Please note these are the actual videorecorded proceedings






















- Slides: 66

Please note, these are the actual videorecorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content. Select slides from the original presentation are omitted where Research To Practice was unable to obtain permission from the publication source and/or author. Links to view the actual reference materials have been provided for your use in place of any omitted slides.

Oncology Tumor Panel Series Lung Cancer Oncologist and Nurse Investigators Consult on Actual Patients from the Practices of the Invited Faculty Thursday, April 23, 2015 12: 00 PM – 1: 30 PM Faculty Julie R Brahmer, MD Ann Culkin, RN, OCN Kelly EH Goodwin, MSN, RN, ANP-BC Anne S Tsao, MD Moderator Neil Love, MD

Oncology Tumor Panel Series

Cases to be Presented • A 52 -Year-Old Woman with EGFR-Mutant Adenocarcinoma of the Lung with Bone, Liver and Brain Metastases (Ms Culkin) • A 55 -Year-Old Man with Metastatic Squamous Cell Carcinoma of the Lung (Ms Goodwin) • A 66 -Year-Old Woman with Metastatic Adenocarcinoma (Ms Culkin) • A 52 -Year-Old Woman with Cancer of Unknown Primary and ALK-Rearranged Metastases (Ms Goodwin)






Theme of this series Applying evidence-based oncology to individual patients

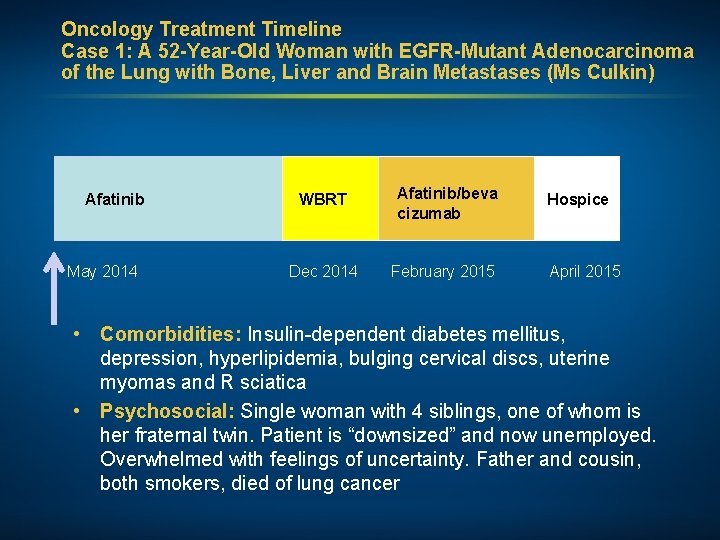
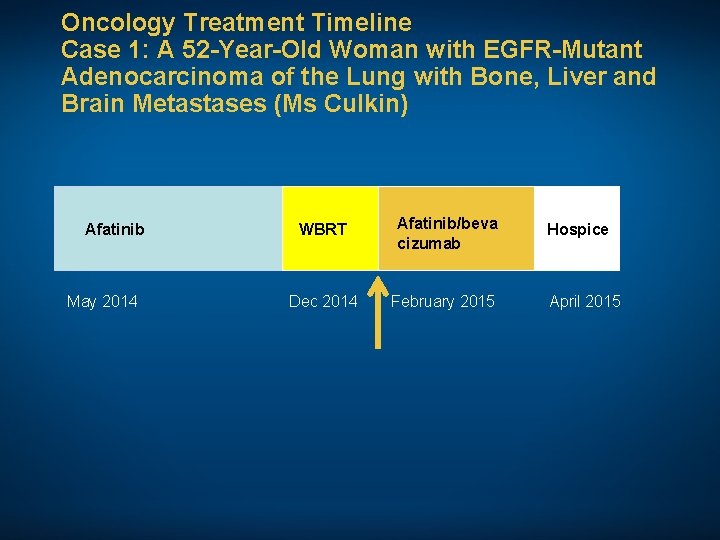
Oncology Treatment Timeline Case 1: A 52 -Year-Old Woman with EGFR-Mutant Adenocarcinoma of the Lung with Bone, Liver and Brain Metastases (Ms Culkin) Afatinib May 2014 WBRT Afatinib/beva cizumab Dec 2014 February 2015 Hospice April 2015 • Comorbidities: Insulin-dependent diabetes mellitus, depression, hyperlipidemia, bulging cervical discs, uterine myomas and R sciatica • Psychosocial: Single woman with 4 siblings, one of whom is her fraternal twin. Patient is “downsized” and now unemployed. Overwhelmed with feelings of uncertainty. Father and cousin, both smokers, died of lung cancer



Patient Education Plan 52 -Year-Old Woman with EGFR-Mutant Adenocarcinoma of the Lung Time point: Initiation of afatinib Goals of treatment Antitumor effects of treatment Dose and schedule Follow-up plan, including restaging Related psychosocial issues Important potential side effects/toxicities – Rash – Diarrhea – Pulmonary toxicity/interstitial lung disease – Other Safe handling

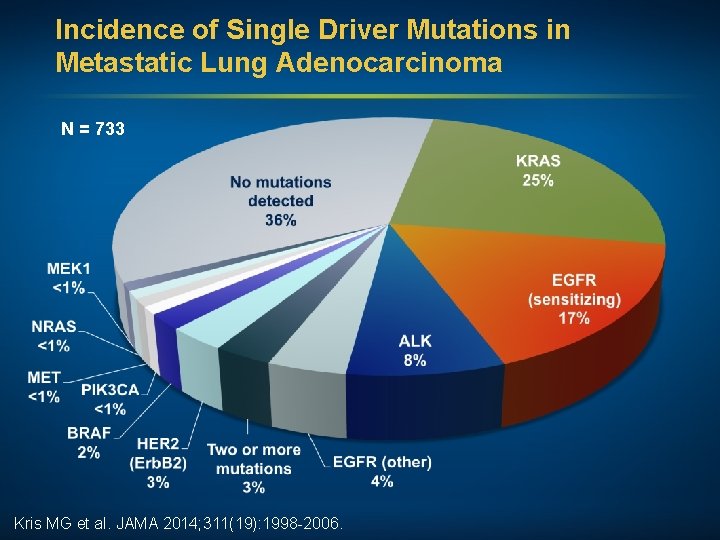
Incidence of Single Driver Mutations in Metastatic Lung Adenocarcinoma N = 733 Kris MG et al. JAMA 2014; 311(19): 1998 -2006.

Tissue Testing in Non-Small Cell Lung Cancer • • Squamous vs nonsquamous Smokers vs nonsmokers Women vs men Asians vs other Adequate tissue? Multiplex vs individual assays (EGFR, ALK) ASCO, CAP, NCCN recommendations

EGFR-Mutant Lung Cancer • Discovered 2004 • Types observed and TKI response – Exon 19 deletion – Exon 21 L 858 R – Exon 20 insertion – Other • Rapidity of response to TKIs • Typical clinical course with TKI

Oncologic Rationale for Treatment Selection: First-Line Treatment Options in EGFR-Mutant Lung Cancer • Chemotherapy • Erlotinib • Afatinib • Erlotinib + bevacizumab Zhou C et al. Lancet Oncol 2011; 12(8): 735 -42. Zhou C et al. Proc ASCO 2012; Abstract LBA 7520. Rosell R et al. Lancet Oncol 2012; 13(3): 239 -46. Seto T et al. Lancet Oncol 2014; 15(11): 1236 -44.

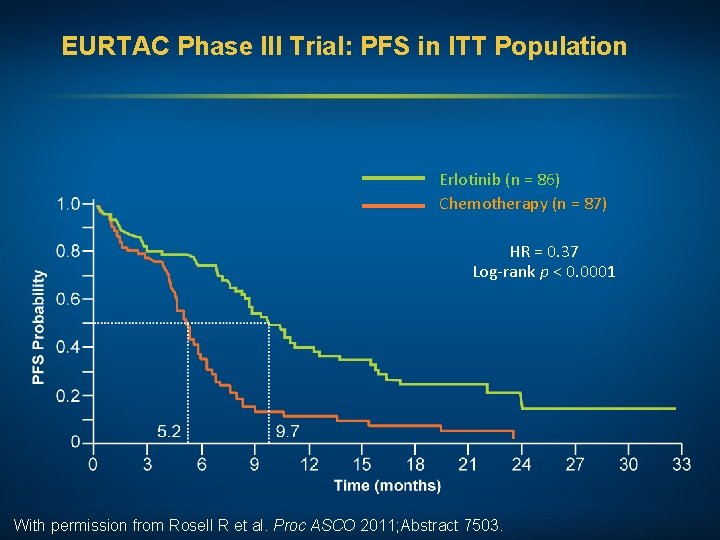
EURTAC Phase III Trial: PFS in ITT Population Erlotinib (n = 86) Chemotherapy (n = 87) HR = 0. 37 Log-rank p < 0. 0001 With permission from Rosell R et al. Proc ASCO 2011; Abstract 7503.

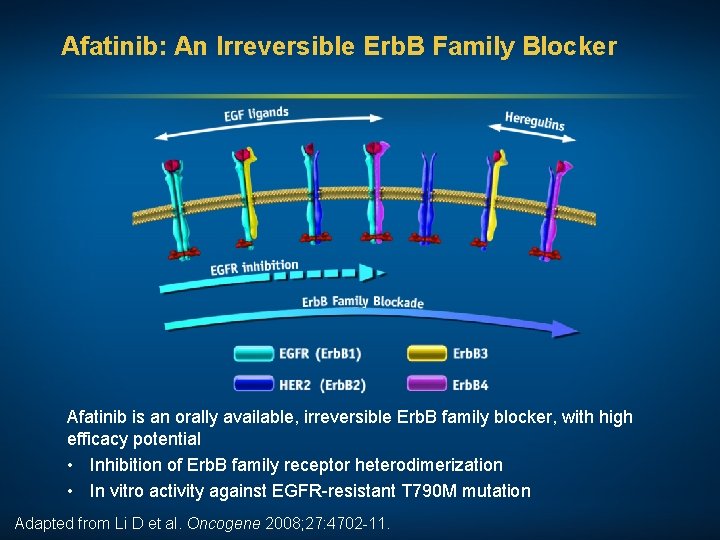
Afatinib: An Irreversible Erb. B Family Blocker Afatinib is an orally available, irreversible Erb. B family blocker, with high efficacy potential • Inhibition of Erb. B family receptor heterodimerization • In vitro activity against EGFR-resistant T 790 M mutation Adapted from Li D et al. Oncogene 2008; 27: 4702 -11.

Afatinib in EGFR-Mutant NSCLC • FDA approval July 12, 2013 for first-line treatment in metastatic NSCLC with exon 19 or exon 21 EGFR mutations • LUX-Lung-3 and LUX-Lung-6 Phase III studies of afatinib versus cisplatin-based chemo – Response and PFS better with EGFR TKI – No difference in survival for overall population – Survival better in combined analysis for exon 19 mutation-positive disease http: //www. cancer. gov/cancertopics/treatment/drugs/fda-afatinibdimaleate. Sequist LV et al. J Clin Oncol 2013; 31(27): 3327 -34; Wu YL et al. Lancet Oncol 2014; 15(2): 213 -22. Yang CH et al. Lancet Oncol 2015; 16: 141 -51.

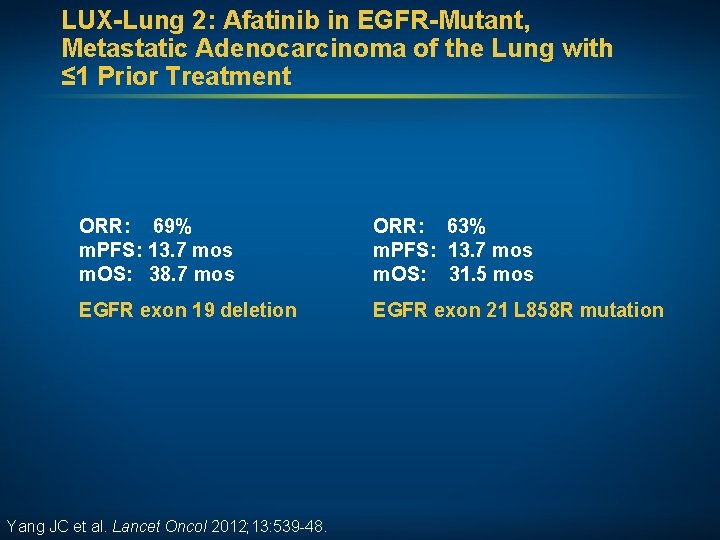
LUX-Lung 2: Afatinib in EGFR-Mutant, Metastatic Adenocarcinoma of the Lung with ≤ 1 Prior Treatment ORR: 69% m. PFS: 13. 7 mos m. OS: 38. 7 mos ORR: 63% m. PFS: 13. 7 mos m. OS: 31. 5 mos EGFR exon 19 deletion EGFR exon 21 L 858 R mutation Yang JC et al. Lancet Oncol 2012; 13: 539 -48.

Skin Rash from Tyrosine Kinase Inhibitors • Most frequent dermatologic side effect reported is acneiform eruption. • Affects mainly face, upper chest and/or back • Also known as acne, acneiform skin reaction/rash, follicular rash and maculopapular skin rash. Ricciardi S et al. Clin Lung Cancer 2009; 10(1): 28 -35.

Prophylaxis of Dermatologic Toxicities with EGFR Inhibitors Within first 6 weeks of EGFR TKI administration • Employ proactive approach • Thick, alcohol-free emollient cream • Sunscreen ≥SPF 15, preferably with zinc oxide or titanium dioxide • Good hygiene Lynch TJ et al. Oncologist 2007; 12: 610 -21.

Management of Dermatologic Toxicities with EGFR Inhibitors • Hydrocortisone 1% or 2. 5% cream • Clindamycin 1% gel • Pimecrolimus 1% cream • Doxycycline 100 mg BID • Minocycline 100 mg BID • Methylprednisolone dose pack • Dose reduction, interruption or discontinuation Lynch TJ et al. Oncologist 2007; 12: 610 -21.

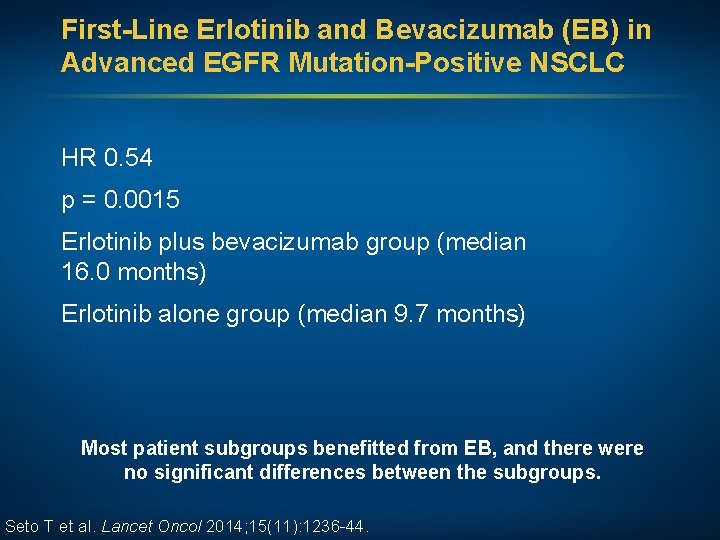
First-Line Erlotinib and Bevacizumab (EB) in Advanced EGFR Mutation-Positive NSCLC HR 0. 54 p = 0. 0015 Erlotinib plus bevacizumab group (median 16. 0 months) Erlotinib alone group (median 9. 7 months) Most patient subgroups benefitted from EB, and there were no significant differences between the subgroups. Seto T et al. Lancet Oncol 2014; 15(11): 1236 -44.

Oncology Treatment Timeline Case 1: A 52 -Year-Old Woman with EGFR-Mutant Adenocarcinoma of the Lung with Bone, Liver and Brain Metastases (Ms Culkin) Afatinib May 2014 WBRT Afatinib/beva cizumab Dec 2014 February 2015 Hospice April 2015

Options for Second-Line Treatment After Progression on an EGFR TKI • Continuation of TKI + chemotherapy • Chemotherapy • Afatinib + cetuximab • Clinical trial of novel EGFR TKIs

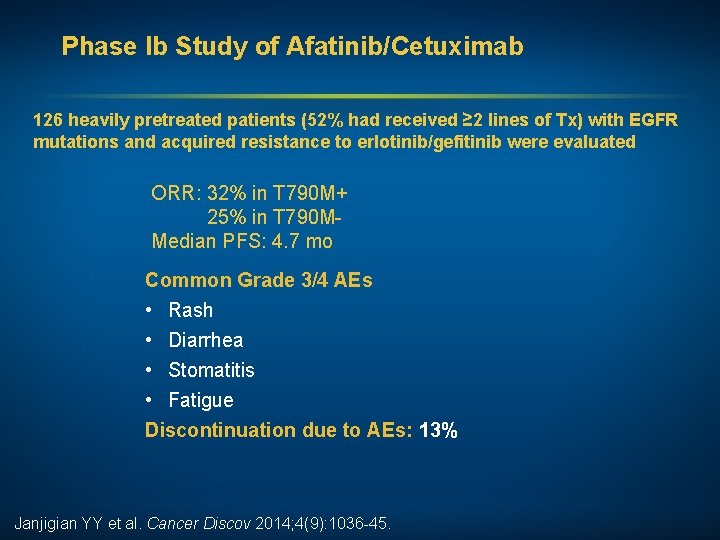
Phase Ib Study of Afatinib/Cetuximab 126 heavily pretreated patients (52% had received ≥ 2 lines of Tx) with EGFR mutations and acquired resistance to erlotinib/gefitinib were evaluated ORR: 32% in T 790 M+ 25% in T 790 MMedian PFS: 4. 7 mo Common Grade 3/4 AEs • Rash • Diarrhea • Stomatitis • Fatigue Discontinuation due to AEs: 13% Janjigian YY et al. Cancer Discov 2014; 4(9): 1036 -45.

AZD 9291 in EGFR TKI-Resistant NSCLC Evaluable patients with T 790 M+ NSCLC, expansion cohorts only (N = 107) ORR: 64% Most Common ≥Grade 3 AEs • Diarrhea • Fatigue • Interstitial lung disease-like events Janne PA et al. Proc ASCO 2014; Abstract 8009.

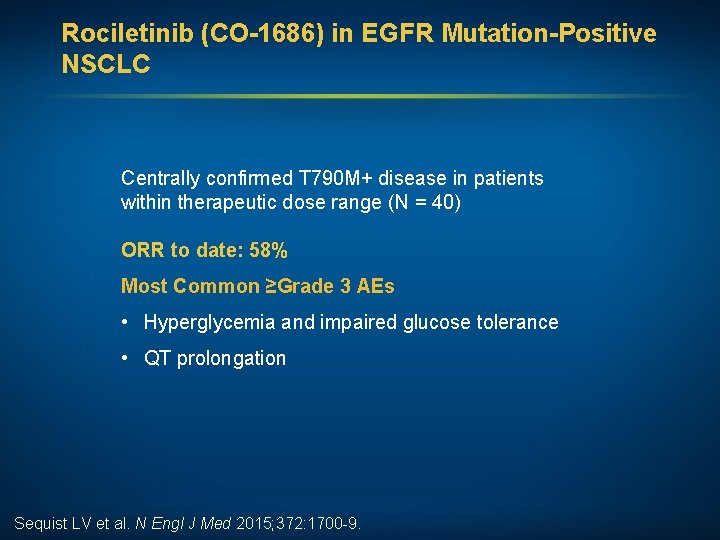
Rociletinib (CO-1686) in EGFR Mutation-Positive NSCLC Centrally confirmed T 790 M+ disease in patients within therapeutic dose range (N = 40) ORR to date: 58% Most Common ≥Grade 3 AEs • Hyperglycemia and impaired glucose tolerance • QT prolongation Sequist LV et al. N Engl J Med 2015; 372: 1700 -9.

Oncology Treatment Timeline Case 2: A 55 -Year-Old Man with Metastatic Squamous Cell Carcinoma of the Lung (Ms Goodwin) Palliative RT May 2014 Carboplatin + gemcitabine Relapse? • Comorbidities: COPD, asthma, hypertension, hepatitis C • Psychosocial: Current smoker with prior alcohol abuse who has been visiting the doctor's office on and off for a few years



Patient Education Plan Case 2: 55 -Year-Old Man with Metastatic Squamous Cell Carcinoma of the Lung Time point: Initiation of carboplatin/gemcitabine Goals of treatment Antitumor effects of treatment Dose and schedule Follow-up plan, including restaging Related psychosocial issues Important potential side effects/toxicities

Oncologic Rationale for Treatment Selection: First-Line Treatment Options • Platinum-based doublets – Carboplatin versus cisplatin – Common partners • Gemcitabine • Paclitaxel • Nab paclitaxel

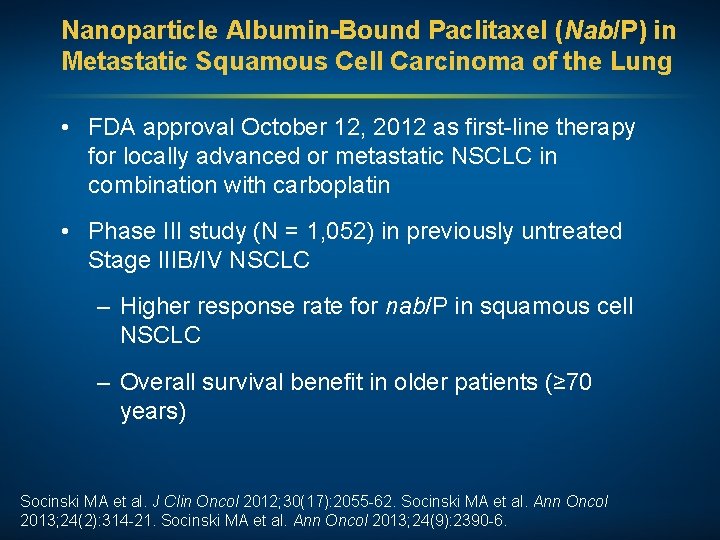
Nanoparticle Albumin-Bound Paclitaxel (Nab/P) in Metastatic Squamous Cell Carcinoma of the Lung • FDA approval October 12, 2012 as first-line therapy for locally advanced or metastatic NSCLC in combination with carboplatin • Phase III study (N = 1, 052) in previously untreated Stage IIIB/IV NSCLC – Higher response rate for nab/P in squamous cell NSCLC – Overall survival benefit in older patients (≥ 70 years) Socinski MA et al. J Clin Oncol 2012; 30(17): 2055 -62. Socinski MA et al. Ann Oncol 2013; 24(2): 314 -21. Socinski MA et al. Ann Oncol 2013; 24(9): 2390 -6.

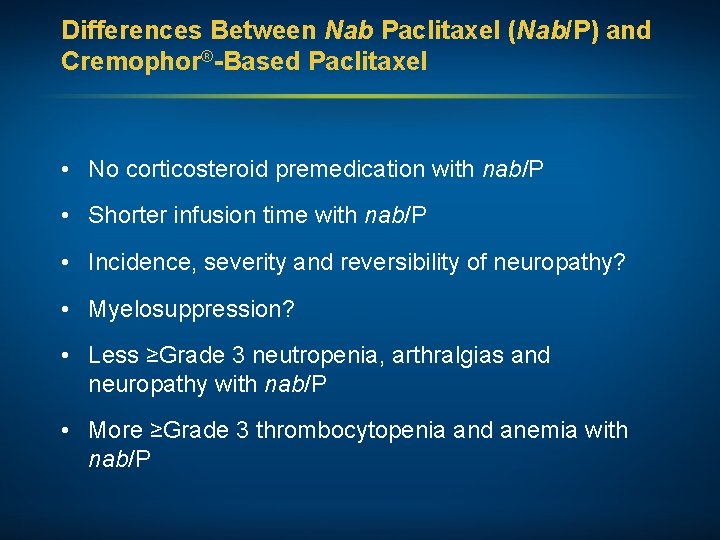
Differences Between Nab Paclitaxel (Nab/P) and Cremophor®-Based Paclitaxel • No corticosteroid premedication with nab/P • Shorter infusion time with nab/P • Incidence, severity and reversibility of neuropathy? • Myelosuppression? • Less ≥Grade 3 neutropenia, arthralgias and neuropathy with nab/P • More ≥Grade 3 thrombocytopenia and anemia with nab/P

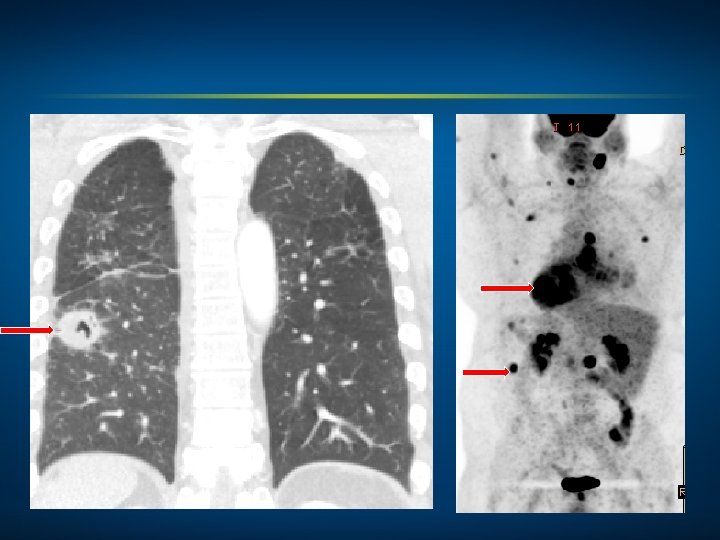
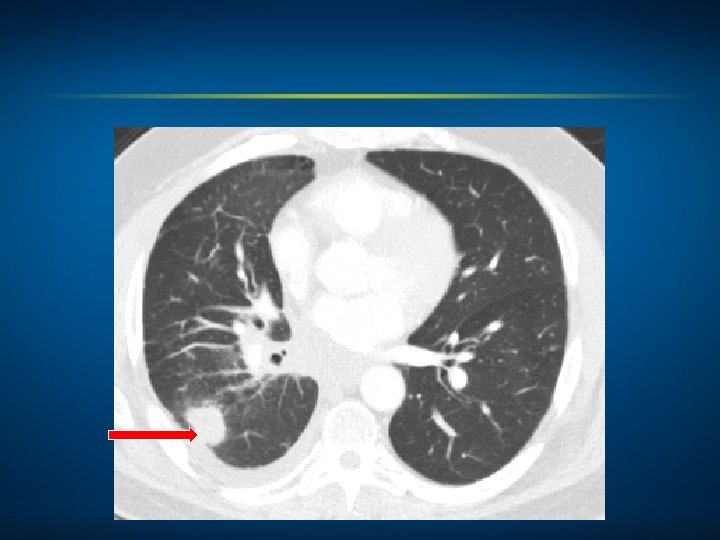
Oncology Treatment Timeline Case 2: A 55 -Year-Old Man with Metastatic Squamous Cell Carcinoma of the Lung (Ms Goodwin) Palliative RT Carboplatin + gemcitabine May 2014 • Optimal treatment at next relapse? Relapse?

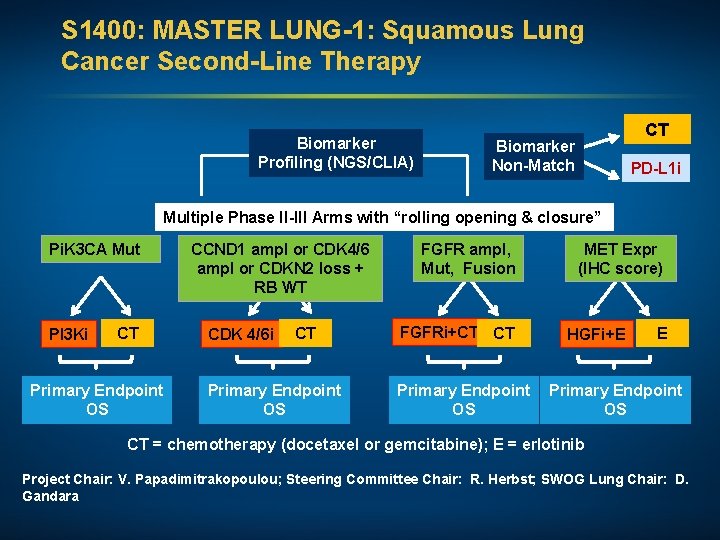
S 1400: MASTER LUNG-1: Squamous Lung Cancer Second-Line Therapy Biomarker Profiling (NGS/CLIA) CT Biomarker Non-Match PD-L 1 i Multiple Phase II-III Arms with “rolling opening & closure” Pi. K 3 CA Mut PI 3 Ki CT Primary Endpoint OS CCND 1 ampl or CDK 4/6 ampl or CDKN 2 loss + RB WT CDK 4/6 i CT Primary Endpoint OS FGFR ampl, Mut, Fusion FGFRi+CT CT Primary Endpoint OS MET Expr (IHC score) HGFi+E E Primary Endpoint OS CT = chemotherapy (docetaxel or gemcitabine); E = erlotinib Project Chair: V. Papadimitrakopoulou; Steering Committee Chair: R. Herbst; SWOG Lung Chair: D. Gandara

FDA Approval of Nivolumab for Previously Treated Metastatic Squamous NSCLC “On March 4, 2015, the US Food and Drug Administration granted approval to nivolumab for the treatment of patients with metastatic squamous non-small cell lung cancer (NSCLC) with progression on or after platinum-based chemotherapy… Approval was based on superior overall survival (OS) for patients who were randomly allocated to either nivolumab or docetaxel in an open-label, multicenter, multinational randomized trial in patients with metastatic squamous NSCLC who had experienced disease progression during or after one prior platinum-based chemotherapy regimen. ” http: //www. fda. gov/Drugs/Information. On. Drugs/Approved. Drugs/ucm 436566. htm

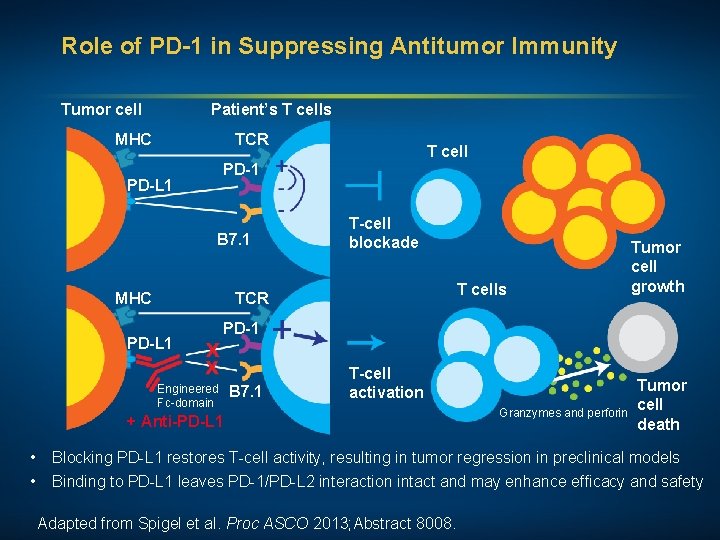
Role of PD-1 in Suppressing Antitumor Immunity Tumor cell Patient’s T cells MHC TCR T cell PD-1 PD-L 1 B 7. 1 MHC T-cell blockade T cells TCR PD-L 1 Tumor cell growth PD-1 Engineered Fc-domain B 7. 1 T-cell activation + Anti-PD-L 1 Granzymes and perforin Tumor cell death • Blocking PD-L 1 restores T-cell activity, resulting in tumor regression in preclinical models • Binding to PD-L 1 leaves PD-1/PD-L 2 interaction intact and may enhance efficacy and safety Adapted from Spigel et al. Proc ASCO 2013; Abstract 8008.

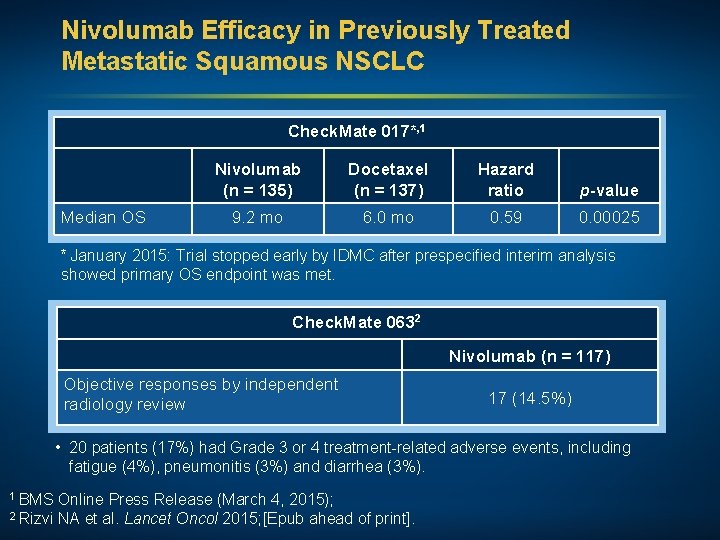
Nivolumab Efficacy in Previously Treated Metastatic Squamous NSCLC Check. Mate 017*, 1 Median OS Nivolumab (n = 135) Docetaxel (n = 137) Hazard ratio p-value 9. 2 mo 6. 0 mo 0. 59 0. 00025 * January 2015: Trial stopped early by IDMC after prespecified interim analysis showed primary OS endpoint was met. Check. Mate 0632 Nivolumab (n = 117) Objective responses by independent radiology review 17 (14. 5%) • 20 patients (17%) had Grade 3 or 4 treatment-related adverse events, including fatigue (4%), pneumonitis (3%) and diarrhea (3%). 1 BMS Online Press Release (March 4, 2015); 2 Rizvi NA et al. Lancet Oncol 2015; [Epub ahead of print].

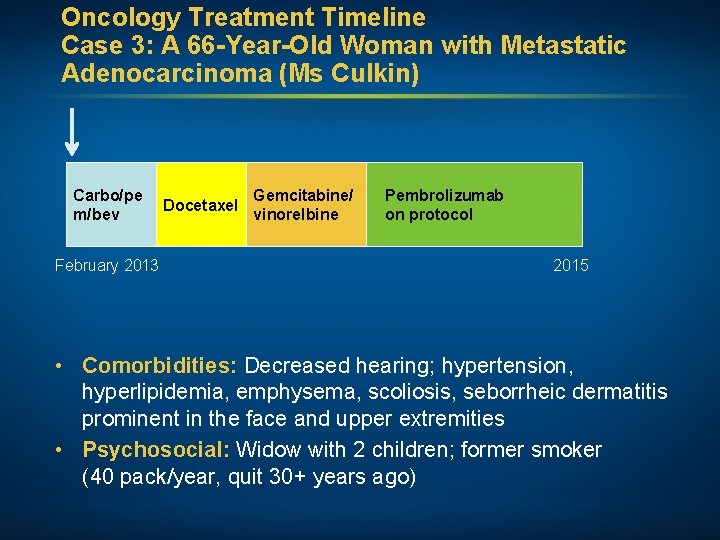
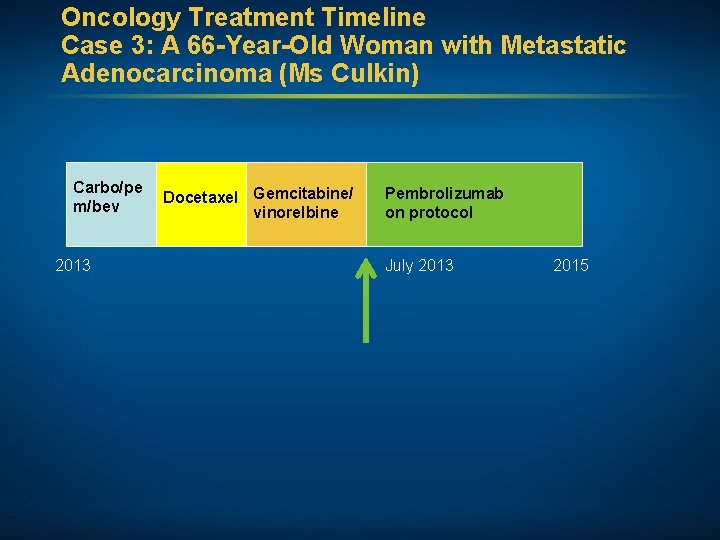
Oncology Treatment Timeline Case 3: A 66 -Year-Old Woman with Metastatic Adenocarcinoma (Ms Culkin) Carbo/pe m/bev February 2013 Docetaxel Gemcitabine/ vinorelbine Pembrolizumab on protocol 2015 • Comorbidities: Decreased hearing; hypertension, hyperlipidemia, emphysema, scoliosis, seborrheic dermatitis prominent in the face and upper extremities • Psychosocial: Widow with 2 children; former smoker (40 pack/year, quit 30+ years ago)

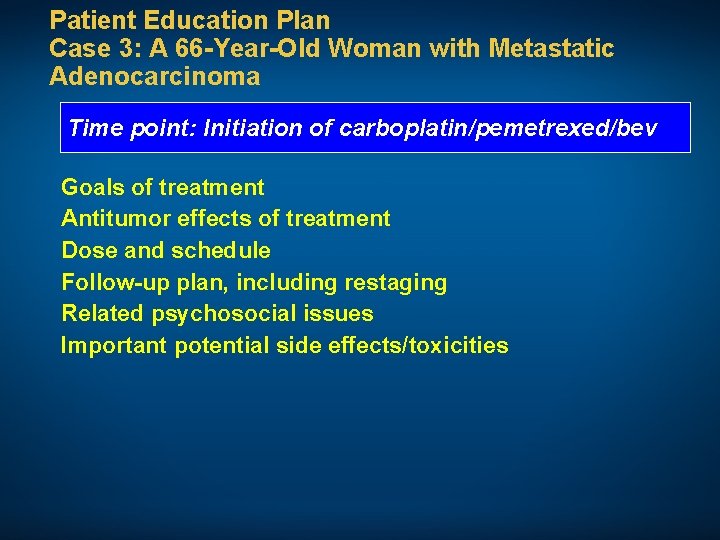
Patient Education Plan Case 3: A 66 -Year-Old Woman with Metastatic Adenocarcinoma Time point: Initiation of carboplatin/pemetrexed/bev Goals of treatment Antitumor effects of treatment Dose and schedule Follow-up plan, including restaging Related psychosocial issues Important potential side effects/toxicities

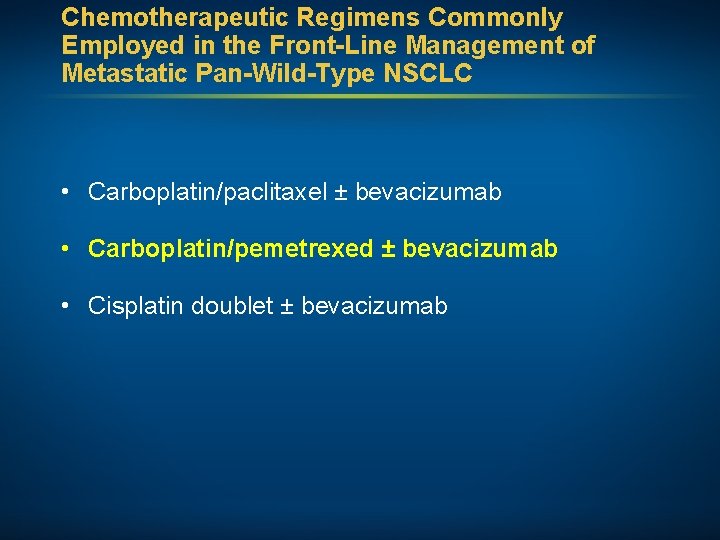
Chemotherapeutic Regimens Commonly Employed in the Front-Line Management of Metastatic Pan-Wild-Type NSCLC • Carboplatin/paclitaxel ± bevacizumab • Carboplatin/pemetrexed ± bevacizumab • Cisplatin doublet ± bevacizumab

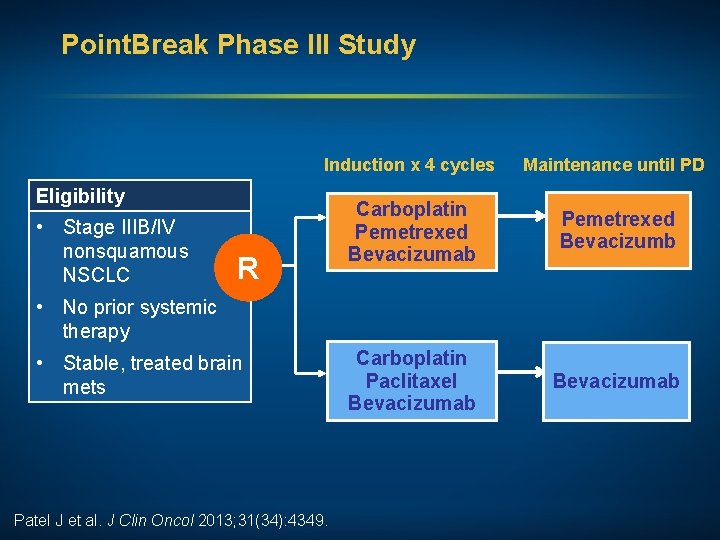
Point. Break Phase III Study Induction x 4 cycles Maintenance until PD Carboplatin Pemetrexed Bevacizumab Pemetrexed Bevacizumb Carboplatin Paclitaxel Bevacizumab Eligibility • Stage IIIB/IV nonsquamous NSCLC R • No prior systemic therapy • Stable, treated brain mets Patel J et al. J Clin Oncol 2013; 31(34): 4349.

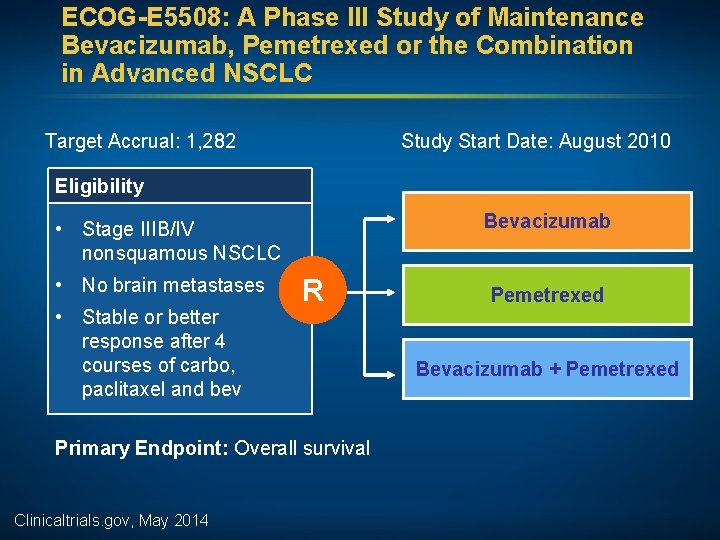
ECOG-E 5508: A Phase III Study of Maintenance Bevacizumab, Pemetrexed or the Combination in Advanced NSCLC Target Accrual: 1, 282 Study Start Date: August 2010 Eligibility Bevacizumab • Stage IIIB/IV nonsquamous NSCLC • No brain metastases • Stable or better response after 4 courses of carbo, paclitaxel and bev R Primary Endpoint: Overall survival Clinicaltrials. gov, May 2014 Pemetrexed Bevacizumab + Pemetrexed

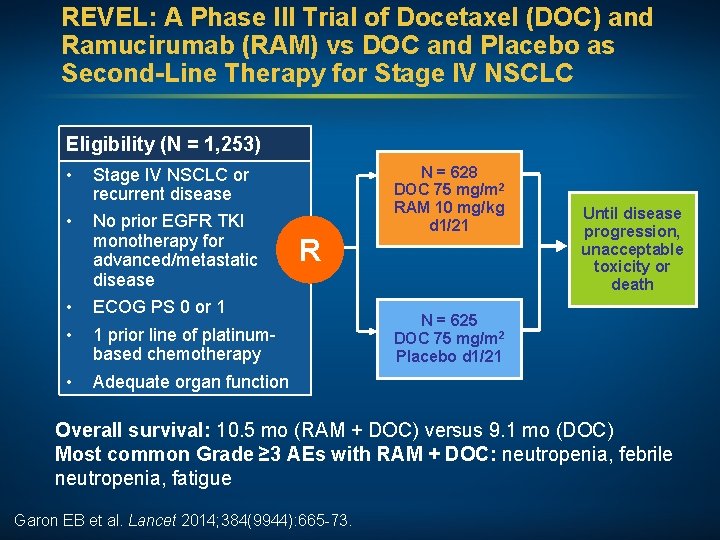
REVEL: A Phase III Trial of Docetaxel (DOC) and Ramucirumab (RAM) vs DOC and Placebo as Second-Line Therapy for Stage IV NSCLC Eligibility (N = 1, 253) • Stage IV NSCLC or recurrent disease • No prior EGFR TKI monotherapy for advanced/metastatic disease • • ECOG PS 0 or 1 • Adequate organ function R 1 prior line of platinumbased chemotherapy N = 628 DOC 75 mg/m 2 RAM 10 mg/kg d 1/21 Until disease progression, unacceptable toxicity or death N = 625 DOC 75 mg/m 2 Placebo d 1/21 Overall survival: 10. 5 mo (RAM + DOC) versus 9. 1 mo (DOC) Most common Grade ≥ 3 AEs with RAM + DOC: neutropenia, febrile neutropenia, fatigue Garon EB et al. Lancet 2014; 384(9944): 665 -73.

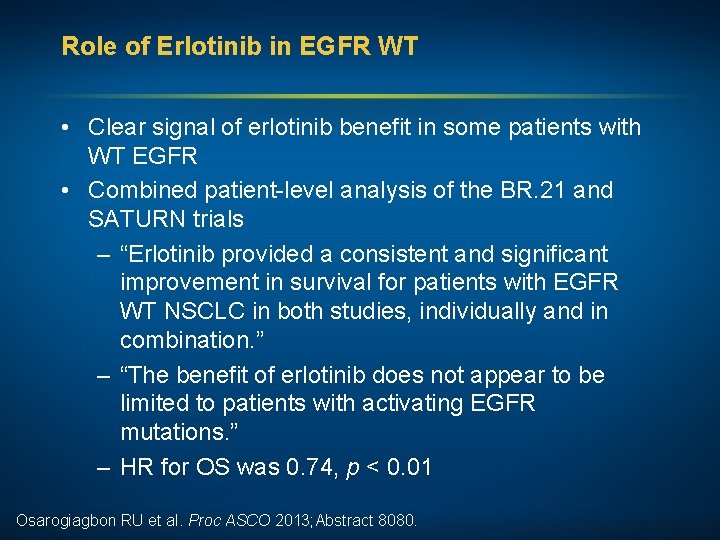
Role of Erlotinib in EGFR WT • Clear signal of erlotinib benefit in some patients with WT EGFR • Combined patient-level analysis of the BR. 21 and SATURN trials – “Erlotinib provided a consistent and significant improvement in survival for patients with EGFR WT NSCLC in both studies, individually and in combination. ” – “The benefit of erlotinib does not appear to be limited to patients with activating EGFR mutations. ” – HR for OS was 0. 74, p < 0. 01 Osarogiagbon RU et al. Proc ASCO 2013; Abstract 8080.

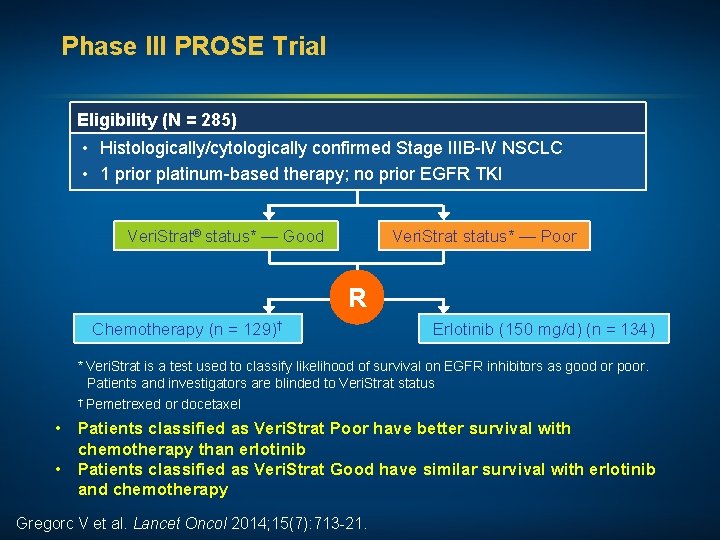
Phase III PROSE Trial Eligibility (N = 285) • Histologically/cytologically confirmed Stage IIIB-IV NSCLC • 1 prior platinum-based therapy; no prior EGFR TKI Veri. Strat® status* — Good Veri. Strat status* — Poor R Chemotherapy (n = 129)† Erlotinib (150 mg/d) (n = 134) * Veri. Strat is a test used to classify likelihood of survival on EGFR inhibitors as good or poor. Patients and investigators are blinded to Veri. Strat status † Pemetrexed or docetaxel • Patients classified as Veri. Strat Poor have better survival with chemotherapy than erlotinib • Patients classified as Veri. Strat Good have similar survival with erlotinib and chemotherapy Gregorc V et al. Lancet Oncol 2014; 15(7): 713 -21.

Oncology Treatment Timeline Case 3: A 66 -Year-Old Woman with Metastatic Adenocarcinoma (Ms Culkin) Carbo/pe m/bev 2013 Docetaxel Gemcitabine/ vinorelbine Pembrolizumab on protocol July 2013 2015

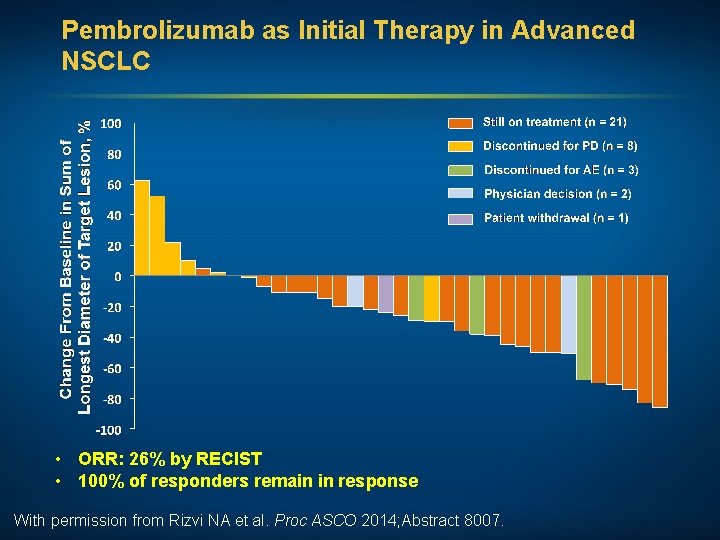
Pembrolizumab as Initial Therapy in Advanced NSCLC • ORR: 26% by RECIST • 100% of responders remain in response With permission from Rizvi NA et al. Proc ASCO 2014; Abstract 8007.

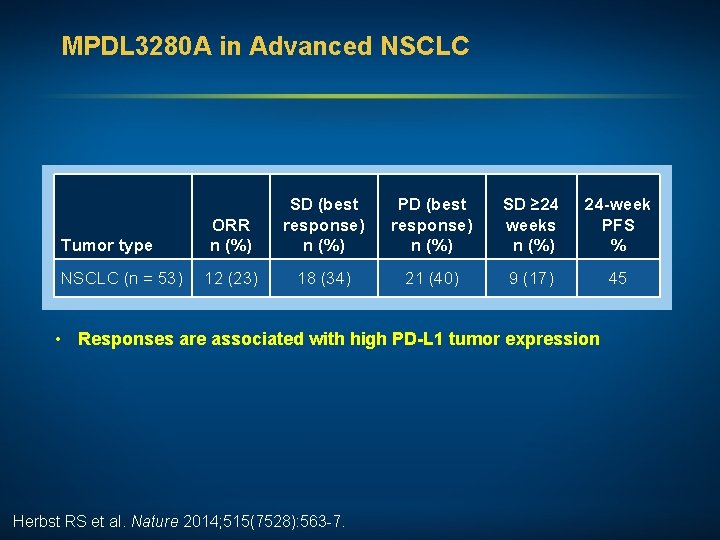
MPDL 3280 A in Advanced NSCLC Tumor type ORR n (%) NSCLC (n = 53) 12 (23) SD (best response) n (%) PD (best response) n (%) SD ≥ 24 weeks n (%) 24 -week PFS % 18 (34) 21 (40) 9 (17) 45 • Responses are associated with high PD-L 1 tumor expression Herbst RS et al. Nature 2014; 515(7528): 563 -7.

Check. Mate-057 Phase III Trial of Nivolumab Versus Docetaxel in Advanced NSCLC April 17, 2015: The open-label, randomized Phase III study evaluating nivolumab versus docetaxel in previously treated patients with advanced NSCLC, Check. Mate-057, was stopped early because an assessment conducted by the independent Data Monitoring Committee (DMC) concluded that the study met its endpoint, demonstrating superior overall survival in patients receiving nivolumab compared to the control arm. http: //news. bms. com/press-release/checkmate-057 -pivotal-phase-iii-opdivo-nivolumab-lungcancer-trial-stopped-early

IRAEs Associated with Immune Checkpoint Inhibitors • Hypophysitis • Thyroiditis • Adrenal insufficiency • Colitis • Dermatitis • Pneumonitis • Hepatitis • Pancreatitis • Motor & sensory neuropathies • Arthritis Less common: hematologic; cardiovascular; ocular; renal

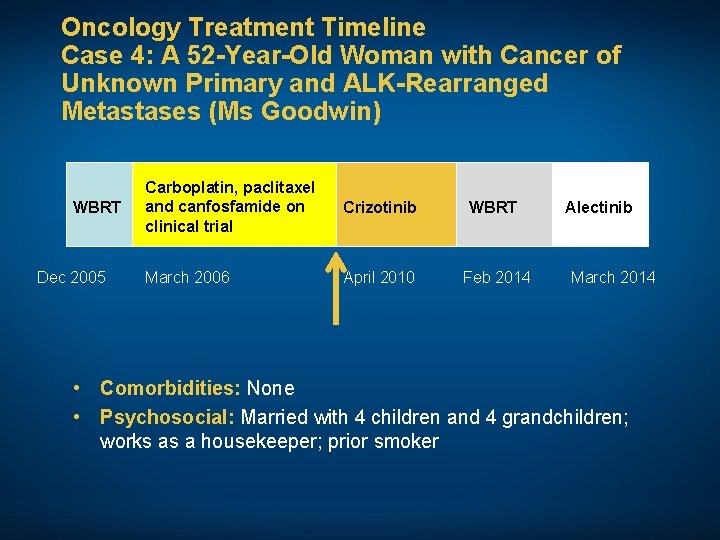
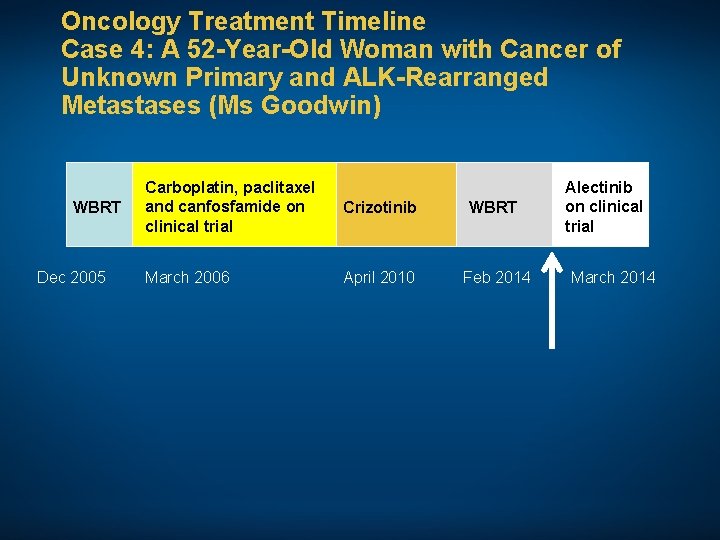
Oncology Treatment Timeline Case 4: A 52 -Year-Old Woman with Cancer of Unknown Primary and ALK-Rearranged Metastases (Ms Goodwin) WBRT Dec 2005 Carboplatin, paclitaxel and canfosfamide on clinical trial Crizotinib WBRT March 2006 April 2010 Feb 2014 Alectinib March 2014 • Comorbidities: None • Psychosocial: Married with 4 children and 4 grandchildren; works as a housekeeper; prior smoker

EML 4 -ALK Alterations in NSCLC • In ~4% of NSCLC cases • Enriched in younger, never or light smokers with adenocarcinoma • Rarely overlaps with EGFR and K-RAS mutations Soda M et al. Nature 2007; 448(7153): 561 -6.

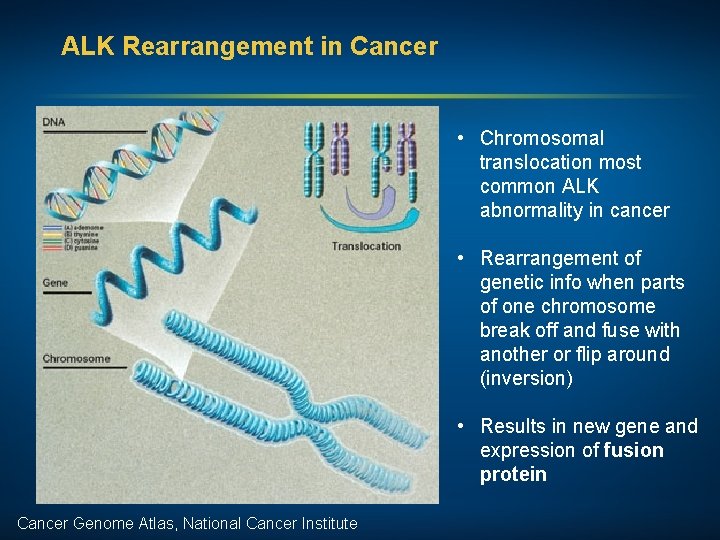
ALK Rearrangement in Cancer • Chromosomal translocation most common ALK abnormality in cancer • Rearrangement of genetic info when parts of one chromosome break off and fuse with another or flip around (inversion) • Results in new gene and expression of fusion protein Cancer Genome Atlas, National Cancer Institute

Patient Education Plan Case 4: A 52 -Year-Old Woman with Cancer of Unknown Primary and ALK-Rearranged Metastases Time point: Initiation of crizotinib Goals of treatment Antitumor effects of treatment Dose and schedule Follow-up plan, including restaging Related psychosocial issues Important potential side effects/toxicities – Visual changes – Hypogonadism (men) – Other

Visual Disorders with Crizotinib “Trails” coming off lights in peripheral vision, in low light conditions Also at edges of vision in low light conditions: • Image persistence • Flashes of light, which don’t appear to be connected to a real light source • High contrast images (eg, stripes) flip registration Camidge DR. The International Journal of Targeted Therapies in Cancer 2012; 6. 12: 30 -3.

Oncology Treatment Timeline Case 4: A 52 -Year-Old Woman with Cancer of Unknown Primary and ALK-Rearranged Metastases (Ms Goodwin) WBRT Dec 2005 Carboplatin, paclitaxel and canfosfamide on clinical trial Crizotinib WBRT March 2006 April 2010 Feb 2014 Alectinib on clinical trial March 2014

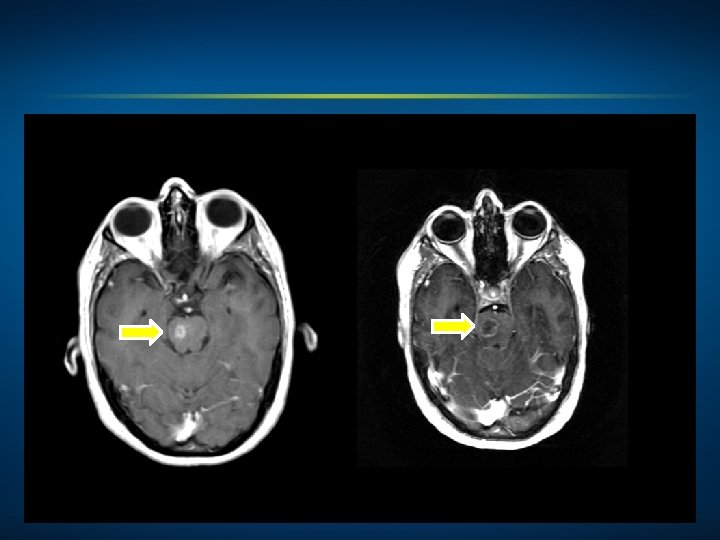
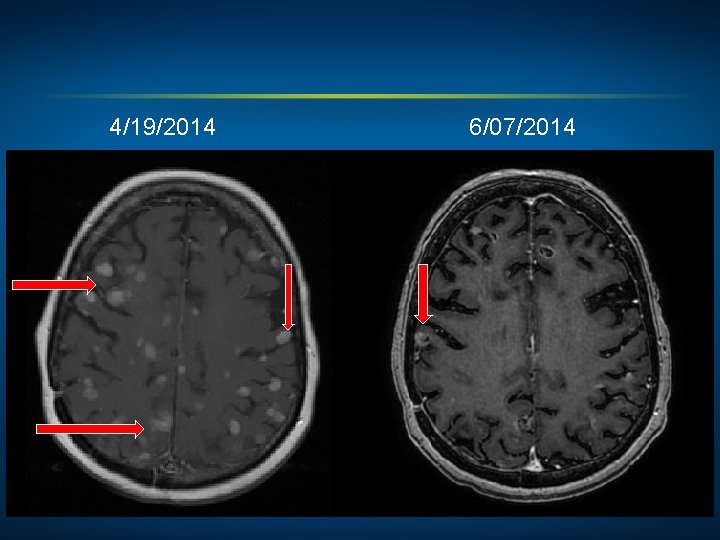
4/19/2014 6/07/2014

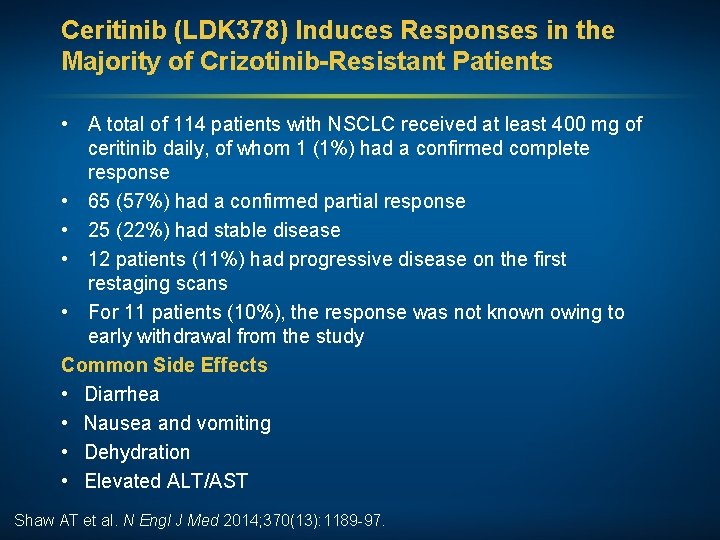
Ceritinib (LDK 378) Induces Responses in the Majority of Crizotinib-Resistant Patients • A total of 114 patients with NSCLC received at least 400 mg of ceritinib daily, of whom 1 (1%) had a confirmed complete response • 65 (57%) had a confirmed partial response • 25 (22%) had stable disease • 12 patients (11%) had progressive disease on the first restaging scans • For 11 patients (10%), the response was not known owing to early withdrawal from the study Common Side Effects • Diarrhea • Nausea and vomiting • Dehydration • Elevated ALT/AST Shaw AT et al. N Engl J Med 2014; 370(13): 1189 -97.

Typical Response to Ceritinib (LDK 378) • Some responses were rapid and dramatic • One patient experienced a 52% reduction in tumor burden after 6 weeks of ceritinib treatment Shaw AT et al. N Engl J Med 2014; 370(13): 1189 -97.

Investigational Agent Alectinib in Advanced ALK+, Crizotinib-Resistant NSCLC Best response (N = 44): 55% Most AEs Grade 1 -2 • Fatigue • Edema • Myalgia ≥Grade 3: Reduction in neutrophils Gadgeel SM et al. Lancet Oncol 2014; 15: 1119 -28.