ORGANIC CHEMISTRY 1 Chapter 6 Part 1 1

![[6. 2 -5] Properties and uses of haloalkanes: (A) Polar C- X bond: (B) [6. 2 -5] Properties and uses of haloalkanes: (A) Polar C- X bond: (B)](https://slidetodoc.com/presentation_image/31848c1bf7eca5d65eab31e17a86b5ed/image-7.jpg)



![[6. 13] A new page – and a new chemistry: The same substrate but [6. 13] A new page – and a new chemistry: The same substrate but](https://slidetodoc.com/presentation_image/31848c1bf7eca5d65eab31e17a86b5ed/image-53.jpg)
![[6. 13] The reaction rate - experimental data: The reaction: (CH 3)3 C-Br + [6. 13] The reaction rate - experimental data: The reaction: (CH 3)3 C-Br +](https://slidetodoc.com/presentation_image/31848c1bf7eca5d65eab31e17a86b5ed/image-54.jpg)
![The more “expensive” [in k. J] the cation, the higher the Ea 1 and The more “expensive” [in k. J] the cation, the higher the Ea 1 and](https://slidetodoc.com/presentation_image/31848c1bf7eca5d65eab31e17a86b5ed/image-58.jpg)





- Slides: 82

ORGANIC CHEMISTRY 1 Chapter 6, Part 1 (1) Haloalkanes, preparation & properties (2) Nucleophilic Substitution Reactions -or: - How to make alcohols, ethers, esters, amines, nitriles, … (3) Elimination Reactions – or: -How to make alkenes from haloalkanes and alcohols Based on Organic Chemistry, by L. G. Wade, 7 th ed; Compiled by: Dr. Peter Ilich, St. John’s University Queens, New York, Spring 2012

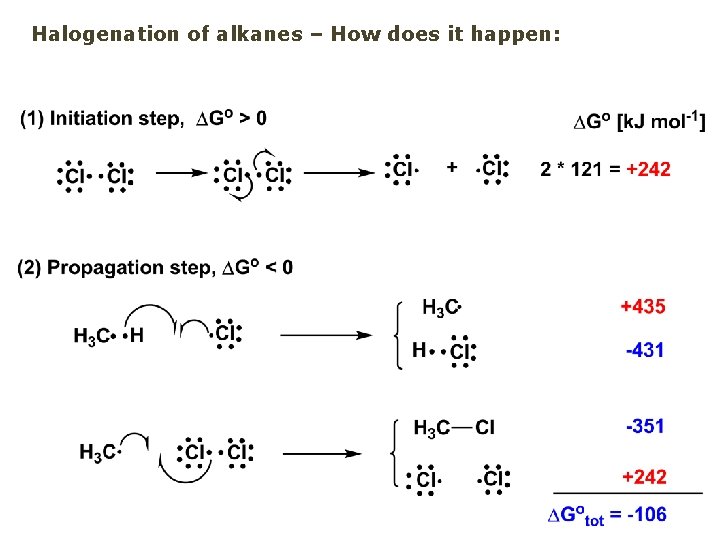
Halogenation of alkanes – How does it happen:

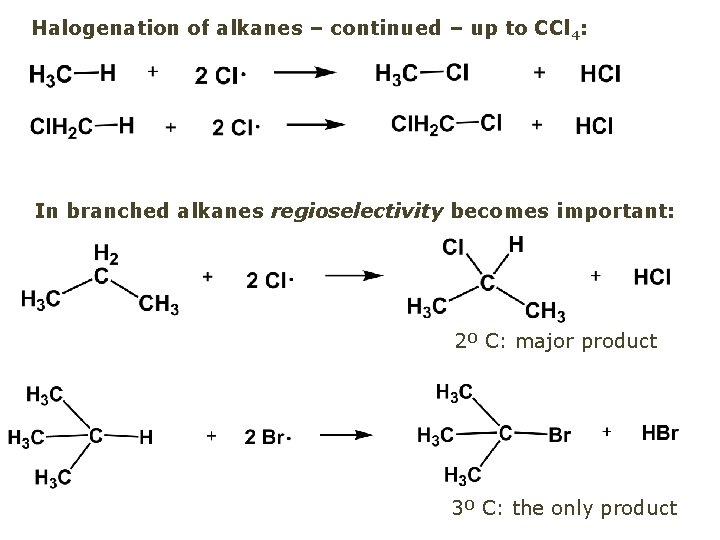
Halogenation of alkanes – continued – up to CCl 4: In branched alkanes regioselectivity becomes important: 2º C: major product 3º C: the only product

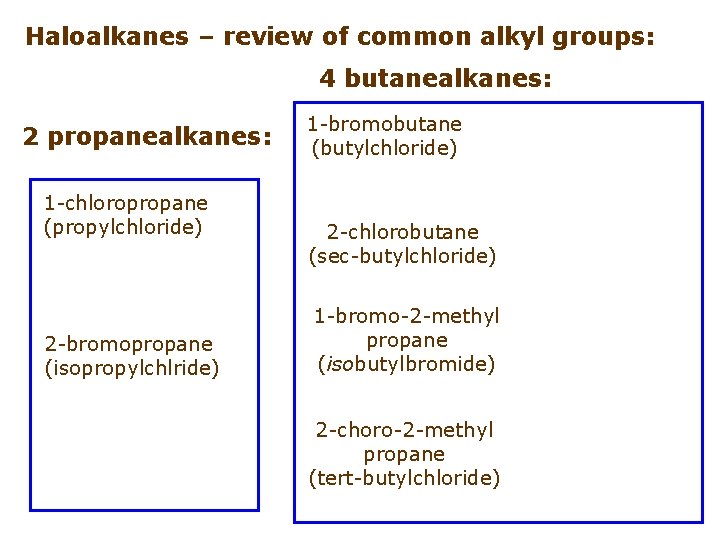
Haloalkanes – review of common alkyl groups: 4 butanealkanes: 2 propanealkanes: 1 -chloropropane (propylchloride) 2 -bromopropane (isopropylchlride) 1 -bromobutane (butylchloride) 2 -chlorobutane (sec-butylchloride) 1 -bromo-2 -methyl propane (isobutylbromide) 2 -choro-2 -methyl propane (tert-butylchloride)

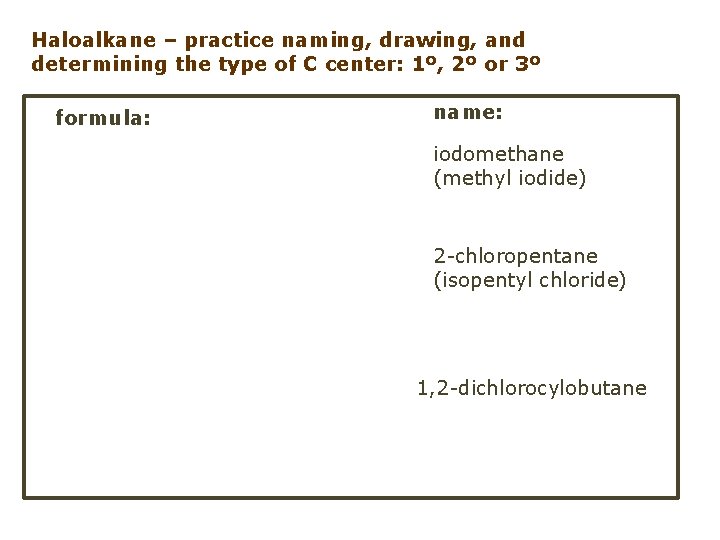
Haloalkane – practice naming, drawing, and determining the type of C center: 1º, 2º or 3º formula: name: iodomethane (methyl iodide) 2 -chloropentane (isopentyl chloride) 1, 2 -dichlorocylobutane

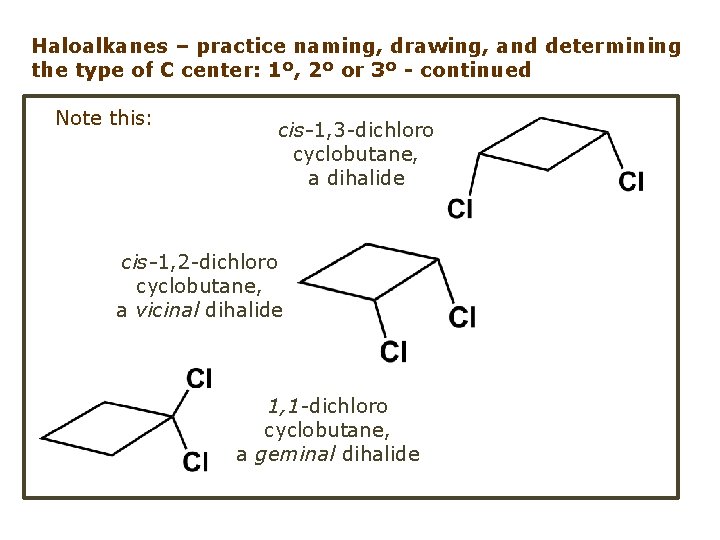
Haloalkanes – practice naming, drawing, and determining the type of C center: 1º, 2º or 3º - continued Note this: cis-1, 3 -dichloro cyclobutane, a dihalide cis-1, 2 -dichloro cyclobutane, a vicinal dihalide 1, 1 -dichloro cyclobutane, a geminal dihalide
![6 2 5 Properties and uses of haloalkanes A Polar C X bond B [6. 2 -5] Properties and uses of haloalkanes: (A) Polar C- X bond: (B)](https://slidetodoc.com/presentation_image/31848c1bf7eca5d65eab31e17a86b5ed/image-7.jpg)
[6. 2 -5] Properties and uses of haloalkanes: (A) Polar C- X bond: (B) Immiscible with but heavier than water: water alkane water haloalkane

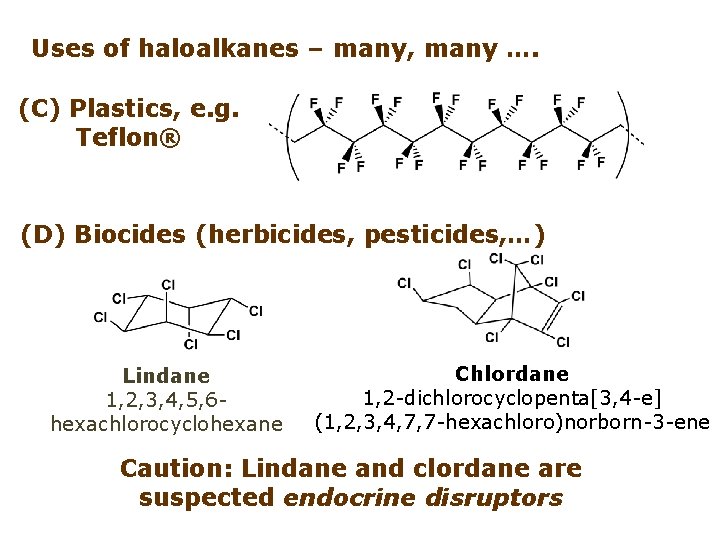
Uses of haloalkanes – many, many …. (C) Plastics, e. g. Teflon® (D) Biocides (herbicides, pesticides, …) Lindane 1, 2, 3, 4, 5, 6 hexachlorocyclohexane Chlordane 1, 2 -dichlorocyclopenta[3, 4 -e] (1, 2, 3, 4, 7, 7 -hexachloro)norborn-3 -ene Caution: Lindane and clordane are suspected endocrine disruptors

