POLARIMETRY T 2 Chapter 5 Organic Chemistry 7








![• [α] depends on the temperature and the wavelength of the light used • [α] depends on the temperature and the wavelength of the light used](https://slidetodoc.com/presentation_image_h/c959f1c323e5357c1535fb678a4f9a70/image-25.jpg)










- Slides: 38

POLARIMETRY • T 2 • Chapter # 5, “Organic Chemistry” (7 th ed. ) by Solomons and Fryhle

Introduction: • It’s a type of qualitative and quantitative technique, used mostly for optically active compounds • the tendency of the molecules to rotate the plane of plane polarized light (clockwise or anticlockwise) and the extent of rotation is measured • these properties are unique for a molecule, thus polarimetry can be used to identify and estimate the compounds

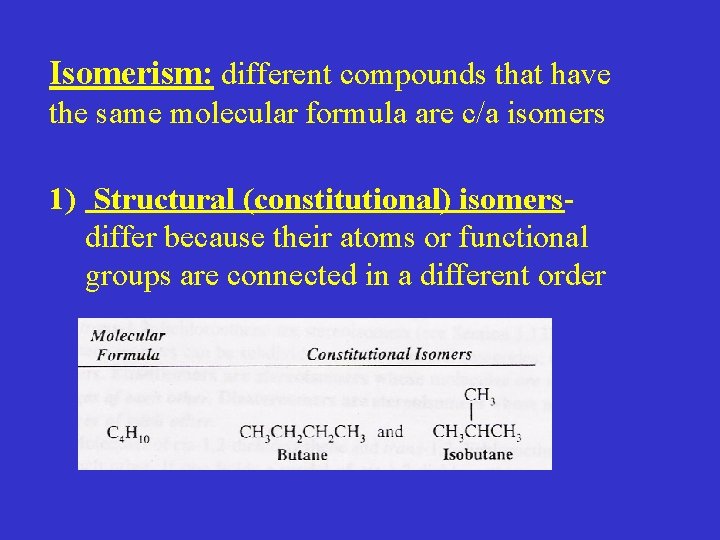
Isomerism: different compounds that have the same molecular formula are c/a isomers 1) Structural (constitutional) isomersdiffer because their atoms or functional groups are connected in a different order

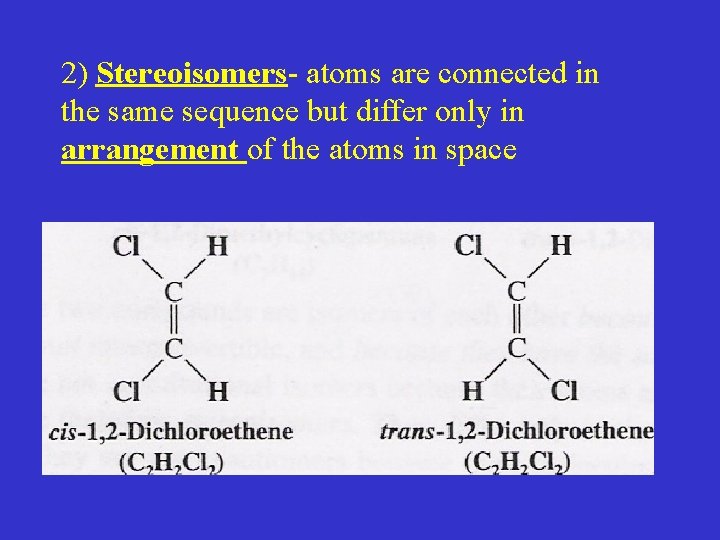
2) Stereoisomers- atoms are connected in the same sequence but differ only in arrangement of the atoms in space

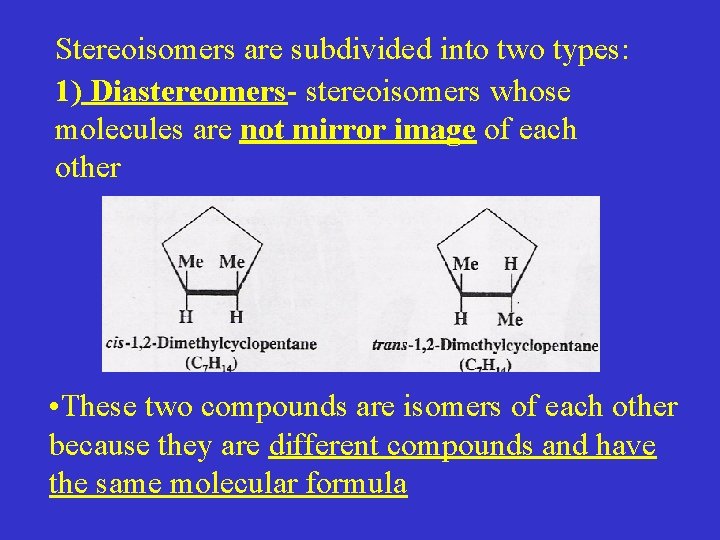
Stereoisomers are subdivided into two types: 1) Diastereomers- stereoisomers whose molecules are not mirror image of each other • These two compounds are isomers of each other because they are different compounds and have the same molecular formula

• Their atoms are joined in the same sequence, therefore not a constitutional (structural) isomer • They differ only in the arrangement of their atoms in the space, therefore stereoisomers • They are not mirror image of each other, therefore not enantiomers but diastereomers Another example of diastereomers


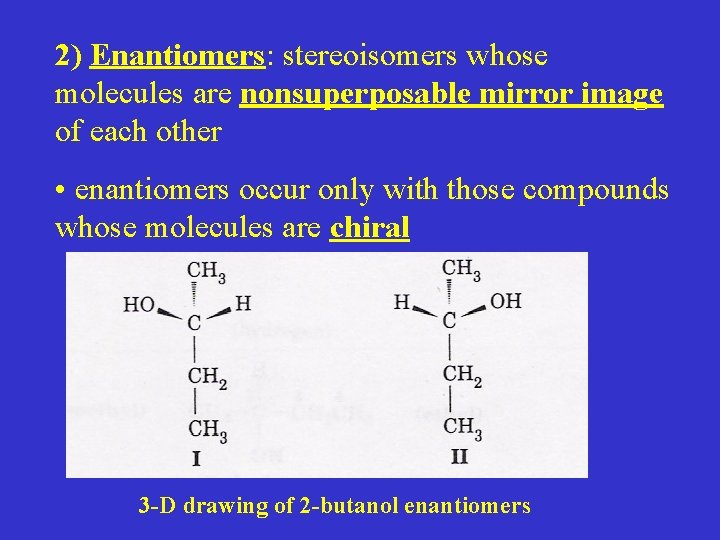
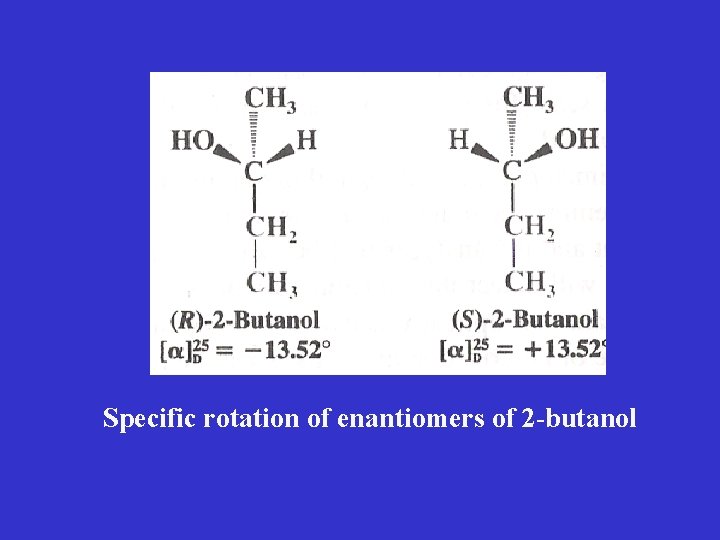
2) Enantiomers: stereoisomers whose molecules are nonsuperposable mirror image of each other • enantiomers occur only with those compounds whose molecules are chiral 3 -D drawing of 2 -butanol enantiomers

