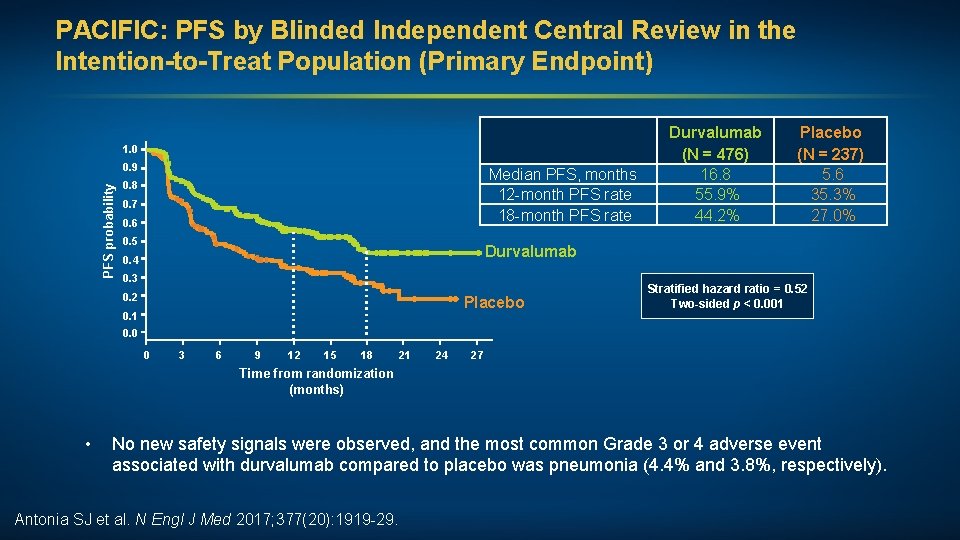
PACIFIC: PFS by Blinded Independent Central Review in the Intention-to-Treat Population (Primary Endpoint) 1. 0 PFS probability 0. 9 Median PFS, months 12 -month PFS rate 18 -month PFS rate 0. 8 0. 7 0. 6 0. 5 Durvalumab (N = 476) 16. 8 55. 9% 44. 2% Placebo (N = 237) 5. 6 35. 3% 27. 0% Durvalumab 0. 4 0. 3 0. 2 Placebo 0. 1 Stratified hazard ratio = 0. 52 Two-sided p < 0. 001 0. 0 0 3 6 9 12 15 18 21 24 27 Time from randomization (months) • No new safety signals were observed, and the most common Grade 3 or 4 adverse event associated with durvalumab compared to placebo was pneumonia (4. 4% and 3. 8%, respectively). Antonia SJ et al. N Engl J Med 2017; 377(20): 1919 -29.

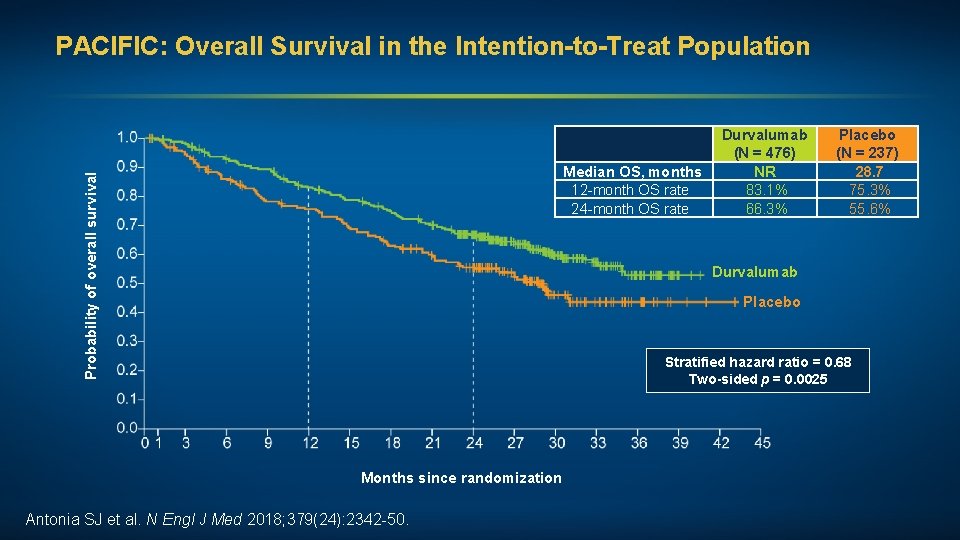
PACIFIC: Overall Survival in the Intention-to-Treat Population Probability of overall survival Median OS, months 12 -month OS rate 24 -month OS rate Durvalumab (N = 476) NR 83. 1% 66. 3% Placebo (N = 237) 28. 7 75. 3% 55. 6% Durvalumab Placebo Stratified hazard ratio = 0. 68 Two-sided p = 0. 0025 Months since randomization Antonia SJ et al. N Engl J Med 2018; 379(24): 2342 -50.

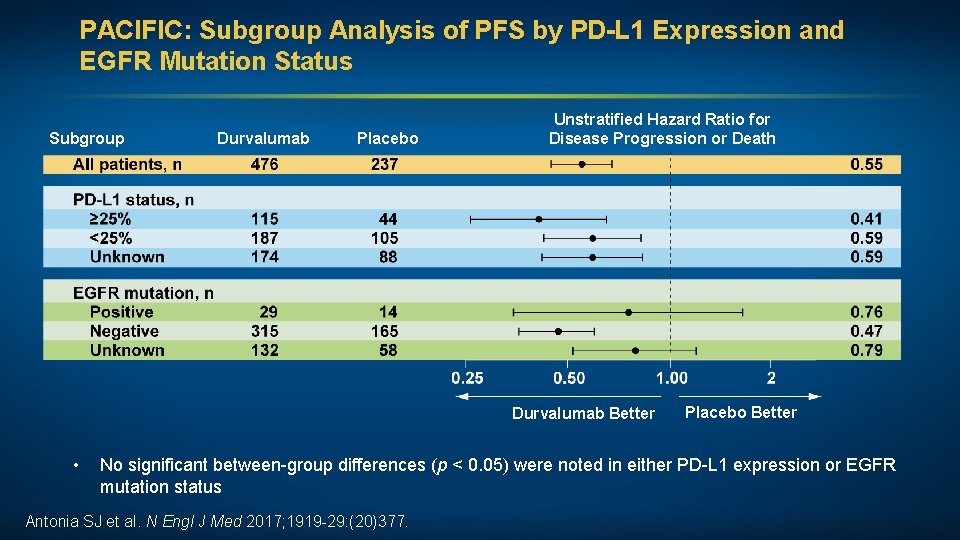
PACIFIC: Subgroup Analysis of PFS by PD-L 1 Expression and EGFR Mutation Status Subgroup Durvalumab Placebo Unstratified Hazard Ratio for Disease Progression or Death Durvalumab Better • Placebo Better No significant between-group differences (p < 0. 05) were noted in either PD-L 1 expression or EGFR mutation status Antonia SJ et al. N Engl J Med 2017; 1919 -29: (20)377.

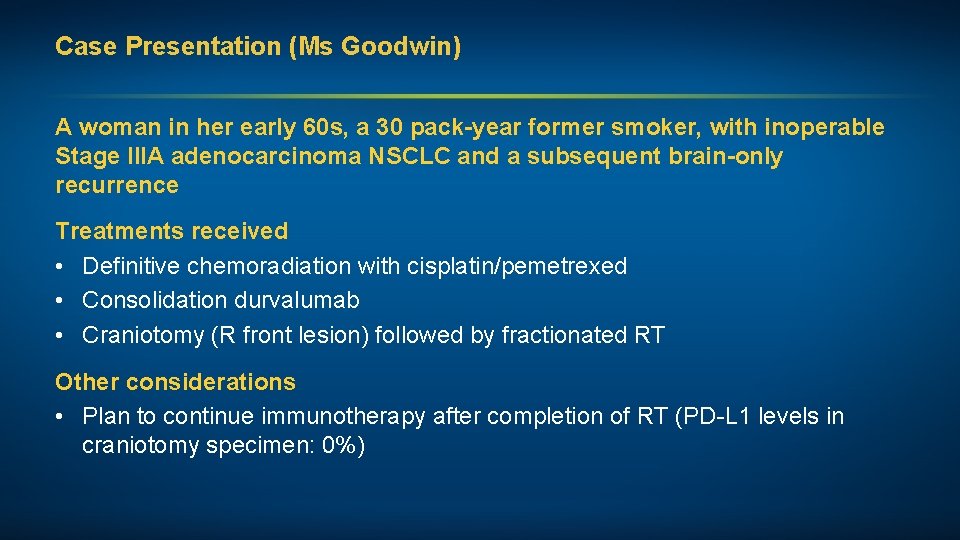
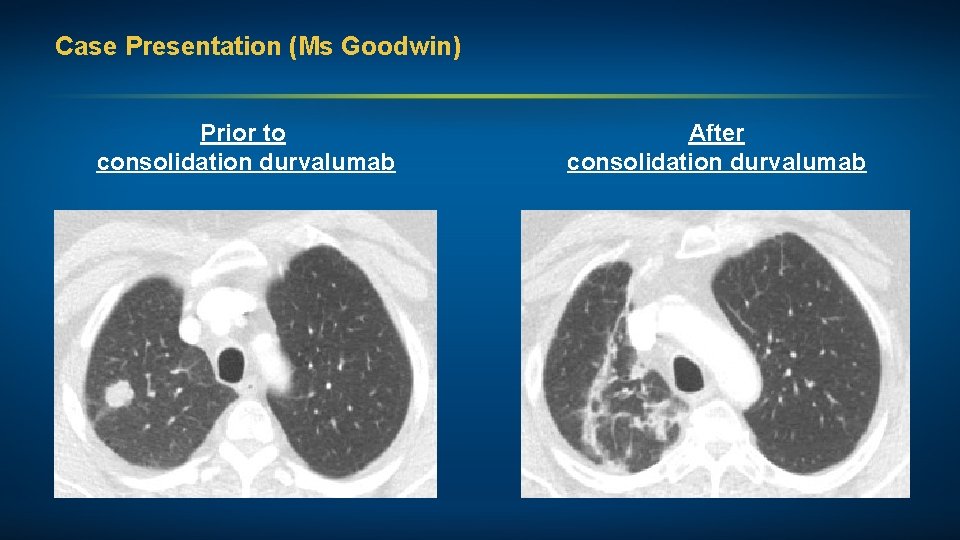
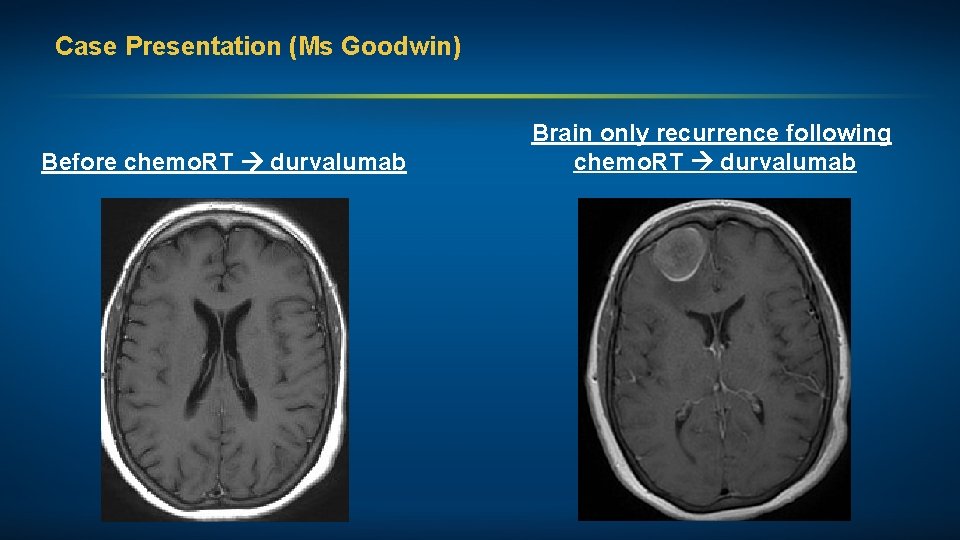
Case Presentation (Ms Goodwin) A woman in her early 60 s, a 30 pack-year former smoker, with inoperable Stage IIIA adenocarcinoma NSCLC and a subsequent brain-only recurrence Treatments received • Definitive chemoradiation with cisplatin/pemetrexed • Consolidation durvalumab • Craniotomy (R front lesion) followed by fractionated RT Other considerations • Plan to continue immunotherapy after completion of RT (PD-L 1 levels in craniotomy specimen: 0%)

Case Presentation (Ms Goodwin) Prior to consolidation durvalumab After consolidation durvalumab

Case Presentation (Ms Goodwin) Before chemo. RT durvalumab Brain only recurrence following chemo. RT durvalumab

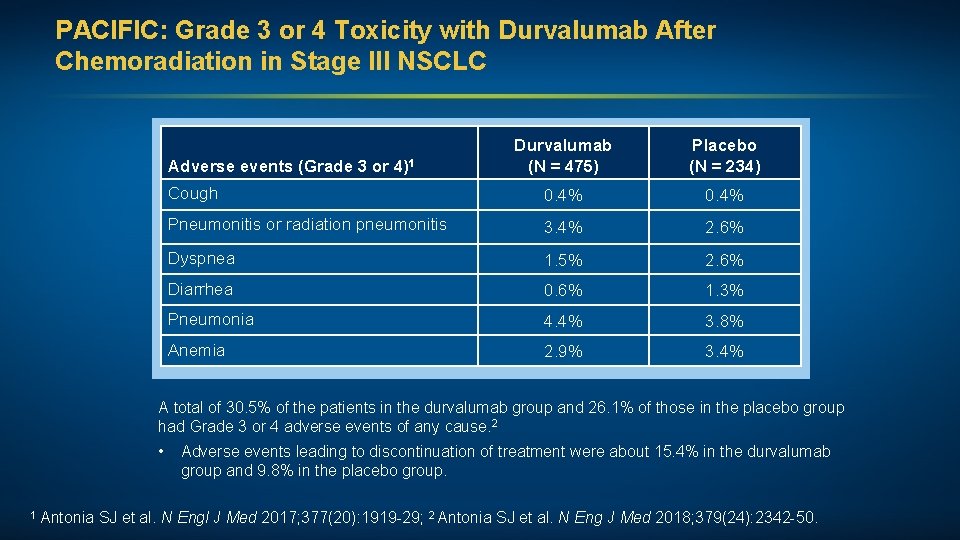
PACIFIC: Grade 3 or 4 Toxicity with Durvalumab After Chemoradiation in Stage III NSCLC Durvalumab (N = 475) Placebo (N = 234) Cough 0. 4% Pneumonitis or radiation pneumonitis 3. 4% 2. 6% Dyspnea 1. 5% 2. 6% Diarrhea 0. 6% 1. 3% Pneumonia 4. 4% 3. 8% Anemia 2. 9% 3. 4% Adverse events (Grade 3 or 4)1 A total of 30. 5% of the patients in the durvalumab group and 26. 1% of those in the placebo group had Grade 3 or 4 adverse events of any cause. 2 • 1 Antonia SJ et al. N Adverse events leading to discontinuation of treatment were about 15. 4% in the durvalumab group and 9. 8% in the placebo group. Engl J Med 2017; 377(20): 1919 -29; 2 Antonia SJ et al. N Eng J Med 2018; 379(24): 2342 -50.

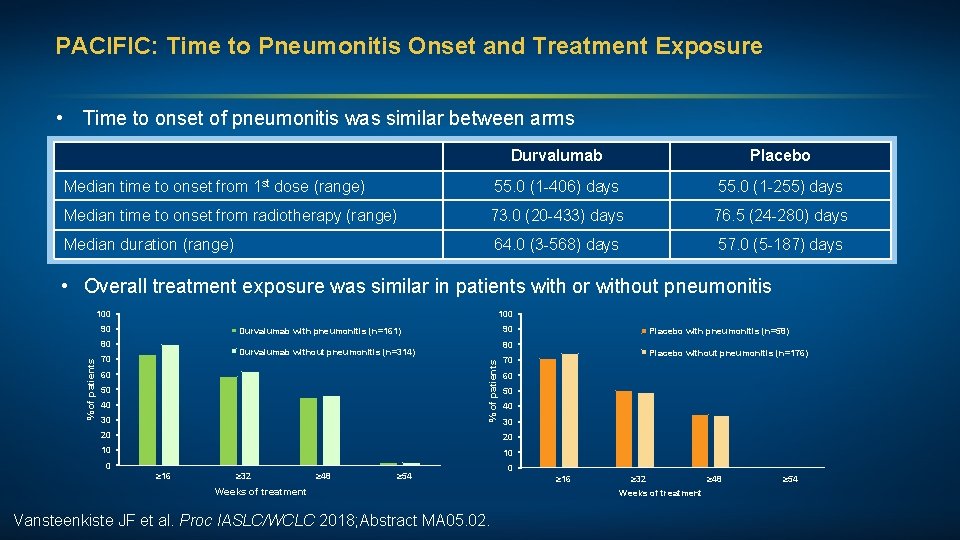
PACIFIC: Time to Pneumonitis Onset and Treatment Exposure • Time to onset of pneumonitis was similar between arms Durvalumab Placebo Median time to onset from 1 st dose (range) 55. 0 (1 -406) days 55. 0 (1 -255) days Median time to onset from radiotherapy (range) 73. 0 (20 -433) days 76. 5 (24 -280) days Median duration (range) 64. 0 (3 -568) days 57. 0 (5 -187) days • Overall treatment exposure was similar in patients with or without pneumonitis 100 90 Durvalumab without pneumonitis (n=314) 70 60 50 40 30 20 10 10 0 ≥ 32 ≥ 48 ≥ 54 Weeks of treatment Vansteenkiste JF et al. Proc IASLC/WCLC 2018; Abstract MA 05. 02. Placebo without pneumonitis (n=176) 70 20 ≥ 16 Placebo with pneumonitis (n=58) 80 % of patients 90 Durvalumab with pneumonitis (n=161) 80 0 ≥ 16 ≥ 32 ≥ 48 Weeks of treatment ≥ 54

Leveraging the Immune System for Therapeutic Benefit in Non-Small Cell Lung Cancer Does 1 + 1 = 2? OR Does 1 + 1 = 10? Immune Checkpoint Inhibitor + • Chemotherapy • Anti-angiogenics • Radiation therapy • Novel agents