Please note these are the actual videorecorded proceedings




















- Slides: 52

Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content.

Oncology Grand Rounds Chronic Lymphocytic Leukemia Nurse and Physician Investigators Discuss New Agents, Novel Therapies and Actual Cases from Practice Thursday, April 11, 2019 12: 15 PM – 1: 45 PM Faculty Paul M Barr, MD Nitin Jain, MD Lynn Rich, RN, MSN, OCN, BC-ANP Josie Bazemore, MSN, AGPCNP-BC Moderator Neil Love, MD

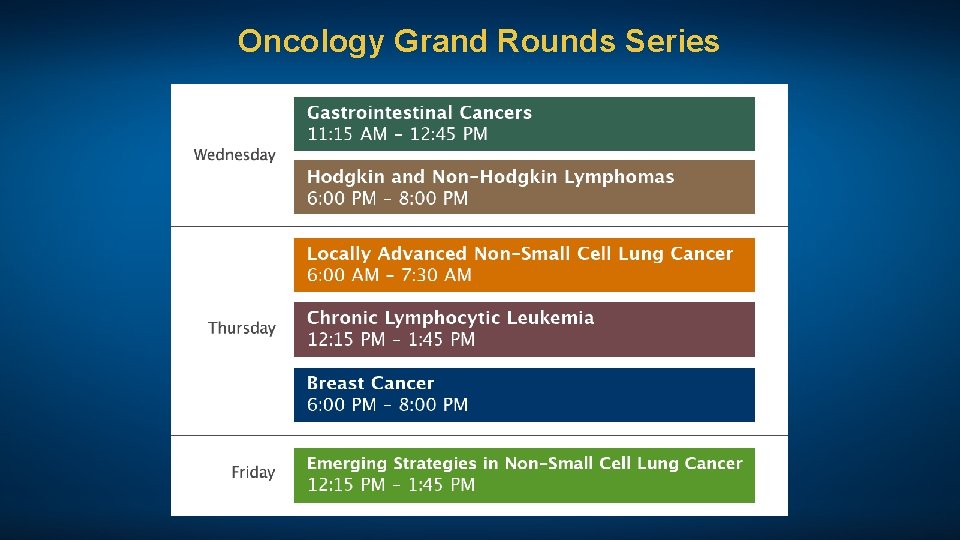
Oncology Grand Rounds Series

Disclosures for Moderator Neil Love, MD Dr Love is president and CEO of Research To Practice receives funds in the form of educational grants to develop CME activities from the following commercial interests: Abb. Vie Inc, Acerta Pharma — A member of the Astra. Zeneca Group, Adaptive Biotechnologies, Agendia Inc, Agios Pharmaceuticals Inc, Amgen Inc, Ariad Pharmaceuticals Inc, Array Bio. Pharma Inc, Astellas Pharma Global Development Inc, Astra. Zeneca Pharmaceuticals LP, Bayer Health. Care Pharmaceuticals, Biodesix Inc, bio. Theranostics Inc, Boehringer Ingelheim Pharmaceuticals Inc, Boston Biomedical Inc, Bristol-Myers Squibb Company, Celgene Corporation, Clovis Oncology, Daiichi Sankyo Inc, Dendreon Pharmaceuticals Inc, Eisai Inc, Exelixis Inc, Foundation Medicine, Genentech, Genmab, Genomic Health Inc, Gilead Sciences Inc, Guardant Health, Halozyme Inc, Immuno. Gen Inc, Incyte Corporation, Infinity Pharmaceuticals Inc, Ipsen Biopharmaceuticals Inc, Janssen Biotech Inc, administered by Janssen Scientific Affairs LLC, Jazz Pharmaceuticals Inc, Kite Pharma Inc, Lexicon Pharmaceuticals Inc, Lilly, Loxo Oncology, Merck, Merrimack Pharmaceuticals Inc, Myriad Genetic Laboratories Inc, Natera Inc, Novartis, Oncopeptides, Pfizer Inc, Pharmacyclics LLC, an Abb. Vie Company, Prometheus Laboratories Inc, Puma Biotechnology Inc, Regeneron Pharmaceuticals Inc, Sandoz Inc, a Novartis Division, Sanofi Genzyme, Seattle Genetics, Sirtex Medical Ltd, Spectrum Pharmaceuticals Inc, Taiho Oncology Inc, Takeda Oncology, Tesaro, Teva Oncology, Tokai Pharmaceuticals Inc and Tolero Pharmaceuticals.

Paul M Barr, MD Medical Director, Clinical Trials Office Associate Professor of Medicine Wilmot Cancer Institute University of Rochester Medical Center Rochester, New York

Disclosures for Dr Barr Advisory Committee Abb. Vie Inc, Astra. Zeneca Pharmaceuticals LP, Celgene Corporation, Genentech, Gilead Sciences Inc, Janssen Biotech Inc, Merck, Pharmacyclics LLC, an Abb. Vie Company Consulting Agreement Seattle Genetics Data and Safety Monitoring Board/Committee TG Therapeutics Inc

Josie Bazemore, MSN, AGPCNP-BC Dana-Farber Cancer Institute Boston, Massachusetts

Disclosures for Ms Bazemore Advisory Committee Janssen Biotech Inc

Nitin Jain, MD Associate Professor of Medicine Department of Leukemia The University of Texas MD Anderson Cancer Center Houston, Texas

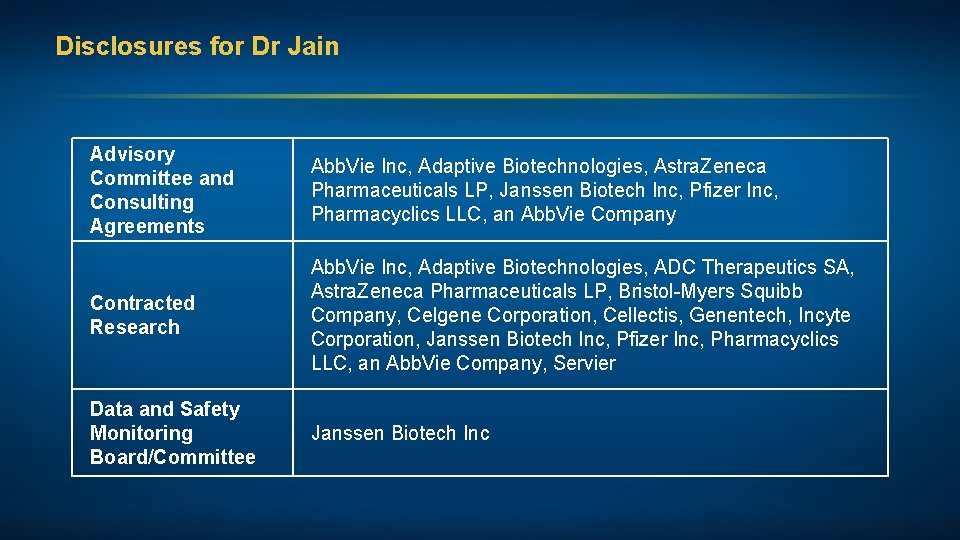
Disclosures for Dr Jain Advisory Committee and Consulting Agreements Abb. Vie Inc, Adaptive Biotechnologies, Astra. Zeneca Pharmaceuticals LP, Janssen Biotech Inc, Pfizer Inc, Pharmacyclics LLC, an Abb. Vie Company Contracted Research Abb. Vie Inc, Adaptive Biotechnologies, ADC Therapeutics SA, Astra. Zeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Celgene Corporation, Cellectis, Genentech, Incyte Corporation, Janssen Biotech Inc, Pfizer Inc, Pharmacyclics LLC, an Abb. Vie Company, Servier Data and Safety Monitoring Board/Committee Janssen Biotech Inc

Lynn Rich, RN, MSN, OCN, BC-ANP University of Rochester Wilmot Cancer Institute Lymphoma Program Rochester, New York

Disclosures for Ms Rich No relevant conflicts of interest to disclose

Chronic Lymphocytic Leukemia: Agenda Newly Diagnosed CLL: Observation versus Treatment First-Line Treatment of CLL Relapsed/Refractory CLL

Case Presentation (Ms Bazemore) A woman in her early 60 s with CLL (17 p deletion, mutated IGHV, trisomy 12) Diagnostic Workup • 6/2015: Bulky diffuse lymphadenopathy with no B symptoms; WBC 150, 000 • 10/2016: WBC 440, 000; Hgb 10; PLT 140, 000

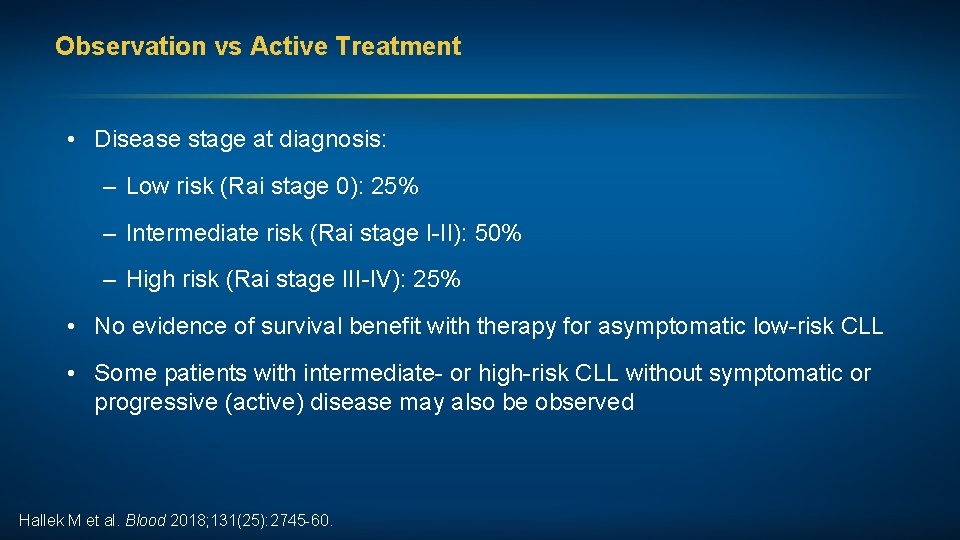
Observation vs Active Treatment • Disease stage at diagnosis: – Low risk (Rai stage 0): 25% – Intermediate risk (Rai stage I-II): 50% – High risk (Rai stage III-IV): 25% • No evidence of survival benefit with therapy for asymptomatic low-risk CLL • Some patients with intermediate- or high-risk CLL without symptomatic or progressive (active) disease may also be observed Hallek M et al. Blood 2018; 131(25): 2745 -60.

Chronic Lymphocytic Leukemia: Agenda Newly Diagnosed CLL: Observation versus Treatment First-Line Treatment of CLL Relapsed/Refractory CLL

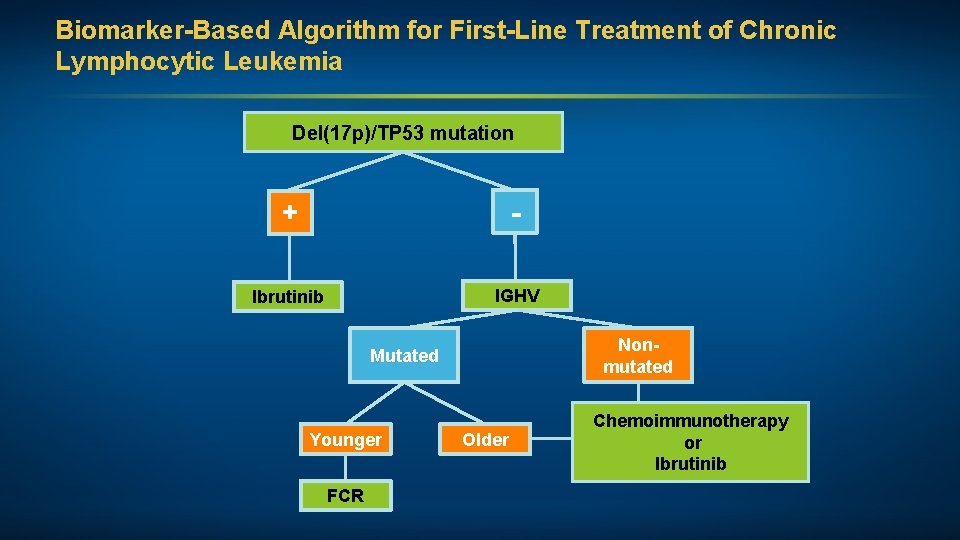
Biomarker-Based Algorithm for First-Line Treatment of Chronic Lymphocytic Leukemia Del(17 p)/TP 53 mutation + - Ibrutinib IGHV Nonmutated Mutated Younger FCR Older Chemoimmunotherapy or Ibrutinib

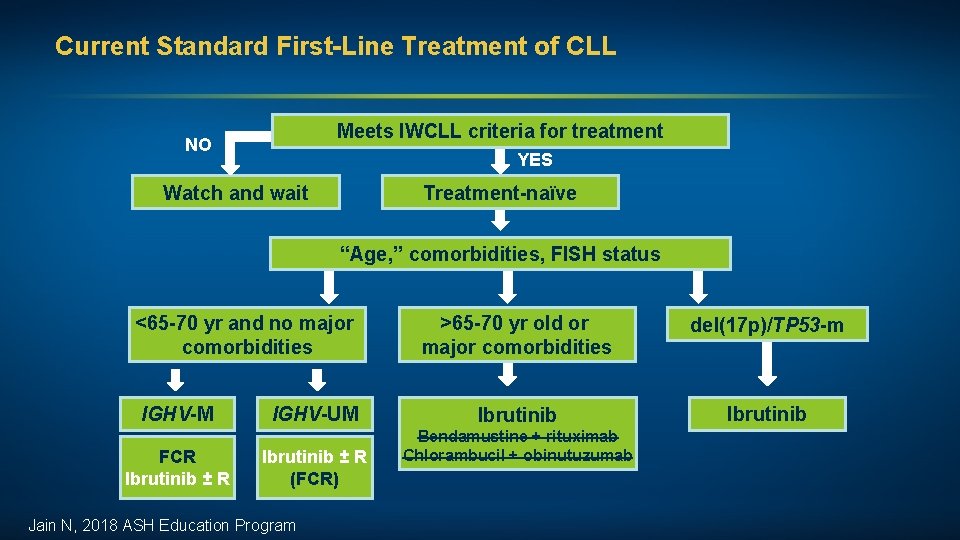
Current Standard First-Line Treatment of CLL Meets IWCLL criteria for treatment NO YES Watch and wait Treatment-naïve “Age, ” comorbidities, FISH status <65 -70 yr and no major comorbidities IGHV-M FCR Ibrutinib ± R IGHV-UM Ibrutinib ± R (FCR) Jain N, 2018 ASH Education Program >65 -70 yr old or major comorbidities del(17 p)/TP 53 -m Ibrutinib Bendamustine + rituximab Chlorambucil + obinutuzumab

Case Presentation (Ms Rich) A woman in her late 30 s with chronic lymphocytic leukemia (CLL) Treatments received • FCR Other considerations • Multiple sinus and respiratory infections (none life-threatening in the last 3 -4 years) • Elementary school teacher (unable to work for years due to continual infections but now has her own classroom)

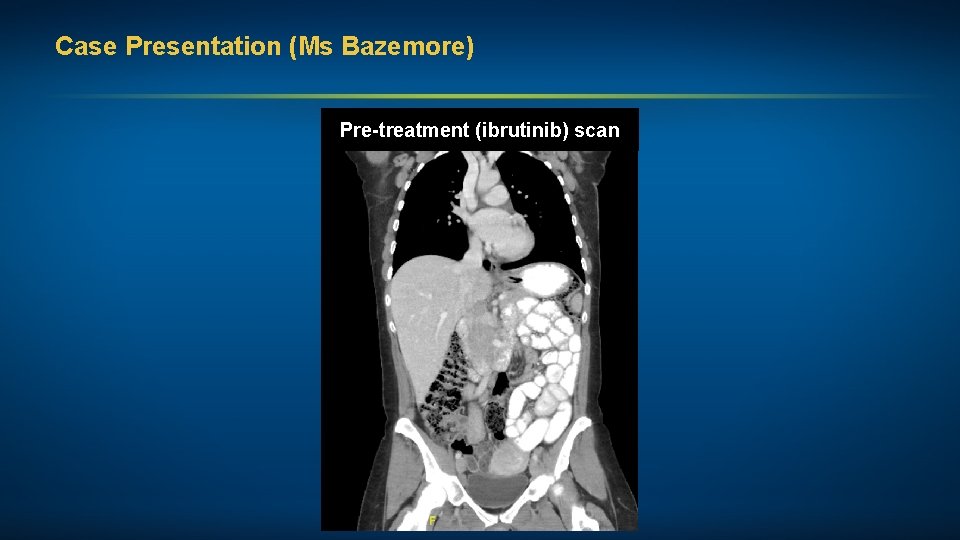
Case Presentation (Ms Bazemore) A woman in her early 60 s with CLL (17 p deletion, mutated IGHV, trisomy 12) Treatments received • Ibrutinib Other considerations • Supports holistic medicine approach to treatment – used high-dose green tea extract prior to and during ibrutinib therapy • Pseudohyperkalemia

Case Presentation (Ms Bazemore) Pre-treatment (ibrutinib) scan

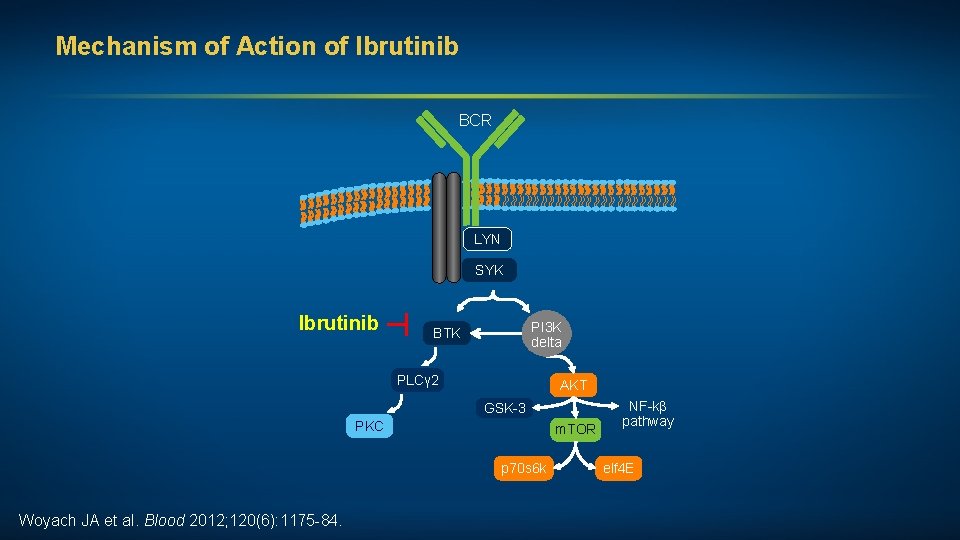
Mechanism of Action of Ibrutinib BCR LYN SYK ┬ Ibrutinib PI 3 K delta BTK PLCγ 2 AKT GSK-3 PKC m. TOR p 70 s 6 k Woyach JA et al. Blood 2012; 120(6): 1175 -84. NF-kβ pathway elf 4 E

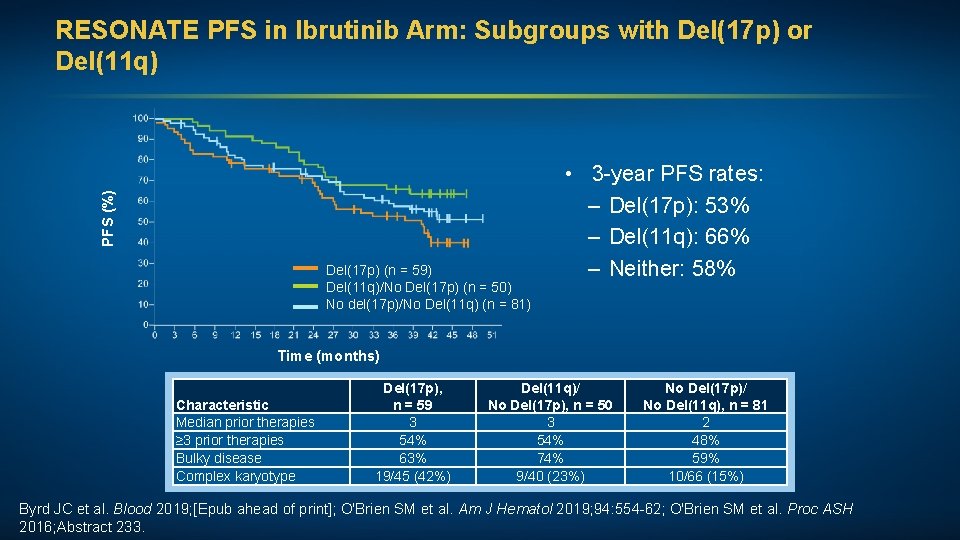
PFS (%) RESONATE PFS in Ibrutinib Arm: Subgroups with Del(17 p) or Del(11 q) Del(17 p) (n = 59) Del(11 q)/No Del(17 p) (n = 50) No del(17 p)/No Del(11 q) (n = 81) • 3 -year PFS rates: – Del(17 p): 53% – Del(11 q): 66% – Neither: 58% Time (months) Characteristic Median prior therapies ≥ 3 prior therapies Bulky disease Complex karyotype Del(17 p), n = 59 3 54% 63% 19/45 (42%) Del(11 q)/ No Del(17 p), n = 50 3 54% 74% 9/40 (23%) No Del(17 p)/ No Del(11 q), n = 81 2 48% 59% 10/66 (15%) Byrd JC et al. Blood 2019; [Epub ahead of print]; O'Brien SM et al. Am J Hematol 2019; 94: 554 -62; O'Brien SM et al. Proc ASH 2016; Abstract 233.

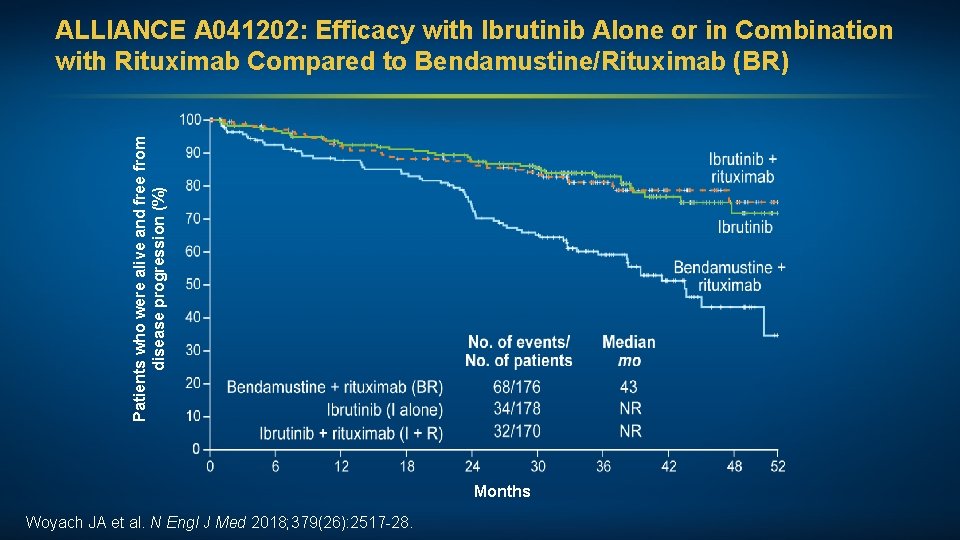
Patients who were alive and free from disease progression (%) ALLIANCE A 041202: Efficacy with Ibrutinib Alone or in Combination with Rituximab Compared to Bendamustine/Rituximab (BR) Months Woyach JA et al. N Engl J Med 2018; 379(26): 2517 -28.

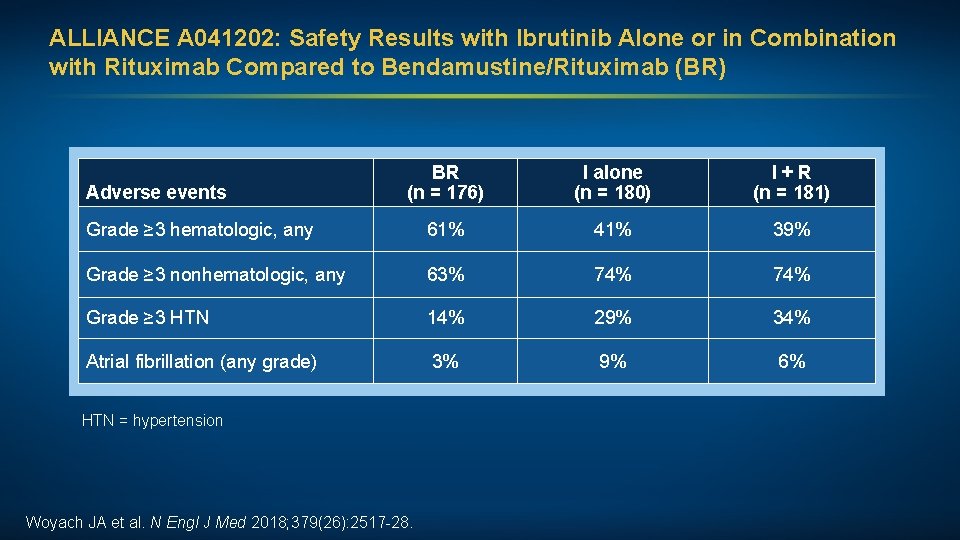
ALLIANCE A 041202: Safety Results with Ibrutinib Alone or in Combination with Rituximab Compared to Bendamustine/Rituximab (BR) BR (n = 176) I alone (n = 180) I + R (n = 181) Grade ≥ 3 hematologic, any 61% 41% 39% Grade ≥ 3 nonhematologic, any 63% 74% Grade ≥ 3 HTN 14% 29% 34% Atrial fibrillation (any grade) 3% 9% 6% Adverse events HTN = hypertension Woyach JA et al. N Engl J Med 2018; 379(26): 2517 -28.

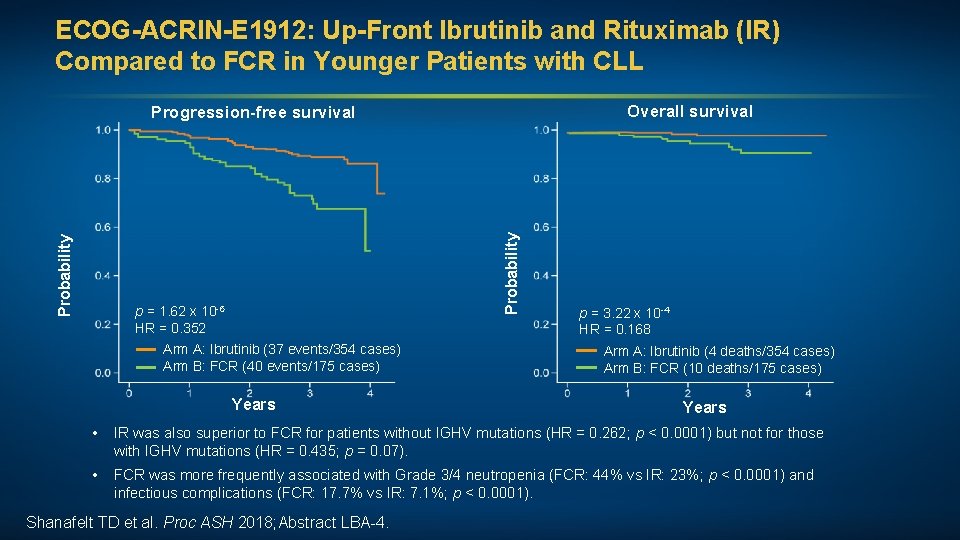
ECOG-ACRIN-E 1912: Up-Front Ibrutinib and Rituximab (IR) Compared to FCR in Younger Patients with CLL Overall survival Probability Progression-free survival p = 1. 62 x 10 -6 HR = 0. 352 Arm A: Ibrutinib (37 events/354 cases) Arm B: FCR (40 events/175 cases) Years p = 3. 22 x 10 -4 HR = 0. 168 Arm A: Ibrutinib (4 deaths/354 cases) Arm B: FCR (10 deaths/175 cases) Years • IR was also superior to FCR for patients without IGHV mutations (HR = 0. 262; p < 0. 0001) but not for those with IGHV mutations (HR = 0. 435; p = 0. 07). • FCR was more frequently associated with Grade 3/4 neutropenia (FCR: 44% vs IR: 23%; p < 0. 0001) and infectious complications (FCR: 17. 7% vs IR: 7. 1%; p < 0. 0001). Shanafelt TD et al. Proc ASH 2018; Abstract LBA-4.

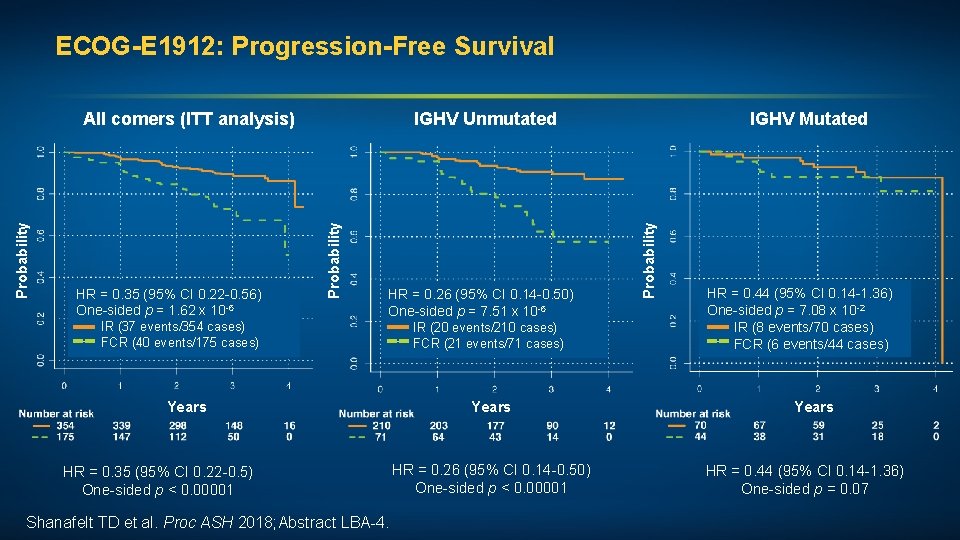
ECOG-E 1912: Progression-Free Survival IR (37 events/354 cases) FCR (40 events/175 cases) HR = 0. 26 (95% CI 0. 14 -0. 50) One-sided p = 7. 51 x 10 -6 IR (20 events/210 cases) FCR (21 events/71 cases) Years HR = 0. 35 (95% CI 0. 22 -0. 5) One-sided p < 0. 00001 Shanafelt TD et al. Proc ASH 2018; Abstract LBA-4. Years HR = 0. 26 (95% CI 0. 14 -0. 50) One-sided p < 0. 00001 IGHV Mutated Probability HR = 0. 35 (95% CI 0. 22 -0. 56) One-sided p = 1. 62 x 10 -6 IGHV Unmutated Probability All comers (ITT analysis) HR = 0. 44 (95% CI 0. 14 -1. 36) One-sided p = 7. 08 x 10 -2 IR (8 events/70 cases) FCR (6 events/44 cases) Years HR = 0. 44 (95% CI 0. 14 -1. 36) One-sided p = 0. 07

FDA Approval of Ibrutinib in Combination with Obinutuzumab in Treatment-Naïve CLL/SLL January 28, 2019 “Today, the US Food and Drug Administration (FDA) approved ibrutinib, a Bruton's tyrosine kinase inhibitor, in combination with obinutuzumab in treatment-naive patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). This is the first approval of a nonchemotherapy combination regimen for treatment-naive patients with CLL/SLL. This approval is based on results from the phase III i. LLUMINATE trial. At a median follow-up of 31 months, ibrutinib plus obinutuzumab showed a significant improvement in independent review committee (IRC)-evaluated progression-free survival compared with chlorambucil plus obinutuzumab (median not evaluable [NE] vs 19 months; hazard ratio [HR] = 0. 23; P <. 0001), with a 77% reduction in risk of progression or death. ” https: //www. ascopost. com/News/59690

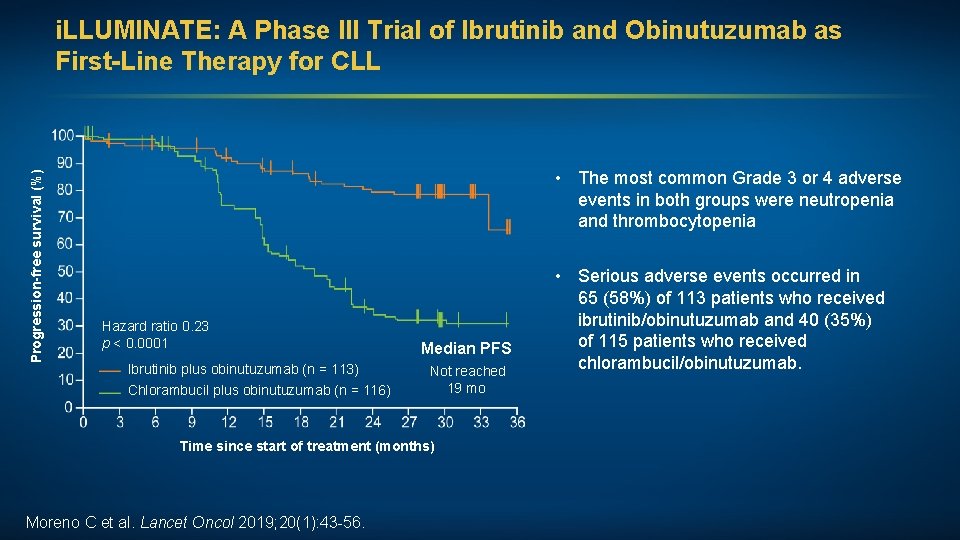
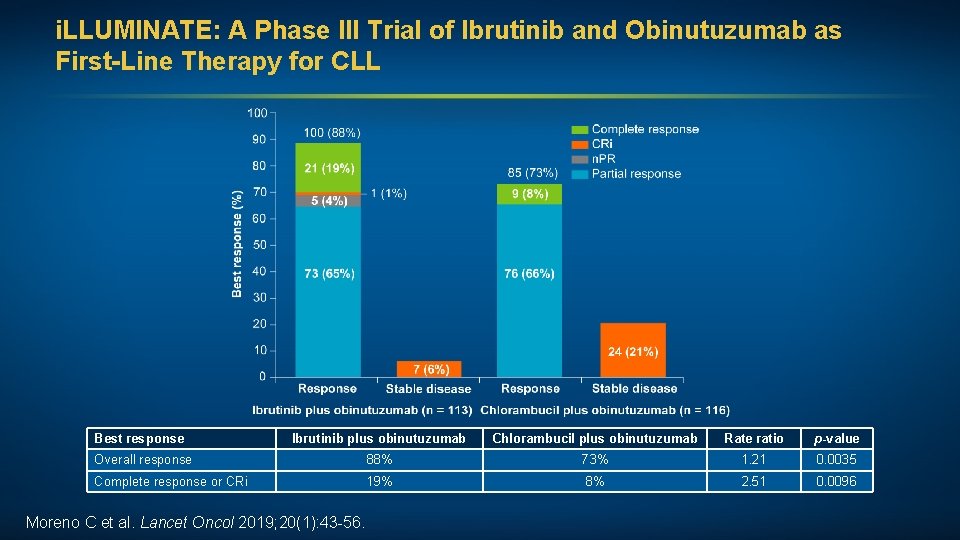
Progression-free survival (%) i. LLUMINATE: A Phase III Trial of Ibrutinib and Obinutuzumab as First-Line Therapy for CLL • The most common Grade 3 or 4 adverse events in both groups were neutropenia and thrombocytopenia Hazard ratio 0. 23 p < 0. 0001 Ibrutinib plus obinutuzumab (n = 113) Chlorambucil plus obinutuzumab (n = 116) Median PFS Not reached 19 mo Time since start of treatment (months) Moreno C et al. Lancet Oncol 2019; 20(1): 43 -56. • Serious adverse events occurred in 65 (58%) of 113 patients who received ibrutinib/obinutuzumab and 40 (35%) of 115 patients who received chlorambucil/obinutuzumab.

i. LLUMINATE: A Phase III Trial of Ibrutinib and Obinutuzumab as First-Line Therapy for CLL Best response Ibrutinib plus obinutuzumab Chlorambucil plus obinutuzumab Rate ratio p-value Overall response 88% 73% 1. 21 0. 0035 Complete response or CRi 19% 8% 2. 51 0. 0096 Moreno C et al. Lancet Oncol 2019; 20(1): 43 -56.

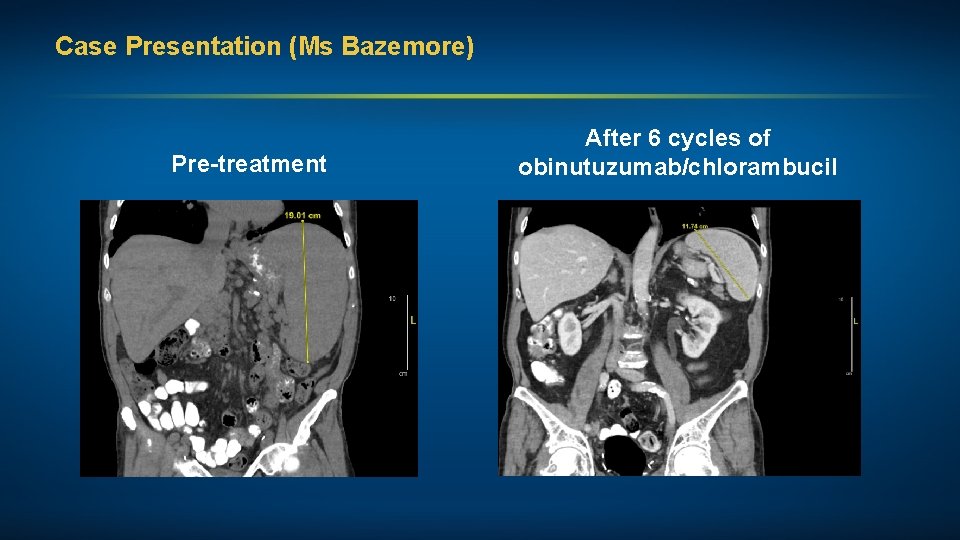
Case Presentation (Ms Bazemore) A man in his early 90 s with CLL (unmutated IGHV and deletion 13 q) Treatments received • Obinutuzumab/chlorambucil Other considerations • Former pediatrician • Financial considerations • TLS prophylaxis

Case Presentation (Ms Bazemore) Pre-treatment After 6 cycles of obinutuzumab/chlorambucil

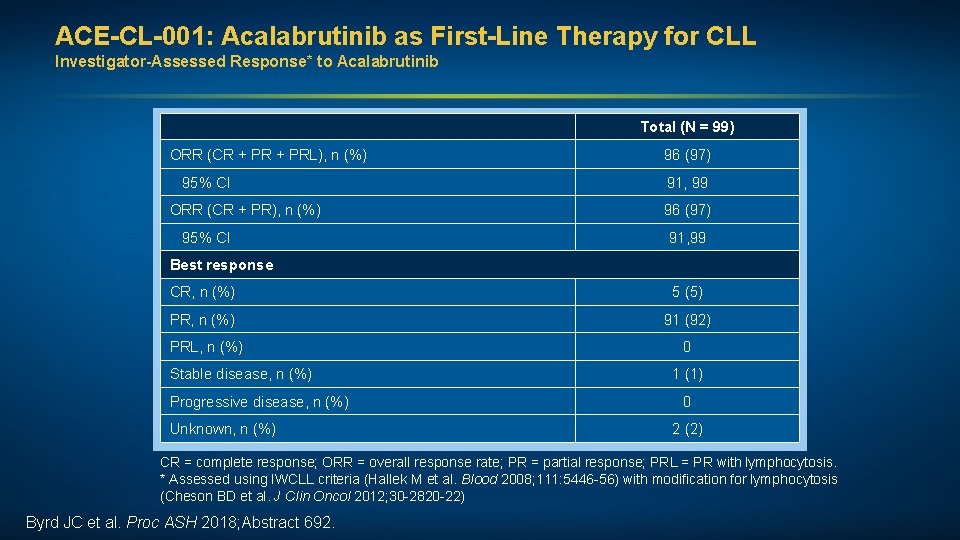
ACE-CL-001: Acalabrutinib as First-Line Therapy for CLL Investigator-Assessed Response* to Acalabrutinib Total (N = 99) ORR (CR + PRL), n (%) 96 (97) 95% CI 91, 99 ORR (CR + PR), n (%) 96 (97) 95% CI 91, 99 Best response CR, n (%) 5 (5) PR, n (%) 91 (92) PRL, n (%) 0 Stable disease, n (%) Progressive disease, n (%) Unknown, n (%) 1 (1) 0 2 (2) CR = complete response; ORR = overall response rate; PR = partial response; PRL = PR with lymphocytosis. * Assessed using IWCLL criteria (Hallek M et al. Blood 2008; 111: 5446 -56) with modification for lymphocytosis (Cheson BD et al. J Clin Oncol 2012; 30 -2820 -22) Byrd JC et al. Proc ASH 2018; Abstract 692.

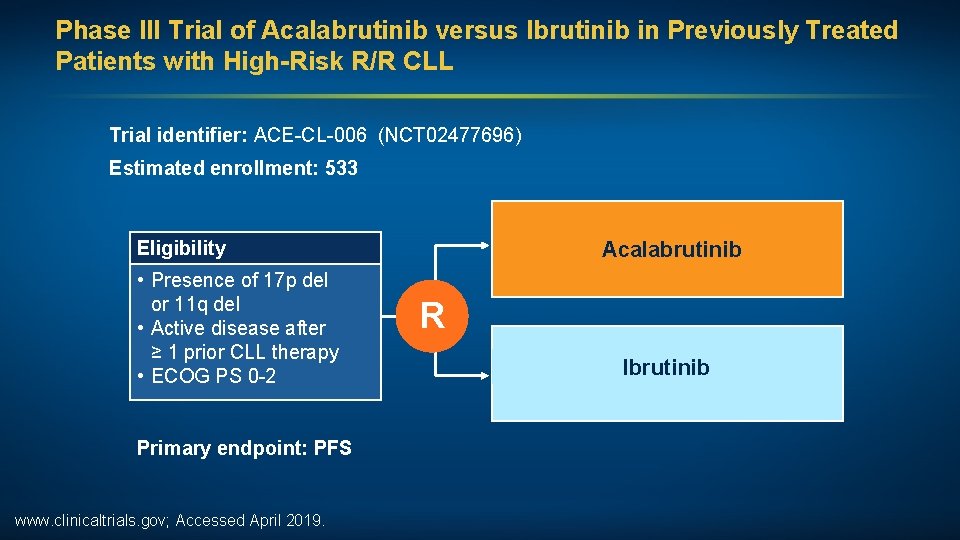
Phase III Trial of Acalabrutinib versus Ibrutinib in Previously Treated Patients with High-Risk R/R CLL Trial identifier: ACE-CL-006 (NCT 02477696) Estimated enrollment: 533 Eligibility • Presence of 17 p del or 11 q del • Active disease after ≥ 1 prior CLL therapy • ECOG PS 0 -2 Primary endpoint: PFS www. clinicaltrials. gov; Accessed April 2019. Acalabrutinib R Ibrutinib

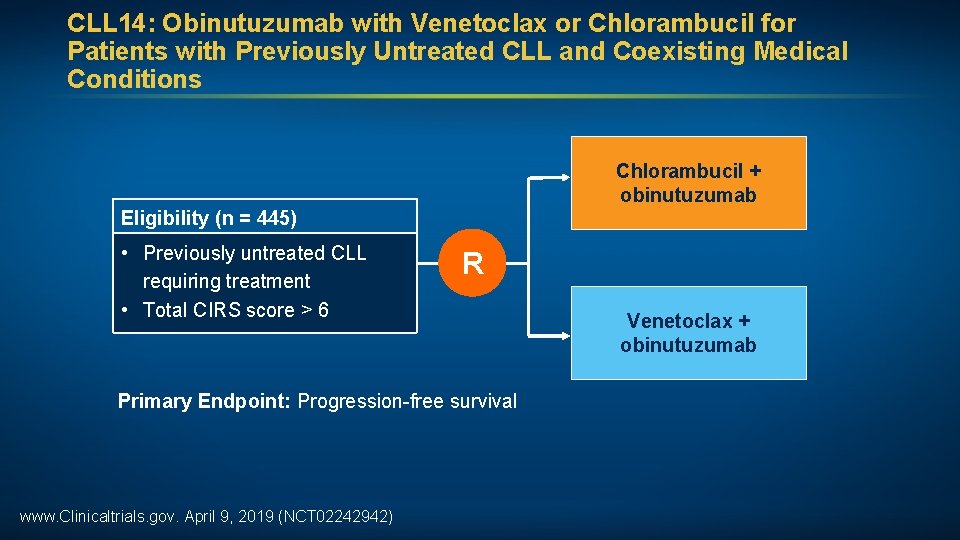
CLL 14: Obinutuzumab with Venetoclax or Chlorambucil for Patients with Previously Untreated CLL and Coexisting Medical Conditions Chlorambucil + obinutuzumab Eligibility (n = 445) • Previously untreated CLL requiring treatment • Total CIRS score > 6 R Primary Endpoint: Progression-free survival www. Clinicaltrials. gov. April 9, 2019 (NCT 02242942) Venetoclax + obinutuzumab

Phase III CLL 14 Trial of Venetoclax with Obinutuzumab Meets Its Primary PFS Endpoint Press Release — October 31, 2018 “The randomized Phase III CLL 14 study, which evaluated fixed-duration venetoclax in combination with obinutuzumab in people with previously untreated chronic lymphocytic leukemia (CLL) and co-existing medical conditions, met its primary endpoint and showed a statistically significant reduction in the risk of disease worsening or death (progression-free survival [PFS] as assessed by investigator) compared to standard-of-care obinutuzumab plus chlorambucil. The results showed that no new safety signals or increase in known toxicities of venetoclax or obinutuzumab were observed with the treatment combination. ” https: //www. businesswire. com/news/home/20181031005831/en/Phase-III-Data-Showed-Venclexta-Gazyva. Reduced

Chronic Lymphocytic Leukemia: Agenda Newly Diagnosed CLL: Observation versus Treatment First-Line Treatment of CLL Relapsed/Refractory CLL

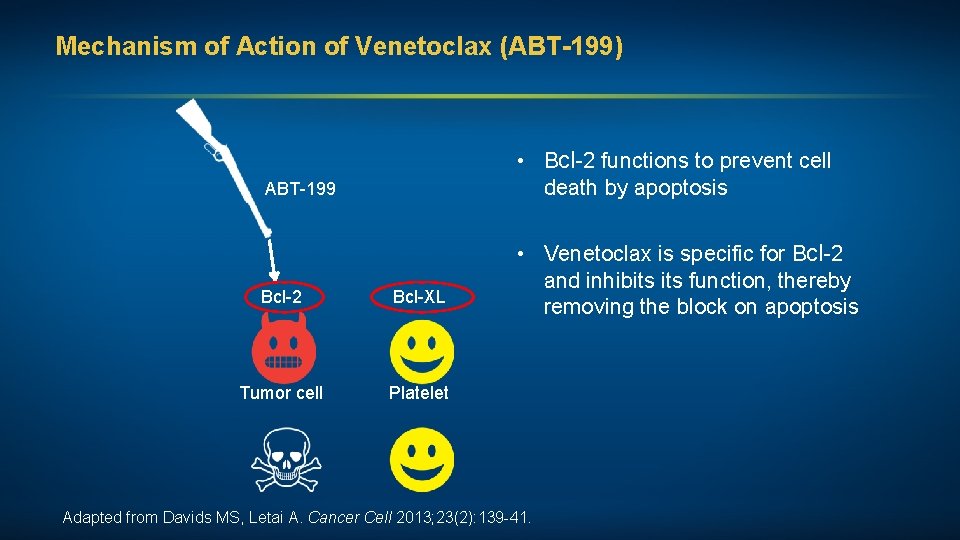
Mechanism of Action of Venetoclax (ABT-199) • Bcl-2 functions to prevent cell death by apoptosis ABT-199 Bcl-2 Bcl-XL Tumor cell Platelet • Venetoclax is specific for Bcl-2 and inhibits function, thereby removing the block on apoptosis Adapted from Davids MS, Letai A. Cancer Cell 2013; 23(2): 139 -41.

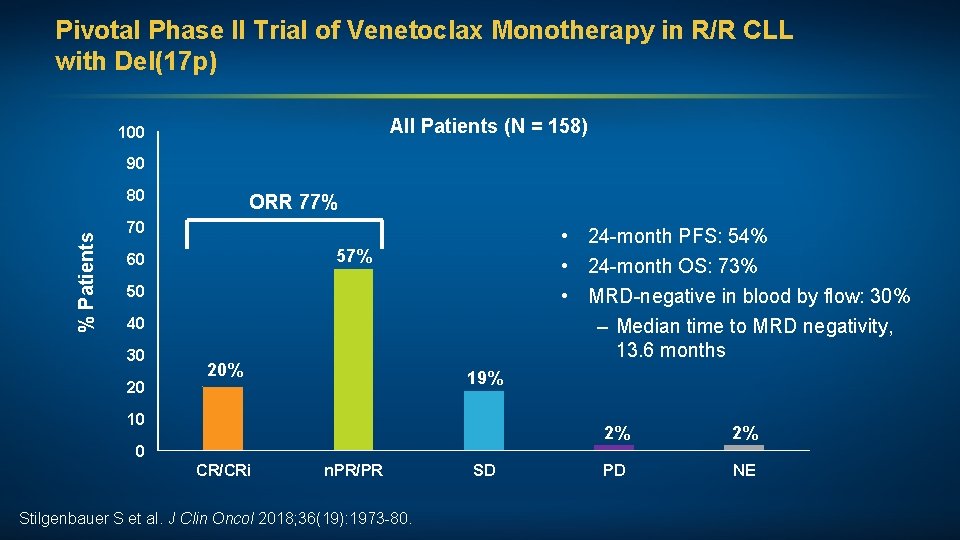
Pivotal Phase II Trial of Venetoclax Monotherapy in R/R CLL with Del(17 p) All Patients (N = 158) 100 90 % Patients 80 ORR 77% 70 • 24 -month PFS: 54% • 24 -month OS: 73% • MRD-negative in blood by flow: 30% – Median time to MRD negativity, 13. 6 months 57% 60 50 40 30 20 20% 19% 10 0 CR/CRi n. PR/PR Stilgenbauer S et al. J Clin Oncol 2018; 36(19): 1973 -80. SD 2% 2% PD NE

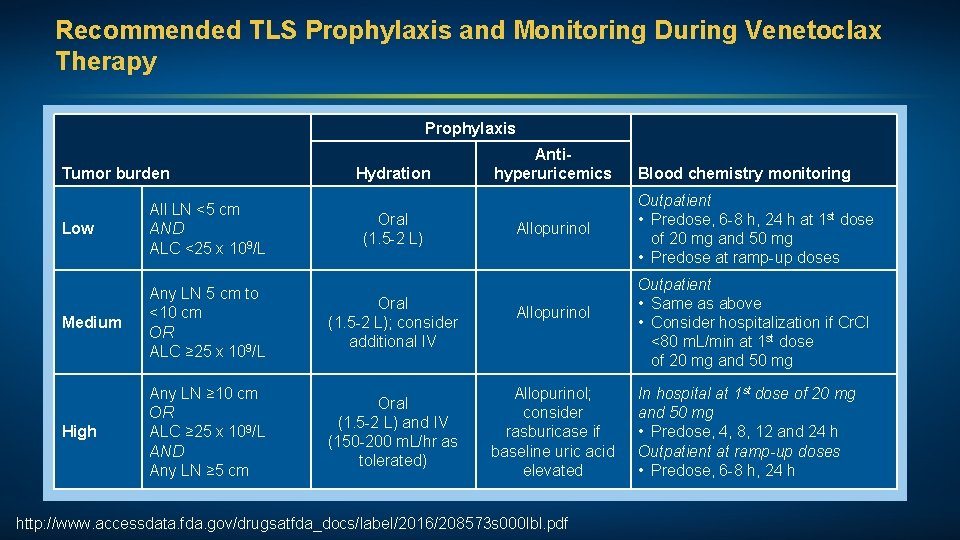
Recommended TLS Prophylaxis and Monitoring During Venetoclax Therapy Prophylaxis Tumor burden Low All LN <5 cm AND ALC <25 x 109/L Medium Any LN 5 cm to <10 cm OR ALC ≥ 25 x 109/L High Any LN ≥ 10 cm OR ALC ≥ 25 x 109/L AND Any LN ≥ 5 cm Hydration Oral (1. 5 -2 L); consider additional IV Oral (1. 5 -2 L) and IV (150 -200 m. L/hr as tolerated) Antihyperuricemics Allopurinol; consider rasburicase if baseline uric acid elevated http: //www. accessdata. fda. gov/drugsatfda_docs/label/2016/208573 s 000 lbl. pdf Blood chemistry monitoring Outpatient • Predose, 6 -8 h, 24 h at 1 st dose of 20 mg and 50 mg • Predose at ramp-up doses Outpatient • Same as above • Consider hospitalization if Cr. Cl <80 m. L/min at 1 st dose of 20 mg and 50 mg In hospital at 1 st dose of 20 mg and 50 mg • Predose, 4, 8, 12 and 24 h Outpatient at ramp-up doses • Predose, 6 -8 h, 24 h

Venetoclax: Drug Interactions • Strong CYP 3 A inhibitors: avoid during escalation, later 75% dose reduction • Moderate CYP 3 A inhibitor or P-gp inhibitors: avoid during escalation, later 50% dose reduction • No contra-indication to anticoagulation in general, but will increase serum warfarin concentration

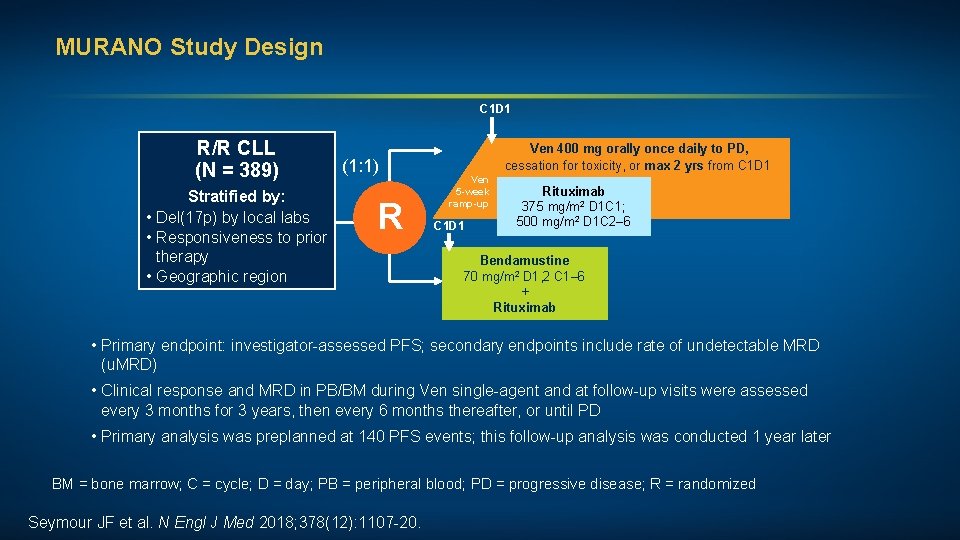
MURANO Study Design C 1 D 1 R/R CLL (N = 389) Stratified by: • Del(17 p) by local labs • Responsiveness to prior therapy • Geographic region (1: 1) R Ven 400 mg orally once daily to PD, cessation for toxicity, or max 2 yrs from C 1 D 1 Ven 5 -week ramp-up C 1 D 1 Rituximab 375 mg/m 2 D 1 C 1; 500 mg/m 2 D 1 C 2– 6 Bendamustine 70 mg/m 2 D 1, 2 C 1– 6 + Rituximab • Primary endpoint: investigator-assessed PFS; secondary endpoints include rate of undetectable MRD (u. MRD) • Clinical response and MRD in PB/BM during Ven single-agent and at follow-up visits were assessed every 3 months for 3 years, then every 6 months thereafter, or until PD • Primary analysis was preplanned at 140 PFS events; this follow-up analysis was conducted 1 year later BM = bone marrow; C = cycle; D = day; PB = peripheral blood; PD = progressive disease; R = randomized Seymour JF et al. N Engl J Med 2018; 378(12): 1107 -20.

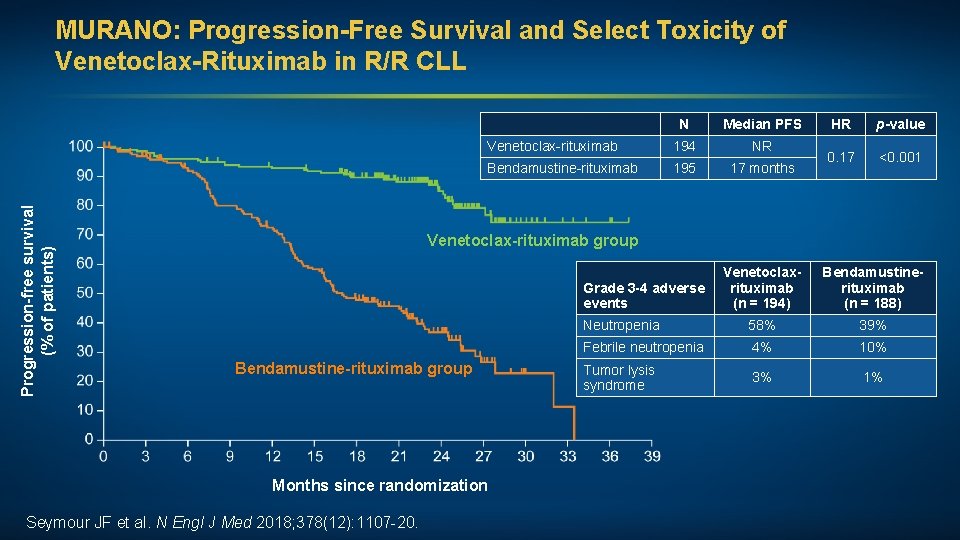
Progression-free survival (% of patients) MURANO: Progression-Free Survival and Select Toxicity of Venetoclax-Rituximab in R/R CLL N Median PFS Venetoclax-rituximab 194 NR Bendamustine-rituximab 195 17 months HR p-value 0. 17 <0. 001 Venetoclax-rituximab group Venetoclaxrituximab (n = 194) Bendamustinerituximab (n = 188) Neutropenia 58% 39% Febrile neutropenia 4% 10% Tumor lysis syndrome 3% 1% Grade 3 -4 adverse events Bendamustine-rituximab group Months since randomization Seymour JF et al. N Engl J Med 2018; 378(12): 1107 -20.

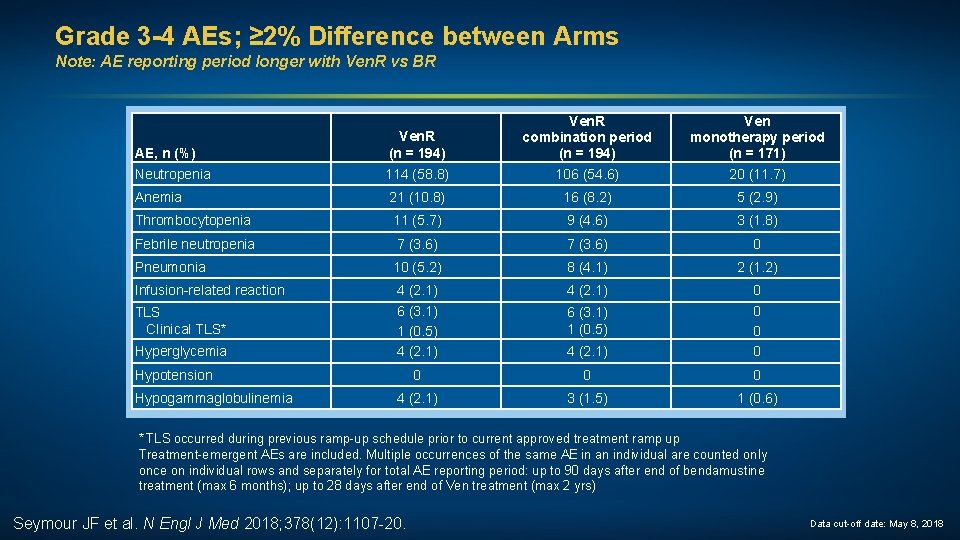
Grade 3 -4 AEs; ≥ 2% Difference between Arms Note: AE reporting period longer with Ven. R vs BR AE, n (%) Neutropenia Ven. R (n = 194) 114 (58. 8) Ven. R combination period (n = 194) 106 (54. 6) Ven monotherapy period (n = 171) 20 (11. 7) Anemia 21 (10. 8) 16 (8. 2) 5 (2. 9) Thrombocytopenia 11 (5. 7) 9 (4. 6) 3 (1. 8) Febrile neutropenia 7 (3. 6) 0 Pneumonia 10 (5. 2) 8 (4. 1) 2 (1. 2) Infusion-related reaction 4 (2. 1) 6 (3. 1) 1 (0. 5) 4 (2. 1) 0 0 0 0 4 (2. 1) 3 (1. 5) 1 (0. 6) TLS Clinical TLS* Hyperglycemia Hypotension Hypogammaglobulinemia 6 (3. 1) 1 (0. 5) * TLS occurred during previous ramp-up schedule prior to current approved treatment ramp up Treatment-emergent AEs are included. Multiple occurrences of the same AE in an individual are counted only once on individual rows and separately for total AE reporting period: up to 90 days after end of bendamustine treatment (max 6 months); up to 28 days after end of Ven treatment (max 2 yrs) Seymour JF et al. N Engl J Med 2018; 378(12): 1107 -20. Data cut-off date: May 8, 2018

Case Presentation (Ms Bazemore) A man in his mid-60 s with CLL (IGVH unmutated, del 17 p/TP 53 mutated) Treatments received • Ibrutinib • Venetoclax Other considerations • Works as a vending machine operator • Widowed now 2 years, emotionally labile since wife died (depressed, noncompliant with meds, easily angered); followed closely by social work

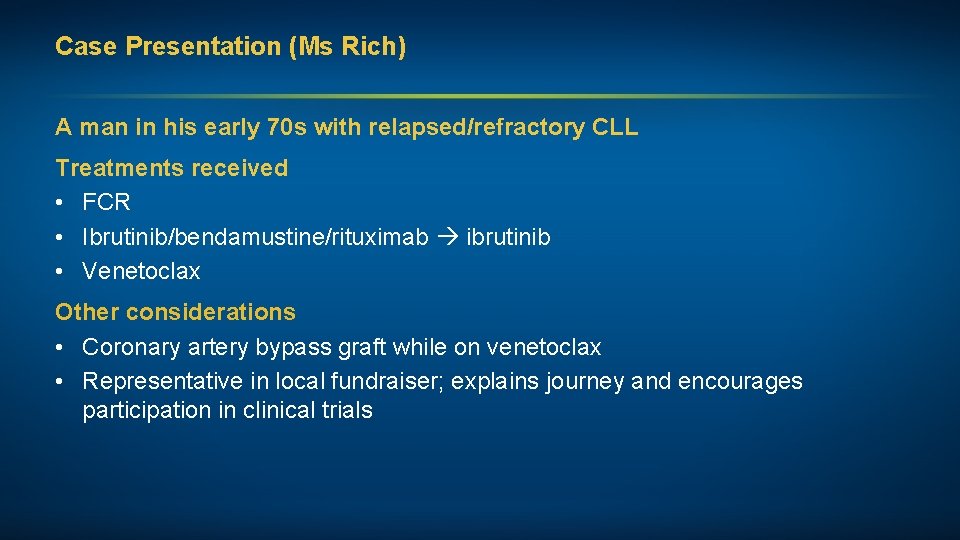
Case Presentation (Ms Rich) A man in his early 70 s with relapsed/refractory CLL Treatments received • FCR • Ibrutinib/bendamustine/rituximab ibrutinib • Venetoclax Other considerations • Coronary artery bypass graft while on venetoclax • Representative in local fundraiser; explains journey and encourages participation in clinical trials

