Chemistry The Molecular Nature of Matter and Change








- Slides: 78

Chemistry The Molecular Nature of Matter and Change Eighth Edition Martin S. Silberberg and Patricia G. Amateis ©Mc. Graw-Hill Education. All rights reserved. Authorized only for instructor use in the classroom. No reproduction or further distribution permitted without the prior written consent of Mc. Graw-Hill Education.

Gases and the Kinetic Molecular Theory • 5. 1 An Overview of the Physical States of Matter • 5. 2 Gas Pressure and Its Measurement • 5. 3 The Gas Laws and Their Experimental Foundations • 5. 4 Rearrangements of the Ideal Gas Law • 5. 5 The Kinetic-Molecular Theory: A Model for Gas Behavior • 5. 6 Real Gases: Deviations from Ideal Behavior ©Mc. Graw-Hill Education.

The Three States of Matter Figure 5. 1 ©Mc. Graw-Hill Education. © Mc. Graw-Hill Education/Stephen Frisch, photographer

An Overview of the Physical States of Matter • Distinguishing gases from liquids and solids. • Gas volume changes significantly with pressure. – Solid and liquid volumes are not greatly affected by pressure. • Gas volume changes significantly with temperature. – Gases expand when heated and shrink when cooled. – The volume change is 50 to 100 times greater for gases than for liquids and solids. • Gases flow very freely. • Gases have relatively low densities. • Gases form a solution in any proportions. – Gases are freely miscible with each other. ©Mc. Graw-Hill Education.

Gas Pressure and its Measurement • ©Mc. Graw-Hill Education.

Effect of Atmospheric Pressure on a Familiar Object Figure 5. 2 ©Mc. Graw-Hill Education. © Mc. Graw-Hill Education/Charles Winters/Timeframe Photography, Inc.

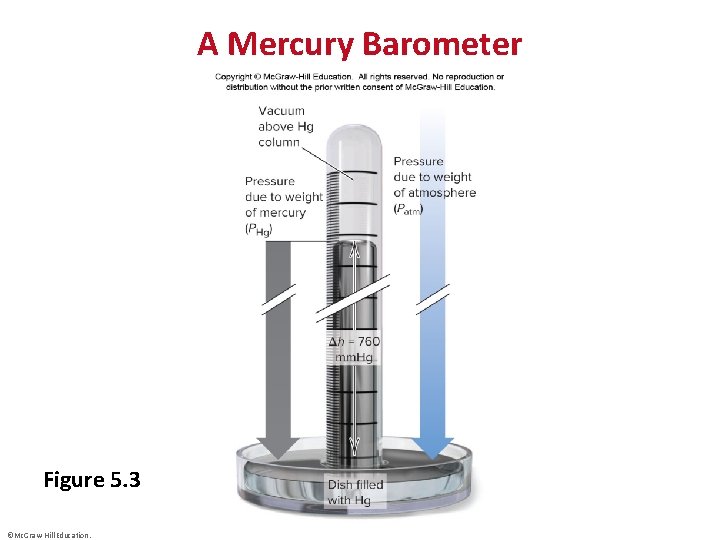
A Mercury Barometer Figure 5. 3 ©Mc. Graw-Hill Education.

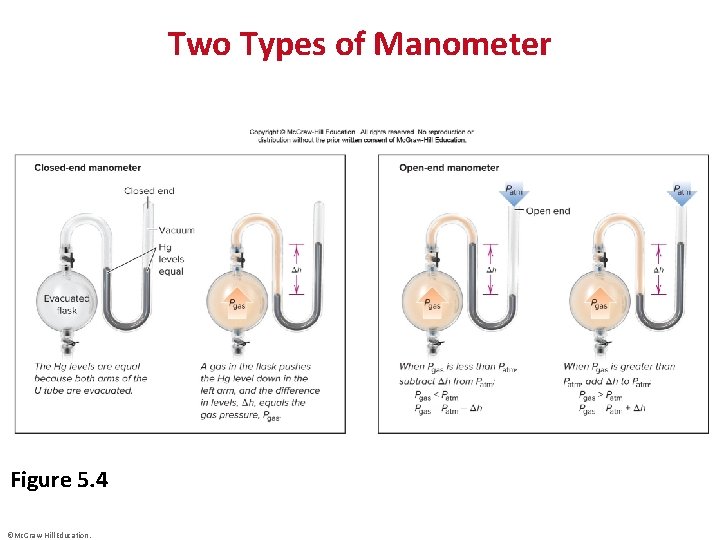
Two Types of Manometer Figure 5. 4 ©Mc. Graw-Hill Education.

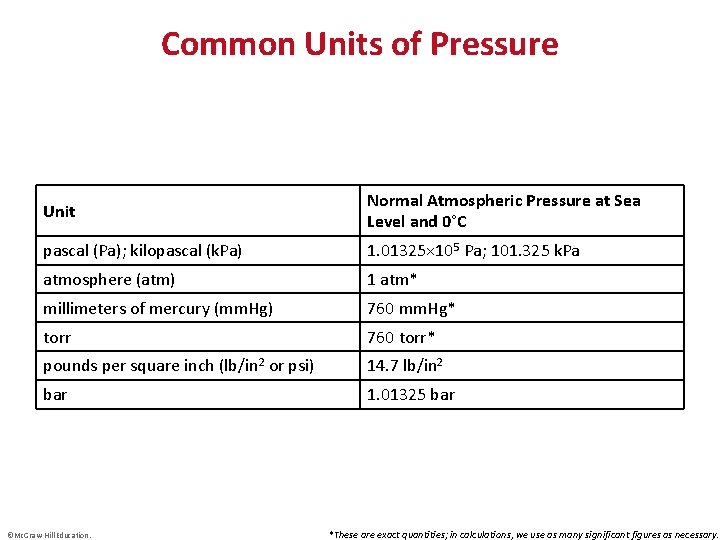
Common Units of Pressure Unit Normal Atmospheric Pressure at Sea Level and 0°C pascal (Pa); kilopascal (k. Pa) 1. 01325× 105 Pa; 101. 325 k. Pa atmosphere (atm) 1 atm* millimeters of mercury (mm. Hg) 760 mm. Hg* torr 760 torr* pounds per square inch (lb/in 2 or psi) 14. 7 lb/in 2 bar 1. 01325 bar ©Mc. Graw-Hill Education. *These are exact quantities; in calculations, we use as many significant figures as necessary.

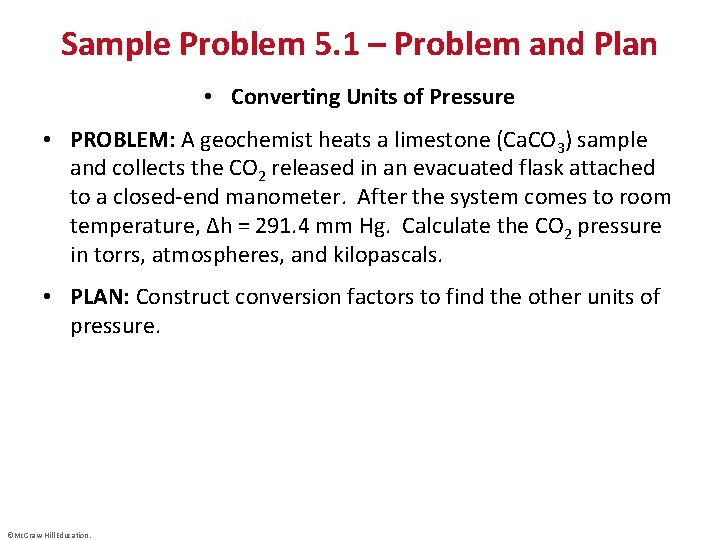
Sample Problem 5. 1 – Problem and Plan • Converting Units of Pressure • PROBLEM: A geochemist heats a limestone (Ca. CO 3) sample and collects the CO 2 released in an evacuated flask attached to a closed-end manometer. After the system comes to room temperature, Δh = 291. 4 mm Hg. Calculate the CO 2 pressure in torrs, atmospheres, and kilopascals. • PLAN: Construct conversion factors to find the other units of pressure. ©Mc. Graw-Hill Education.

Sample Problem 5. 1 –Solution • ©Mc. Graw-Hill Education.

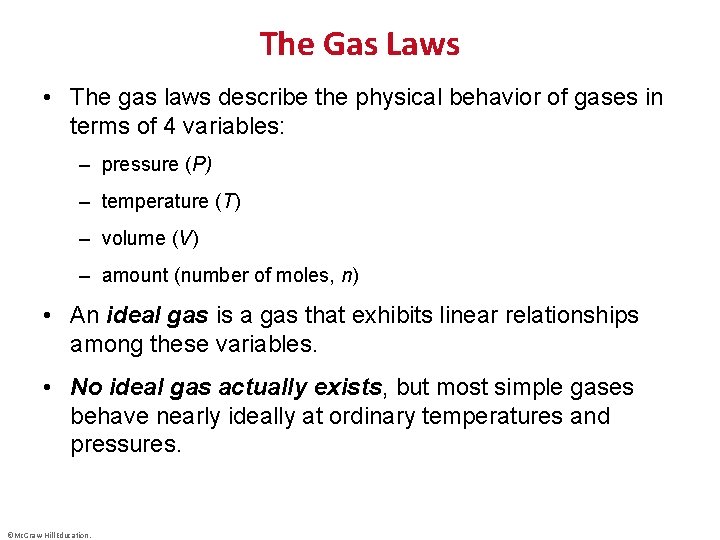
The Gas Laws • The gas laws describe the physical behavior of gases in terms of 4 variables: – pressure (P) – temperature (T) – volume (V) – amount (number of moles, n) • An ideal gas is a gas that exhibits linear relationships among these variables. • No ideal gas actually exists, but most simple gases behave nearly ideally at ordinary temperatures and pressures. ©Mc. Graw-Hill Education.

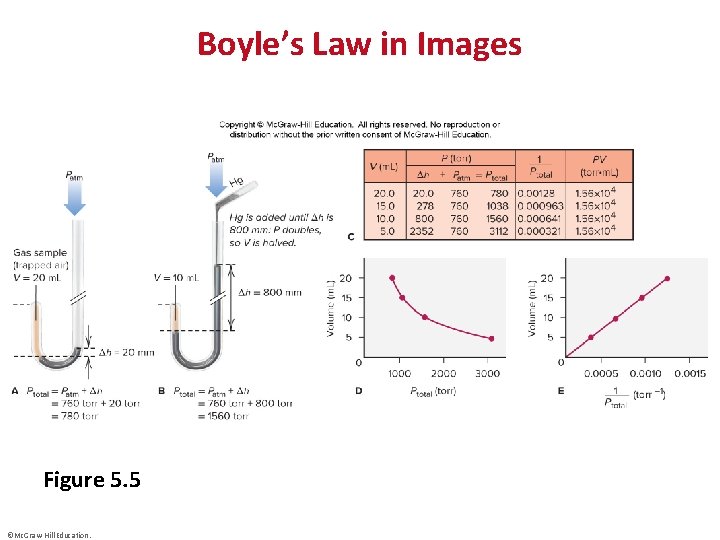
Boyle’s Law in Images Figure 5. 5 ©Mc. Graw-Hill Education.

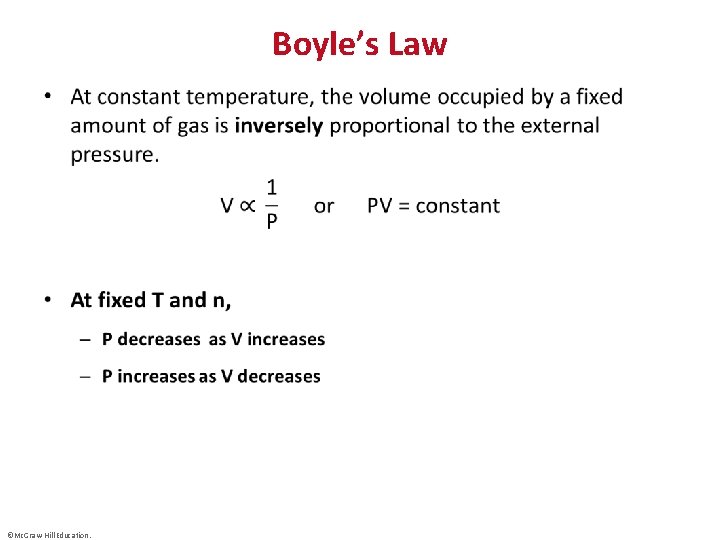
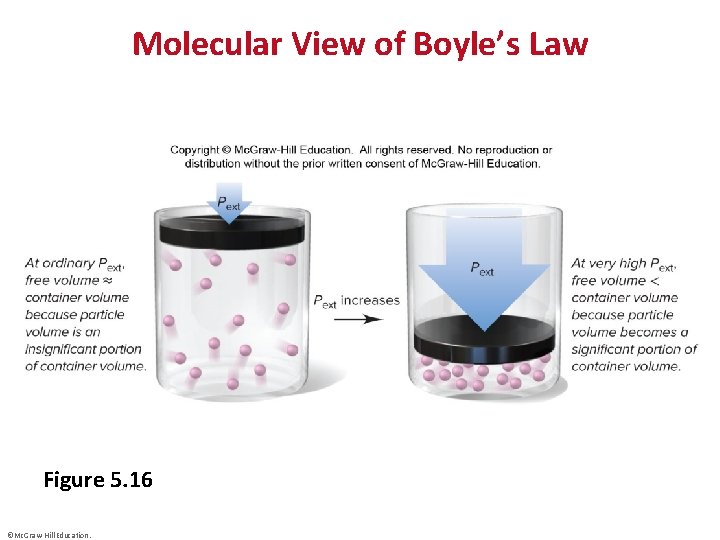
Boyle’s Law • ©Mc. Graw-Hill Education.

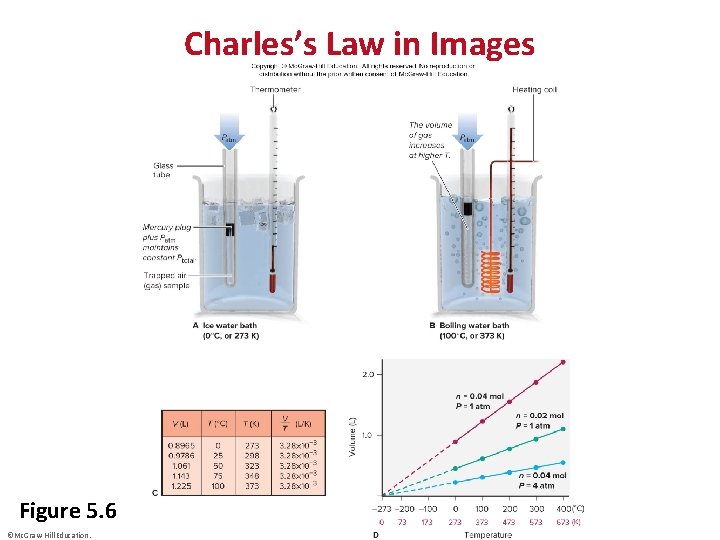
Charles’s Law in Images Figure 5. 6 ©Mc. Graw-Hill Education.

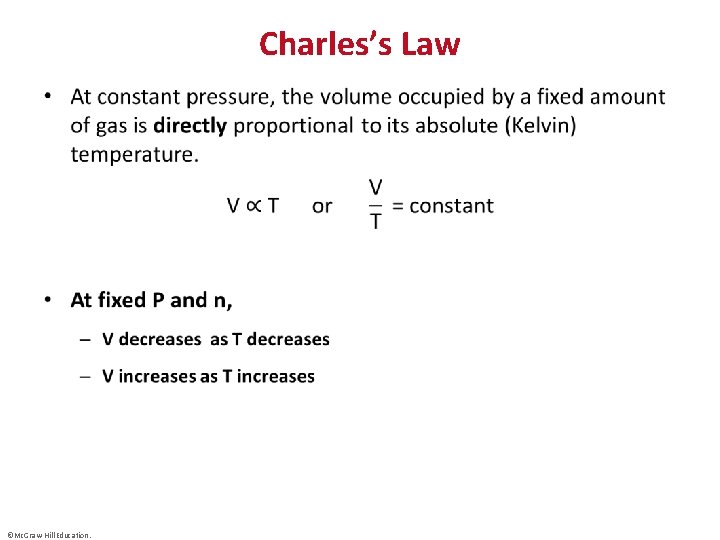
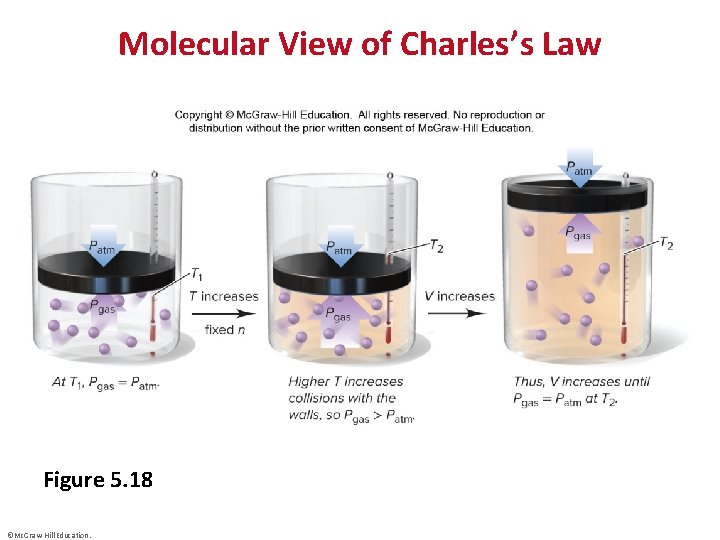
Charles’s Law • ©Mc. Graw-Hill Education.

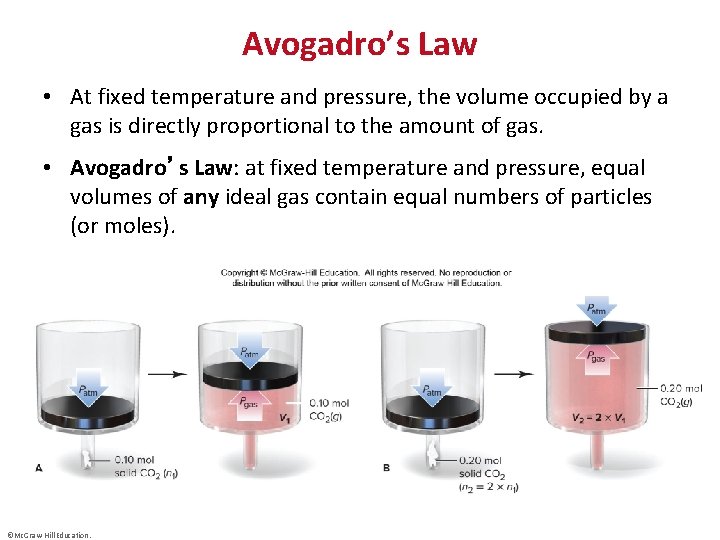
Avogadro’s Law • At fixed temperature and pressure, the volume occupied by a gas is directly proportional to the amount of gas. • Avogadro’s Law: at fixed temperature and pressure, equal volumes of any ideal gas contain equal numbers of particles (or moles). ©Mc. Graw-Hill Education.

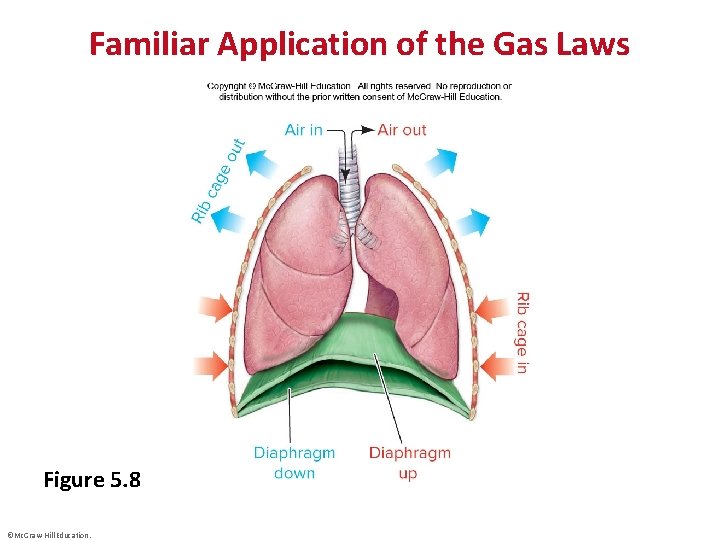
Familiar Application of the Gas Laws Figure 5. 8 ©Mc. Graw-Hill Education.

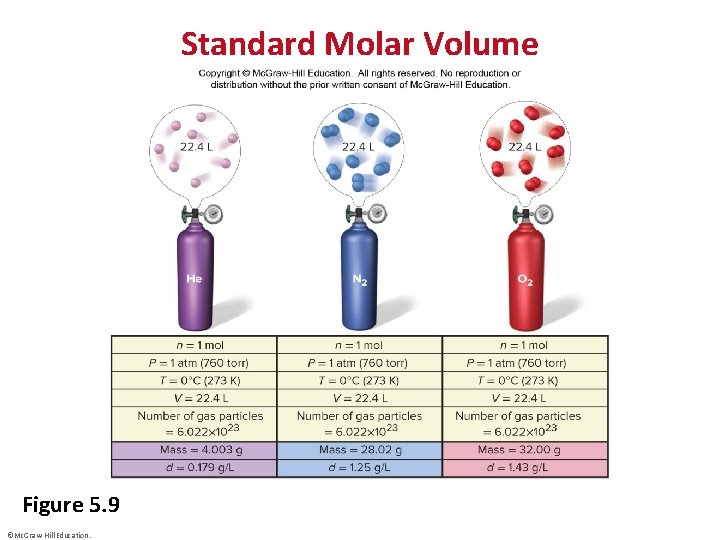
Gas Behavior at Standard Conditions • STP or standard temperature and pressure specifies a pressure of 1 atm (760 torr) and a temperature of 0°C ( 273. 15 K). • The standard molar volume is the volume of 1 mol of an ideal gas at STP. • Standard molar volume = 22. 4141 L or 22. 4 L ©Mc. Graw-Hill Education.

Standard Molar Volume Figure 5. 9 ©Mc. Graw-Hill Education.

Volume of 1 mol of Some Familiar Objects Figure 5. 10 ©Mc. Graw-Hill Education. © Mc. Graw-Hill Education/Charles Winters/Timeframe Photography, Inc.

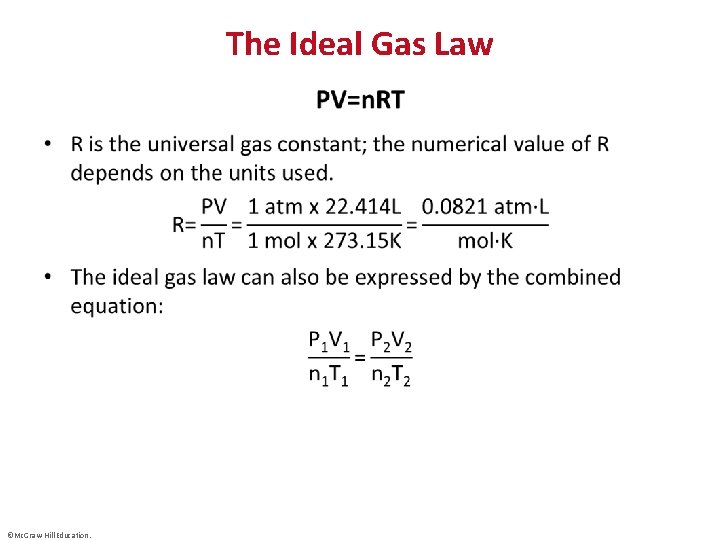
The Ideal Gas Law • ©Mc. Graw-Hill Education.

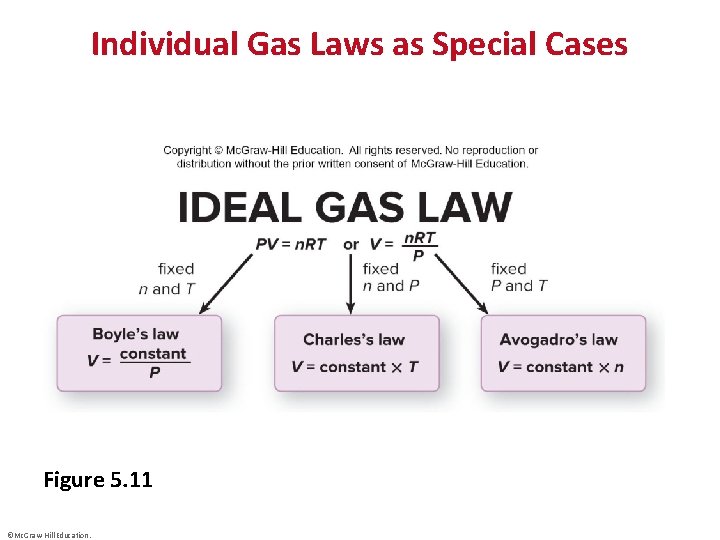
Individual Gas Laws as Special Cases Figure 5. 11 ©Mc. Graw-Hill Education.