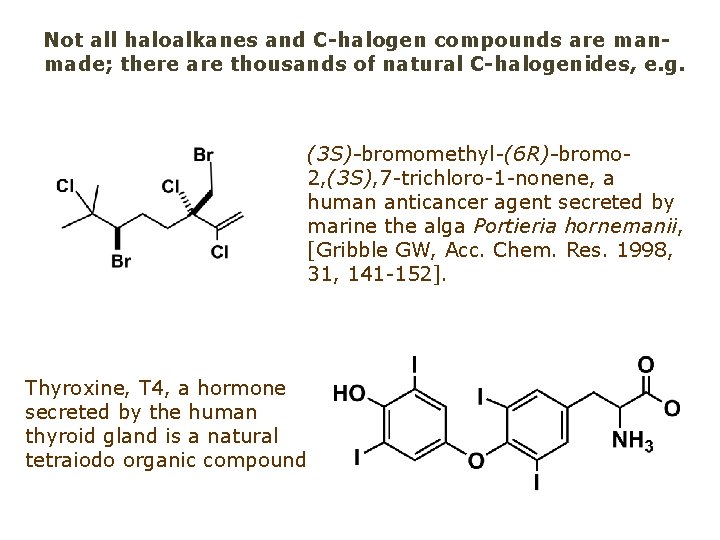
Not all haloalkanes and C-halogen compounds are manmade; there are thousands of natural C-halogenides, e. g. (3 S)-bromomethyl-(6 R)-bromo 2, (3 S), 7 -trichloro-1 -nonene, a human anticancer agent secreted by marine the alga Portieria hornemanii, [Gribble GW, Acc. Chem. Res. 1998, 31, 141 -152]. Thyroxine, T 4, a hormone secreted by the human thyroid gland is a natural tetraiodo organic compound
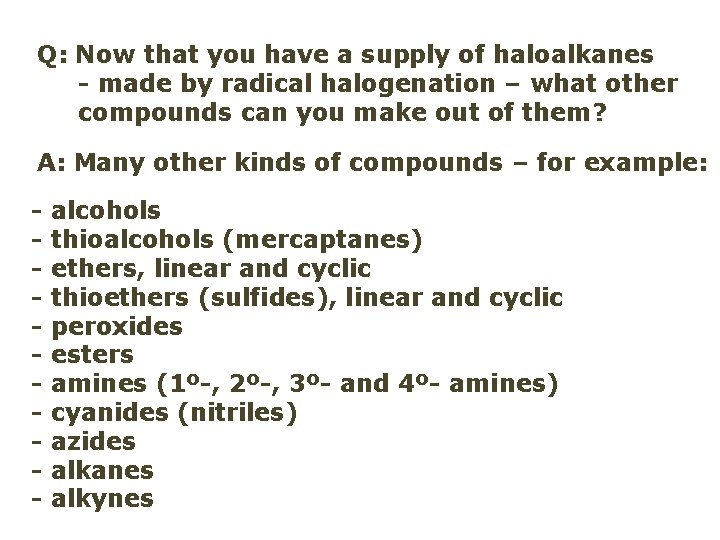
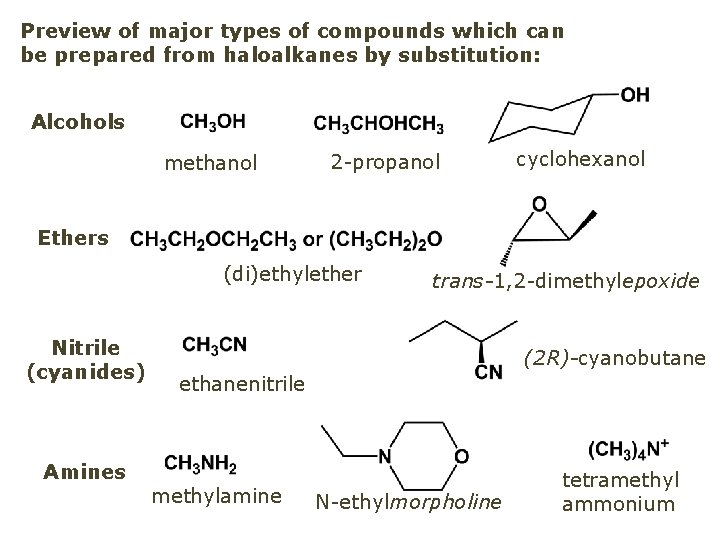
Q: Now that you have a supply of haloalkanes - made by radical halogenation – what other compounds can you make out of them? A: Many other kinds of compounds – for example: - alcohols thioalcohols (mercaptanes) ethers, linear and cyclic thioethers (sulfides), linear and cyclic peroxides esters amines (1º-, 2º-, 3º- and 4º- amines) cyanides (nitriles) azides alkanes alkynes

Preview of major types of compounds which can be prepared from haloalkanes by substitution: Alcohols methanol 2 -propanol cyclohexanol Ethers (di)ethylether Nitrile (cyanides) Amines trans-1, 2 -dimethylepoxide (2 R)-cyanobutane ethanenitrile methylamine N-ethylmorpholine tetramethyl ammonium


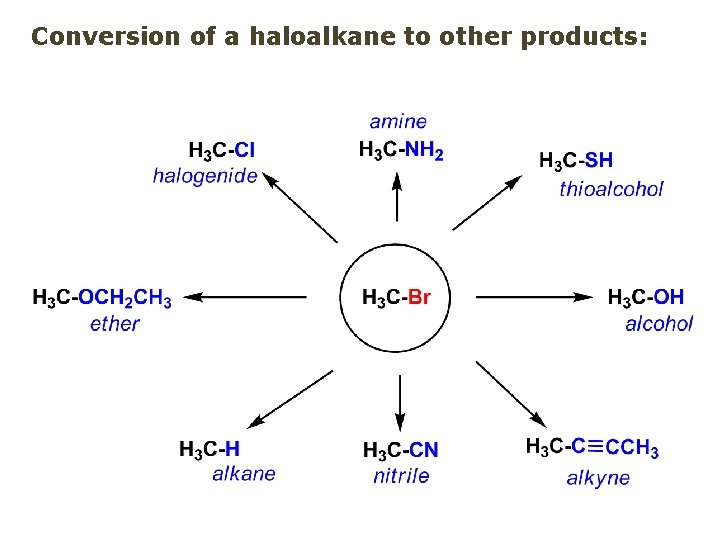
Conversion of a haloalkane to other products:

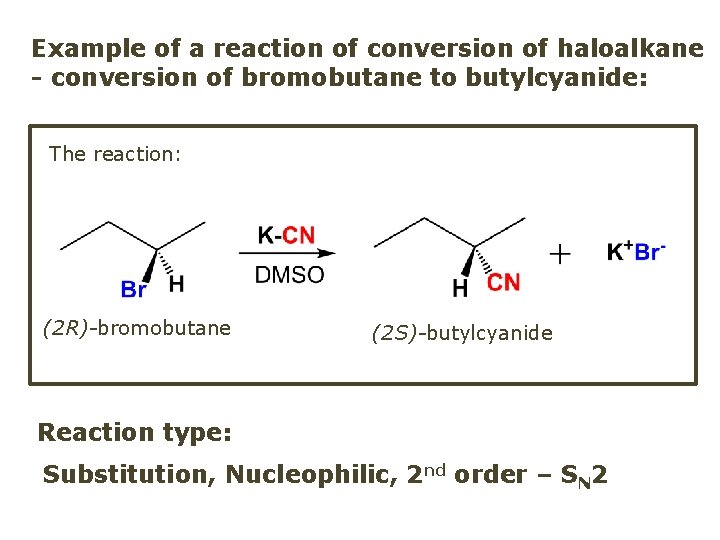
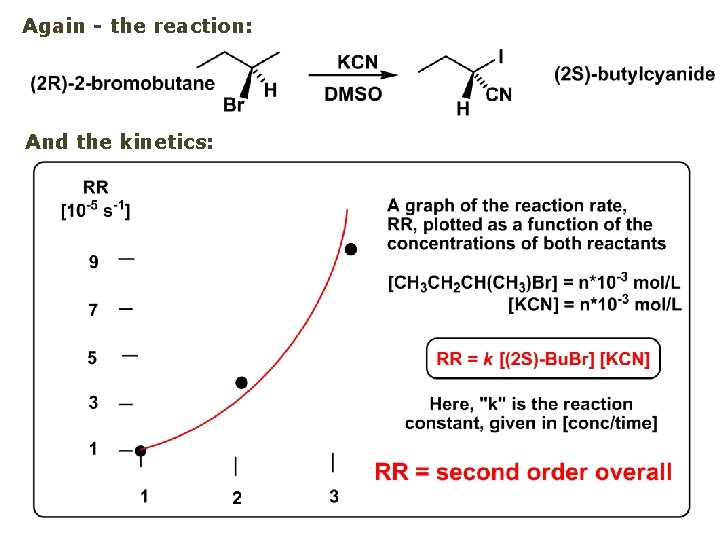
Example of a reaction of conversion of haloalkane - conversion of bromobutane to butylcyanide: The reaction: (2 R)-bromobutane (2 S)-butylcyanide Reaction type: Substitution, Nucleophilic, 2 nd order – SN 2

Again - the reaction: And the kinetics:

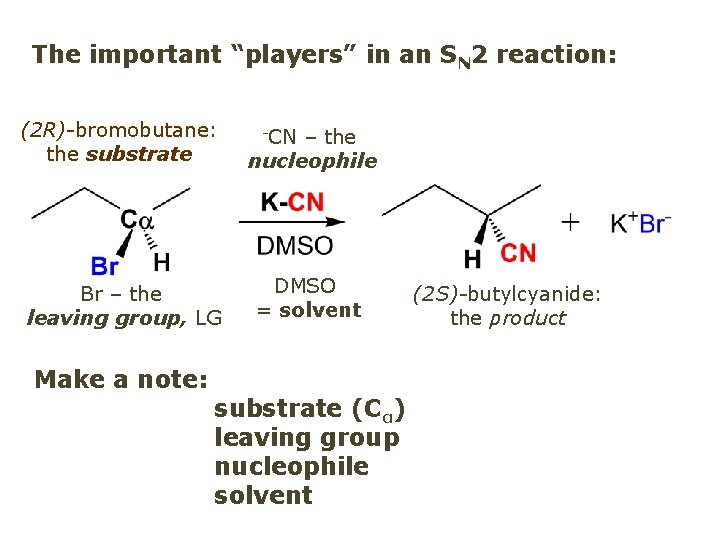
The important “players” in an SN 2 reaction: (2 R)-bromobutane: the substrate – the nucleophile Br – the leaving group, LG DMSO = solvent Make a note: -CN substrate (Cα) leaving group nucleophile solvent (2 S)-butylcyanide: the product

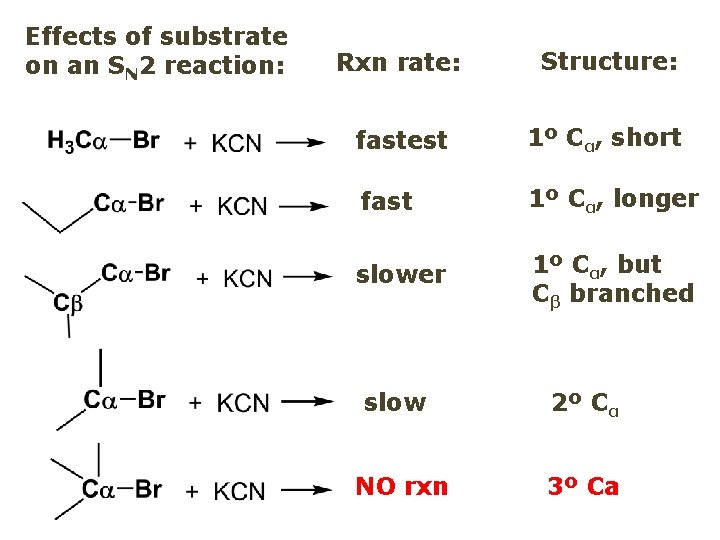
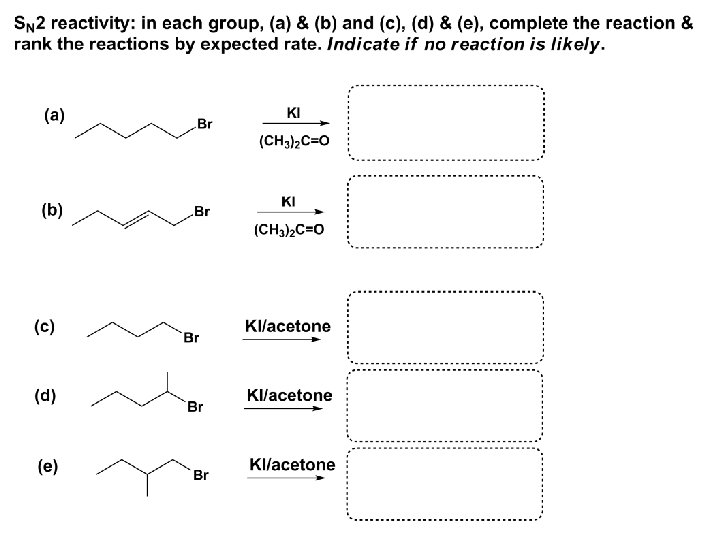
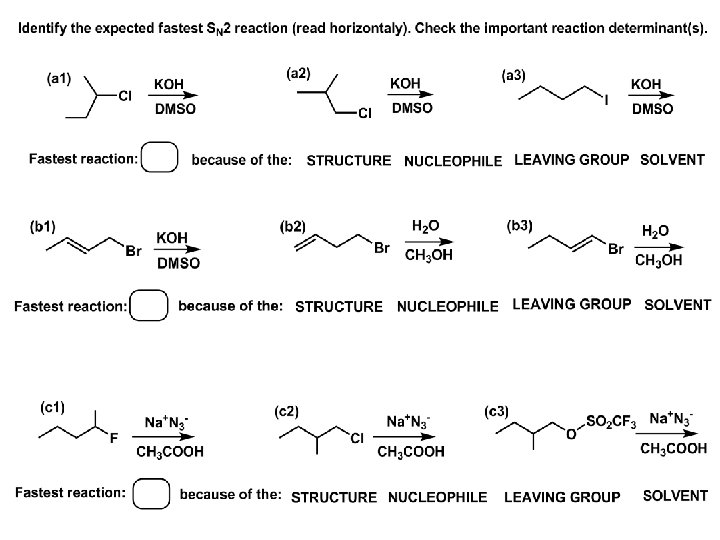
Effects of substrate on an SN 2 reaction: Rxn rate: Structure: fastest 1º Cα, short fast 1º Cα, longer slower 1º Cα, but Cβ branched slow NO rxn 2º Cα 3º Ca