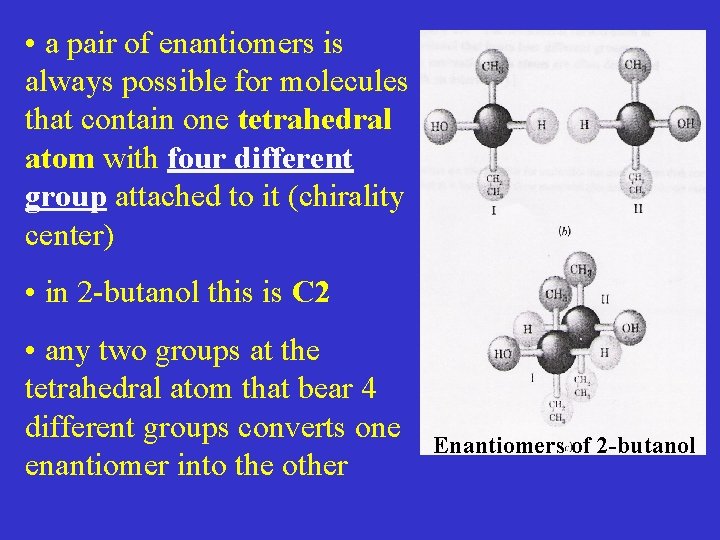
• a pair of enantiomers is always possible for molecules that contain one tetrahedral atom with four different group attached to it (chirality center) • in 2 -butanol this is C 2 • any two groups at the tetrahedral atom that bear 4 different groups converts one enantiomer into the other Enantiomers of 2 -butanol


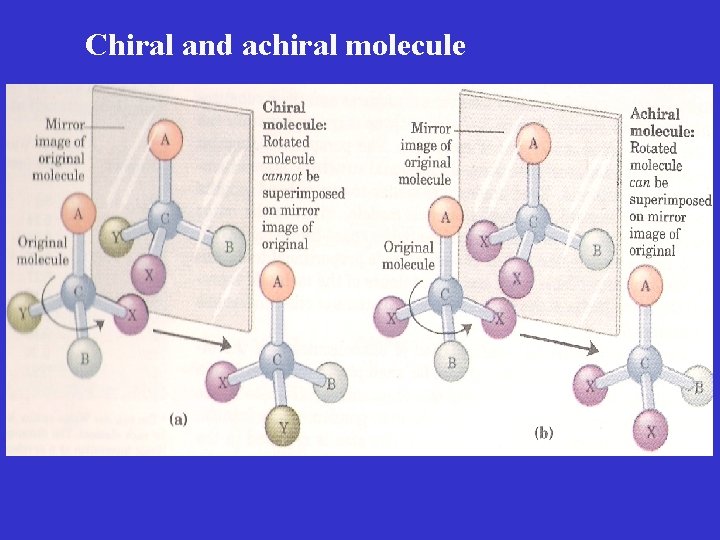
Achiral molecule When there are only three dissimilar groups around the carbon atom (ie. the same group occur twice), the molecule is • symmetric • superimposable on its mirror image • achiral

Chiral and achiral molecule

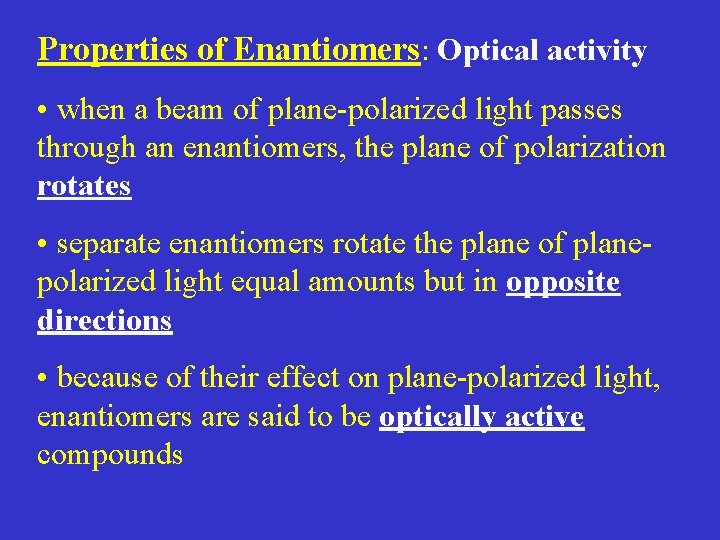
Properties of Enantiomers: Optical activity • when a beam of plane-polarized light passes through an enantiomers, the plane of polarization rotates • separate enantiomers rotate the plane of planepolarized light equal amounts but in opposite directions • because of their effect on plane-polarized light, enantiomers are said to be optically active compounds

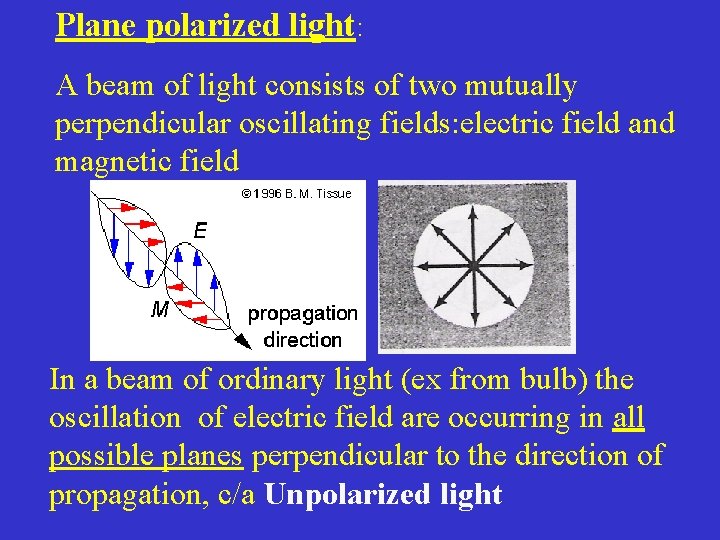
Plane polarized light: A beam of light consists of two mutually perpendicular oscillating fields: electric field and magnetic field In a beam of ordinary light (ex from bulb) the oscillation of electric field are occurring in all possible planes perpendicular to the direction of propagation, c/a Unpolarized light

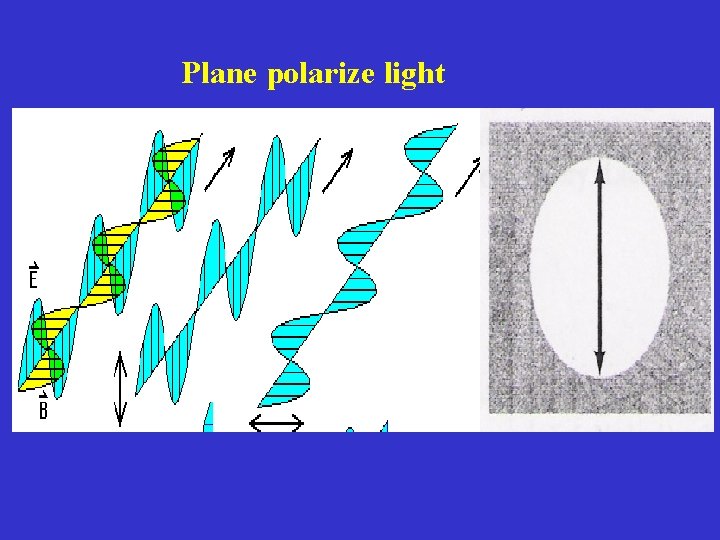
When an unpolarized light is passed through a polarizer, the polarizer interacts with the electrical field • The resultant light which emerge from the polarizer has their electric field vector oscillating in only one direction • Such light is c/a plane-polarized light • Plane polarized light can be polarized in different directions

Plane polarize light

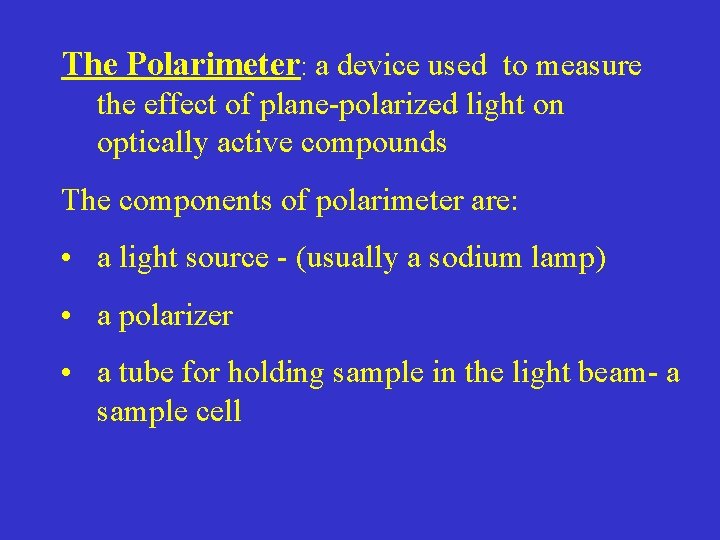
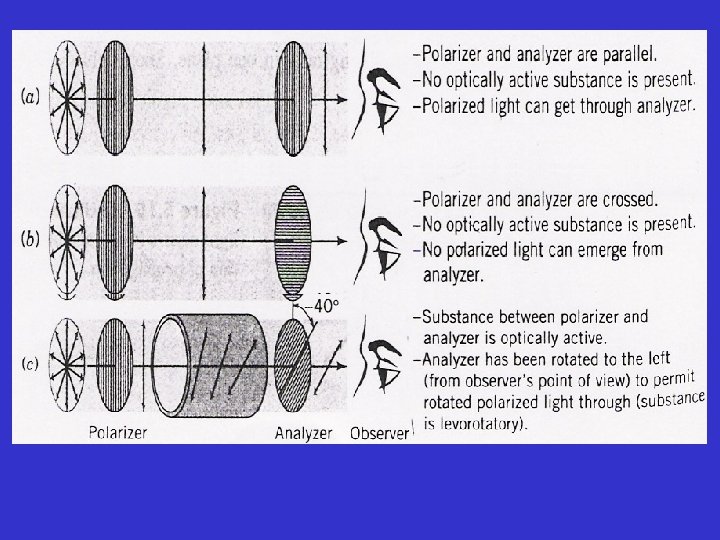
The Polarimeter: a device used to measure the effect of plane-polarized light on optically active compounds The components of polarimeter are: • a light source - (usually a sodium lamp) • a polarizer • a tube for holding sample in the light beam- a sample cell

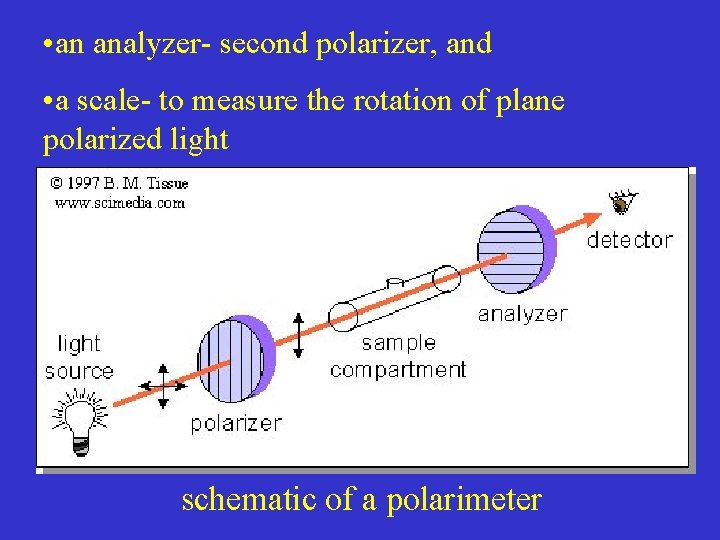
• an analyzer- second polarizer, and • a scale- to measure the rotation of plane polarized light schematic of a polarimeter