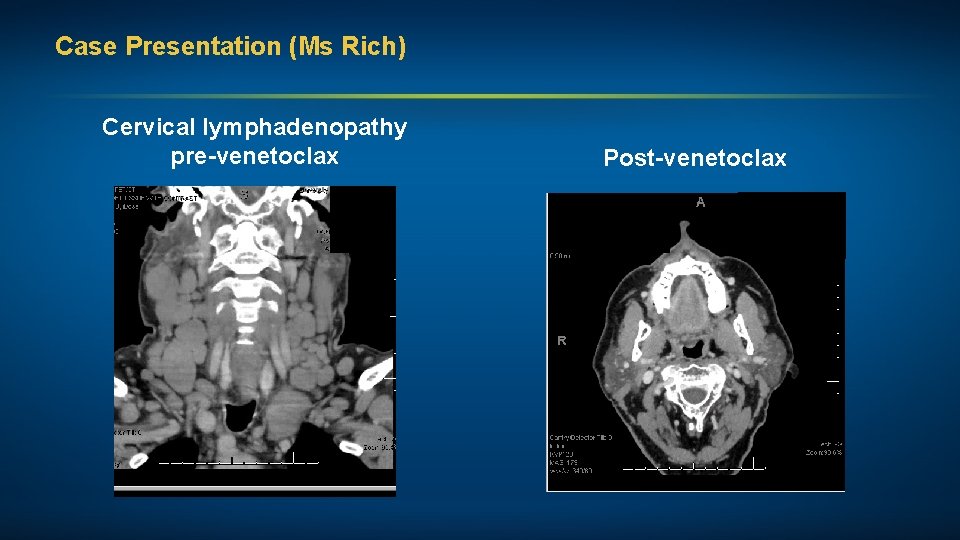
Case Presentation (Ms Rich) Cervical lymphadenopathy pre-venetoclax Post-venetoclax

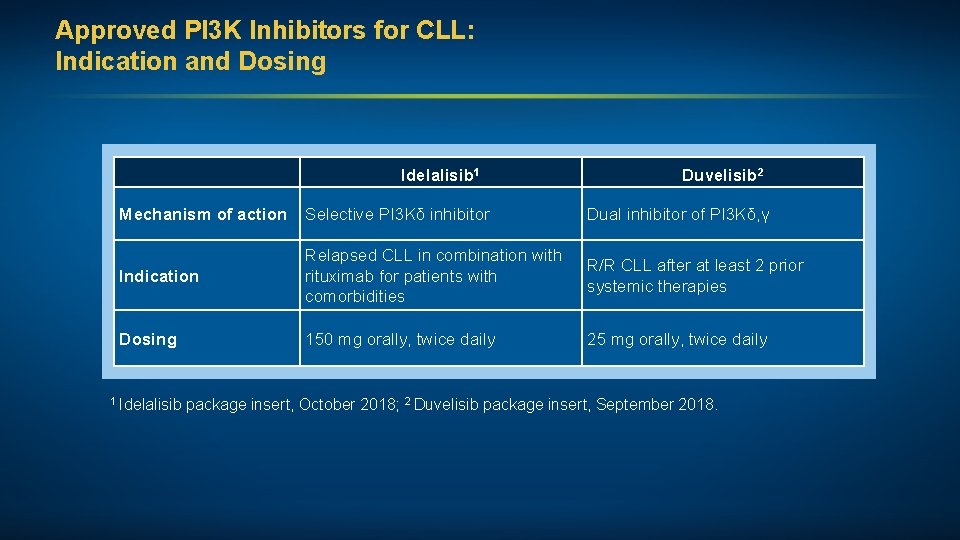
Approved PI 3 K Inhibitors for CLL: Indication and Dosing Idelalisib 1 Duvelisib 2 Mechanism of action Selective PI 3 Kδ inhibitor Dual inhibitor of PI 3 Kδ, γ Indication Relapsed CLL in combination with rituximab for patients with comorbidities R/R CLL after at least 2 prior systemic therapies Dosing 150 mg orally, twice daily 25 mg orally, twice daily 1 Idelalisib package insert, October 2018; 2 Duvelisib package insert, September 2018.

Case Presentation (Ms Rich) A man in his mid-70 s with CLL Treatments received • Lenalidomide • Ibrutinib • Idelalisib Other considerations • Off treatment and off hospice care with mild medical issues • Loves to restore cars, which he continued to do throughout cancer journey

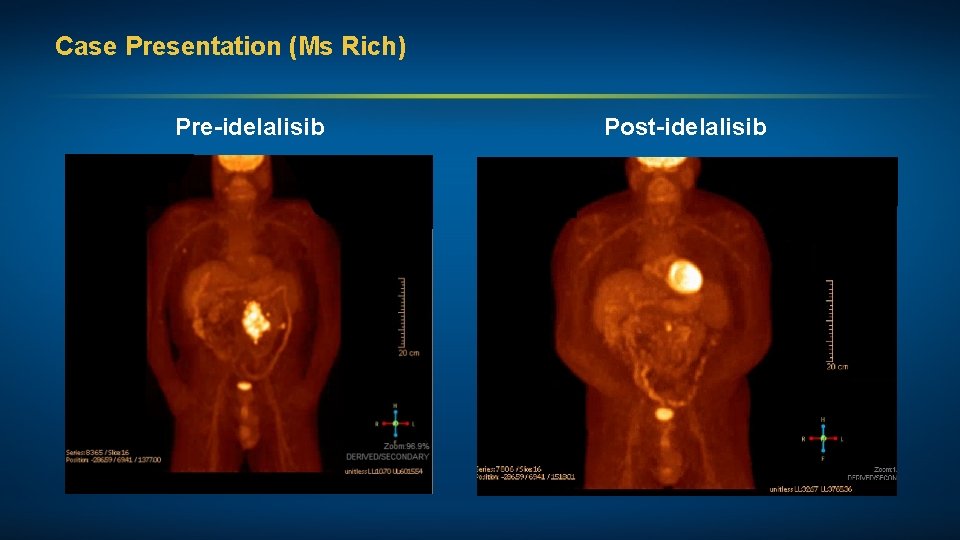
Case Presentation (Ms Rich) Pre-idelalisib Post-idelalisib

Serious Adverse Events Associated with Idelalisib • Hepatotoxicity (elevations in ALT/AST) • Diarrhea and colitis • Dyspnea and pneumonitis • GI perforation • See Risk Evaluation and Mitigation Strategy (REMS) to avoid serious or fatal toxicity http: //www. accessdata. fda. gov/drugsatfda_docs/rems/Zydelig_2014 -07 -23_FACT%20 SHEET. pdf

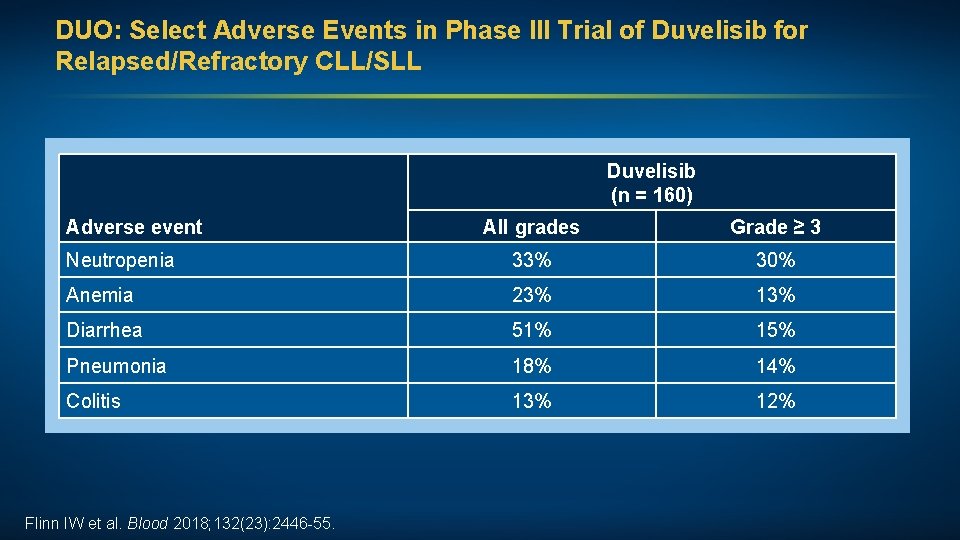
DUO: Select Adverse Events in Phase III Trial of Duvelisib for Relapsed/Refractory CLL/SLL Duvelisib (n = 160) Adverse event All grades Grade ≥ 3 Neutropenia 33% 30% Anemia 23% 13% Diarrhea 51% 15% Pneumonia 18% 14% Colitis 13% 12% Flinn IW et al. Blood 2018; 132(23): 2446 -55.
 Insidan region jh
Insidan region jh How to get divorce
How to get divorce Rule on juveniles in conflict with the law
Rule on juveniles in conflict with the law Meeting adjournment
Meeting adjournment To stop proceedings temporarily; move to another place
To stop proceedings temporarily; move to another place Actual-theoretical/actual
Actual-theoretical/actual Actual grace and sanctifying grace
Actual grace and sanctifying grace Please be quiet. i (try) to sleep
Please be quiet. i (try) to sleep Please note that comma
Please note that comma Difference between note making and note taking
Difference between note making and note taking Simple interest note vs simple discount note
Simple interest note vs simple discount note Financial documents order
Financial documents order Difference between note making and note taking
Difference between note making and note taking What is debit note and credit note
What is debit note and credit note Note making and note taking difference
Note making and note taking difference Note making advantages
Note making advantages What is a debit memo
What is a debit memo Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Thể thơ truyền thống
Thể thơ truyền thống Hát lên người ơi alleluia
Hát lên người ơi alleluia Hổ đẻ mỗi lứa mấy con
Hổ đẻ mỗi lứa mấy con Diễn thế sinh thái là
Diễn thế sinh thái là đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính thế năng
Công thức tính thế năng 101012 bằng
101012 bằng Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Phản ứng thế ankan
Phản ứng thế ankan Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Phối cảnh
Phối cảnh điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Ng-html
Ng-html Sơ đồ cơ thể người
Sơ đồ cơ thể người Số nguyên là gì
Số nguyên là gì đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới