Please note these are the actual videorecorded proceedings


- Slides: 17

Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content.

Immune Therapy and Targeting EGFR Roy S. Herbst, MD, Ph. D Ensign Professor of Medicine, Chief of Medical Oncology, Associate Director for Translational Research Yale School of Medicine | Yale Cancer Center | Smilow Cancer Hospital at Yale-New Haven

Disclosures Consulting Agreements Astra. Zeneca Pharmaceuticals LP, Genentech Bio. Oncology, Lilly, Merck, Pfizer Inc Contracted Research Genentech Bio. Oncology, Merck

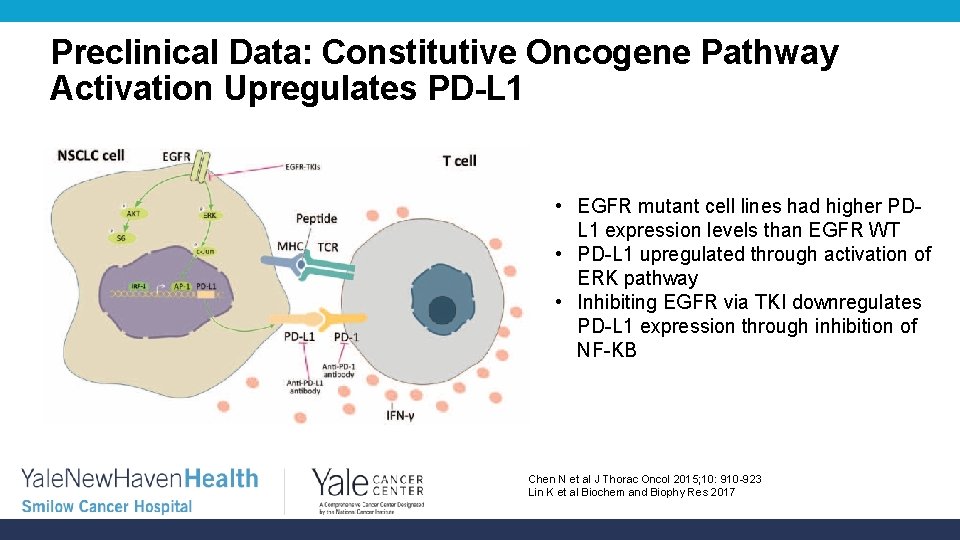
Preclinical Data: Constitutive Oncogene Pathway Activation Upregulates PD-L 1 • EGFR mutant cell lines had higher PDL 1 expression levels than EGFR WT • PD-L 1 upregulated through activation of ERK pathway • Inhibiting EGFR via TKI downregulates PD-L 1 expression through inhibition of NF-KB Chen N et al J Thorac Oncol 2015; 10: 910 -923 Lin K et al Biochem and Biophy Res 2017

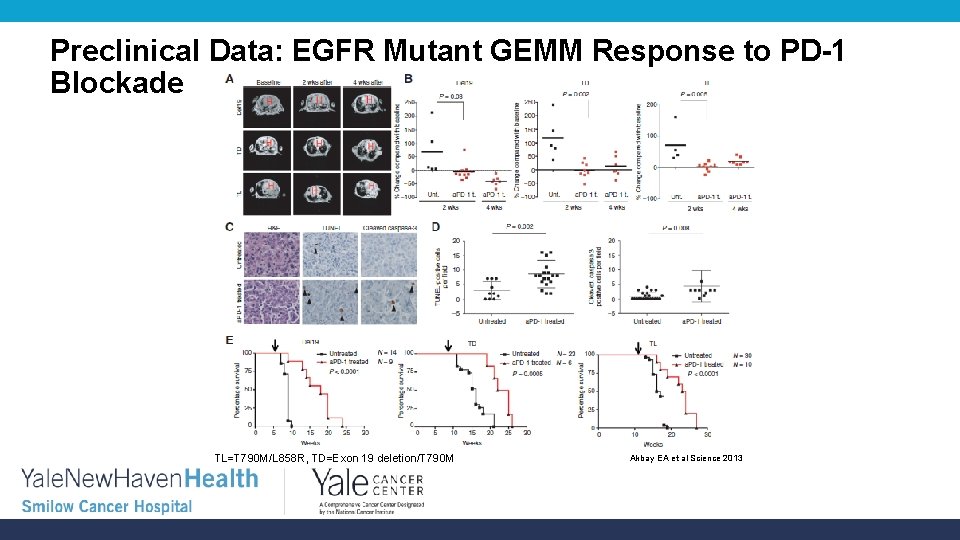
Preclinical Data: EGFR Mutant GEMM Response to PD-1 Blockade TL=T 790 M/L 858 R, TD=Exon 19 deletion/T 790 M Akbay EA et al Science 2013

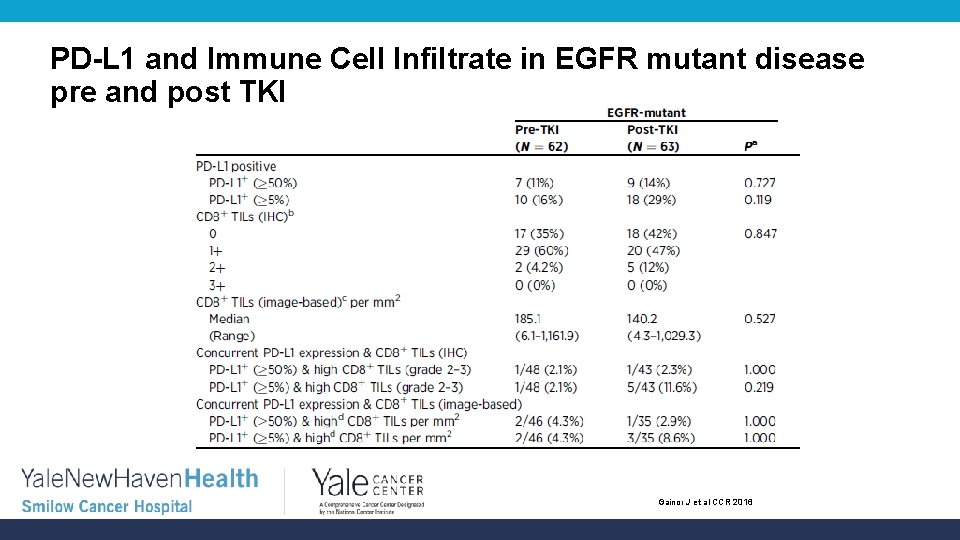
PD-L 1 and Immune Cell Infiltrate in EGFR mutant disease pre and post TKI Gainor J et al CCR 2016

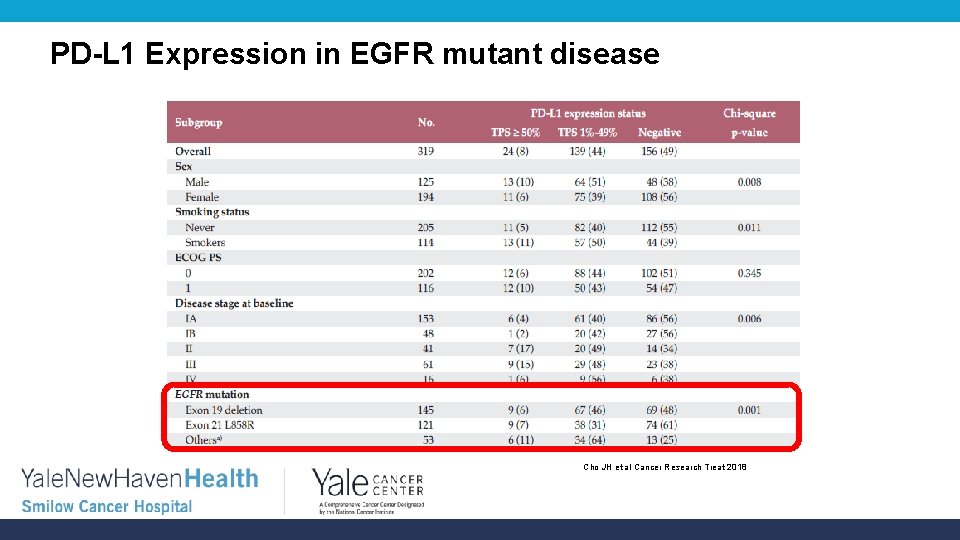
PD-L 1 Expression in EGFR mutant disease Cho JH et al Cancer Research Treat 2018

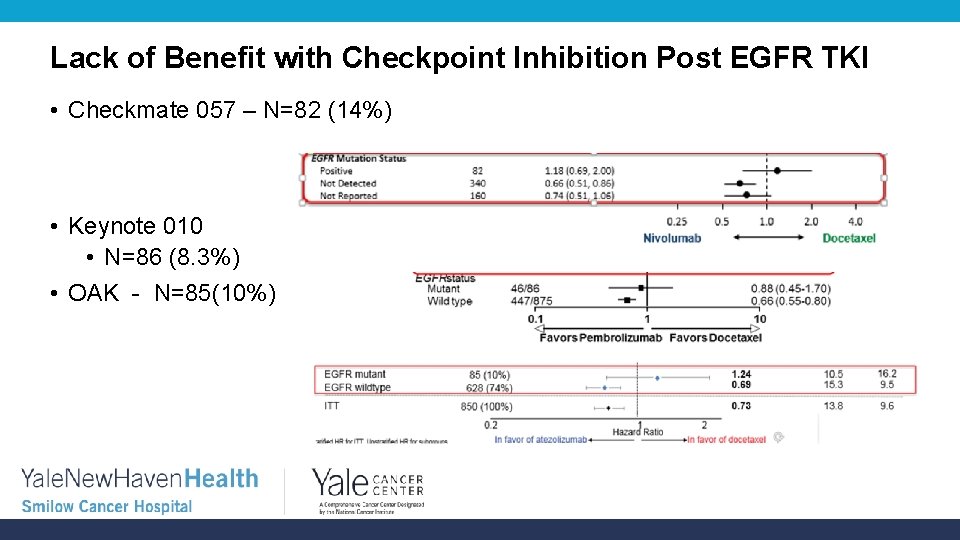
Lack of Benefit with Checkpoint Inhibition Post EGFR TKI • Checkmate 057 – N=82 (14%) • Keynote 010 • N=86 (8. 3%) • OAK - N=85(10%)

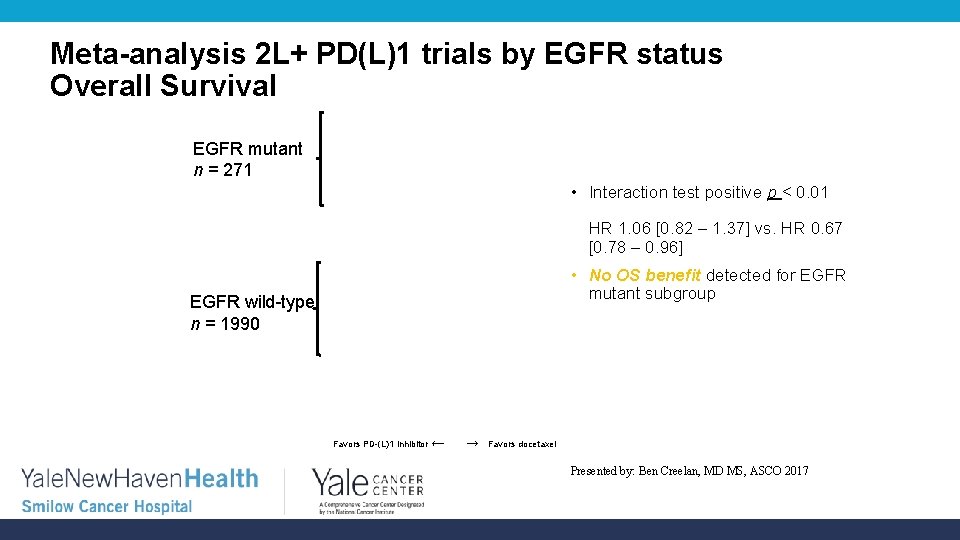
Meta-analysis 2 L+ PD(L)1 trials by EGFR status Overall Survival EGFR mutant n = 271 • Interaction test positive p < 0. 01 HR 1. 06 [0. 82 – 1. 37] vs. HR 0. 67 [0. 78 – 0. 96] • No OS benefit detected for EGFR mutant subgroup EGFR wild-type n = 1990 Favors PD-(L)1 inhibitor ← → Favors docetaxel Presented by: Ben Creelan, MD MS, ASCO 2017

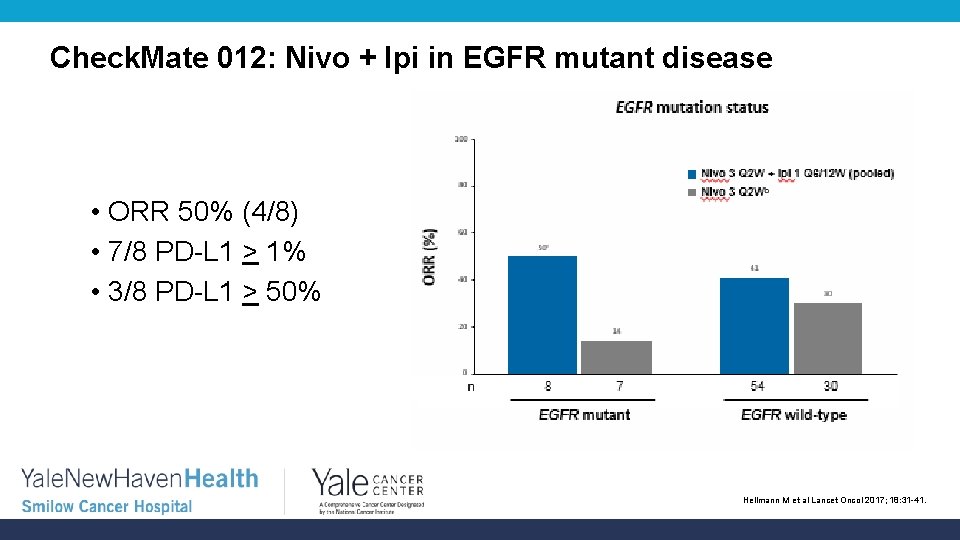
Check. Mate 012: Nivo + Ipi in EGFR mutant disease • ORR 50% (4/8) • 7/8 PD-L 1 > 1% • 3/8 PD-L 1 > 50% Hellmann M et al Lancet Oncol 2017; 18: 31 -41.

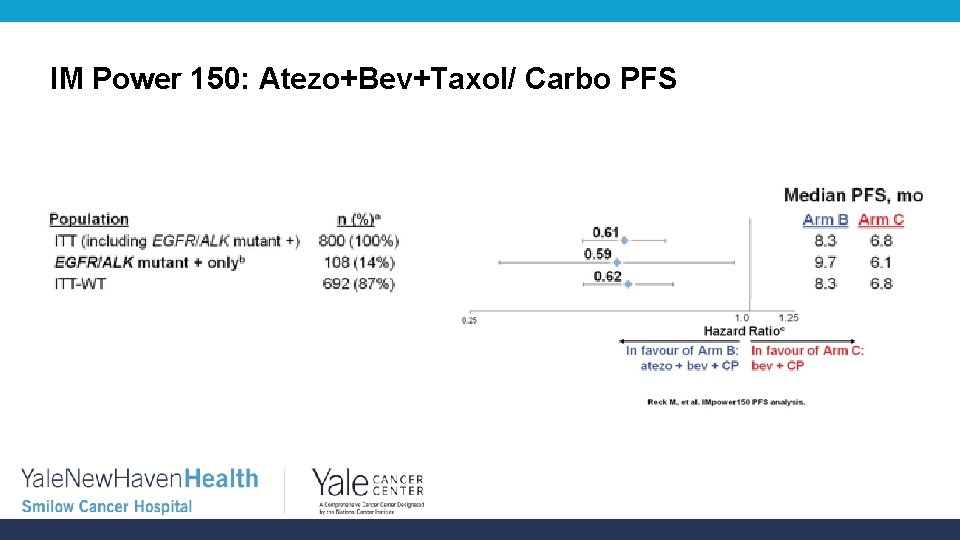
IM Power 150: Atezo+Bev+Taxol/ Carbo PFS

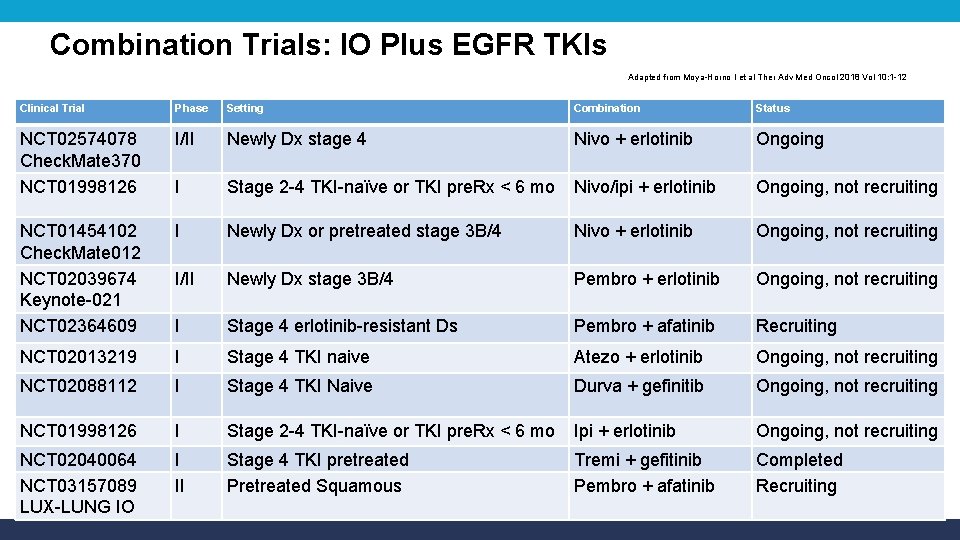
Combination Trials: IO Plus EGFR TKIs Adapted from Moya-Horno I et al Ther Adv Med Oncol 2018 Vol 10: 1 -12 Clinical Trial Phase Setting Combination Status NCT 02574078 Check. Mate 370 NCT 01998126 I/II Newly Dx stage 4 Nivo + erlotinib Ongoing I Stage 2 -4 TKI-naïve or TKI pre. Rx < 6 mo Nivo/ipi + erlotinib Ongoing, not recruiting NCT 01454102 Check. Mate 012 NCT 02039674 Keynote-021 NCT 02364609 I Newly Dx or pretreated stage 3 B/4 Nivo + erlotinib Ongoing, not recruiting I/II Newly Dx stage 3 B/4 Pembro + erlotinib Ongoing, not recruiting I Stage 4 erlotinib-resistant Ds Pembro + afatinib Recruiting NCT 02013219 I Stage 4 TKI naive Atezo + erlotinib Ongoing, not recruiting NCT 02088112 I Stage 4 TKI Naive Durva + gefinitib Ongoing, not recruiting NCT 01998126 I Stage 2 -4 TKI-naïve or TKI pre. Rx < 6 mo Ipi + erlotinib Ongoing, not recruiting NCT 02040064 NCT 03157089 LUX-LUNG IO I II Stage 4 TKI pretreated Pretreated Squamous Tremi + gefitinib Pembro + afatinib Completed Recruiting

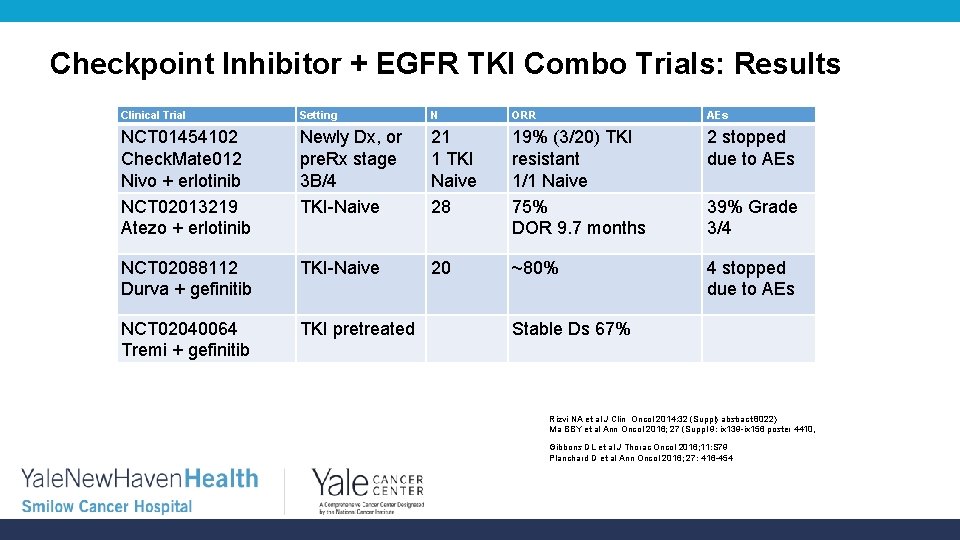
Checkpoint Inhibitor + EGFR TKI Combo Trials: Results Clinical Trial Setting N ORR AEs NCT 01454102 Check. Mate 012 Nivo + erlotinib NCT 02013219 Atezo + erlotinib Newly Dx, or pre. Rx stage 3 B/4 TKI-Naive 21 1 TKI Naive 28 19% (3/20) TKI resistant 1/1 Naive 75% DOR 9. 7 months 2 stopped due to AEs NCT 02088112 Durva + gefinitib TKI-Naive 20 ~80% 4 stopped due to AEs NCT 02040064 Tremi + gefinitib TKI pretreated 39% Grade 3/4 Stable Ds 67% Rizvi NA et al J Clin Oncol 2014: 32 (Suppl) abstract 8022) Ma BBY et al Ann Oncol 2016; 27 (Suppl 9: ix 139 -ix 156 poster 4410, Gibbons DL et al J Thorac Oncol 2016; 11: S 79 Planchard D et al Ann Oncol 2016; 27: 416 -454

Data from Existing EGFR TKI and IO Combination Trials • Lessons from TATTON • Osi + Durva • ORR ~ 40% • Stopped due to increase of pulmonary toxicity • Interstitial lung Dx in 26% of TKI pretreated and 64% in TKI naive Ahn MJ et al J Thorac Oncol 2016; 11: S 115

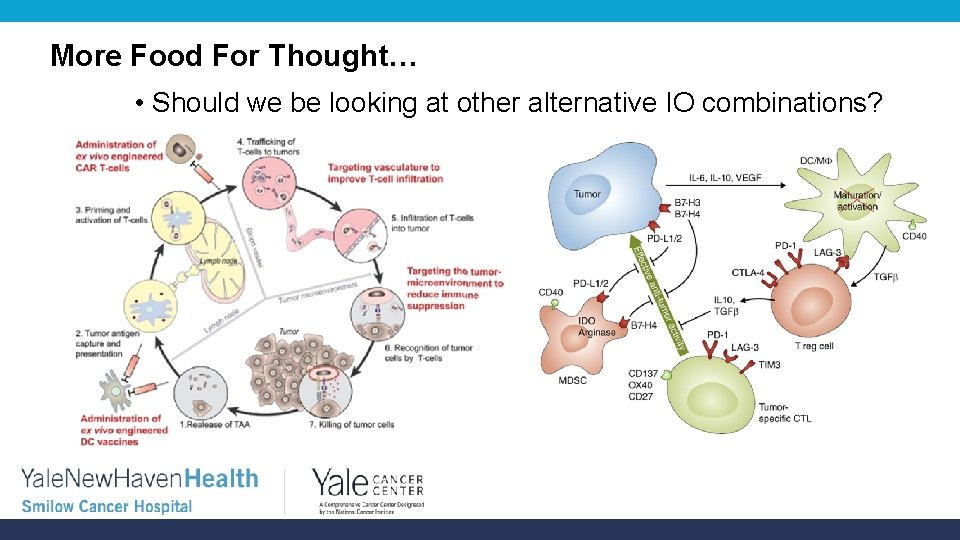
More Food For Thought… • Should we be looking at other alternative IO combinations?

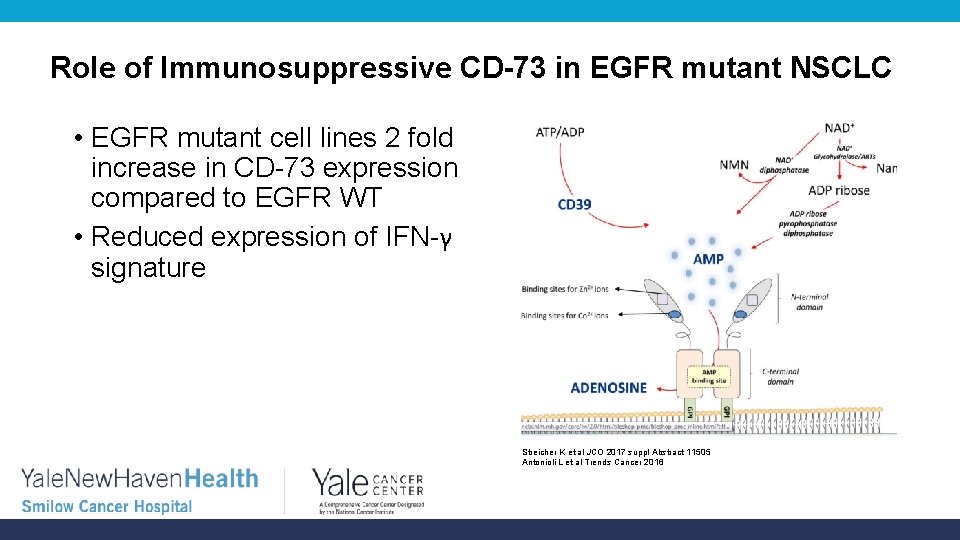
Role of Immunosuppressive CD-73 in EGFR mutant NSCLC • EGFR mutant cell lines 2 fold increase in CD-73 expression compared to EGFR WT • Reduced expression of IFN-γ signature Streicher K et al JCO 2017 suppl Abstract 11505 Antonioli L et al Trends Cancer 2016

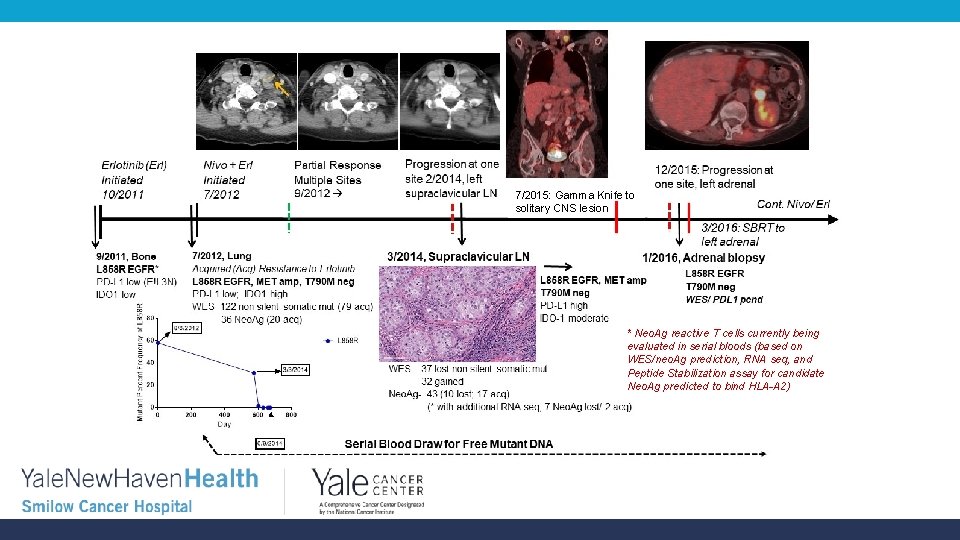
7/2015: Gamma Knife to solitary CNS lesion * Neo. Ag reactive T cells currently being evaluated in serial bloods (based on WES/neo. Ag prediction, RNA seq, and Peptide Stabilization assay for candidate Neo. Ag predicted to bind HLA-A 2)