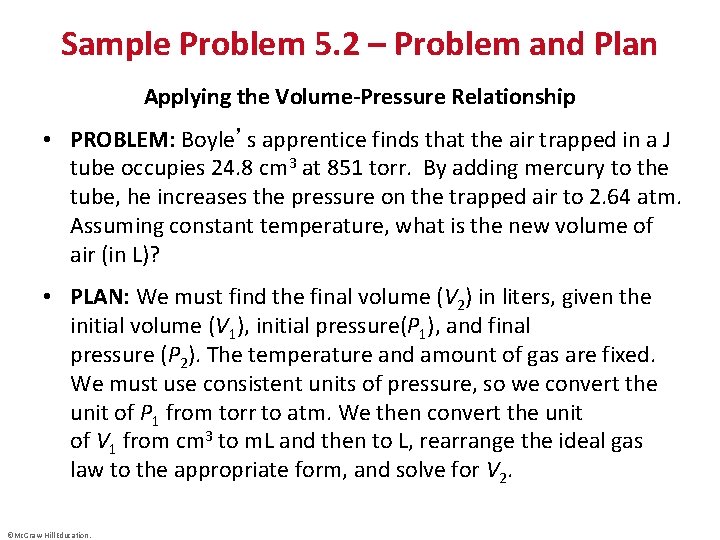
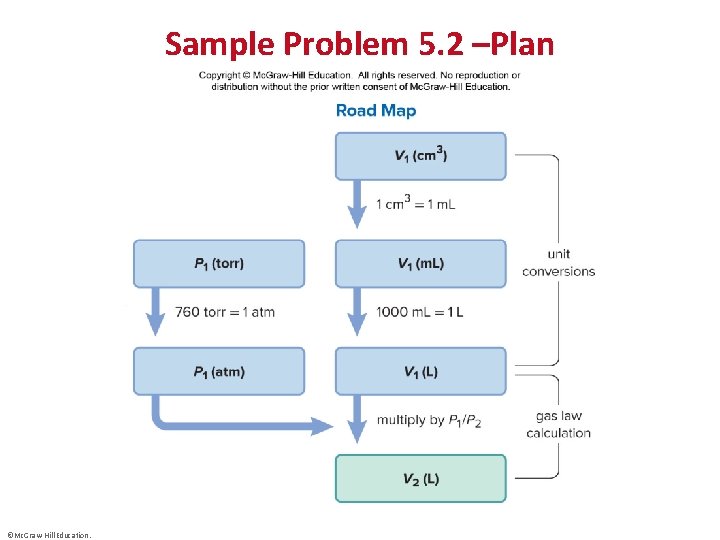
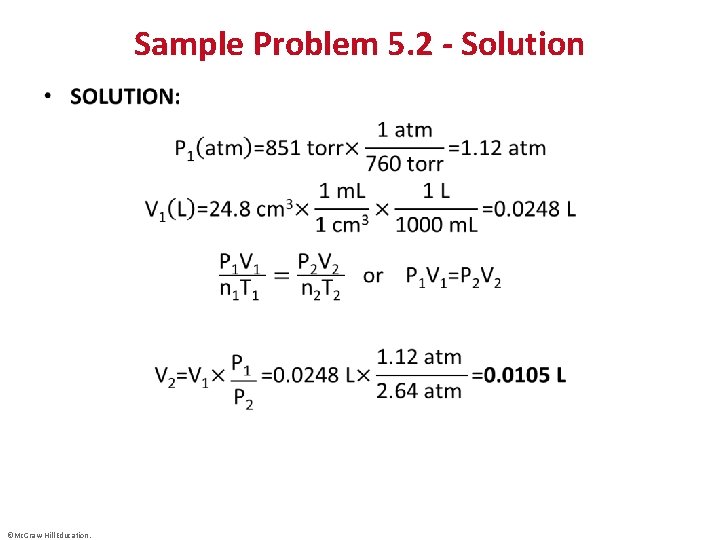
Sample Problem 5. 2 – Problem and Plan Applying the Volume-Pressure Relationship • PROBLEM: Boyle’s apprentice finds that the air trapped in a J tube occupies 24. 8 cm 3 at 851 torr. By adding mercury to the tube, he increases the pressure on the trapped air to 2. 64 atm. Assuming constant temperature, what is the new volume of air (in L)? • PLAN: We must find the final volume (V 2) in liters, given the initial volume (V 1), initial pressure(P 1), and final pressure (P 2). The temperature and amount of gas are fixed. We must use consistent units of pressure, so we convert the unit of P 1 from torr to atm. We then convert the unit of V 1 from cm 3 to m. L and then to L, rearrange the ideal gas law to the appropriate form, and solve for V 2. ©Mc. Graw-Hill Education.

Sample Problem 5. 2 –Plan ©Mc. Graw-Hill Education.

Sample Problem 5. 2 - Solution • ©Mc. Graw-Hill Education.

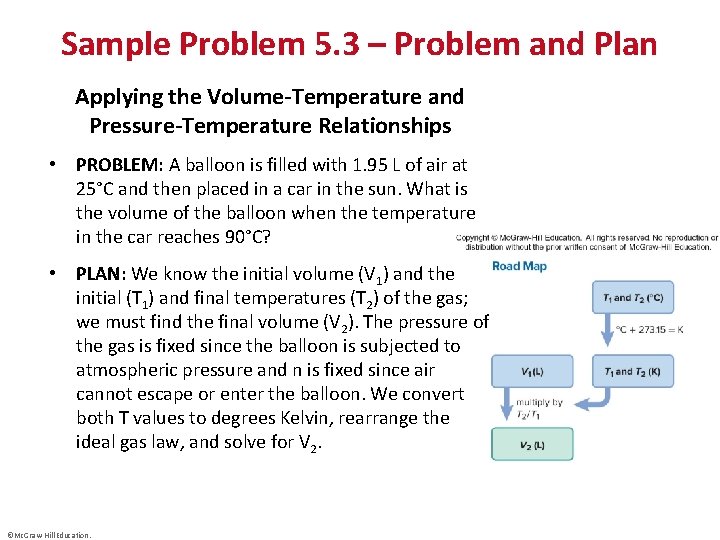
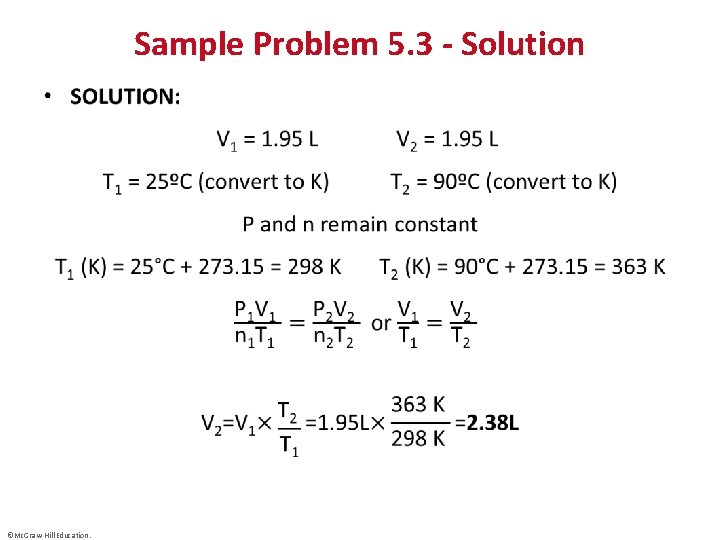
Sample Problem 5. 3 – Problem and Plan Applying the Volume-Temperature and Pressure-Temperature Relationships • PROBLEM: A balloon is filled with 1. 95 L of air at 25°C and then placed in a car in the sun. What is the volume of the balloon when the temperature in the car reaches 90°C? • PLAN: We know the initial volume (V 1) and the initial (T 1) and final temperatures (T 2) of the gas; we must find the final volume (V 2). The pressure of the gas is fixed since the balloon is subjected to atmospheric pressure and n is fixed since air cannot escape or enter the balloon. We convert both T values to degrees Kelvin, rearrange the ideal gas law, and solve for V 2. ©Mc. Graw-Hill Education.

Sample Problem 5. 3 - Solution • ©Mc. Graw-Hill Education.

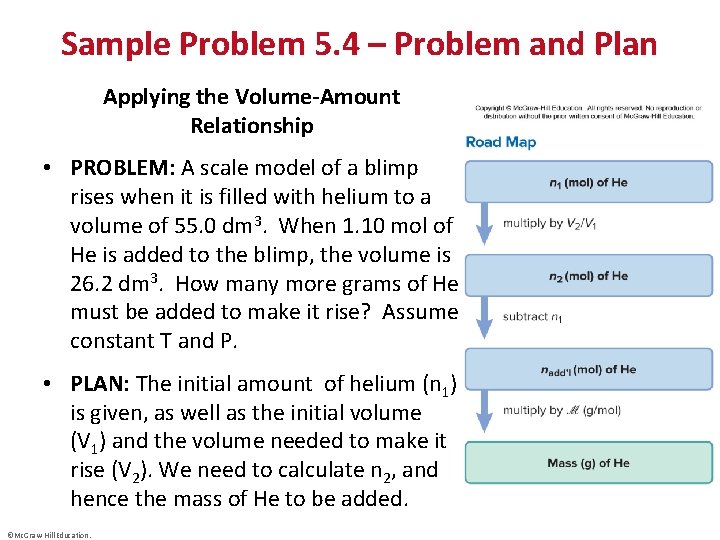
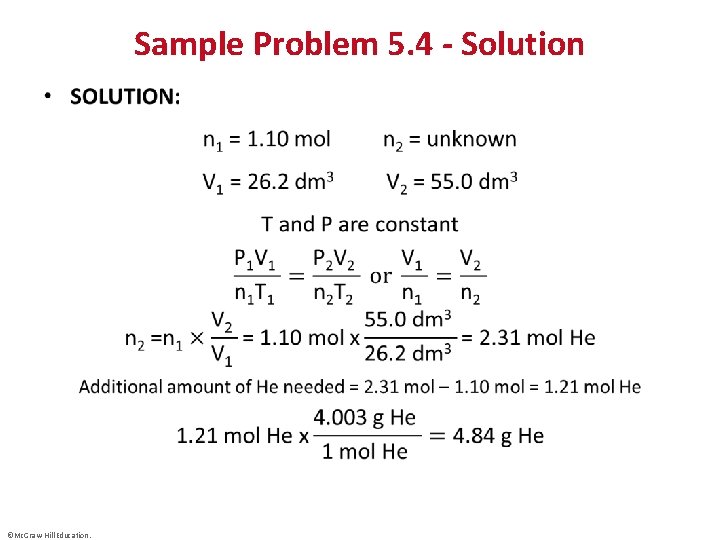
Sample Problem 5. 4 – Problem and Plan Applying the Volume-Amount Relationship • PROBLEM: A scale model of a blimp rises when it is filled with helium to a volume of 55. 0 dm 3. When 1. 10 mol of He is added to the blimp, the volume is 26. 2 dm 3. How many more grams of He must be added to make it rise? Assume constant T and P. • PLAN: The initial amount of helium (n 1) is given, as well as the initial volume (V 1) and the volume needed to make it rise (V 2). We need to calculate n 2, and hence the mass of He to be added. ©Mc. Graw-Hill Education.

Sample Problem 5. 4 - Solution • ©Mc. Graw-Hill Education.

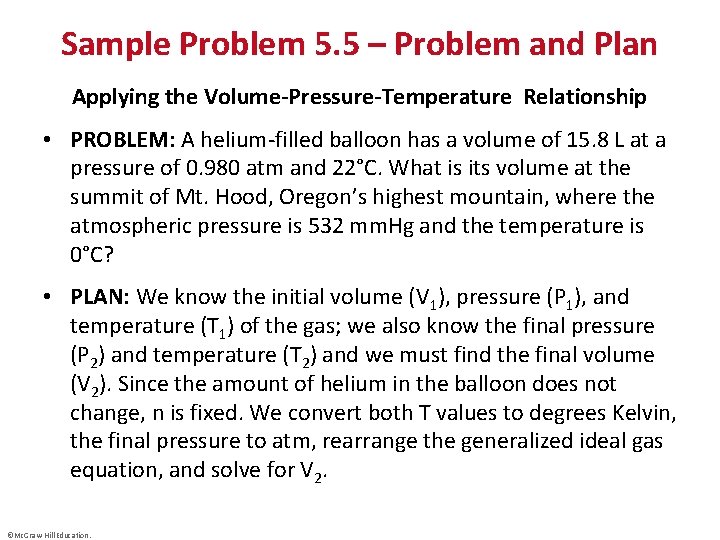
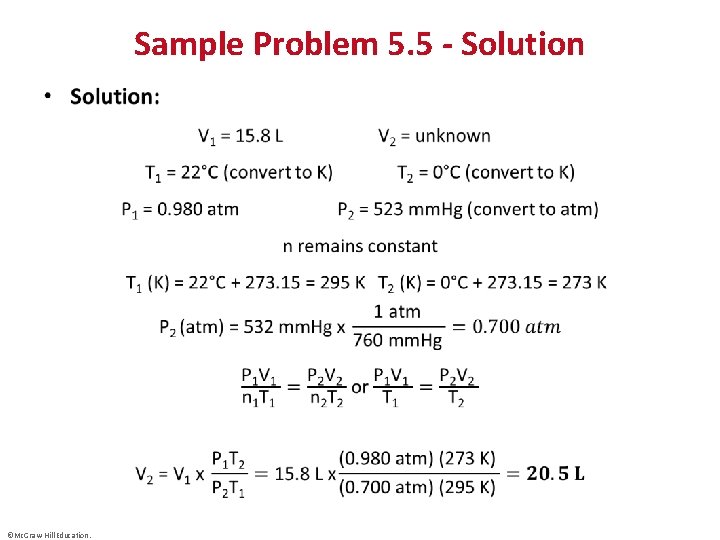
Sample Problem 5. 5 – Problem and Plan Applying the Volume-Pressure-Temperature Relationship • PROBLEM: A helium-filled balloon has a volume of 15. 8 L at a pressure of 0. 980 atm and 22°C. What is its volume at the summit of Mt. Hood, Oregon’s highest mountain, where the atmospheric pressure is 532 mm. Hg and the temperature is 0°C? • PLAN: We know the initial volume (V 1), pressure (P 1), and temperature (T 1) of the gas; we also know the final pressure (P 2) and temperature (T 2) and we must find the final volume (V 2). Since the amount of helium in the balloon does not change, n is fixed. We convert both T values to degrees Kelvin, the final pressure to atm, rearrange the generalized ideal gas equation, and solve for V 2. ©Mc. Graw-Hill Education.

Sample Problem 5. 5 - Solution • ©Mc. Graw-Hill Education.

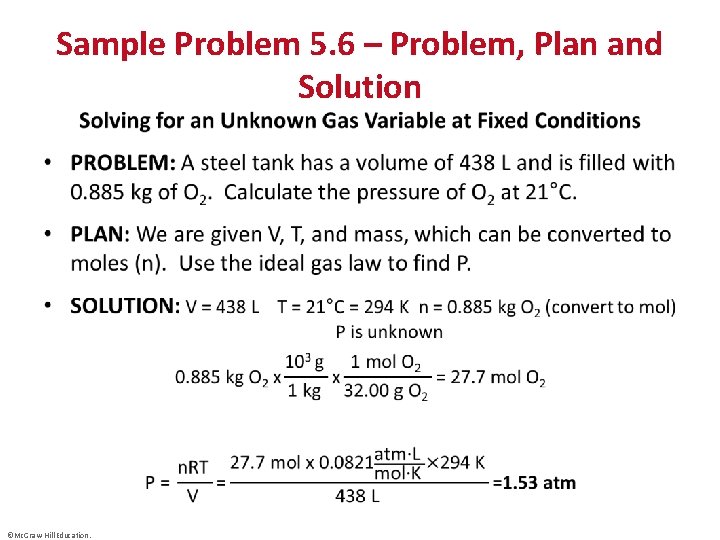
• Sample Problem 5. 6 – Problem, Plan and Solution ©Mc. Graw-Hill Education.

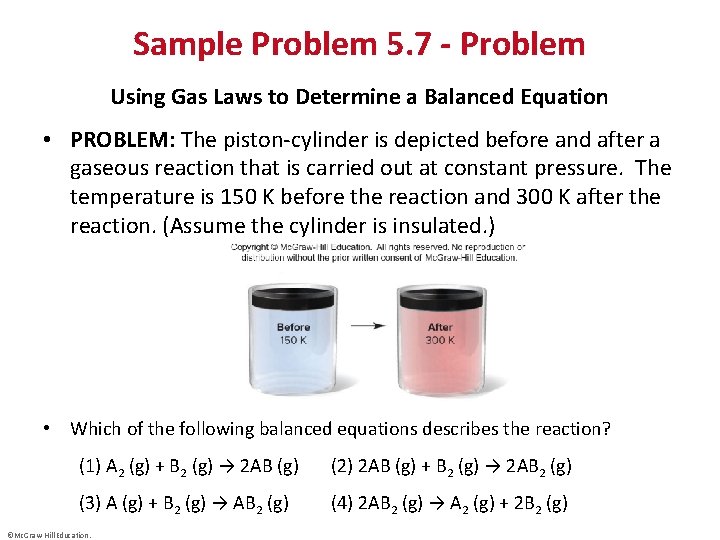
Sample Problem 5. 7 - Problem Using Gas Laws to Determine a Balanced Equation • PROBLEM: The piston-cylinder is depicted before and after a gaseous reaction that is carried out at constant pressure. The temperature is 150 K before the reaction and 300 K after the reaction. (Assume the cylinder is insulated. ) • Which of the following balanced equations describes the reaction? (1) A 2 (g) + B 2 (g) → 2 AB (g) (2) 2 AB (g) + B 2 (g) → 2 AB 2 (g) (3) A (g) + B 2 (g) → AB 2 (g) (4) 2 AB 2 (g) → A 2 (g) + 2 B 2 (g) ©Mc. Graw-Hill Education.

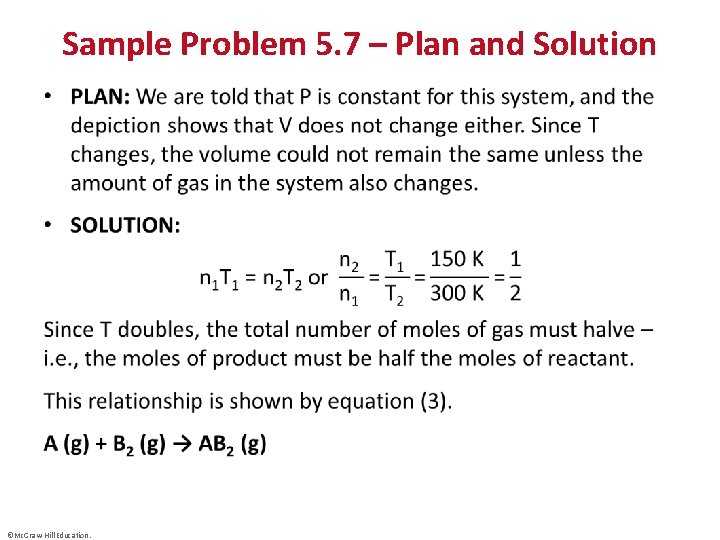
Sample Problem 5. 7 – Plan and Solution • ©Mc. Graw-Hill Education.

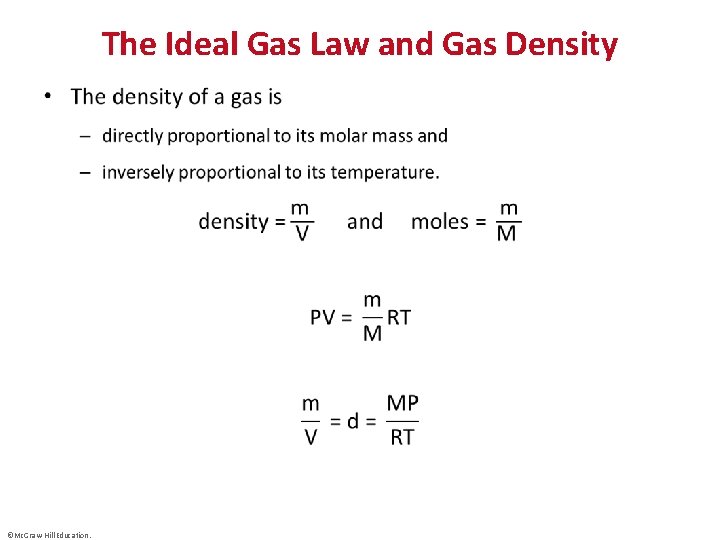
The Ideal Gas Law and Gas Density • ©Mc. Graw-Hill Education.

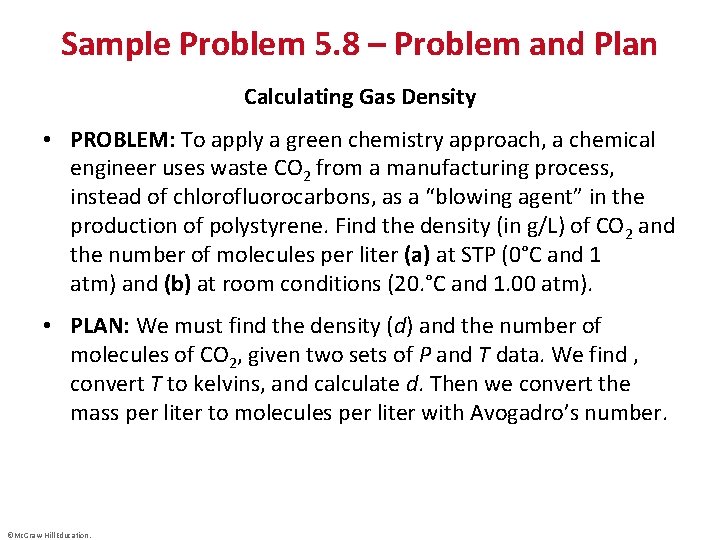
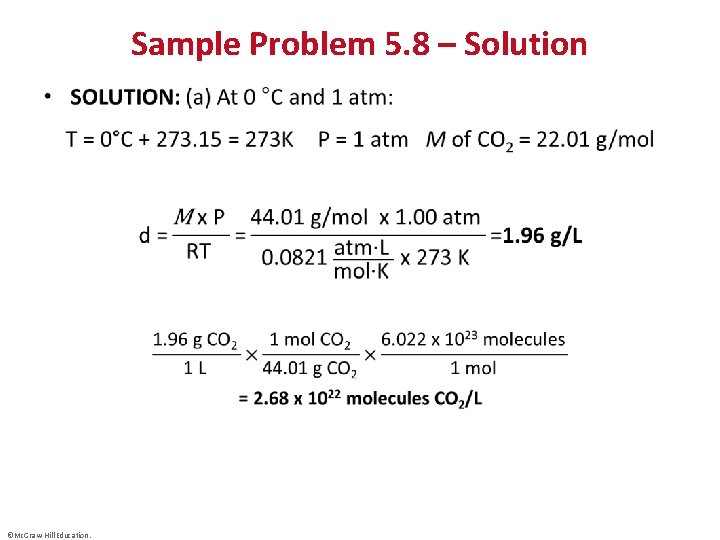
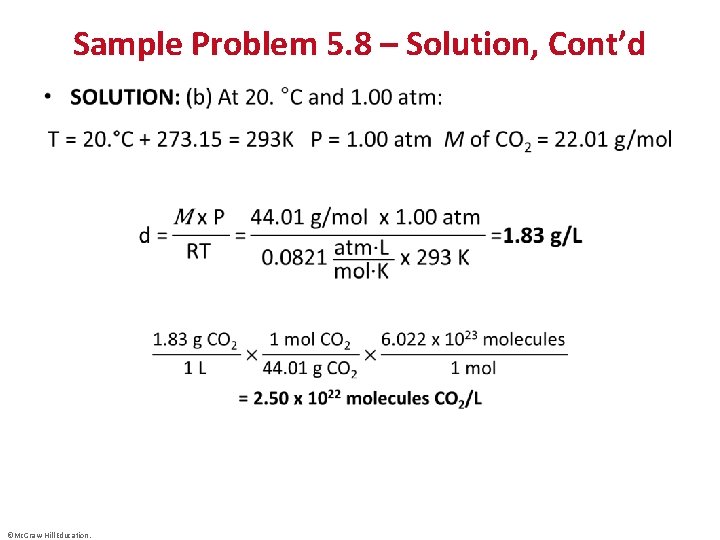
Sample Problem 5. 8 – Problem and Plan Calculating Gas Density • PROBLEM: To apply a green chemistry approach, a chemical engineer uses waste CO 2 from a manufacturing process, instead of chlorofluorocarbons, as a “blowing agent” in the production of polystyrene. Find the density (in g/L) of CO 2 and the number of molecules per liter (a) at STP (0°C and 1 atm) and (b) at room conditions (20. °C and 1. 00 atm). • PLAN: We must find the density (d) and the number of molecules of CO 2, given two sets of P and T data. We find , convert T to kelvins, and calculate d. Then we convert the mass per liter to molecules per liter with Avogadro’s number. ©Mc. Graw-Hill Education.

Sample Problem 5. 8 – Solution • ©Mc. Graw-Hill Education.

Sample Problem 5. 8 – Solution, Cont’d • ©Mc. Graw-Hill Education.

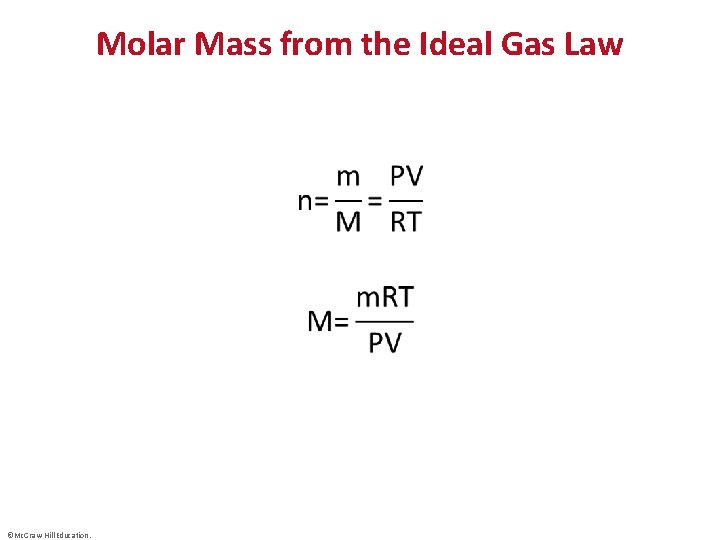
Molar Mass from the Ideal Gas Law • ©Mc. Graw-Hill Education.

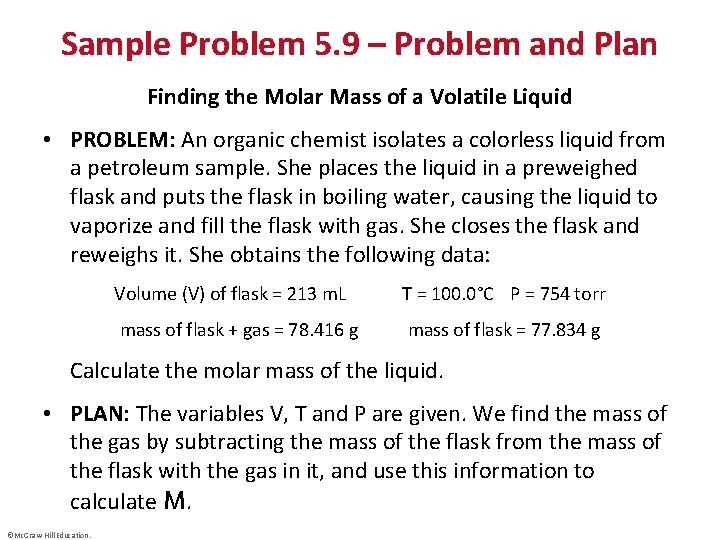
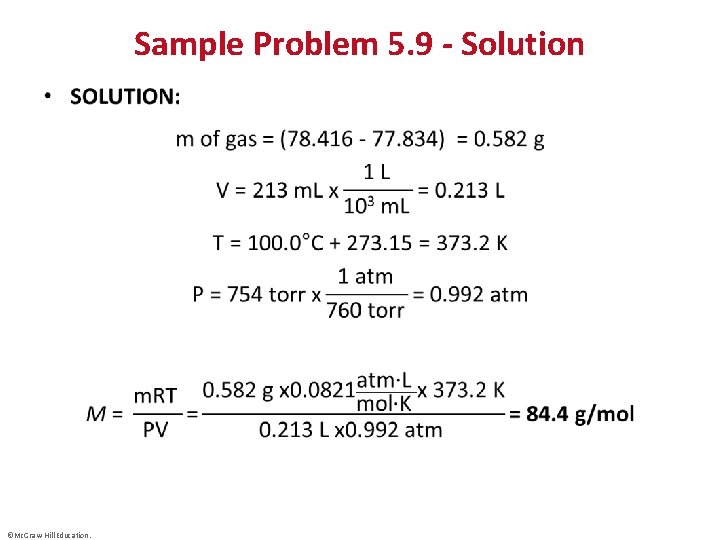
Sample Problem 5. 9 – Problem and Plan Finding the Molar Mass of a Volatile Liquid • PROBLEM: An organic chemist isolates a colorless liquid from a petroleum sample. She places the liquid in a preweighed flask and puts the flask in boiling water, causing the liquid to vaporize and fill the flask with gas. She closes the flask and reweighs it. She obtains the following data: Volume (V) of flask = 213 m. L T = 100. 0°C P = 754 torr mass of flask + gas = 78. 416 g mass of flask = 77. 834 g Calculate the molar mass of the liquid. • PLAN: The variables V, T and P are given. We find the mass of the gas by subtracting the mass of the flask from the mass of the flask with the gas in it, and use this information to calculate M. ©Mc. Graw-Hill Education.

Sample Problem 5. 9 - Solution • ©Mc. Graw-Hill Education.

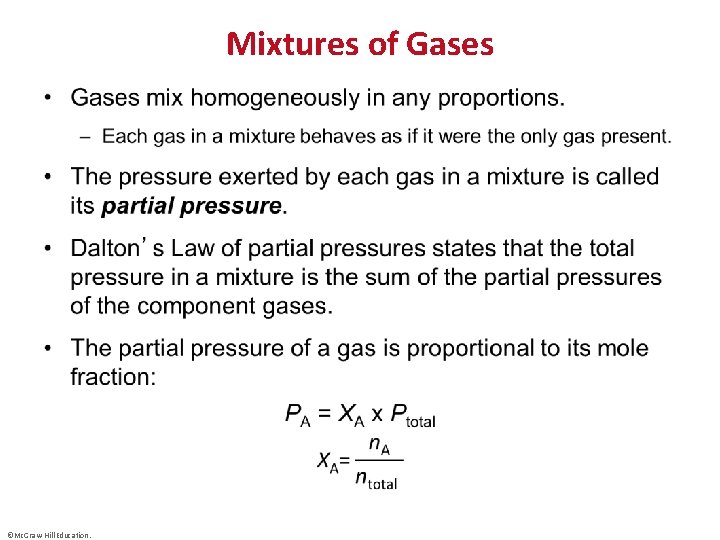
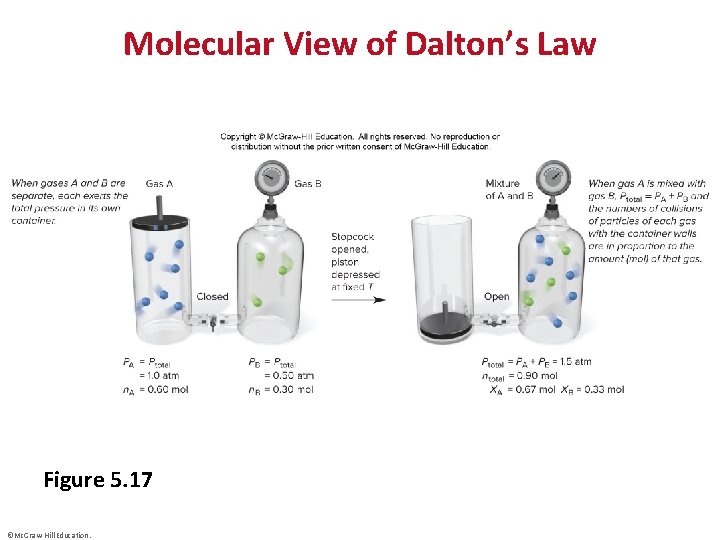
Mixtures of Gases • ©Mc. Graw-Hill Education.

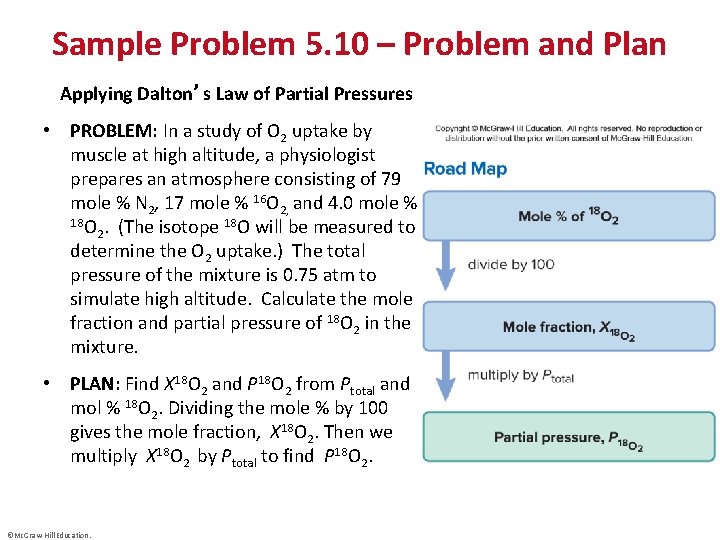
Sample Problem 5. 10 – Problem and Plan Applying Dalton’s Law of Partial Pressures • PROBLEM: In a study of O 2 uptake by muscle at high altitude, a physiologist prepares an atmosphere consisting of 79 mole % N 2, 17 mole % 16 O 2, and 4. 0 mole % 18 O. (The isotope 18 O will be measured to 2 determine the O 2 uptake. ) The total pressure of the mixture is 0. 75 atm to simulate high altitude. Calculate the mole fraction and partial pressure of 18 O 2 in the mixture. • PLAN: Find X 18 O 2 and P 18 O 2 from Ptotal and mol % 18 O 2. Dividing the mole % by 100 gives the mole fraction, X 18 O 2. Then we multiply X 18 O 2 by Ptotal to find P 18 O 2. ©Mc. Graw-Hill Education.

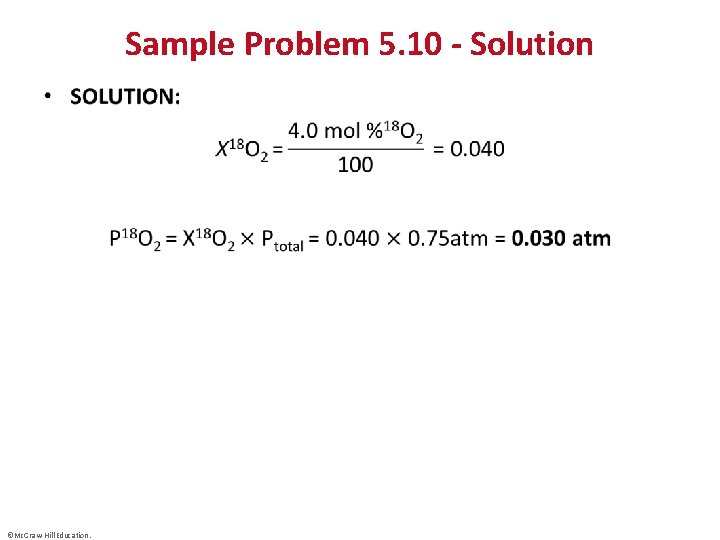
Sample Problem 5. 10 - Solution • ©Mc. Graw-Hill Education.

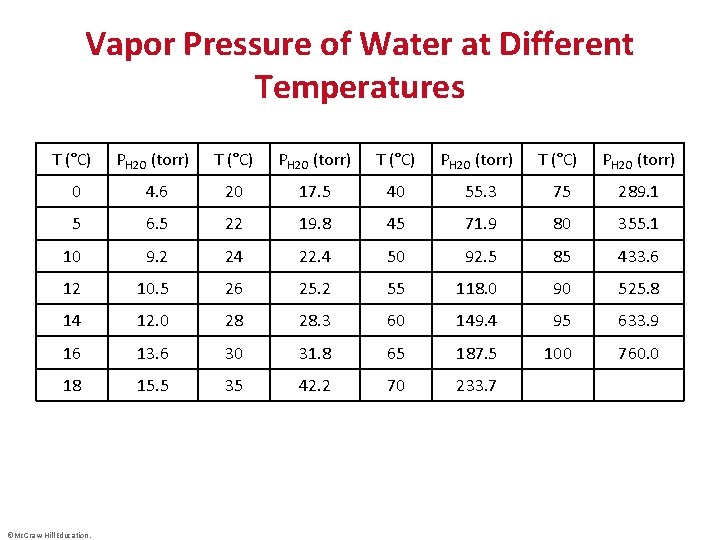
Vapor Pressure of Water at Different Temperatures T (°C) PH 2 O (torr) 0 4. 6 20 17. 5 40 55. 3 75 289. 1 5 6. 5 22 19. 8 45 71. 9 80 355. 1 10 9. 2 24 22. 4 50 92. 5 85 433. 6 12 10. 5 26 25. 2 55 118. 0 90 525. 8 14 12. 0 28 28. 3 60 149. 4 95 633. 9 16 13. 6 30 31. 8 65 187. 5 100 760. 0 18 15. 5 35 42. 2 70 233. 7 ©Mc. Graw-Hill Education.

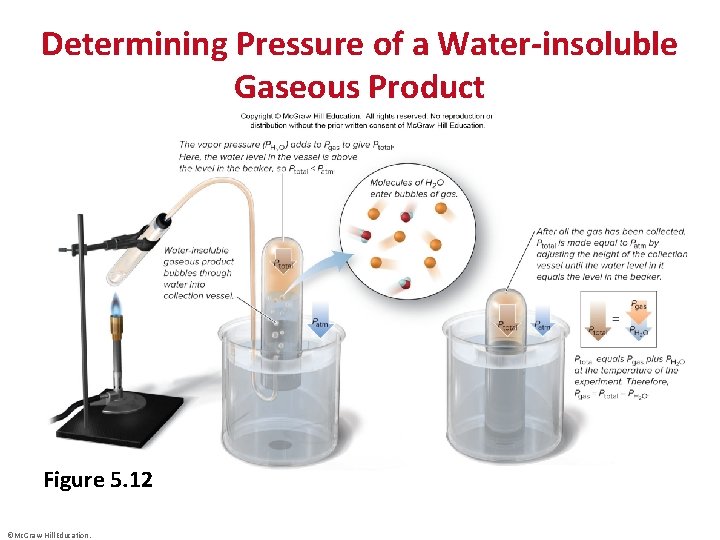
Determining Pressure of a Water-insoluble Gaseous Product Figure 5. 12 ©Mc. Graw-Hill Education.

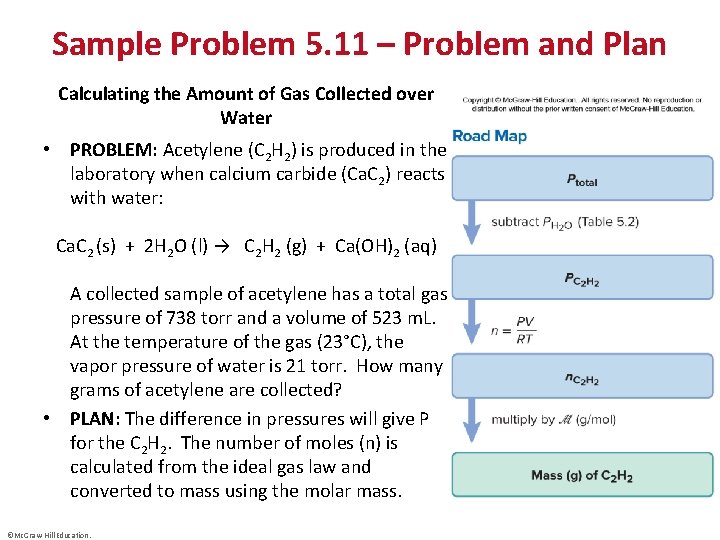
Sample Problem 5. 11 – Problem and Plan Calculating the Amount of Gas Collected over Water • PROBLEM: Acetylene (C 2 H 2) is produced in the laboratory when calcium carbide (Ca. C 2) reacts with water: Ca. C 2 (s) + 2 H 2 O (l) → C 2 H 2 (g) + Ca(OH)2 (aq) A collected sample of acetylene has a total gas pressure of 738 torr and a volume of 523 m. L. At the temperature of the gas (23°C), the vapor pressure of water is 21 torr. How many grams of acetylene are collected? • PLAN: The difference in pressures will give P for the C 2 H 2. The number of moles (n) is calculated from the ideal gas law and converted to mass using the molar mass. ©Mc. Graw-Hill Education.

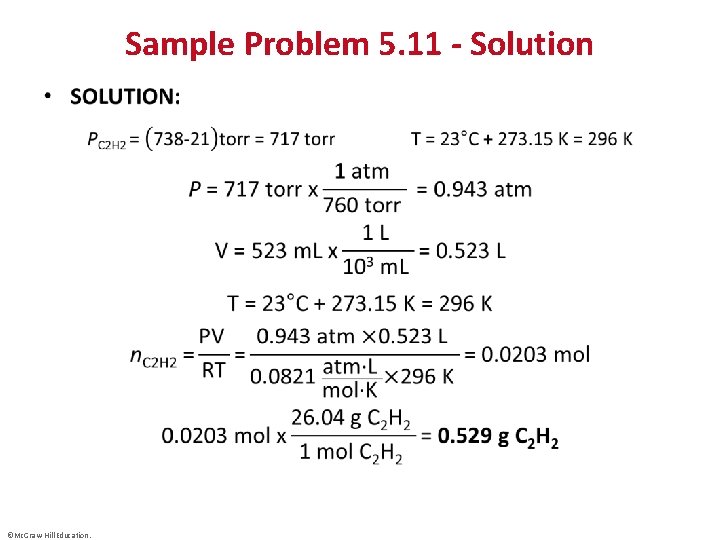
Sample Problem 5. 11 - Solution • ©Mc. Graw-Hill Education.

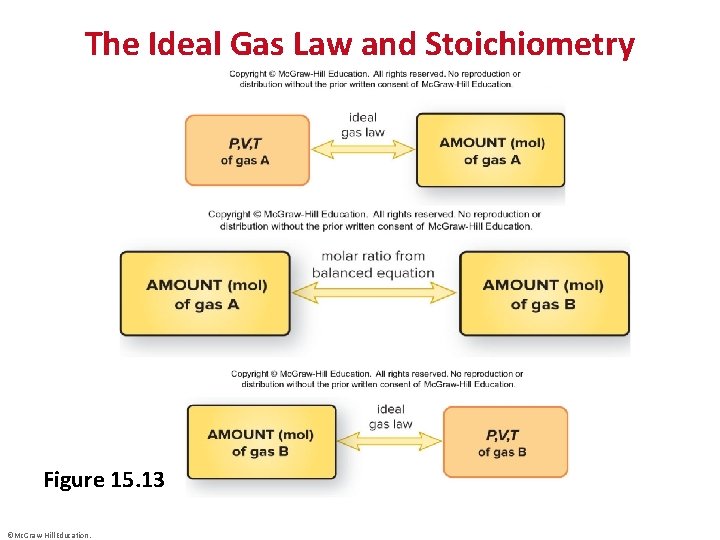
The Ideal Gas Law and Stoichiometry Figure 15. 13 ©Mc. Graw-Hill Education.

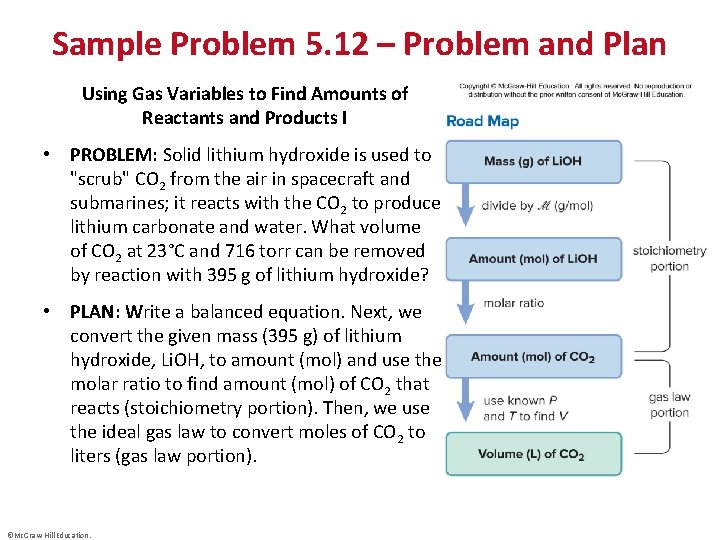
Sample Problem 5. 12 – Problem and Plan Using Gas Variables to Find Amounts of Reactants and Products I • PROBLEM: Solid lithium hydroxide is used to "scrub" CO 2 from the air in spacecraft and submarines; it reacts with the CO 2 to produce lithium carbonate and water. What volume of CO 2 at 23°C and 716 torr can be removed by reaction with 395 g of lithium hydroxide? • PLAN: Write a balanced equation. Next, we convert the given mass (395 g) of lithium hydroxide, Li. OH, to amount (mol) and use the molar ratio to find amount (mol) of CO 2 that reacts (stoichiometry portion). Then, we use the ideal gas law to convert moles of CO 2 to liters (gas law portion). ©Mc. Graw-Hill Education.

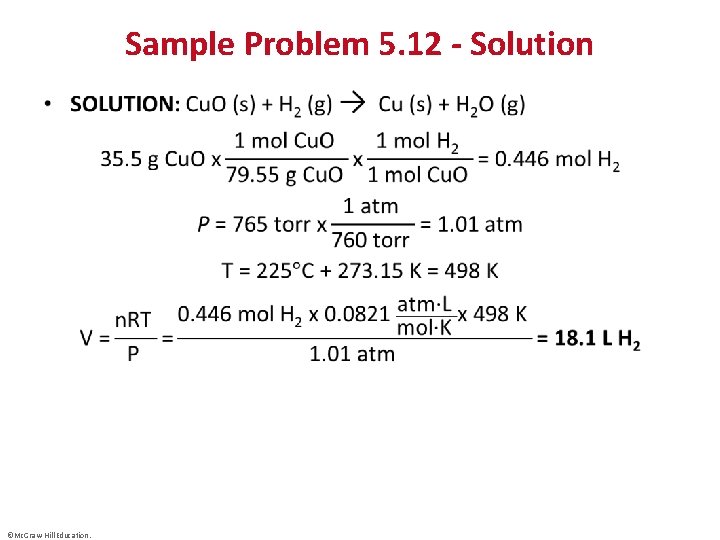
Sample Problem 5. 12 - Solution • ©Mc. Graw-Hill Education.

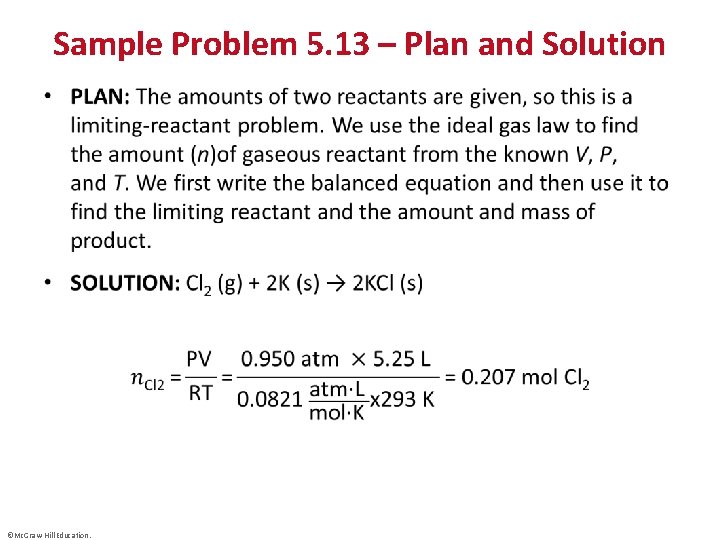
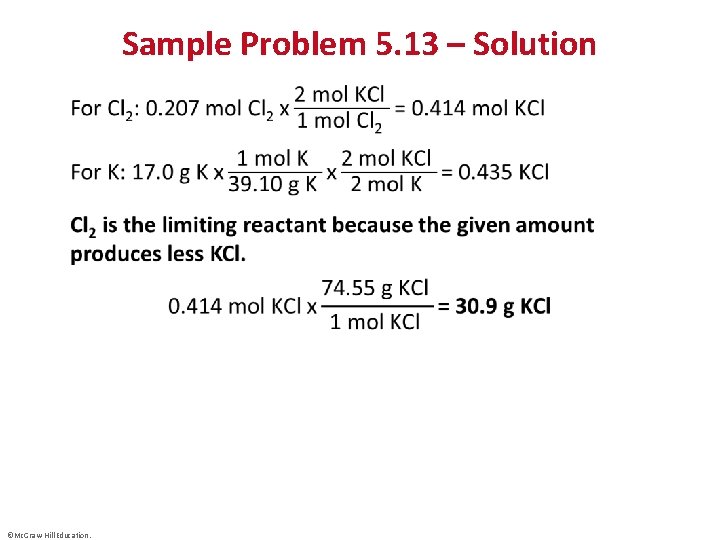
Sample Problem 5. 13 – Problem • Using Gas Variables to Find Amounts of Reactants and Products II • PROBLEM: The alkali metals [Group 1 A(1)]react with the halogens [Group 7 A(17)] to form ionic metal halides. What mass of potassium chloride forms when 5. 25 L of chlorine gas at 0. 950 atm and 293 K reacts with 17. 0 g of potassium? ©Mc. Graw-Hill Education. © Mc. Graw-Hill Education/Stephen Frisch, photographer

Sample Problem 5. 13 – Plan and Solution • ©Mc. Graw-Hill Education.

Sample Problem 5. 13 – Solution • ©Mc. Graw-Hill Education.

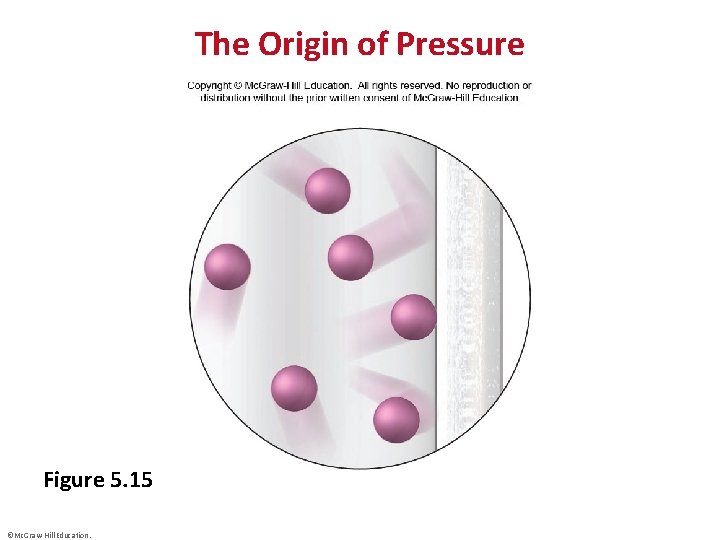
Kinetic-Molecular Theory • Postulate 1: Gas particles are tiny with large spaces between them. The volume of each particle is so small compared to the total volume of the gas that it is assumed to be zero. • Postulate 2: Gas particles are in constant, random, straightline motion except when they collide with each other or with the container walls. • Postulate 3: Collisions are elastic, meaning that colliding particles exchange energy but do not lose any energy due to friction. Their total kinetic energy is constant. Between collisions the particles do not influence each other by attractive or repulsive forces. ©Mc. Graw-Hill Education.

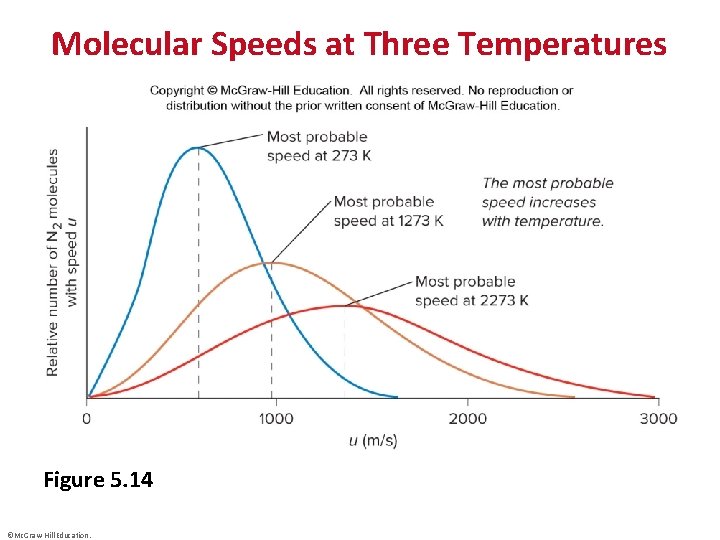
Molecular Speeds at Three Temperatures Figure 5. 14 ©Mc. Graw-Hill Education.

The Origin of Pressure Figure 5. 15 ©Mc. Graw-Hill Education.

Molecular View of Boyle’s Law Figure 5. 16 ©Mc. Graw-Hill Education.

Molecular View of Dalton’s Law Figure 5. 17 ©Mc. Graw-Hill Education.

Molecular View of Charles’s Law Figure 5. 18 ©Mc. Graw-Hill Education.

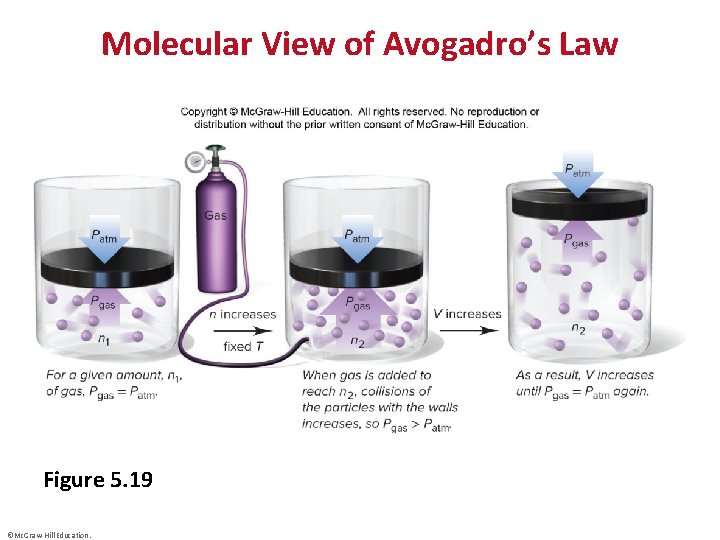
Molecular View of Avogadro’s Law Figure 5. 19 ©Mc. Graw-Hill Education.

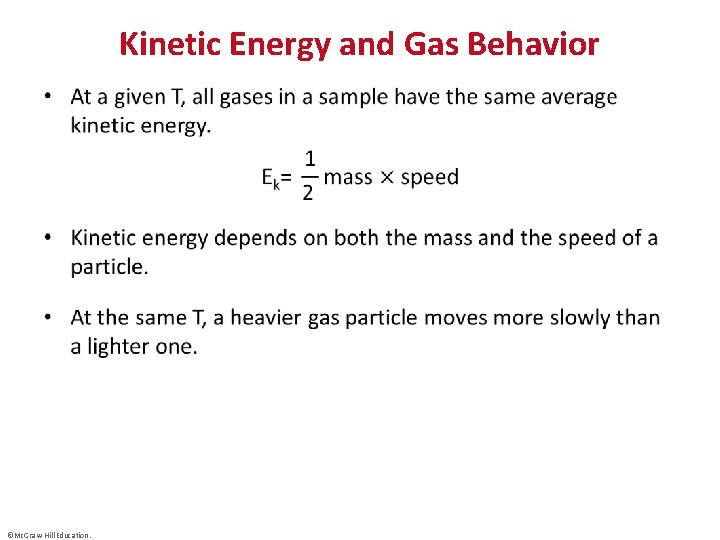
Kinetic Energy and Gas Behavior • ©Mc. Graw-Hill Education.

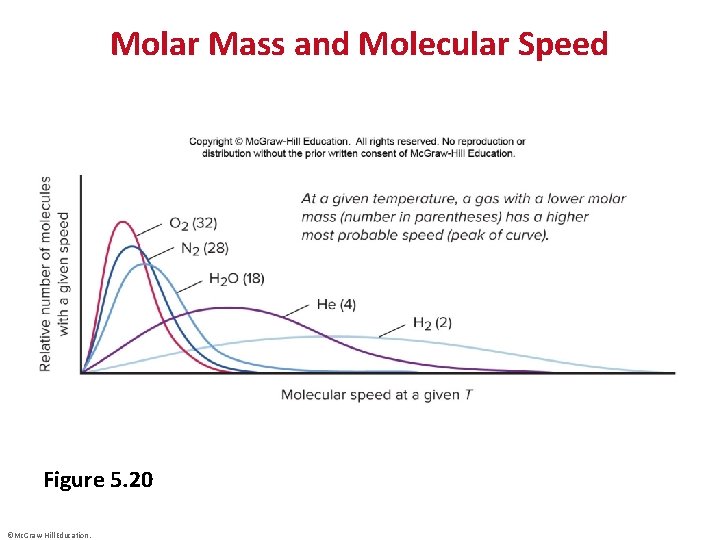
Molar Mass and Molecular Speed Figure 5. 20 ©Mc. Graw-Hill Education.

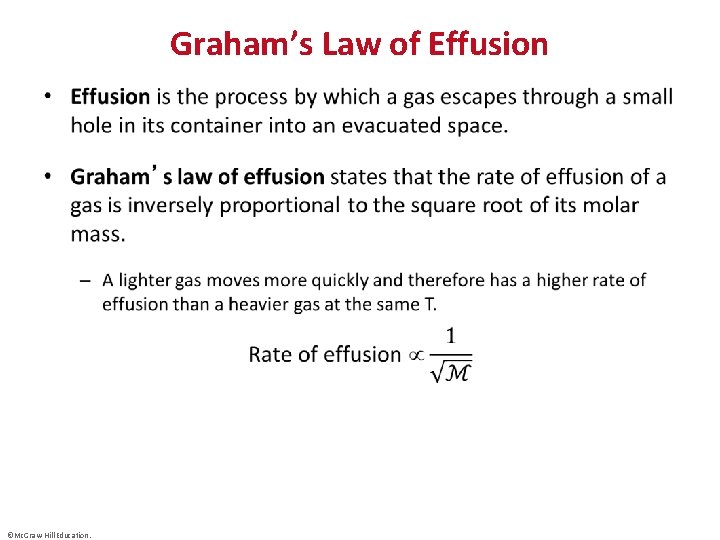
Graham’s Law of Effusion • ©Mc. Graw-Hill Education.

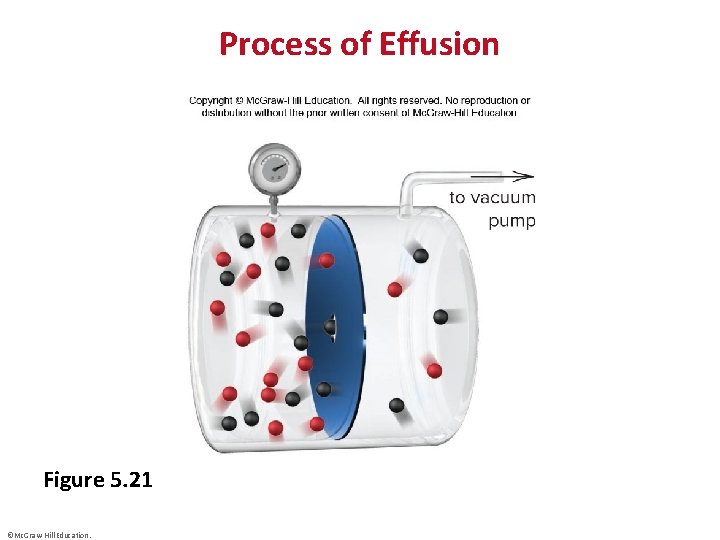
Process of Effusion Figure 5. 21 ©Mc. Graw-Hill Education.

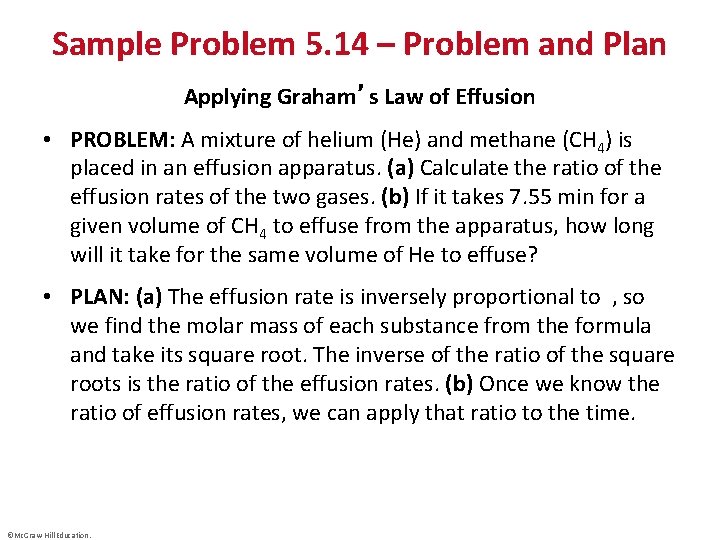
Sample Problem 5. 14 – Problem and Plan Applying Graham’s Law of Effusion • PROBLEM: A mixture of helium (He) and methane (CH 4) is placed in an effusion apparatus. (a) Calculate the ratio of the effusion rates of the two gases. (b) If it takes 7. 55 min for a given volume of CH 4 to effuse from the apparatus, how long will it take for the same volume of He to effuse? • PLAN: (a) The effusion rate is inversely proportional to , so we find the molar mass of each substance from the formula and take its square root. The inverse of the ratio of the square roots is the ratio of the effusion rates. (b) Once we know the ratio of effusion rates, we can apply that ratio to the time. ©Mc. Graw-Hill Education.

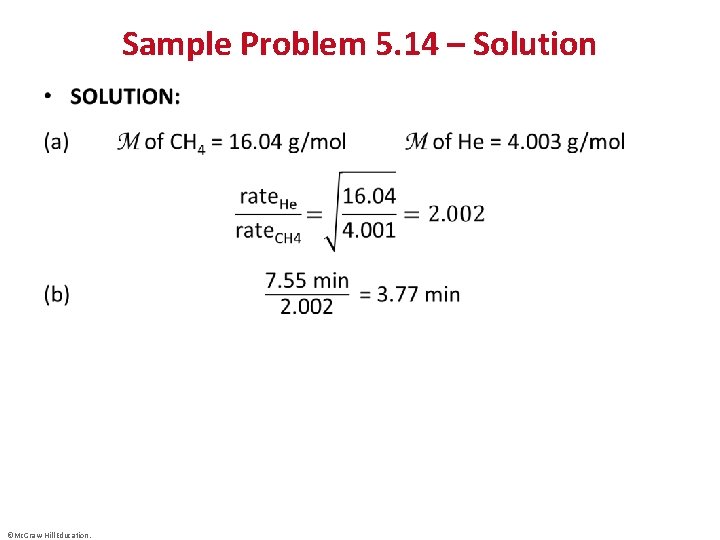
Sample Problem 5. 14 – Solution • ©Mc. Graw-Hill Education.

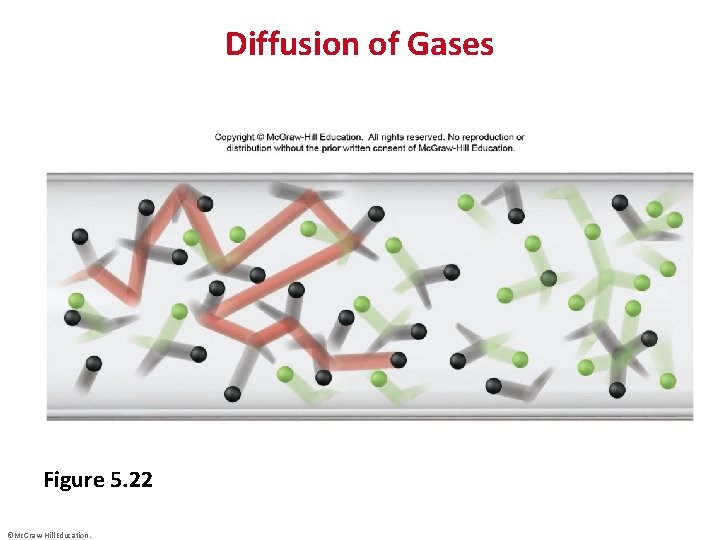
Diffusion of Gases Figure 5. 22 ©Mc. Graw-Hill Education.

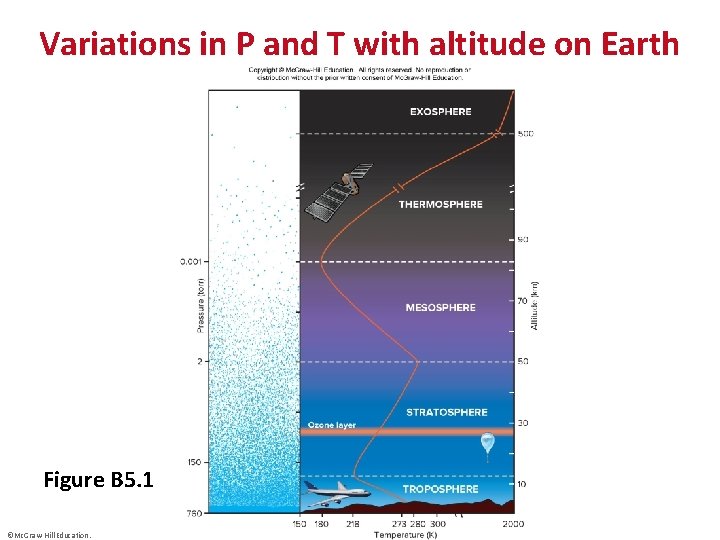
Variations in P and T with altitude on Earth Figure B 5. 1 ©Mc. Graw-Hill Education.

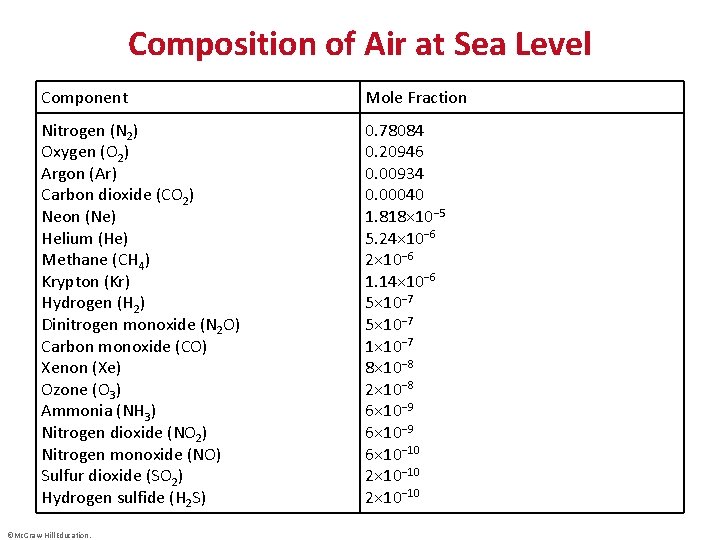
Composition of Air at Sea Level Component Mole Fraction Nitrogen (N 2) Oxygen (O 2) Argon (Ar) Carbon dioxide (CO 2) Neon (Ne) Helium (He) Methane (CH 4) Krypton (Kr) Hydrogen (H 2) Dinitrogen monoxide (N 2 O) Carbon monoxide (CO) Xenon (Xe) Ozone (O 3) Ammonia (NH 3) Nitrogen dioxide (NO 2) Nitrogen monoxide (NO) Sulfur dioxide (SO 2) Hydrogen sulfide (H 2 S) 0. 78084 0. 20946 0. 00934 0. 00040 1. 818× 10− 5 5. 24× 10− 6 2× 10− 6 1. 14× 10− 6 5× 10− 7 1× 10− 7 8× 10− 8 2× 10− 8 6× 10− 9 6× 10− 10 2× 10− 10 ©Mc. Graw-Hill Education.

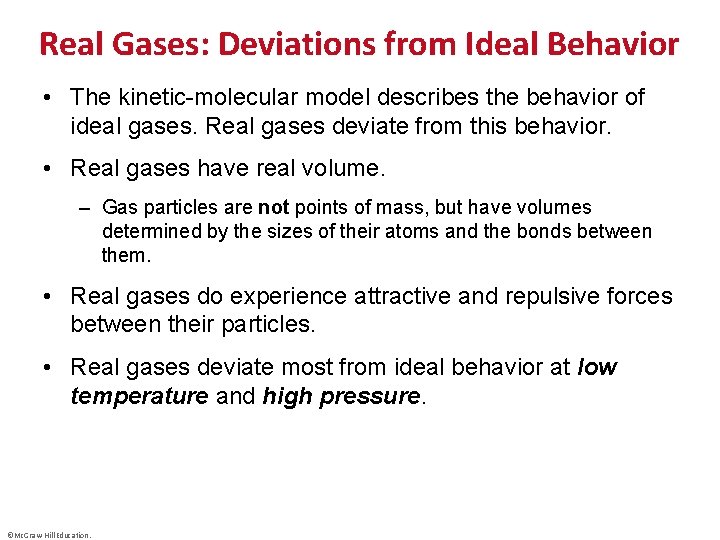
Real Gases: Deviations from Ideal Behavior • The kinetic-molecular model describes the behavior of ideal gases. Real gases deviate from this behavior. • Real gases have real volume. – Gas particles are not points of mass, but have volumes determined by the sizes of their atoms and the bonds between them. • Real gases do experience attractive and repulsive forces between their particles. • Real gases deviate most from ideal behavior at low temperature and high pressure. ©Mc. Graw-Hill Education.

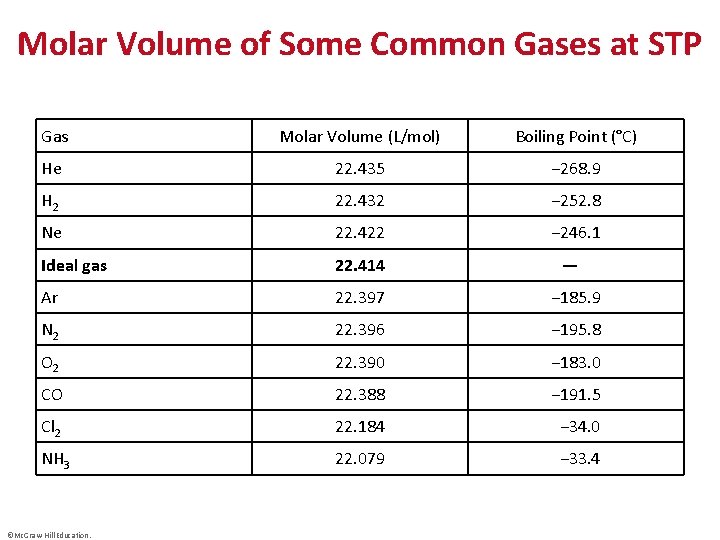
Molar Volume of Some Common Gases at STP Gas Molar Volume (L/mol) Boiling Point (°C) He 22. 435 − 268. 9 H 2 22. 432 − 252. 8 Ne 22. 422 − 246. 1 Ideal gas 22. 414 Ar 22. 397 − 185. 9 N 2 22. 396 − 195. 8 O 2 22. 390 − 183. 0 CO 22. 388 − 191. 5 Cl 2 22. 184 − 34. 0 NH 3 22. 079 − 33. 4 ©Mc. Graw-Hill Education. —

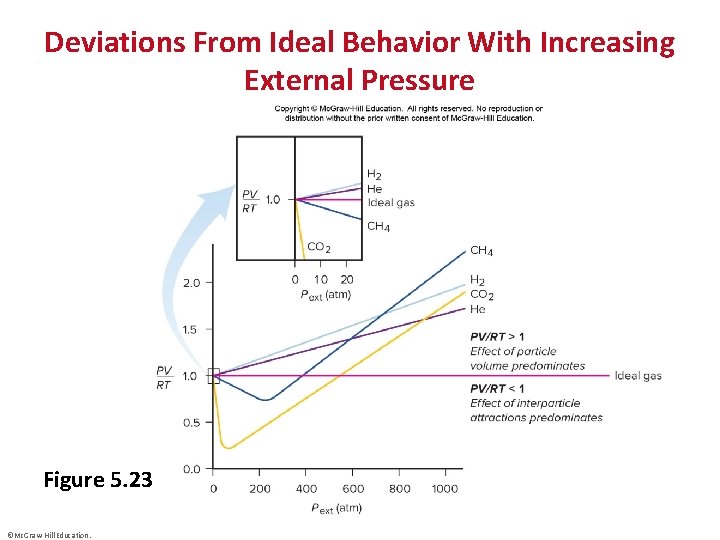
Deviations From Ideal Behavior With Increasing External Pressure Figure 5. 23 ©Mc. Graw-Hill Education.

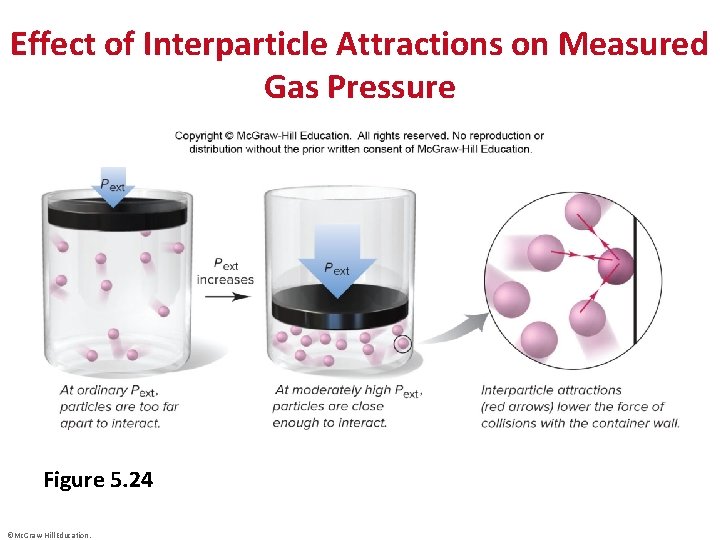
Effect of Interparticle Attractions on Measured Gas Pressure Figure 5. 24 ©Mc. Graw-Hill Education.

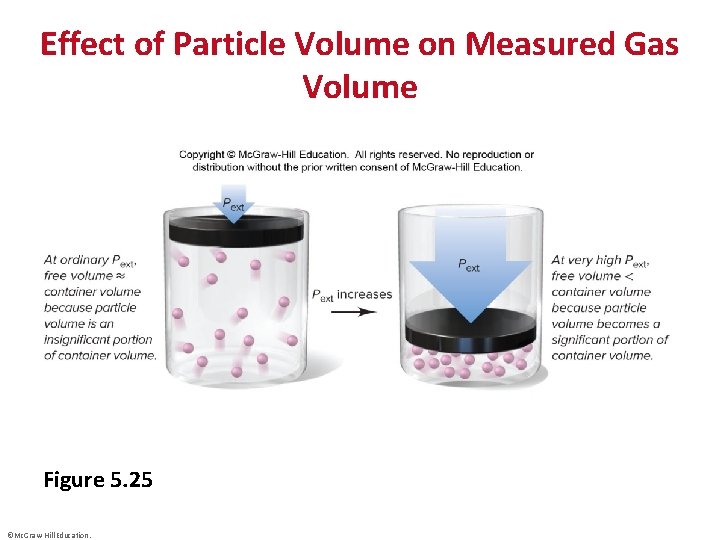
Effect of Particle Volume on Measured Gas Volume Figure 5. 25 ©Mc. Graw-Hill Education.

Van der Waals Equation • ©Mc. Graw-Hill Education.

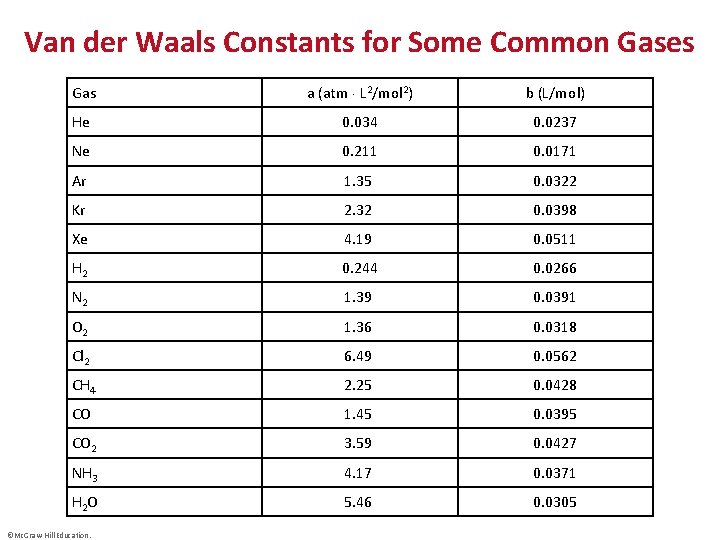
Van der Waals Constants for Some Common Gases Gas a (atm L 2/mol 2) b (L/mol) He 0. 034 0. 0237 Ne 0. 211 0. 0171 Ar 1. 35 0. 0322 Kr 2. 32 0. 0398 Xe 4. 19 0. 0511 H 2 0. 244 0. 0266 N 2 1. 39 0. 0391 O 2 1. 36 0. 0318 Cl 2 6. 49 0. 0562 CH 4 2. 25 0. 0428 CO 1. 45 0. 0395 CO 2 3. 59 0. 0427 NH 3 4. 17 0. 0371 H 2 O 5. 46 0. 0305 ©Mc. Graw-Hill Education.
 Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chemistry matter and change answer key chapter 2
Chemistry matter and change answer key chapter 2 Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
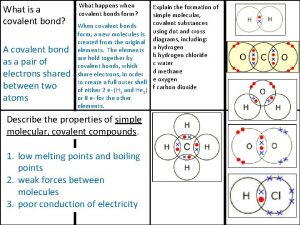
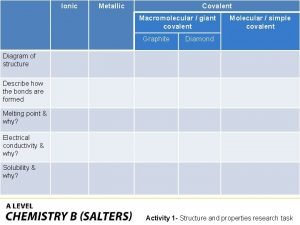
Basic food chemistry the nature of matter Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Grey matter in nervous system
Grey matter in nervous system Arbor vitae
Arbor vitae Gray matter and white matter
Gray matter and white matter What is gray matter
What is gray matter Chemistry matter and its changes
Chemistry matter and its changes Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Ap chemistry molecular geometry
Ap chemistry molecular geometry Nature and nature's laws lay hid in night
Nature and nature's laws lay hid in night Section 1 composition of matter
Section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Chapter 1 review matter and change
Chapter 1 review matter and change Big idea 9 changes in matter
Big idea 9 changes in matter Unit 2 matter and change
Unit 2 matter and change Circle the solute and underline the solvent
Circle the solute and underline the solvent Chapter 8 lesson 1
Chapter 8 lesson 1 The nature of matter chapter 2
The nature of matter chapter 2 Examples of matter in chemistry
Examples of matter in chemistry Mixture flow chart
Mixture flow chart Flowchart undissolved solids
Flowchart undissolved solids Mixture graphic organizer
Mixture graphic organizer Determinace lidské psychiky
Determinace lidské psychiky Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Changes of state of matter
Changes of state of matter Types of change in matter
Types of change in matter Chemical changes example
Chemical changes example Absolute change and relative change formula
Absolute change and relative change formula Difference between physical change and chemical change
Difference between physical change and chemical change Change in supply and change in quantity supplied
Change in supply and change in quantity supplied What is example of physical change
What is example of physical change Rocks change due to temperature and pressure change
Rocks change due to temperature and pressure change Whats physical change
Whats physical change First order change
First order change Theories of social change
Theories of social change The nature of planned change
The nature of planned change Heating and cooling curves
Heating and cooling curves Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Glasgow thang điểm
Glasgow thang điểm Chúa yêu trần thế
Chúa yêu trần thế Môn thể thao bắt đầu bằng chữ f
Môn thể thao bắt đầu bằng chữ f Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế chỉ nói điều hay thôi
Cái miệng nó xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Giọng cùng tên là
Giọng cùng tên là Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Phối cảnh
Phối cảnh Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế