Please note these are the actual videorecorded proceedings





- Slides: 17

Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content. Select slides from the original presentation are omitted where Research To Practice was unable to obtain permission from the publication source and/or author. Links to view the actual reference materials have been provided for your use in place of any omitted slides.



Novel Strategies for Targeting the EGFR Pathway Pasi A. Jänne, M. D. , Ph. D. Lowe Center for Thoracic Oncology Dana Farber Cancer Institute

EGFR Targeted Therapies • First generation TKIs – Erlotinib, gefitinib, lapatinib • Second generation TKIs – Neratinib, afatinib (BIBW 2992), dacomitinib (PF 299804) • Third generation – WZ 4002 – preclinical only • Combinations – Afatinib/cetuximab

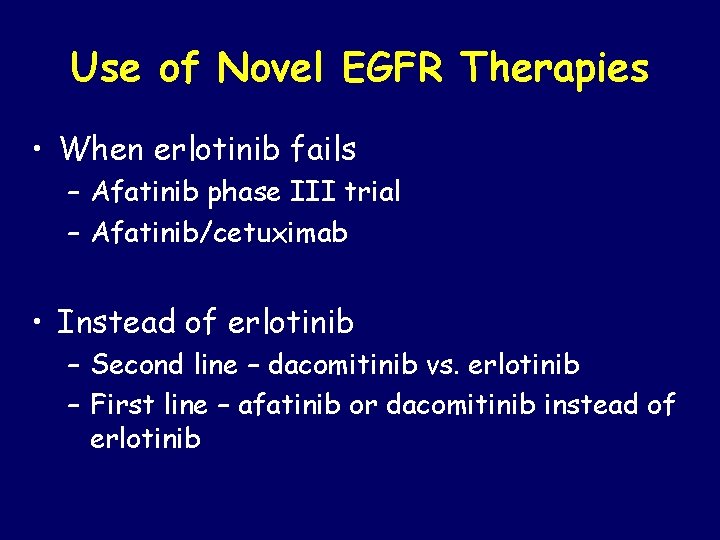
Use of Novel EGFR Therapies • When erlotinib fails – Afatinib phase III trial – Afatinib/cetuximab • Instead of erlotinib – Second line – dacomitinib vs. erlotinib – First line – afatinib or dacomitinib instead of erlotinib

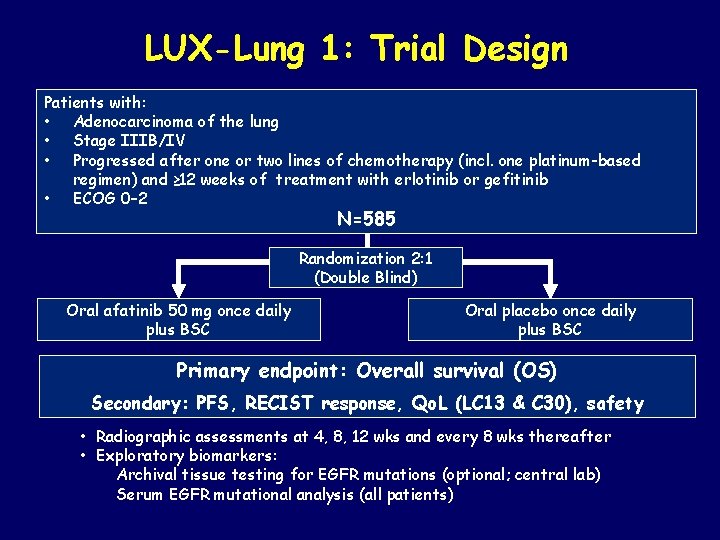
LUX-Lung 1: Trial Design Patients with: • Adenocarcinoma of the lung • Stage IIIB/IV • Progressed after one or two lines of chemotherapy (incl. one platinum-based regimen) and ≥ 12 weeks of treatment with erlotinib or gefitinib • ECOG 0– 2 N=585 Randomization 2: 1 (Double Blind) Oral afatinib 50 mg once daily plus BSC Oral placebo once daily plus BSC Primary endpoint: Overall survival (OS) Secondary: PFS, RECIST response, Qo. L (LC 13 & C 30), safety • Radiographic assessments at 4, 8, 12 wks and every 8 wks thereafter • Exploratory biomarkers: Archival tissue testing for EGFR mutations (optional; central lab) Serum EGFR mutational analysis (all patients)

