MATTER Chapter 8 Lesson 1 What is matter








- Slides: 10

MATTER Chapter 8 Lesson 1

What is matter made of? • Scientists know of more than 112 elements which are classified as metals, nonmetals, or metalloids. • Metals are shiny, conduct heat and electricity, and bend easily Matter is made of building blocks called chemical elements • Nonmetals are elements that are dull, poor conductors of heat and electricity, and brittle - material that cannot be broken down into anything simpler • Metalloids have some properties of metals and some of nonmetals • - anything that has mass and takes up space • Matter is all around you! You are matter and so it this book and the air you breathe • •

What is matter made of? INSIDE ATOMS • In 1803, John Dalton proposed that elements are made of tiny particles called atoms • - the smallest unit of an element that still retains the properties of that element • Atoms are made of even smaller particles • Proton- particle with one unit of positive • • • electric charge Electrons- smaller particles with one unit of negative electric charge. They move within the space outside the nucleus Neutrons- particle with no electric chargeit is neutral Atomic Number- number of protons in an atom Atomic Mass Unit (amu)- Protons and neutrons have about the same mass. In 1913, Niels Bohr pictured an atom’s electrons moving around the nucleus like planets moving around a star

What are compounds? • - forms when two or more elements combine • All compounds have chemical names, and many have common names as well • You’ve probably used table salt before which is made from two elements: sodium and chlorine. • The chemical name indicates which elements make up the compound: rust = iron oxide. • Compounds have properties different from their individual elements. The joining of the atoms is what gives the sodium chloride new and different properties

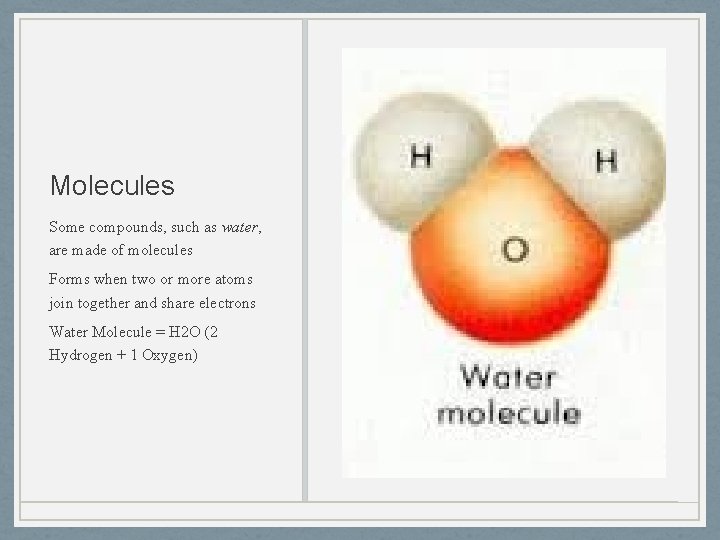
Molecules Some compounds, such as water, are made of molecules Forms when two or more atoms join together and share electrons Water Molecule = H 2 O (2 Hydrogen + 1 Oxygen)

How are elements grouped?

What forms can matter have? • • A state of matter is one of the three common forms that matter can take: solid, liquid, or gas You interact with these states every day and each state has its own properties • Solids stay in a definite shape with a definite volume no matter what container it is in • A rock is a solid. • Liquids move more freely than a solid. They can take shape of its container • Water is a liquid • Gas particles have no definite shape or volume. At room temperature, gas moves around to fill their container. It can expand fill things such as tires or balloons

Changes in states of matter • Elements can change states of matter • Water is a liquid. When frozen it can change to a solid in the form of ice. When it is heated (boiled) it turns into gas (steam).

Let’s take a look… Which term defines any substance that cannot be broken down into a simpler substance? Turn and talk with a partner before you answer A. Atom B. Compound C. Element D. Molecule Answer: C-Element

A few more… • • When 2 oxygen atoms bond chemically with 2 hydrogen atoms, hydrogen peroxide is formed. Which phrase defines this substance? A. An element B. A molecule C. A compound D. A mixture Answer: C-Compounds are formed when atoms are chemically bonded together • As water evaporates, which best explains the change that is occurring? A. A gas changes to a liquid B. A gas changes to a solid C. A liquid changes to a gas D. A liquid changes to a solid Answer: C- as water is heated, it begins to evaporate and is in gas form (usually as steam).