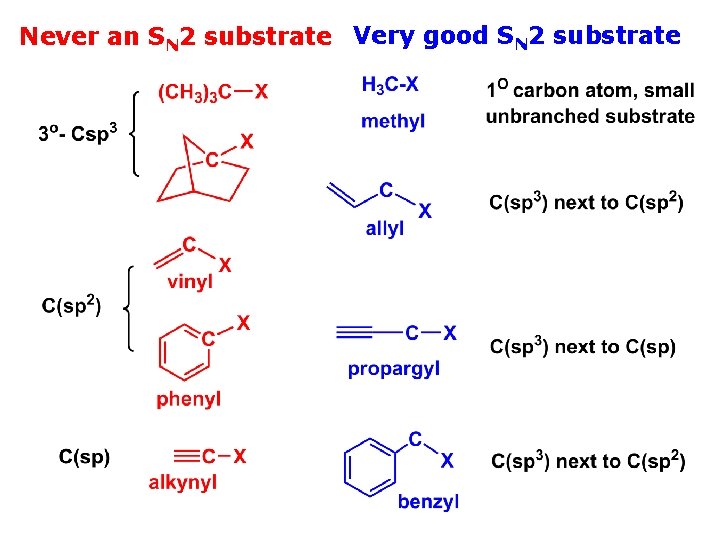
Never an SN 2 substrate Very good SN 2 substrate

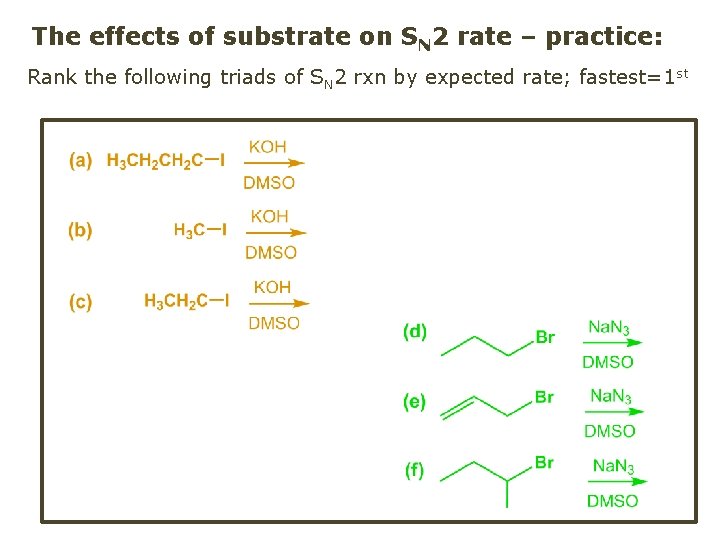
The effects of substrate on SN 2 rate – practice: Rank the following triads of SN 2 rxn by expected rate; fastest=1 st

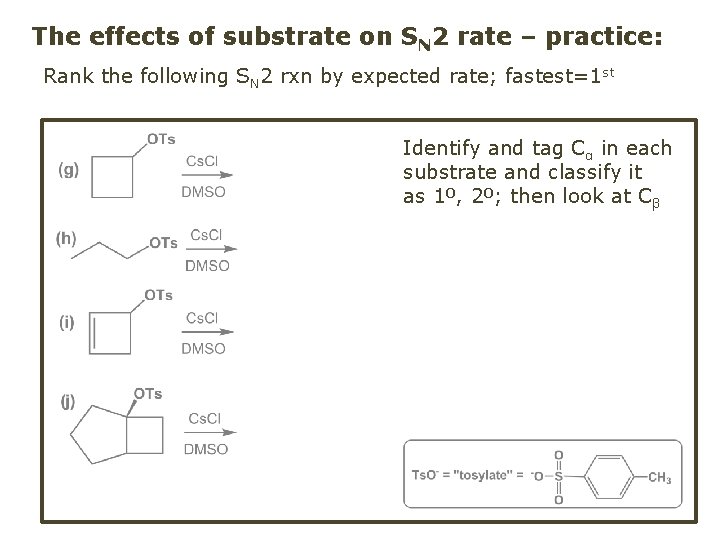
The effects of substrate on SN 2 rate – practice: Rank the following SN 2 rxn by expected rate; fastest=1 st Identify and tag Cα in each substrate and classify it as 1º, 2º; then look at Cβ

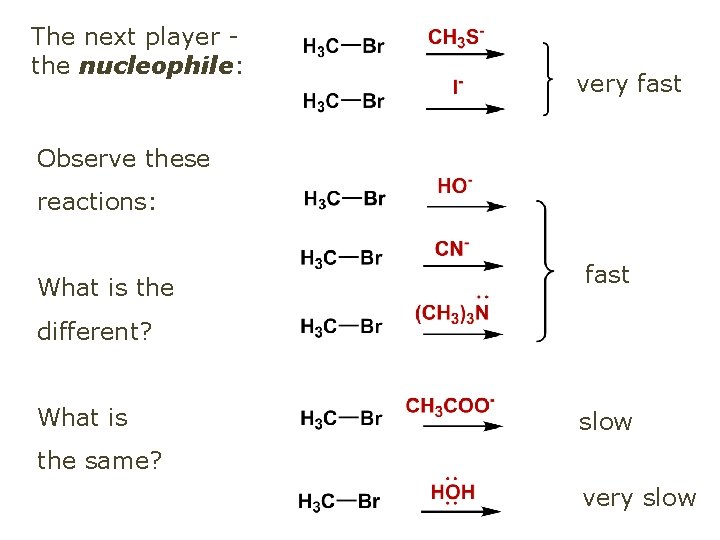
The next player the nucleophile: very fast Observe these reactions: What is the fast different? What is slow the same? very slow

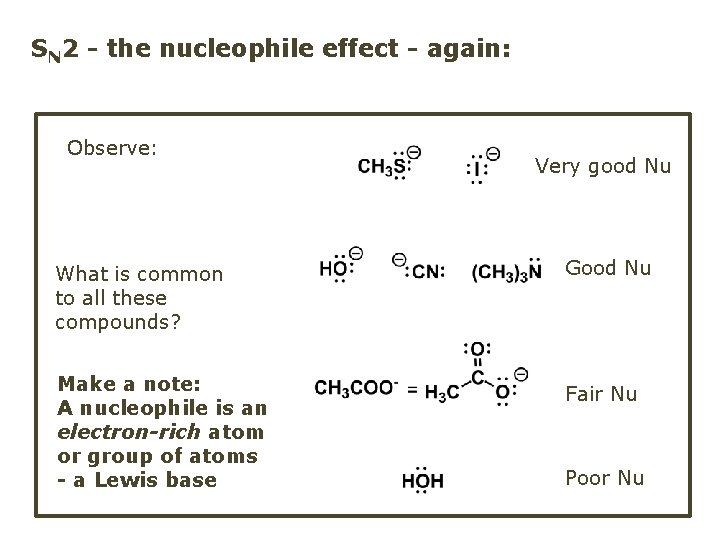
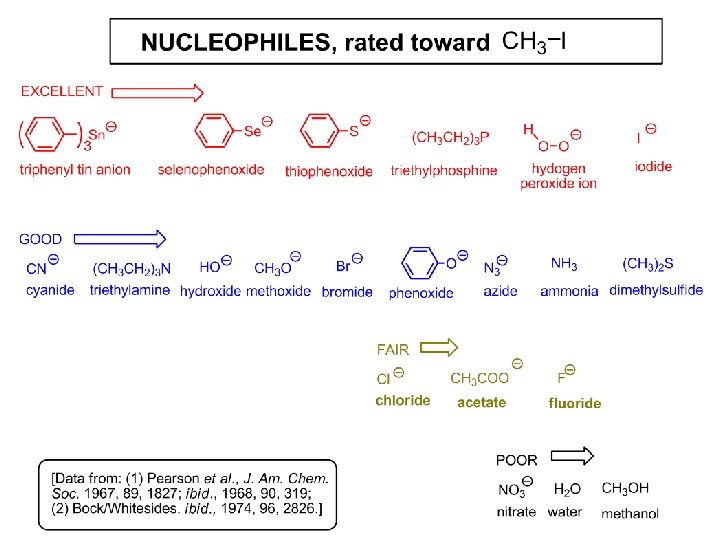
SN 2 - the nucleophile effect - again: Observe: What is common to all these compounds? Make a note: A nucleophile is an electron-rich atom or group of atoms - a Lewis base Very good Nu Good Nu Fair Nu Poor Nu

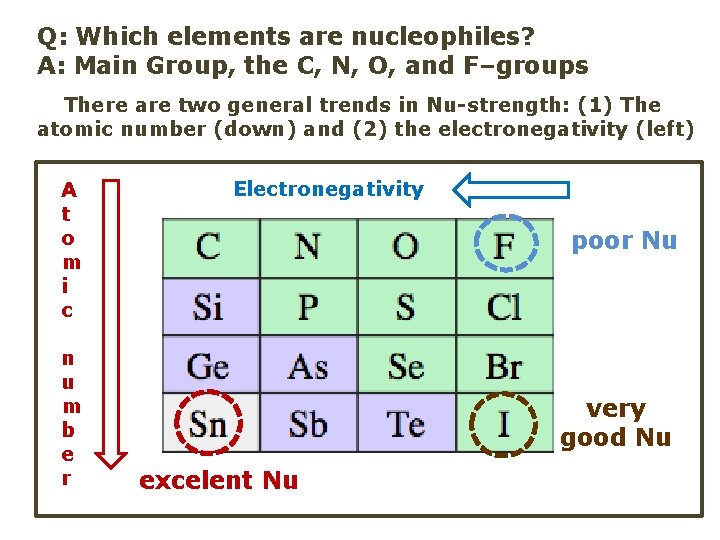
Q: Which elements are nucleophiles? A: Main Group, the C, N, O, and F–groups There are two general trends in Nu-strength: (1) The atomic number (down) and (2) the electronegativity (left) A t o m i c n u m b e r Electronegativity poor Nu very good Nu excelent Nu

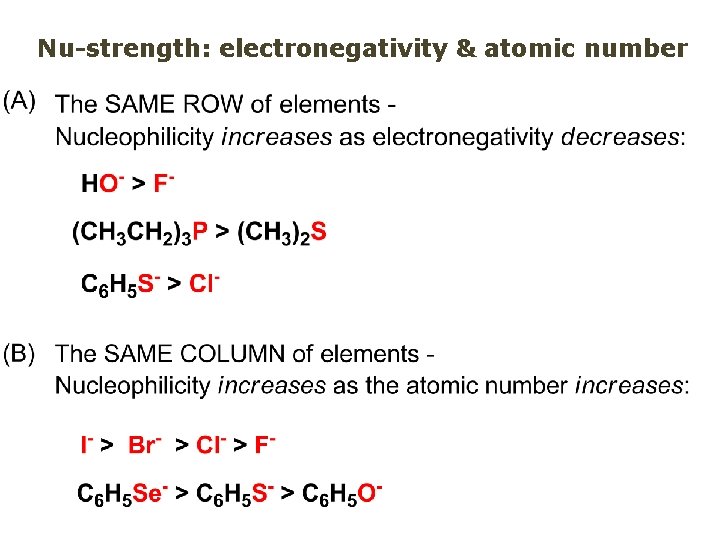
Nu-strength: electronegativity & atomic number


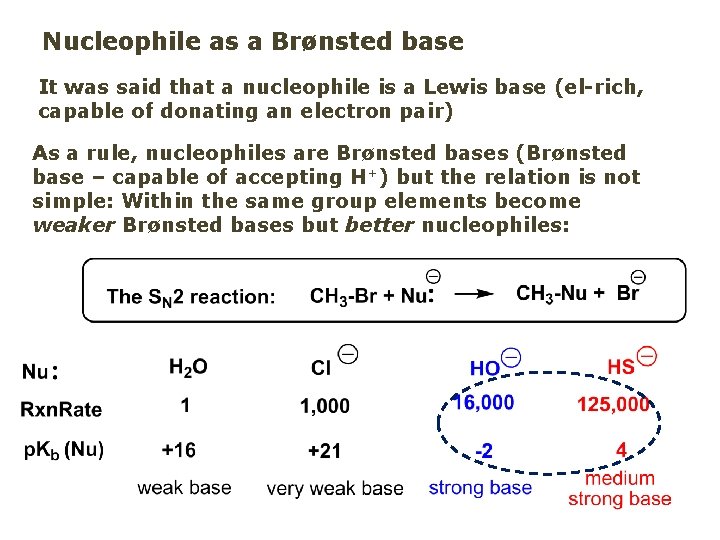
Nucleophile as a Brønsted base It was said that a nucleophile is a Lewis base (el-rich, capable of donating an electron pair) As a rule, nucleophiles are Brønsted bases (Brønsted base – capable of accepting H+) but the relation is not simple: Within the same group elements become weaker Brønsted bases but better nucleophiles:

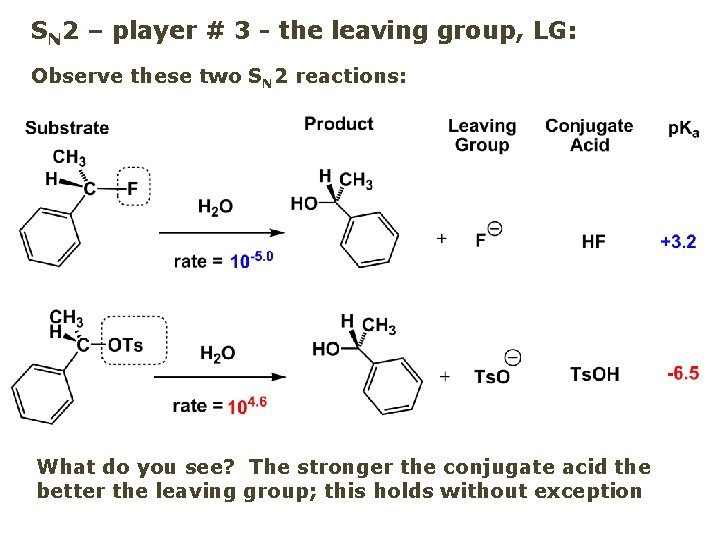
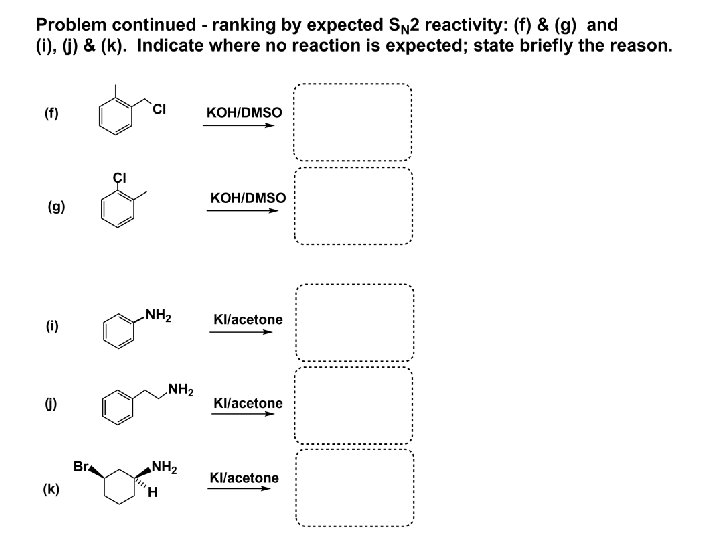
SN 2 – player # 3 - the leaving group, LG: Observe these two SN 2 reactions: What do you see? The stronger the conjugate acid the better the leaving group; this holds without exception

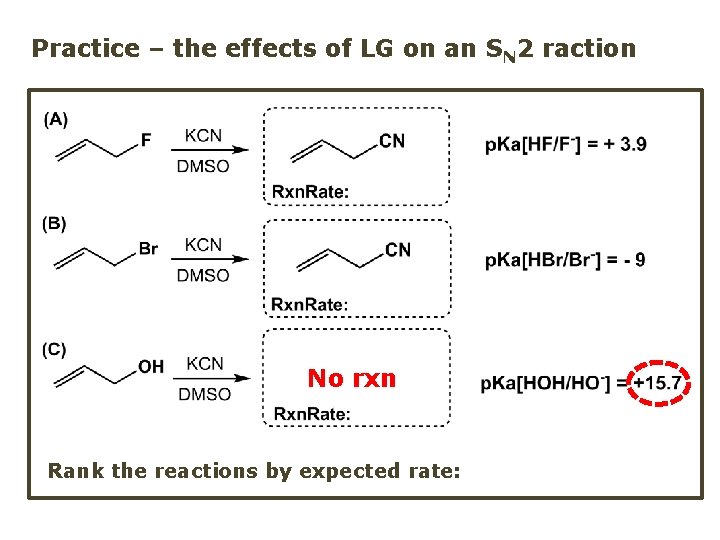
Practice – the effects of LG on an SN 2 raction No rxn Rank the reactions by expected rate:

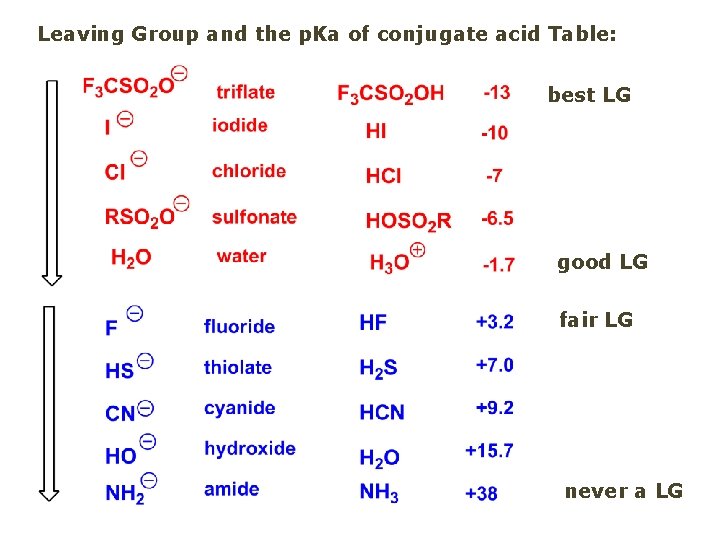
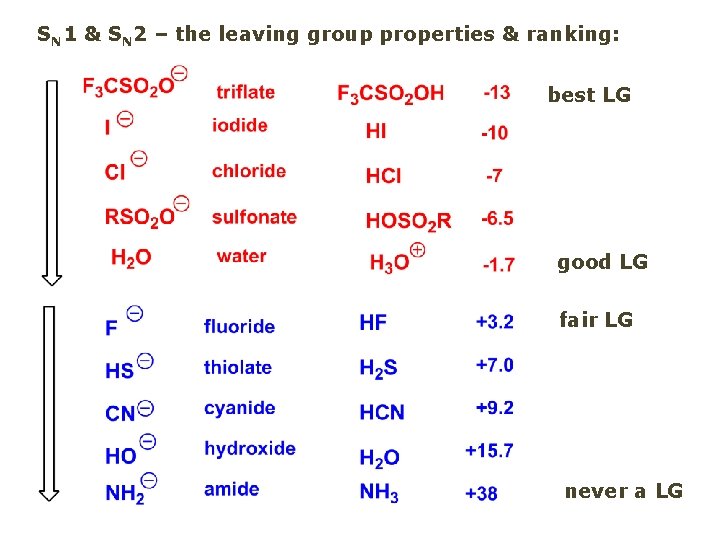
Leaving Group and the p. Ka of conjugate acid Table: best LG good LG fair LG never a LG

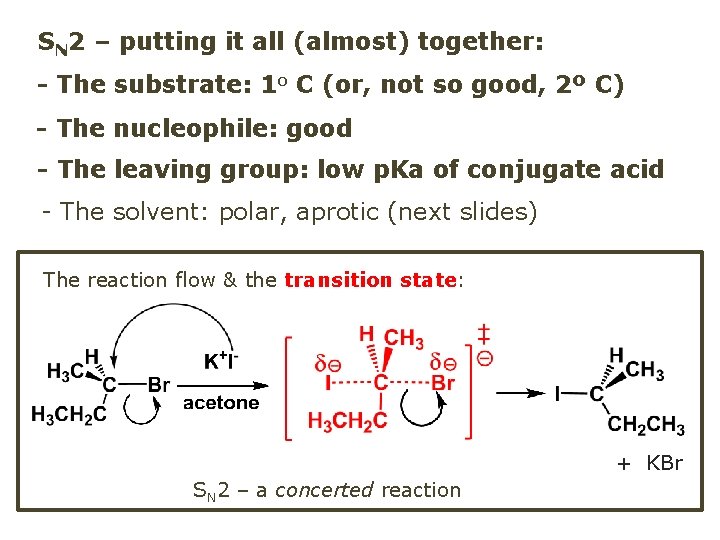
SN 2 – putting it all (almost) together: - The substrate: 1 o C (or, not so good, 2º C) - The nucleophile: good - The leaving group: low p. Ka of conjugate acid - The solvent: polar, aprotic (next slides) The reaction flow & the transition state: + KBr SN 2 – a concerted reaction

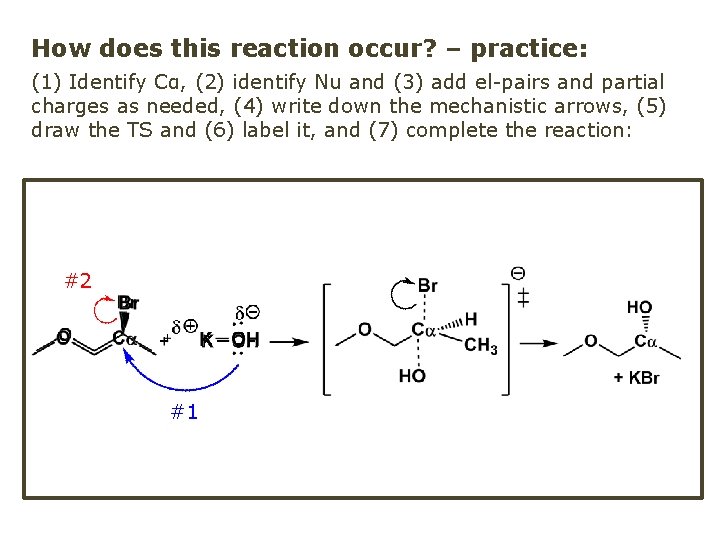
How does this reaction occur? – practice: (1) Identify Cα, (2) identify Nu and (3) add el-pairs and partial charges as needed, (4) write down the mechanistic arrows, (5) draw the TS and (6) label it, and (7) complete the reaction: #2 #1

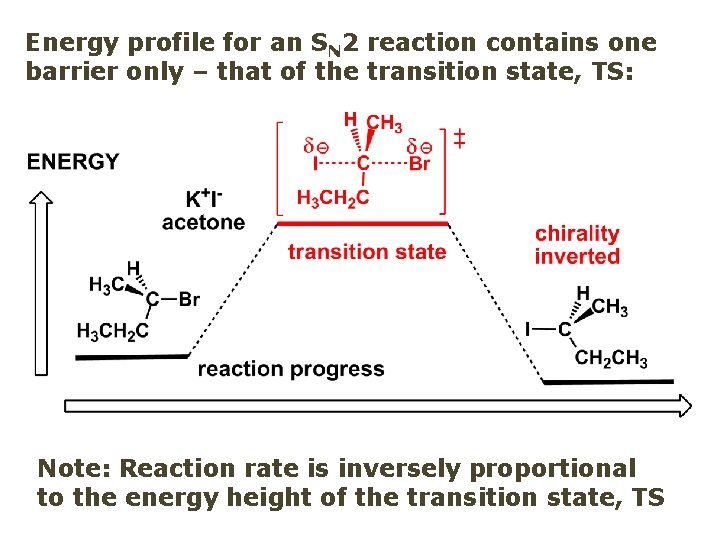
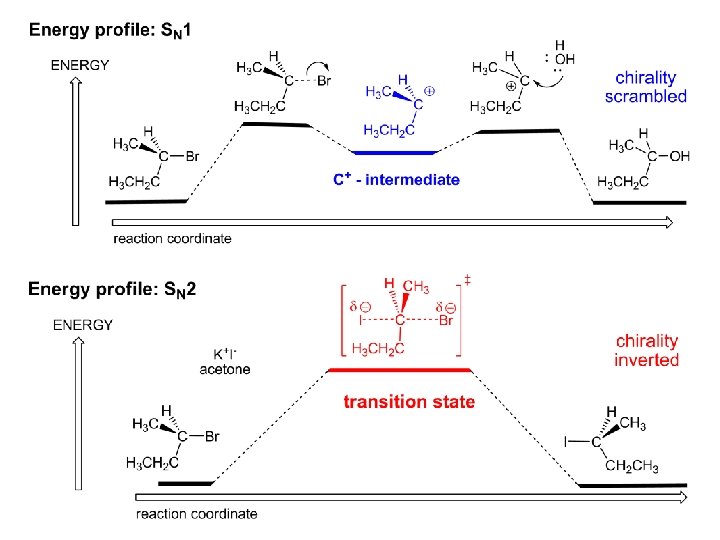
Energy profile for an SN 2 reaction contains one barrier only – that of the transition state, TS: Note: Reaction rate is inversely proportional to the energy height of the transition state, TS

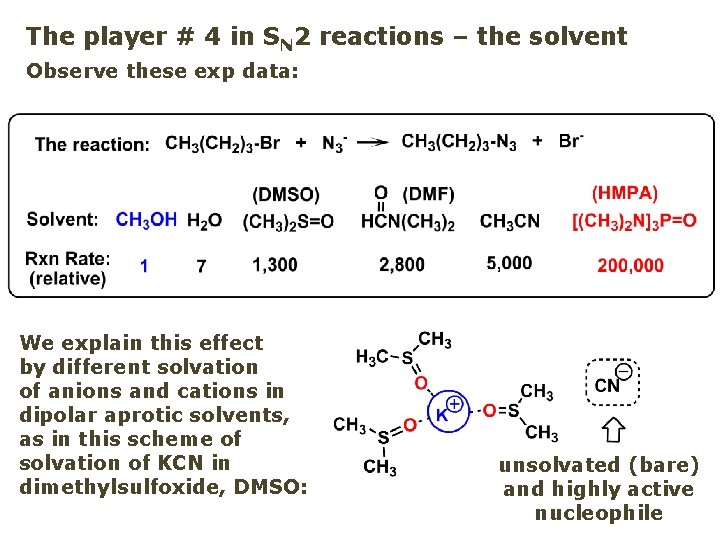
The player # 4 in SN 2 reactions – the solvent Observe these exp data: We explain this effect by different solvation of anions and cations in dipolar aprotic solvents, as in this scheme of solvation of KCN in dimethylsulfoxide, DMSO: unsolvated (bare) and highly active nucleophile

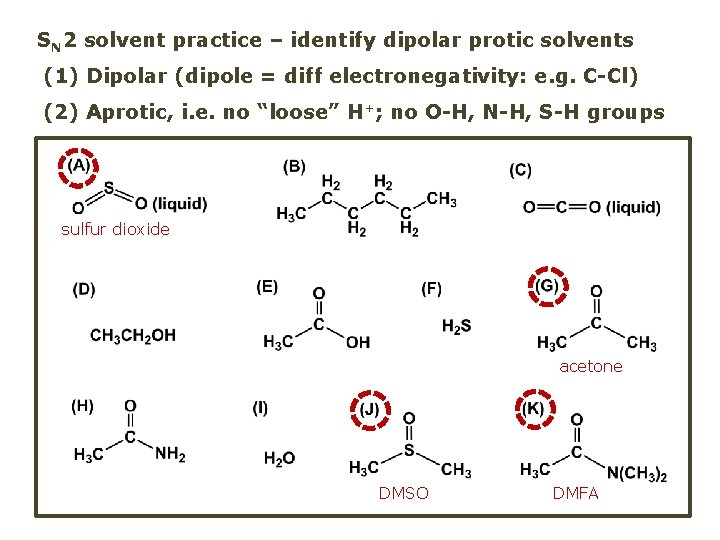
SN 2 solvent practice – identify dipolar protic solvents (1) Dipolar (dipole = diff electronegativity: e. g. C-Cl) (2) Aprotic, i. e. no “loose” H+; no O-H, N-H, S-H groups sulfur dioxide acetone DMSO DMFA

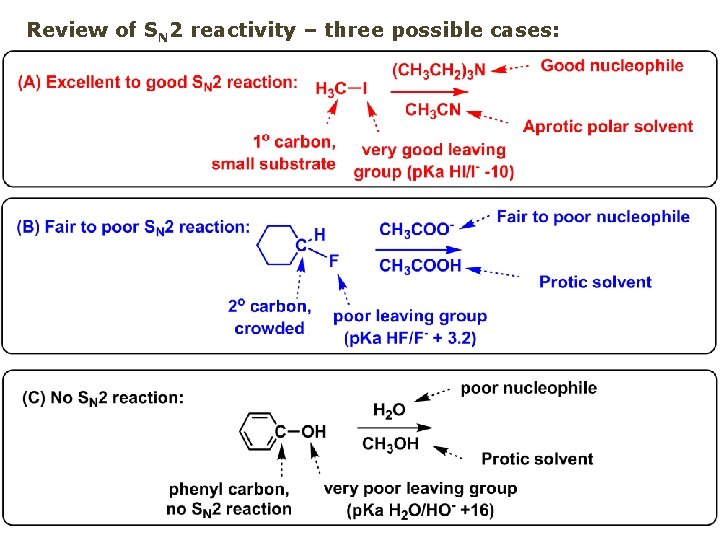
Review of SN 2 reactivity – three possible cases:

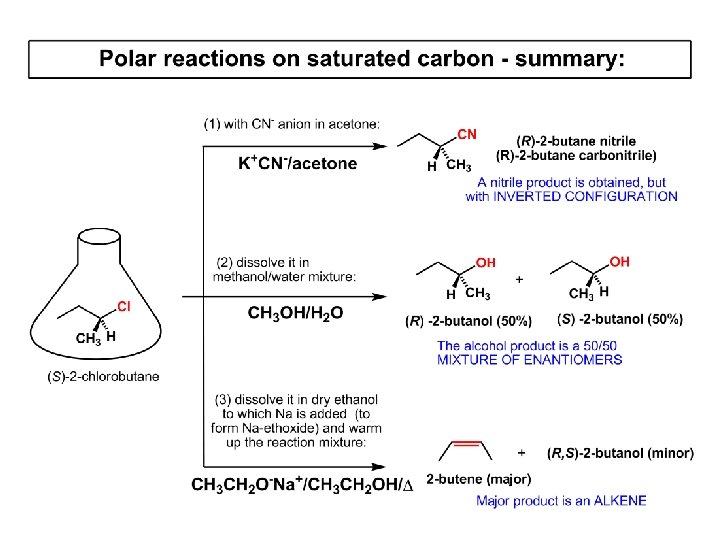
Summary of the Ch. 6, part 1 – What have we learned today? - Haloalkanes can be converted to alcohols, thioalcohols, ethers, thioethers, amines, nitriles, azides, peroxides, . . . and a number of other type compounds - The predominant reaction types in these conversions are second order nulcleophilic substitutions, SN 2 - SN 2 reaction can be shown to depend on substrate, leaving group, nucleophile and solvent - Optimal reaction parameters and conditions are established and the SN 2 mechanism is derived

ORGANIC CHEMISTRY 1 Chapter 6, Part 2 (1) Uses of SN 2 reactivity in synthesis (2) Other SN reactions: SN 1 - SN 1 variables: substrate, nucleophile, leaving group, solvent - The carbocation intermediate paths (3) Elimination, 1 st order & competition with SN 1 (4) Eliminatino, 2 nd order Based on Organic Chemistry, by L. G. Wade, 7 th ed; Compiled by: Dr. Peter Ilich, St. John’s University Queens, New York, Spring 2012

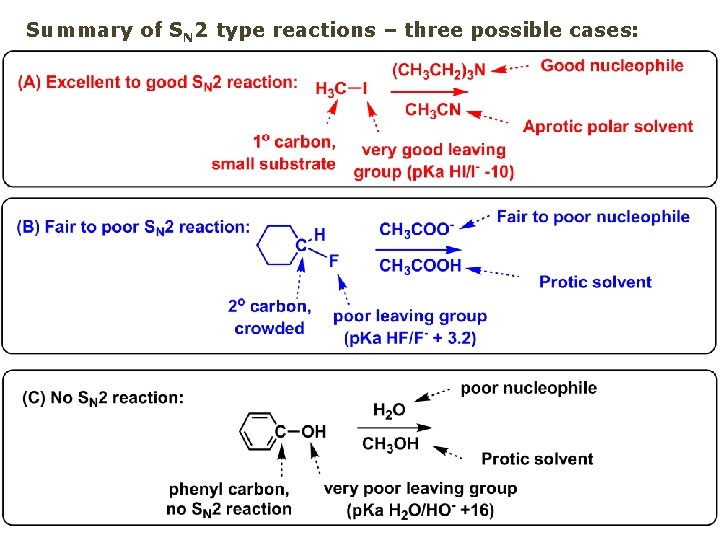
Summary of SN 2 type reactions – three possible cases:

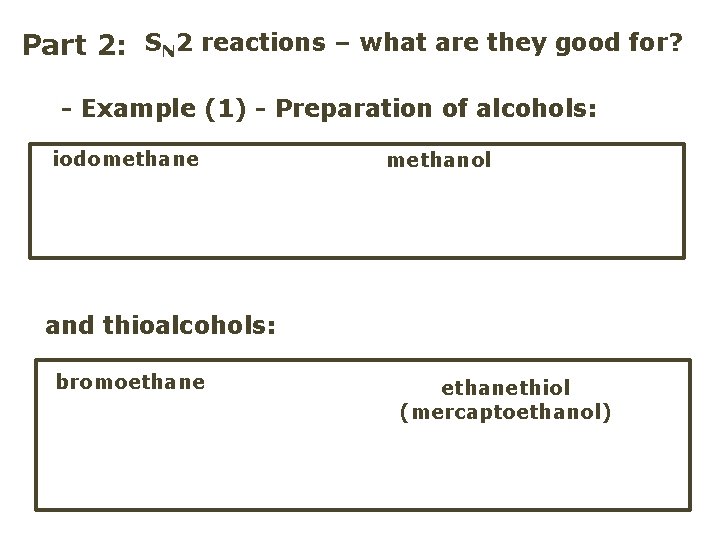
Part 2: SN 2 reactions – what are they good for? - Example (1) - Preparation of alcohols: iodomethane methanol and thioalcohols: bromoethanethiol (mercaptoethanol)

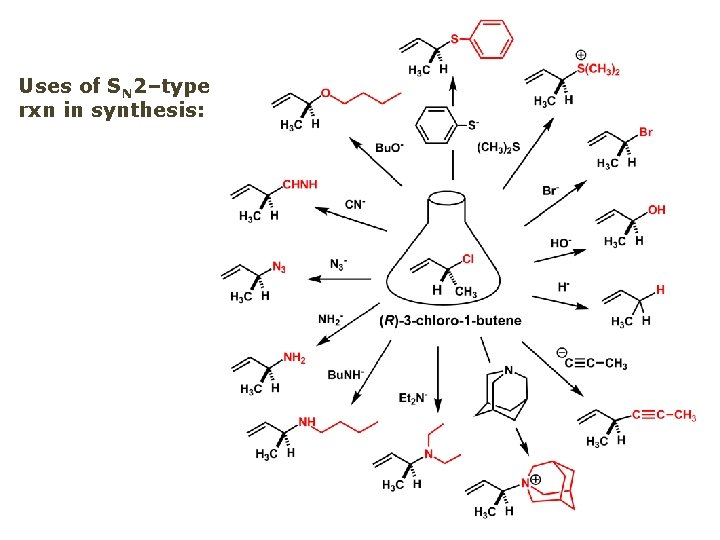
Uses of SN 2–type rxn in synthesis:

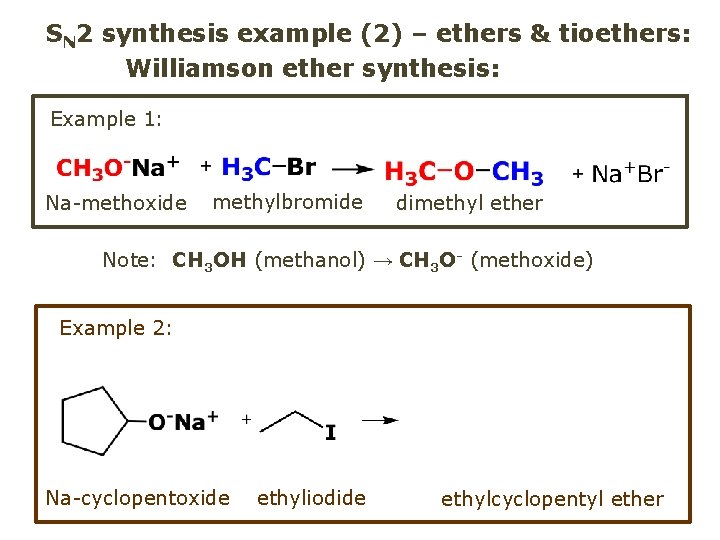
SN 2 synthesis example (2) – ethers & tioethers: Williamson ether synthesis: Example 1: Na-methoxide methylbromide dimethyl ether Note: CH 3 OH (methanol) → CH 3 O- (methoxide) Example 2: Na-cyclopentoxide ethyliodide ethylcyclopentyl ether

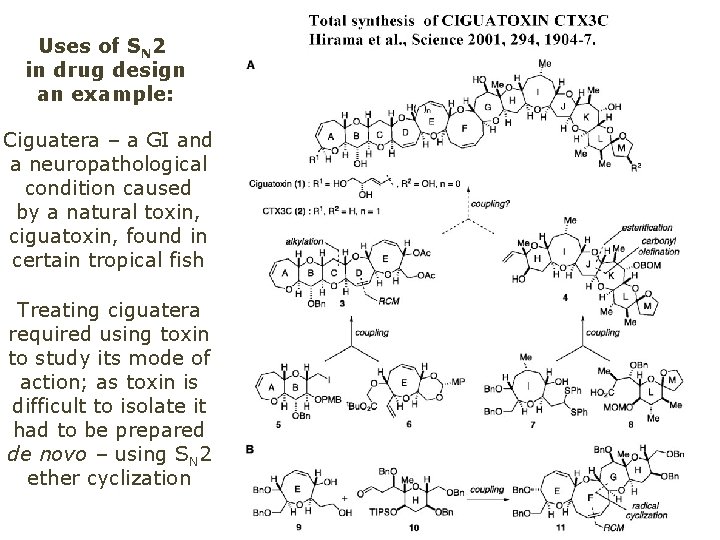
Uses of SN 2 in drug design an example: Ciguatera – a GI and a neuropathological condition caused by a natural toxin, ciguatoxin, found in certain tropical fish Treating ciguatera required using toxin to study its mode of action; as toxin is difficult to isolate it had to be prepared de novo – using SN 2 ether cyclization

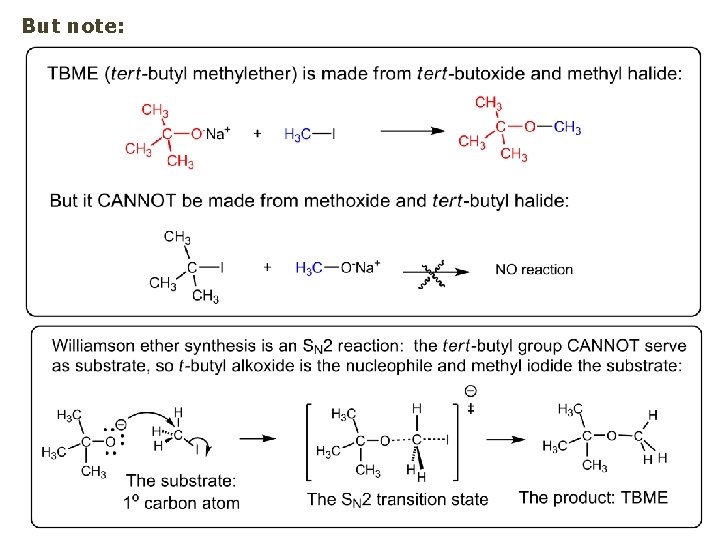
But note:

SN 2 in synthesis – practice Williamson synthesis:

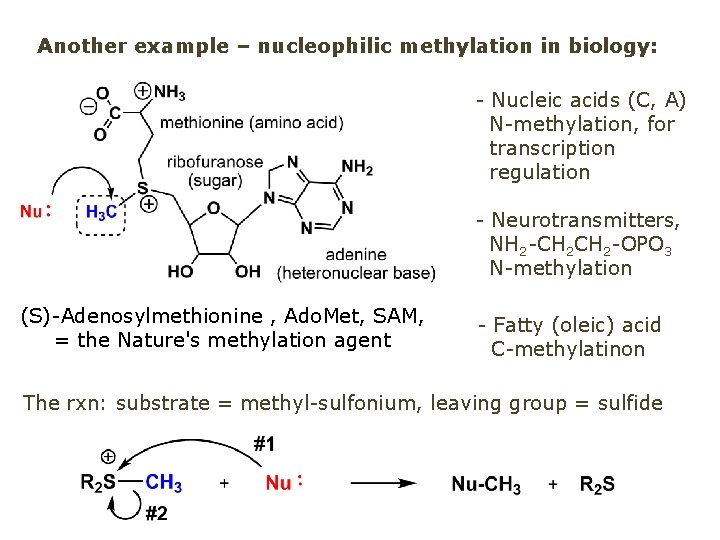
Another example – nucleophilic methylation in biology: - Nucleic acids (C, A) N-methylation, for transcription regulation - Neurotransmitters, NH 2 -CH 2 -OPO 3 N-methylation (S)-Adenosylmethionine , Ado. Met, SAM, = the Nature's methylation agent - Fatty (oleic) acid C-methylatinon The rxn: substrate = methyl-sulfonium, leaving group = sulfide

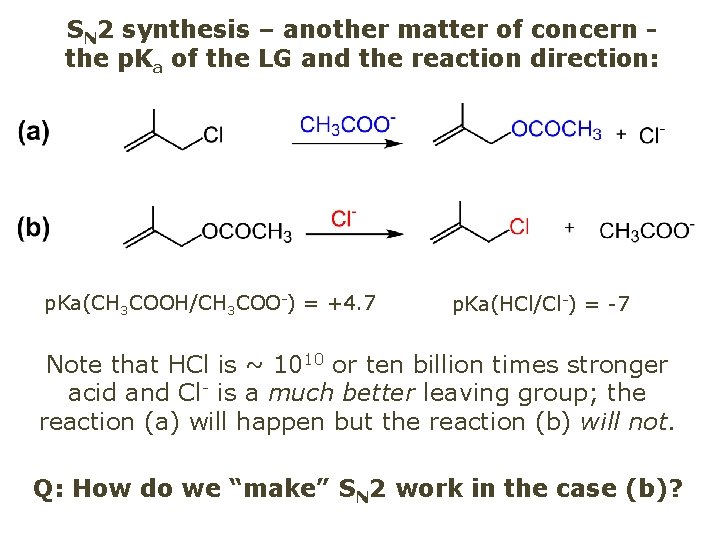
SN 2 synthesis – another matter of concern the p. Ka of the LG and the reaction direction: p. Ka(CH 3 COOH/CH 3 COO-) = +4. 7 p. Ka(HCl/Cl-) = -7 Note that HCl is ~ 1010 or ten billion times stronger acid and Cl- is a much better leaving group; the reaction (a) will happen but the reaction (b) will not. Q: How do we “make” SN 2 work in the case (b)?

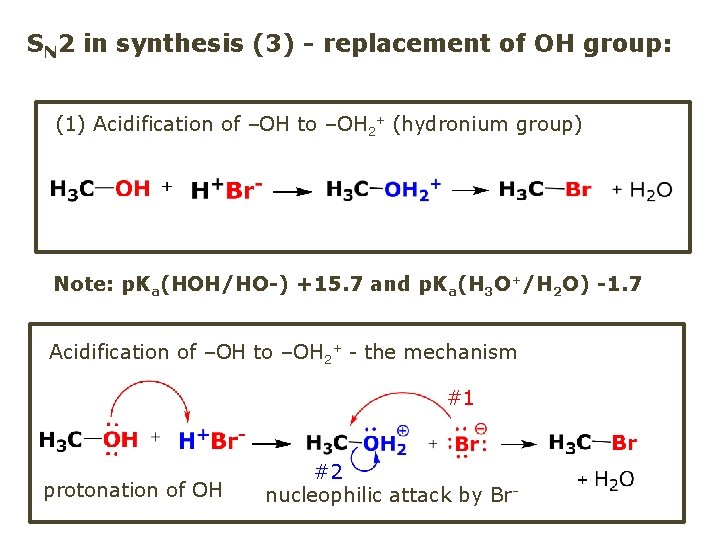
SN 2 in synthesis (3) - replacement of OH group: (1) Acidification of –OH to –OH 2+ (hydronium group) Note: p. Ka(HOH/HO-) +15. 7 and p. Ka(H 3 O+/H 2 O) -1. 7 Acidification of –OH to –OH 2+ - the mechanism #1 protonation of OH #2 nucleophilic attack by Br-

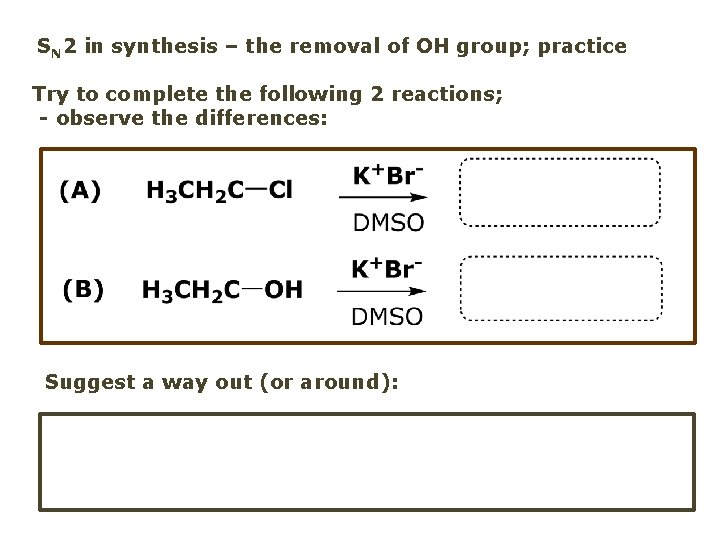
SN 2 in synthesis – the removal of OH group; practice Try to complete the following 2 reactions; - observe the differences: Suggest a way out (or around):

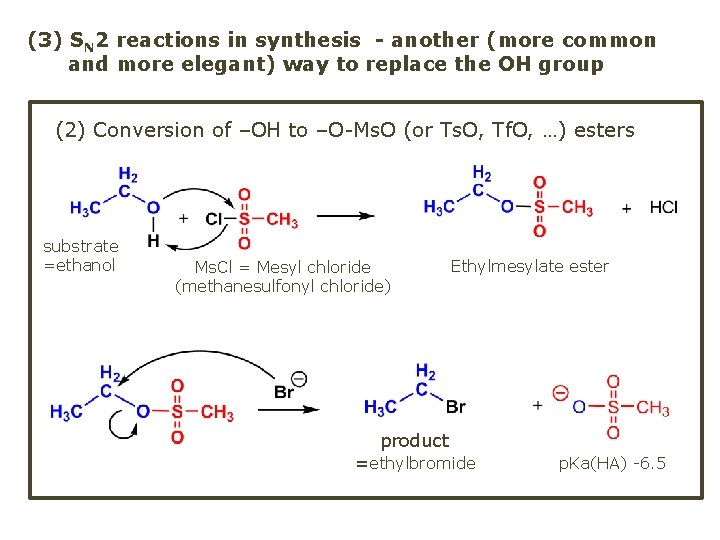
(3) SN 2 reactions in synthesis - another (more common and more elegant) way to replace the OH group (2) Conversion of –OH to –O-Ms. O (or Ts. O, Tf. O, …) esters substrate =ethanol Ms. Cl = Mesyl chloride (methanesulfonyl chloride) Ethylmesylate ester product =ethylbromide p. Ka(HA) -6. 5



![6 13 A new page and a new chemistry The same substrate but [6. 13] A new page – and a new chemistry: The same substrate but](https://slidetodoc.com/presentation_image/31848c1bf7eca5d65eab31e17a86b5ed/image-53.jpg)
[6. 13] A new page – and a new chemistry: The same substrate but a different reaction Substitution, nucleophilic - but a different one: The reaction: (2 R)-bromobutane (2 S)-butanol (2 R)-butanol (Optically inactive racemic mixture)
![6 13 The reaction rate experimental data The reaction CH 33 CBr [6. 13] The reaction rate - experimental data: The reaction: (CH 3)3 C-Br +](https://slidetodoc.com/presentation_image/31848c1bf7eca5d65eab31e17a86b5ed/image-54.jpg)
[6. 13] The reaction rate - experimental data: The reaction: (CH 3)3 C-Br + HOH → (CH 3)3 C-OH + K+Br- The concentration vs. time - exp data: The rate of the reaction changes with the conc. of the substrate, (CH 3)3 CBr, but is independent on the concentration of water, the nucleophile: RR ∝ [HOH]º RR = k [(CH 3)3 CBr]1 [HOH]0 = 1 st order substitution = SN 1

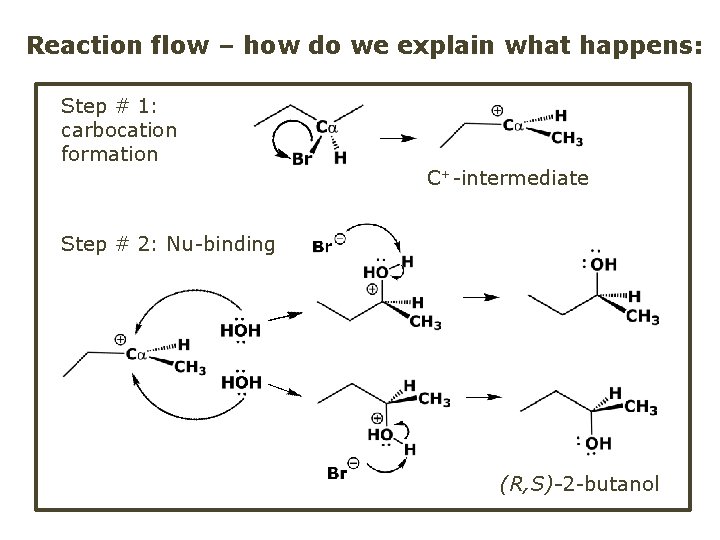
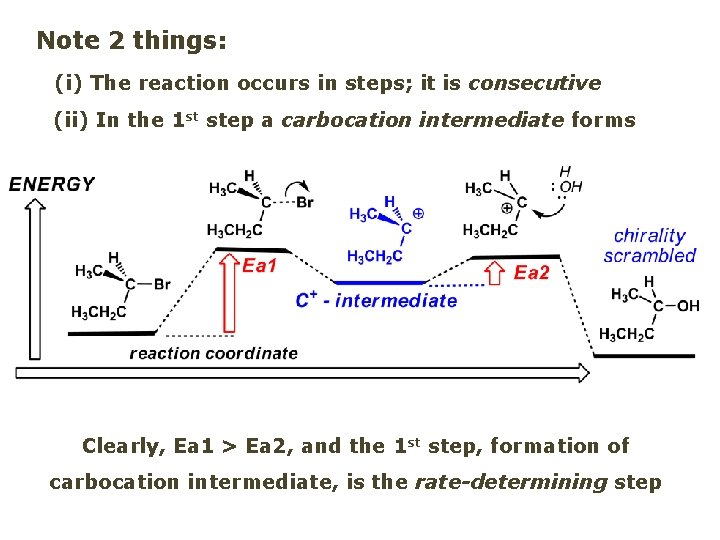
Reaction flow – how do we explain what happens: Step # 1: carbocation formation C+-intermediate Step # 2: Nu-binding (R, S)-2 -butanol

Note 2 things: (i) The reaction occurs in steps; it is consecutive (ii) In the 1 st step a carbocation intermediate forms Clearly, Ea 1 > Ea 2, and the 1 st step, formation of carbocation intermediate, is the rate-determining step

![The more expensive in k J the cation the higher the Ea 1 and The more “expensive” [in k. J] the cation, the higher the Ea 1 and](https://slidetodoc.com/presentation_image/31848c1bf7eca5d65eab31e17a86b5ed/image-58.jpg)
The more “expensive” [in k. J] the cation, the higher the Ea 1 and the more difficult the reaction ΔE [k. Jmol-1] (tropilium-C+) Me-cation 473 ! does not form ! 1º-cation 301 2º-cation 192 3º-cation 125 least unstable

SN 1 – the substrate effects; practice:

SN 1 & SN 2 – the leaving group properties & ranking: best LG good LG fair LG never a LG

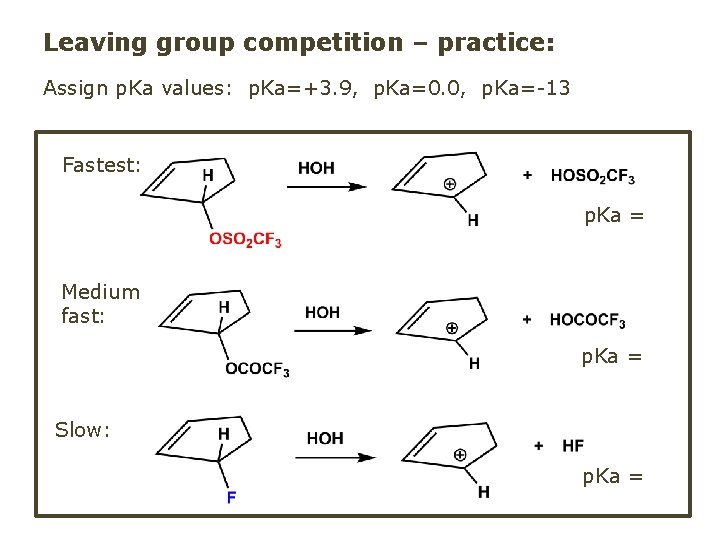
Leaving group competition – practice: Assign p. Ka values: p. Ka=+3. 9, p. Ka=0. 0, p. Ka=-13 Fastest: p. Ka = Medium fast: p. Ka = Slow: p. Ka =

Review of the SN 1 reaction determinants: - The substrate – Csp 3 crowded, a good C+ - The nucleophile - It does not matter - The Leaving Group – same as in SN 2 (p. Ka!) - The solvent in SN 1 reactions – Protic solvents

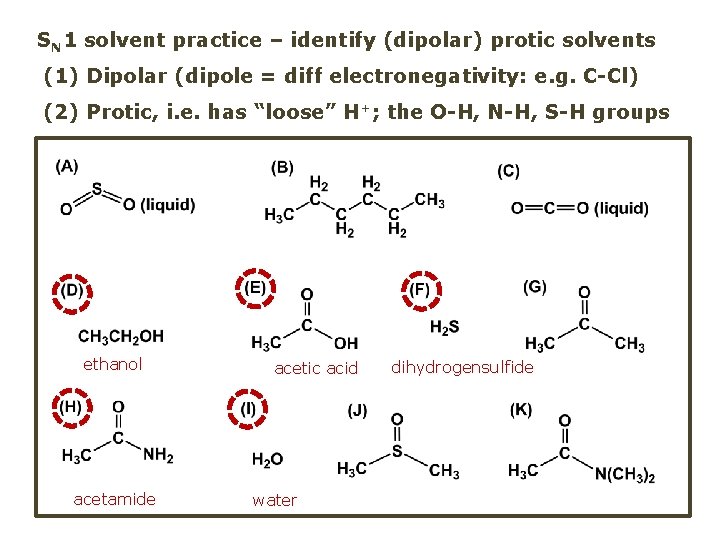
SN 1 solvent practice – identify (dipolar) protic solvents (1) Dipolar (dipole = diff electronegativity: e. g. C-Cl) (2) Protic, i. e. has “loose” H+; the O-H, N-H, S-H groups ethanol acetamide acetic acid water dihydrogensulfide

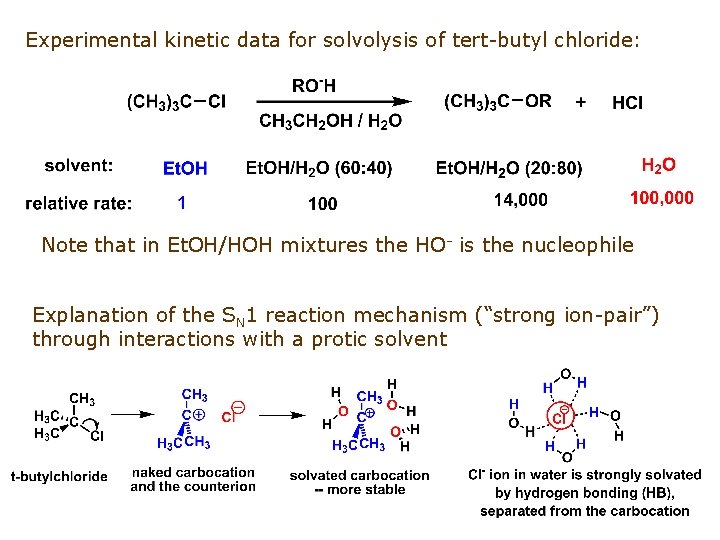
Experimental kinetic data for solvolysis of tert-butyl chloride: Note that in Et. OH/HOH mixtures the HO- is the nucleophile Explanation of the SN 1 reaction mechanism (“strong ion-pair”) through interactions with a protic solvent

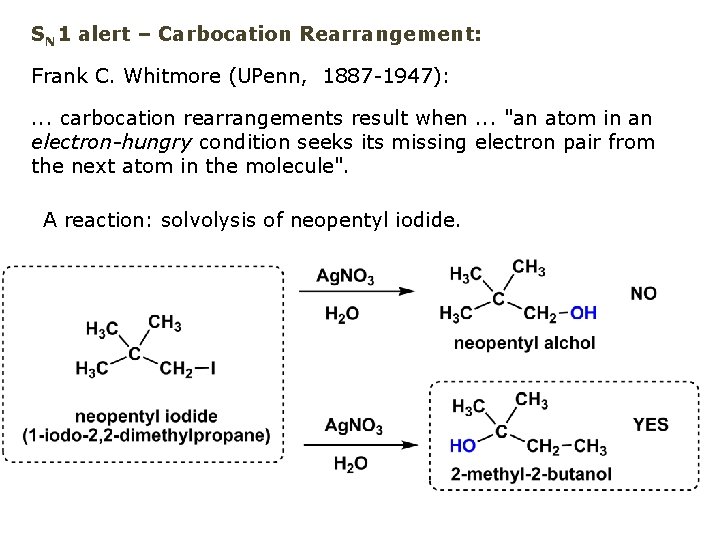
SN 1 alert – Carbocation Rearrangement: Frank C. Whitmore (UPenn, 1887 -1947): . . . carbocation rearrangements result when. . . "an atom in an electron-hungry condition seeks its missing electron pair from the next atom in the molecule". A reaction: solvolysis of neopentyl iodide.

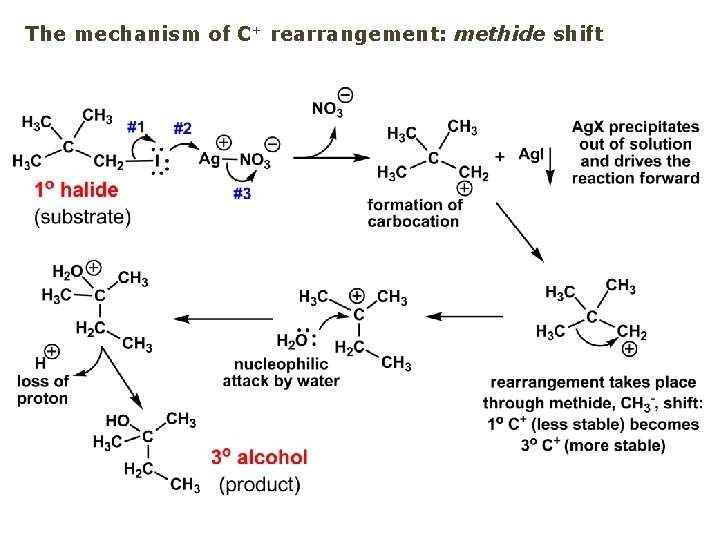
The mechanism of C+ rearrangement: methide shift

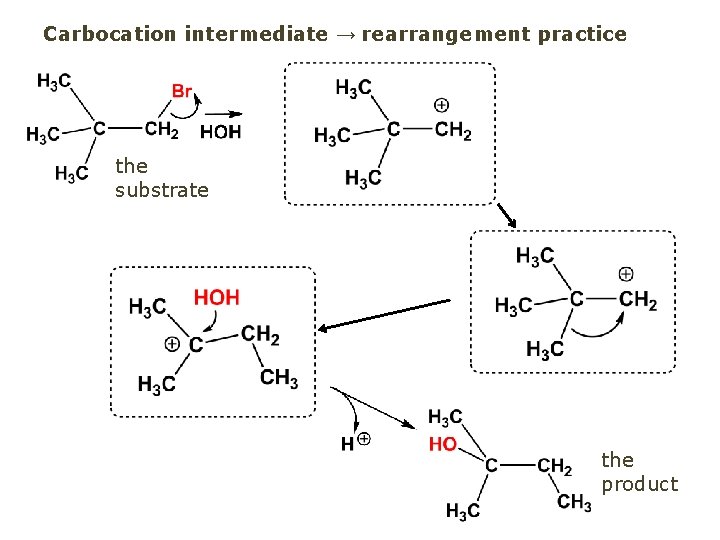
Carbocation intermediate → rearrangement practice the substrate the product

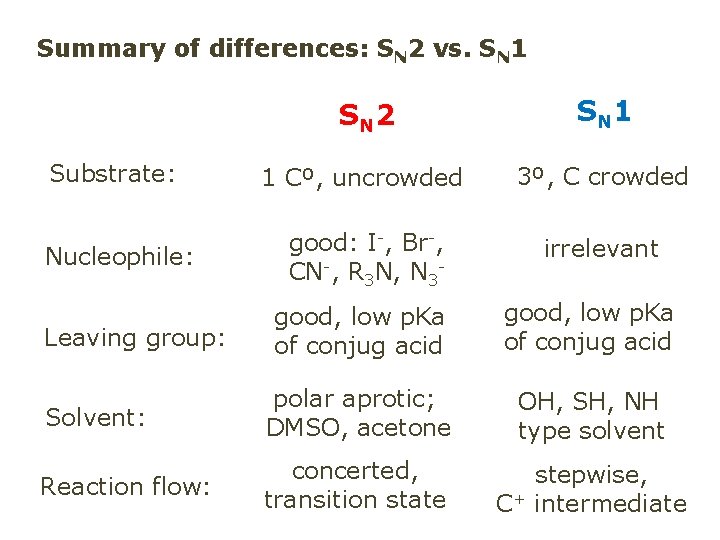
Summary of differences: SN 2 vs. SN 1 SN 2 SN 1 Substrate: 1 Cº, uncrowded 3º, C crowded Nucleophile: good: I-, Br-, CN-, R 3 N, N 3 - irrelevant good, low p. Ka of conjug acid Solvent: polar aprotic; DMSO, acetone OH, SH, NH type solvent Reaction flow: concerted, transition state stepwise, C+ intermediate Leaving group:

SN 2 vs. SN 1 “game” – practice field:

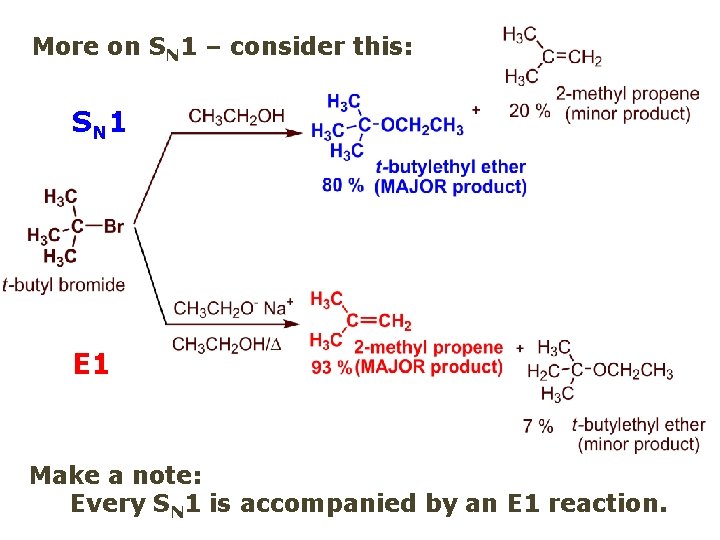
More on SN 1 – consider this: SN 1 E 1 Make a note: Every SN 1 is accompanied by an E 1 reaction.

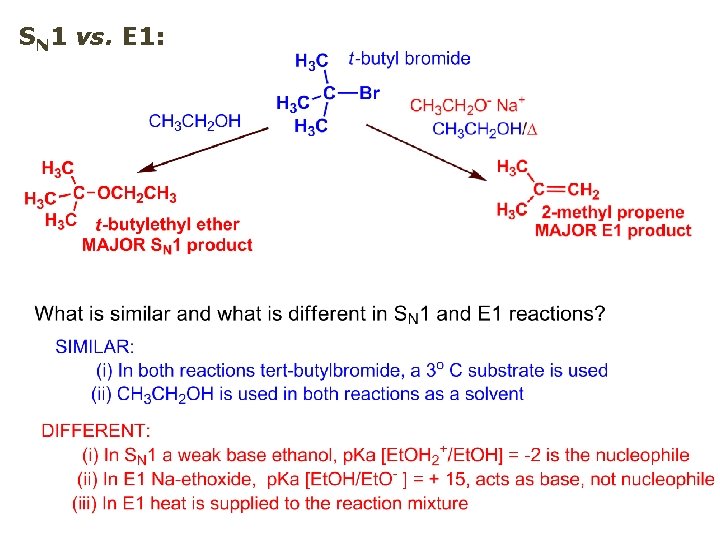
SN 1 vs. E 1:

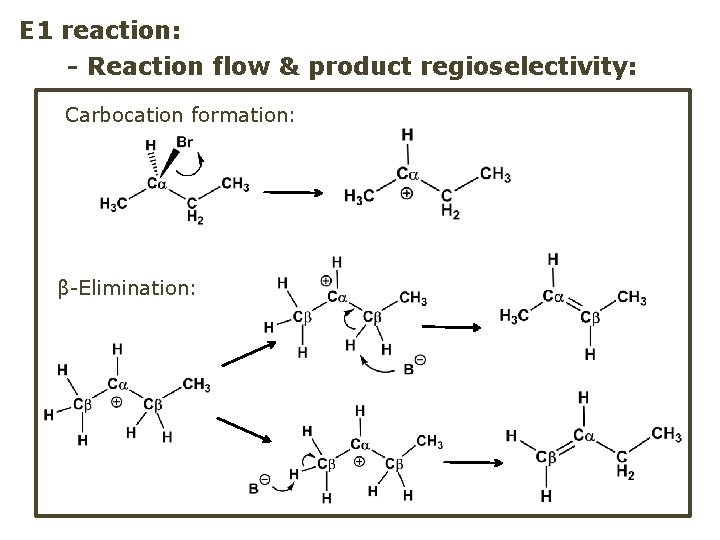
E 1 reaction: - Reaction flow & product regioselectivity: Carbocation formation: β-Elimination:

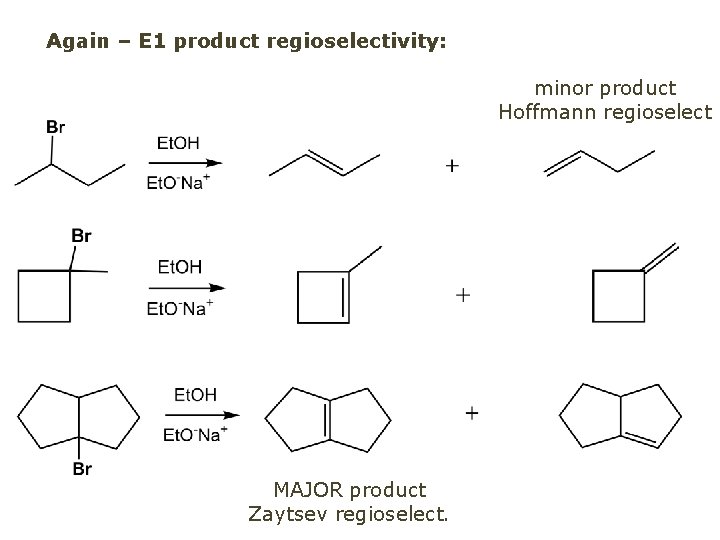
Again – E 1 product regioselectivity: minor product Hoffmann regioselect MAJOR product Zaytsev regioselect.

What a carbocation can do? (4 things) (1) Go forward & form a racemic mixture of products (2) Go backward & form a racemic mixture of the reactant (3) Undergo β-elimination and from an alkene (4) Rearrange and do (1), (2), (3)

E 1 + carbocation rearrangement – practice:


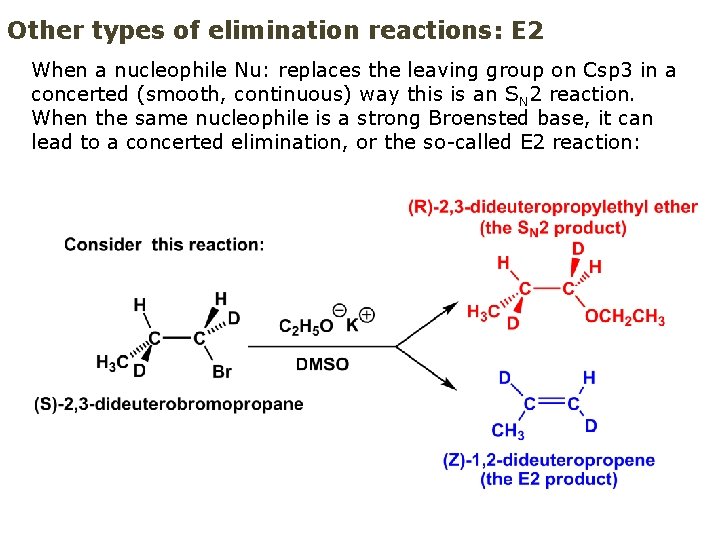
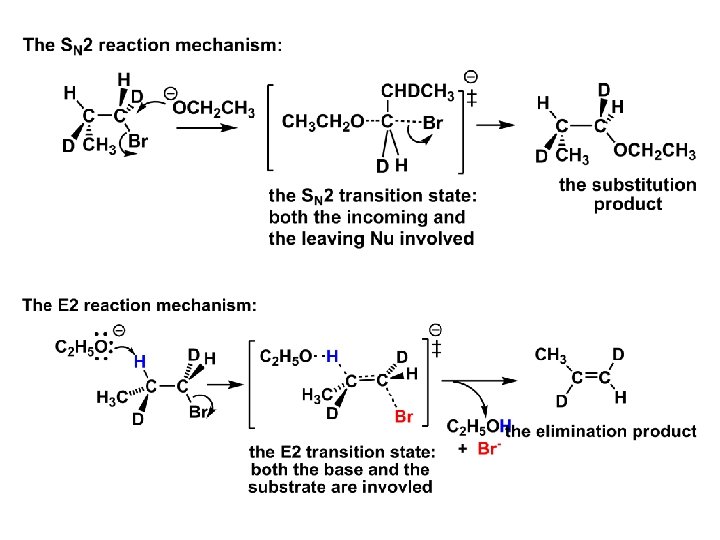
Other types of elimination reactions: E 2 When a nucleophile Nu: replaces the leaving group on Csp 3 in a concerted (smooth, continuous) way this is an S N 2 reaction. When the same nucleophile is a strong Broensted base, it can lead to a concerted elimination, or the so-called E 2 reaction:


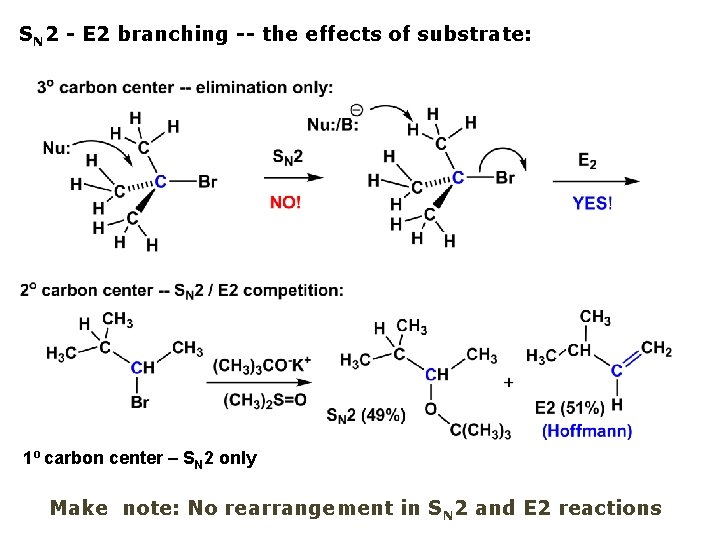
SN 2 - E 2 branching -- the effects of substrate: 1º carbon center – SN 2 only Make note: No rearrangement in SN 2 and E 2 reactions

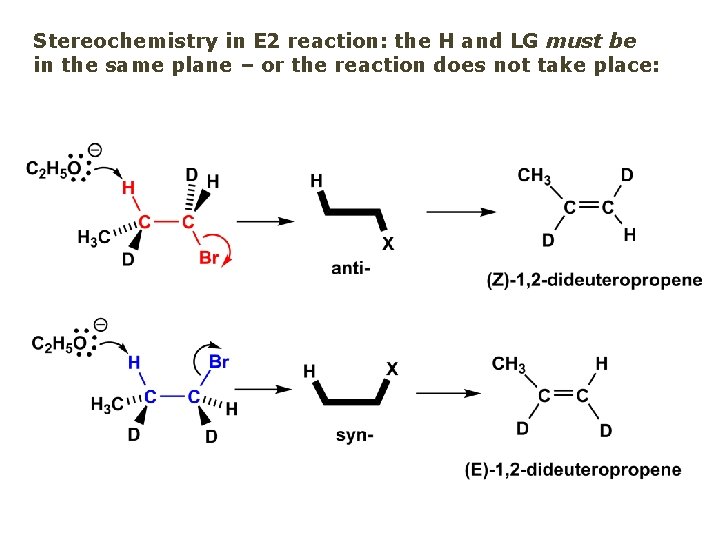
Stereochemistry in E 2 reaction: the H and LG must be in the same plane – or the reaction does not take place:

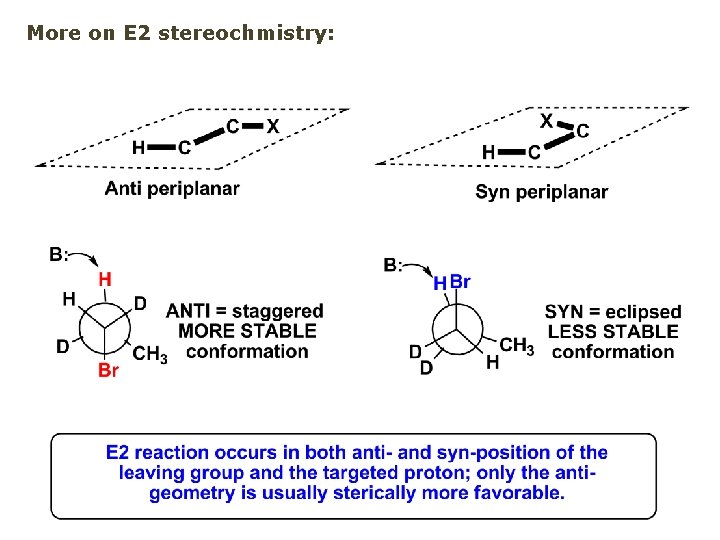
More on E 2 stereochmistry:

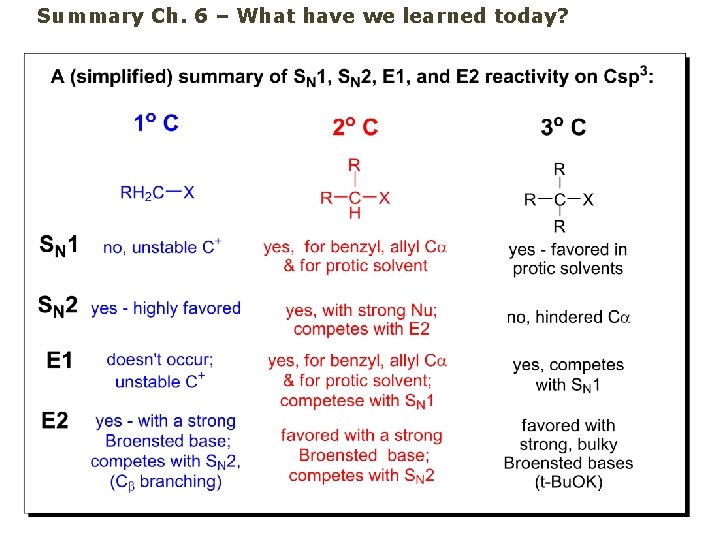
Summary Ch. 6 – What have we learned today?