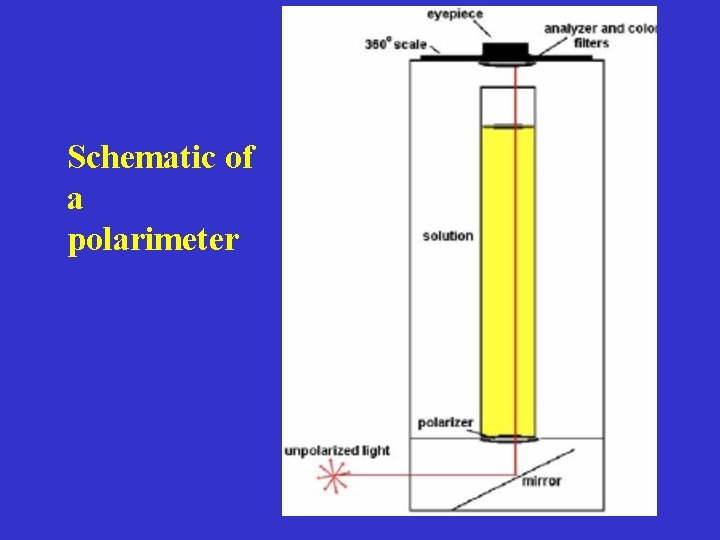
Schematic of a polarimeter


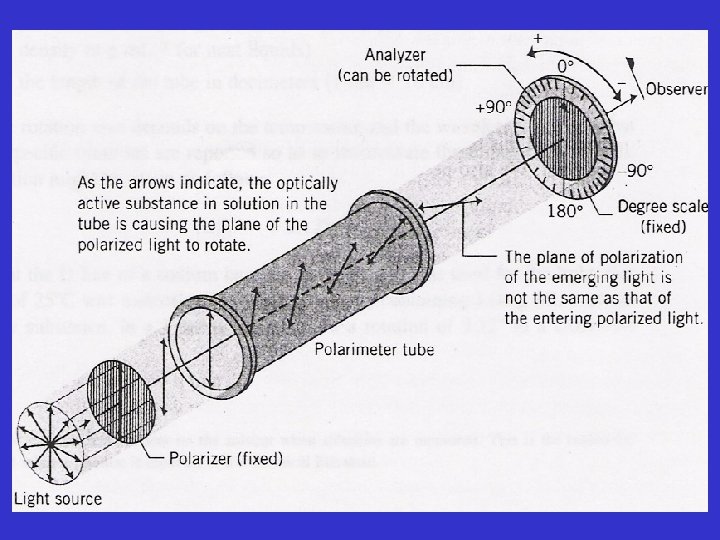
• if no or optically inactive sample is present in the tube and the instrument is reading zero (0 o), the axes of plane polarized light and the analyzer is exactly parallel • the observer will detect maximum amount (100 % transmittance) of light passing through. • if the sample is optically active the plane of PPL will be rotated as it pass through the tube

• in order to detect the maximum brightness of the light (ie. 100% transmittance) observer will have to rotate the axis of the analyzer in either clockwise or counterclockwise direction • if the analyzer is rotated in a clockwise direction, the rotation (α in degree) is said to be positive (+), and such substance are c/a dextrorotatory • if the rotation is counterclockwise, the α is –ve, and such substances are c/a levorotatory


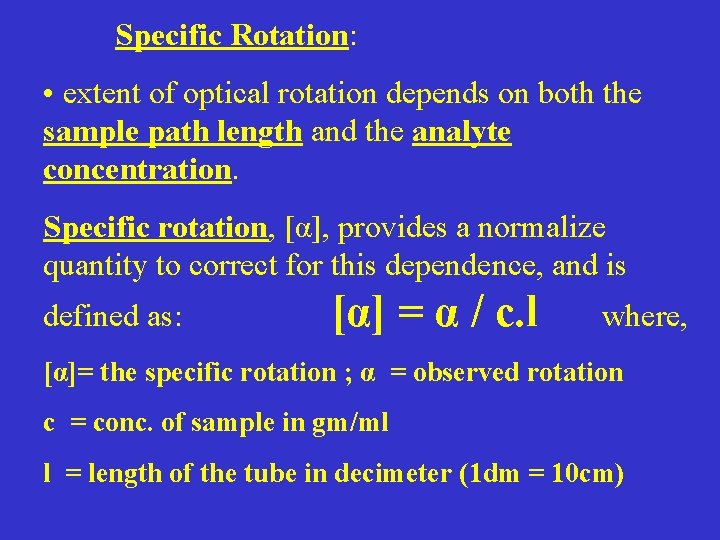
Specific Rotation: • extent of optical rotation depends on both the sample path length and the analyte concentration. Specific rotation, [α], provides a normalize quantity to correct for this dependence, and is defined as: [α] = α / c. l where, [α]= the specific rotation ; α = observed rotation c = conc. of sample in gm/ml l = length of the tube in decimeter (1 dm = 10 cm)
![α depends on the temperature and the wavelength of the light used • [α] depends on the temperature and the wavelength of the light used](https://slidetodoc.com/presentation_image_h/c959f1c323e5357c1535fb678a4f9a70/image-25.jpg)
• [α] depends on the temperature and the wavelength of the light used • these quantities are also incorporated while reporting [α]D = 25 o +3. 12 • means D line of a sodium lamp (λ=589. 6 nm) is used for the light at a temperature of 25 o. C, and that a sample containing 1. 00 g/ml of the optically active substance, in a 1 -dm tube, produces a rotation of 3. 12 o in a clockwise direction

Specific rotation of enantiomers of 2 -butanol

Racemic Mixture: • an equimolar mixture of two enantiomers is c/a a racemic mixture • a racemic mixture is optically inactive and shows no rotation of plane-polarized light • it is often designated as being (±) ex (±)-2 -Butanol

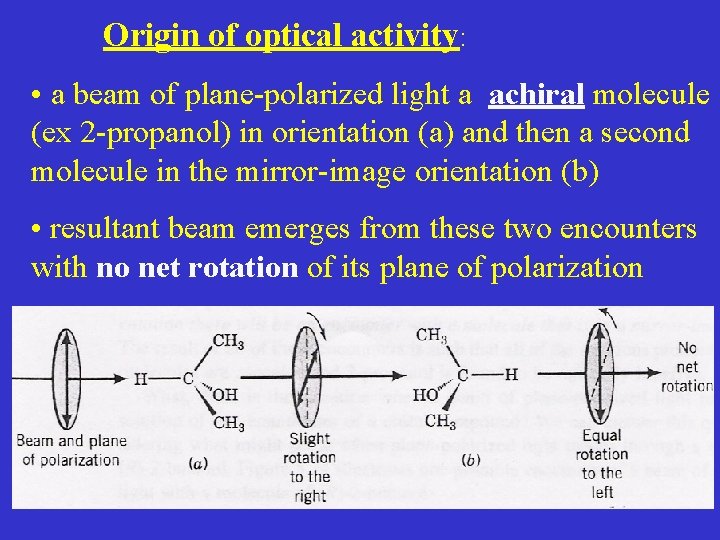
Origin of optical activity: • a beam of plane-polarized light a achiral molecule (ex 2 -propanol) in orientation (a) and then a second molecule in the mirror-image orientation (b) • resultant beam emerges from these two encounters with no net rotation of its plane of polarization

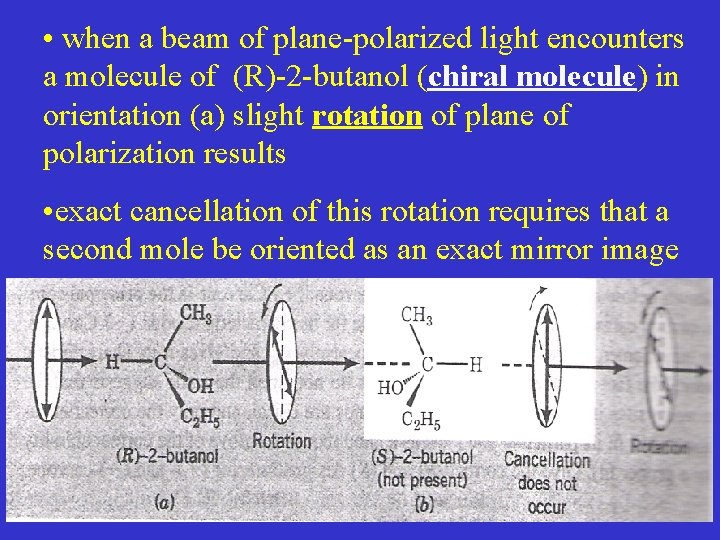
• when a beam of plane-polarized light encounters a molecule of (R)-2 -butanol (chiral molecule) in orientation (a) slight rotation of plane of polarization results • exact cancellation of this rotation requires that a second mole be oriented as an exact mirror image

• this cancellation does not occur because the only molecule that could ever be oriented as an exact mirror image at the first encounter is a molecule of (s)-2 -butanol, which is not present • as a result a net rotation of the plane of polarization occurs

Biological importance of chirality


Application • polarimetric method is a simple and accurate means for determination of structure in micro analysis of expensive and non-duplicable samples. • it is employed in quality control, process control and research in the pharmaceutical, chemical, essential oil, flavor and food industries. • it is so well established that the United States Pharmacopoeia and the Food & Drug Administration include polarimetric specifications for numerous substances.

Research Applications Research applications for polarimetry are found in industry, research institutes and universities as a means of: • isolating and identifying unknowns, crystallized from various solvents or separated by HPLC. • evaluating and characterizing optically active compounds by measuring their specific rotation and comparing this value with theoretical values found in literature.

• investigating kinetic reactions by measuring optical rotation as a function of time. • monitoring changes in concentration of an optically active component in a reaction mixture, as in enzymatic cleavage. • analyzing molecular structure by plotting optical rotatory dispersion (ORD) curves over a wide range of wavelengths. • distinguishing between optical isomers.

Pharmaceutical Applications Determines product purity by measuring specific rotation and optical rotation of: Amino acids, Amino sugars, Analgesics, Antibiotics Cocaine, Dextrose Diuretics Serums Steroids Tranquilizers Vitamins Utilizes polarimetry for incoming raw materials inspection of: Camphors, Citric acid, Glyceric acid Gums Lavender oil, Lemon oil Orange oil Spearmint oil

Ensures product quality by measuring the concentration and purity of the following compounds in sugar based foods, cereals and syrups: Carbohydrates Fructose Glucose Lactose Levulose Maltose Raffinose Sucrose Various Starches Natural monosaccharides Analyzes optical rotation as a means of identifying and characterizing: Natural polymers, Biopolymers, Synthetic polymers

Summary: • enantiomers • polarimeter • origin of optical activity • Biological importance of chirality • Application Next time Gas Chromatography!!!!
 Polarimetry organic chemistry
Polarimetry organic chemistry Polarimetry definition in chemistry
Polarimetry definition in chemistry Biots law polarimetry
Biots law polarimetry Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Organic chemistry chapter 1
Organic chemistry chapter 1 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Organic chemistry chapter 9
Organic chemistry chapter 9 Chapter 7 chemistry review
Chapter 7 chemistry review Chapter 3 organic chemistry
Chapter 3 organic chemistry Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Halohydrin
Halohydrin Numbering carbon chains
Numbering carbon chains Chemistry of soap making
Chemistry of soap making Ester organic chemistry
Ester organic chemistry What is organic chemistry
What is organic chemistry Transition state energy diagram
Transition state energy diagram Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition What is the leveling effect organic chemistry
What is the leveling effect organic chemistry Priority of functional groups in iupac nomenclature
Priority of functional groups in iupac nomenclature Organic chemistry lab report format
Organic chemistry lab report format Britannica.com
Britannica.com Grade 10 organic chemistry
Grade 10 organic chemistry Organic chemistry
Organic chemistry Kiliani fischer synthesis
Kiliani fischer synthesis Eth meth prop but pent hex
Eth meth prop but pent hex Alkane cracking
Alkane cracking Eth meth prop but pent
Eth meth prop but pent Organic chemistry myanmar
Organic chemistry myanmar Hhcchh
Hhcchh M+1 peak
M+1 peak Hono organic chemistry
Hono organic chemistry Father of organic chemistry
Father of organic chemistry Topic 11 organic chemistry
Topic 11 organic chemistry