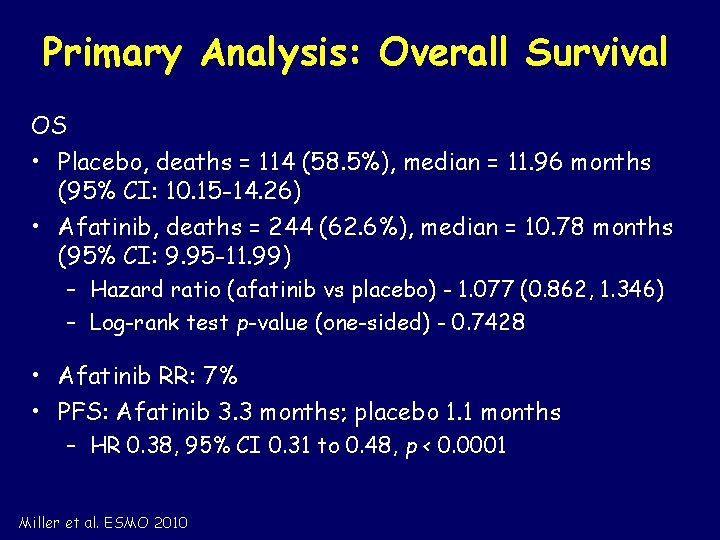
Primary Analysis: Overall Survival OS • Placebo, deaths = 114 (58. 5%), median = 11. 96 months (95% CI: 10. 15 -14. 26) • Afatinib, deaths = 244 (62. 6%), median = 10. 78 months (95% CI: 9. 95 -11. 99) – Hazard ratio (afatinib vs placebo) - 1. 077 (0. 862, 1. 346) – Log-rank test p-value (one-sided) - 0. 7428 • Afatinib RR: 7% • PFS: Afatinib 3. 3 months; placebo 1. 1 months – HR 0. 38, 95% CI 0. 31 to 0. 48, p < 0. 0001 Miller et al. ESMO 2010

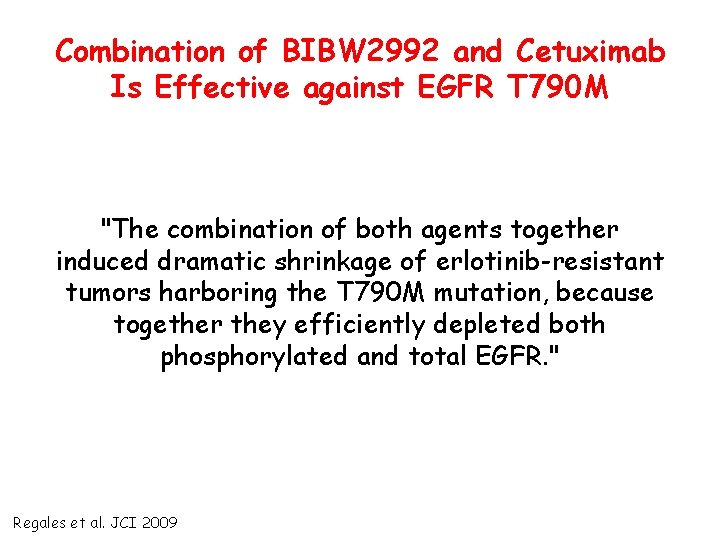
Combination of BIBW 2992 and Cetuximab Is Effective against EGFR T 790 M "The combination of both agents together induced dramatic shrinkage of erlotinib-resistant tumors harboring the T 790 M mutation, because together they efficiently depleted both phosphorylated and total EGFR. " Regales et al. JCI 2009

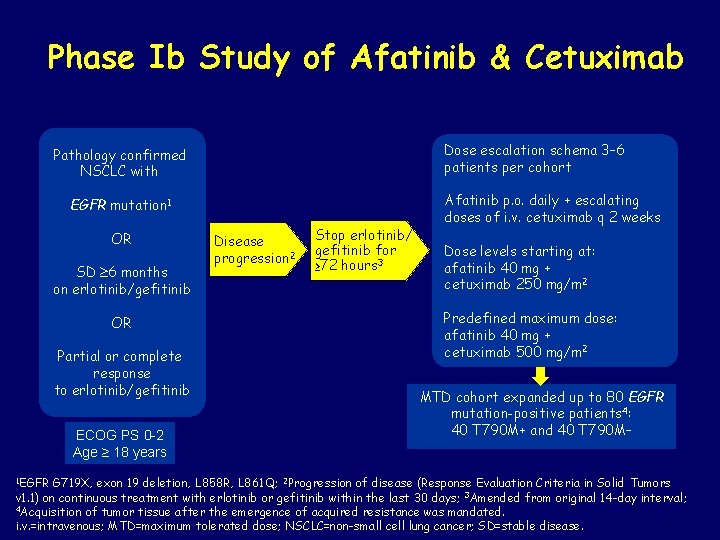
Phase Ib Study of Afatinib & Cetuximab Dose escalation schema 3– 6 patients per cohort Pathology confirmed NSCLC with EGFR mutation 1 OR SD 6 months on erlotinib/gefitinib OR Partial or complete response to erlotinib/gefitinib ECOG PS 0 2 Age ≥ 18 years 1 EGFR Disease progression 2 Stop erlotinib/ gefitinib for ≥ 72 hours 3 Afatinib p. o. daily + escalating doses of i. v. cetuximab q 2 weeks Dose levels starting at: afatinib 40 mg + cetuximab 250 mg/m 2 Predefined maximum dose: afatinib 40 mg + cetuximab 500 mg/m 2 MTD cohort expanded up to 80 EGFR mutation-positive patients 4: 40 T 790 M+ and 40 T 790 M– G 719 X, exon 19 deletion, L 858 R, L 861 Q; 2 Progression of disease (Response Evaluation Criteria in Solid Tumors v 1. 1) on continuous treatment with erlotinib or gefitinib within the last 30 days; 3 Amended from original 14 -day interval; 4 Acquisition of tumor tissue after the emergence of acquired resistance was mandated. i. v. =intravenous; MTD=maximum tolerated dose; NSCLC=non-small cell lung cancer; SD=stable disease.

Research To Practice could not obtain permission to reproduce this slide at the time of publication. To access the following abstract, please visit our Select Publications page: Groen JL et al. Proc IASLC 2011; Abstract O 19. 07.

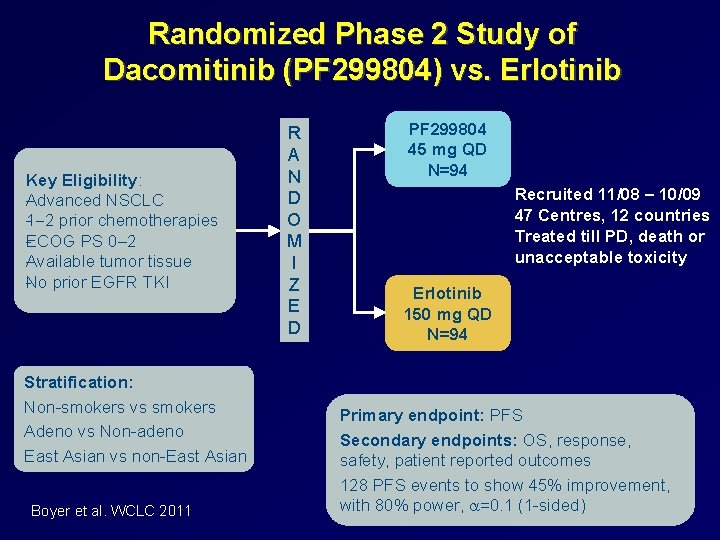
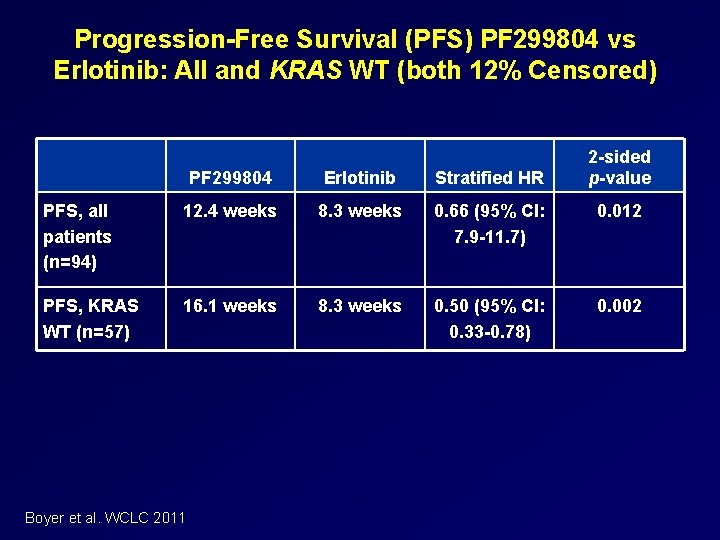
Randomized Phase 2 Study of Dacomitinib (PF 299804) vs. Erlotinib Key Eligibility: Advanced NSCLC 1 2 prior chemotherapies ECOG PS 0‒ 2 Available tumor tissue No prior EGFR TKI Stratification: Non smokers vs smokers Adeno vs Non adeno East Asian vs non East Asian Boyer et al. WCLC 2011 R A N D O M I Z E D PF 299804 45 mg QD N=94 Recruited 11/08 – 10/09 47 Centres, 12 countries Treated till PD, death or unacceptable toxicity Erlotinib 150 mg QD N=94 Primary endpoint: PFS Secondary endpoints: OS, response, safety, patient reported outcomes 128 PFS events to show 45% improvement, with 80% power, a=0. 1 (1 sided)

Progression-Free Survival (PFS) PF 299804 vs Erlotinib: All and KRAS WT (both 12% Censored) 2 -sided p-value PF 299804 Erlotinib Stratified HR PFS, all patients (n=94) 12. 4 weeks 8. 3 weeks 0. 66 (95% CI: 7. 9 -11. 7) 0. 012 PFS, KRAS WT (n=57) 16. 1 weeks 8. 3 weeks 0. 50 (95% CI: 0. 33 -0. 78) 0. 002 Boyer et al. WCLC 2011

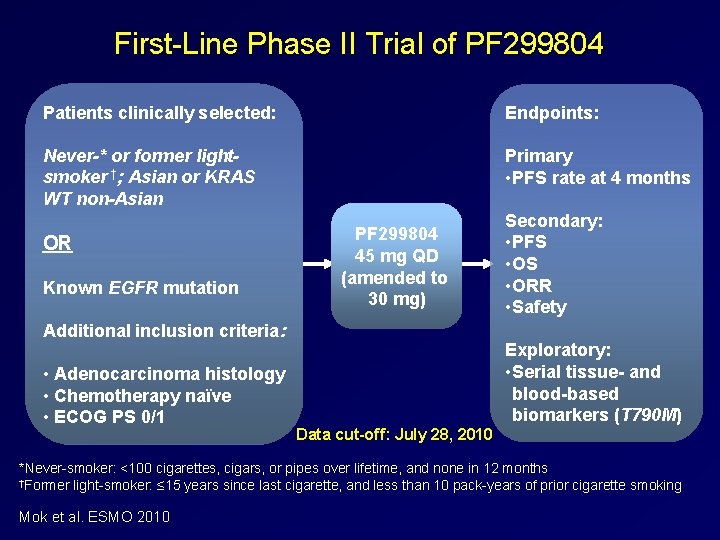
First Line Phase II Trial of PF 299804 Patients clinically selected: Endpoints: Never-* or former lightsmoker †; Asian or KRAS WT non-Asian Primary • PFS rate at 4 months OR Known EGFR mutation PF 299804 45 mg QD (amended to 30 mg) Additional inclusion criteria: • Adenocarcinoma histology • Chemotherapy naϊve • ECOG PS 0/1 Secondary: • PFS • ORR • Safety Exploratory: • Serial tissue- and blood-based biomarkers (T 790 M) Data cut-off: July 28, 2010 *Never smoker: <100 cigarettes, cigars, or pipes over lifetime, and none in 12 months †Former light smoker: ≤ 15 years since last cigarette, and less than 10 pack years of prior cigarette smoking Mok et al. ESMO 2010

Best Tumor Change in Target Lesions: Overall Population Treated with PF 299804 40 Best change from baseline (%) 20 0 – 20 – 40 – 60 – 80 – 100 45 mg, RDI ≥ 70% 45 mg, RDI <70% 30 mg, RDI ≥ 70% 30 mg, RDI <70% N=71 With permission from Mok et al. ESMO 2010

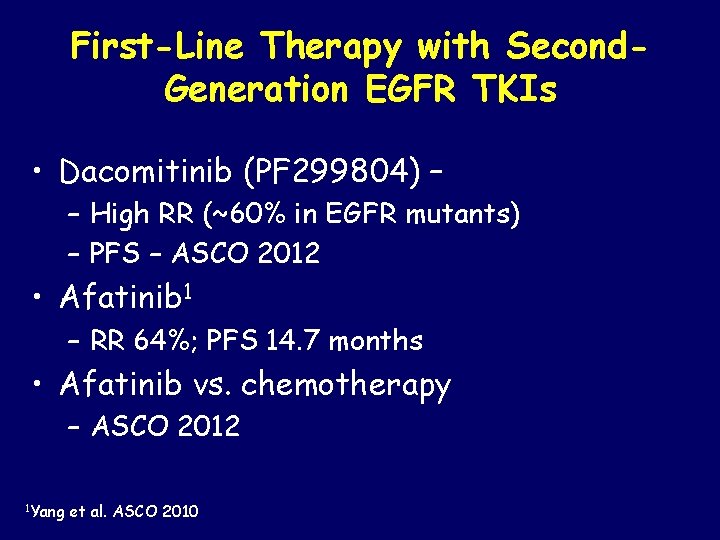
First-Line Therapy with Second. Generation EGFR TKIs • Dacomitinib (PF 299804) – – High RR (~60% in EGFR mutants) – PFS – ASCO 2012 • Afatinib 1 – RR 64%; PFS 14. 7 months • Afatinib vs. chemotherapy – ASCO 2012 1 Yang et al. ASCO 2010

Saturday, February 11, 2012 Hollywood, Florida Co-Chairs Rogerio C Lilenbaum, MD Mark A Socinski, MD Co-Chair and Moderator Neil Love, MD Faculty Chandra P Belani, MD John Heymach, MD, Ph. D Pasi A Jänne, MD, Ph. D Thomas J Lynch Jr, MD Heather Wakelee, MD