CHEMISTRY The Molecular Nature of Matter and Change











































- Slides: 62

CHEMISTRY The Molecular Nature of Matter and Change 3 rd Edition Chapter 7 Lecture Notes: Quantum Theory and Atomic Structure Chem 150 - Ken Marr - Winter 2006

Welcome to Chem 150!! Below are a few due dates and other useful information 1. 2. 3. Do the Prelab Preparation for tomorrow's lab activity, Atomic Spectrum of Hydrogen. Turn in the prelab questions at the start of lab and complete in your lab notebook the following sections of the report for this lab exercise: Title, Introduction, Materials/Methods and Data Tables. The completed report for lab 1 is due on Monday January 9, 2005. Due Friday January 6, 2006: ALE 1

Quantum Theory and Atomic Structure 7. 1 The Nature of Light 7. 2 Atomic Spectra 7. 3 The Wave-Particle Duality of Matter and Energy 7. 4 The Quantum-Mechanical Model of the Atom

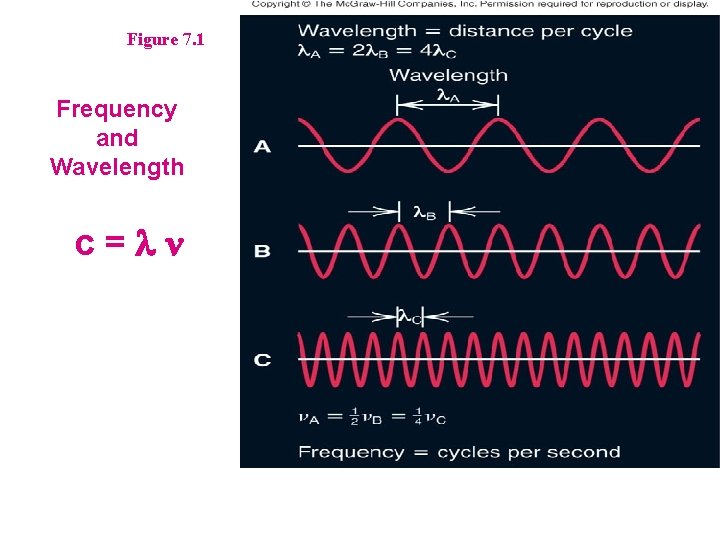
Section 7. 1 The Nature of Light (Electromagnetic Radiation) • • Light consists of waves with electrical and magnetic components Waves have a specific Frequency and Wavelength » Symbol and Units of Each? c = n l = 3. 00 X 10 8 m/s C = 2. 99792 X 108 m/s

Figure 7. 1 Frequency and Wavelength c=ln

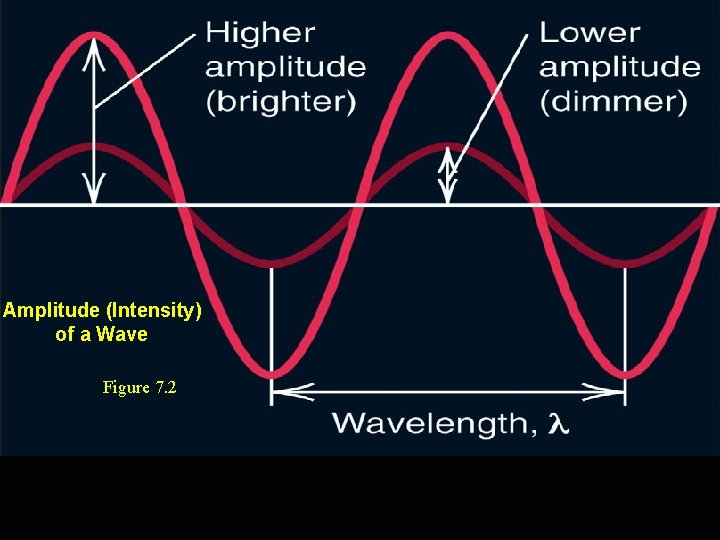
Amplitude (Intensity) of a Wave Figure 7. 2

Figure 7. 3 Regions of the Electromagnetic Spectrum Increasing Wavelength Increasing Frequency, S-1


Practice Problems: Interconverting Frequency and Wavelength 1. 2. Calculate the frequency in hertz of green light with a wavelength of 550 nm. Calculate the broadcast wavelength in meters of an FM radio station that broadcasts at 104. 3 MHz. Answers: 1. 5. 4 x 1014 hertz 2. 2. 876 m

Wave-Particle Duality of Light: in some cases light behaves as waves, in other times as photons (particles) 1. Evidence for Wave Behavior of light » Refraction of light » Diffraction of light 2. Evidence for Particle Behavior of light » Blackbody Radiation » Photoelectric Effect

Fig. 7. 4 Different Behaviors of Waves and Particles Refraction of Light Speed changes when pebble enters H 2 O Diffraction of Light

Evidence for the wave nature of light Diffraction of Light—

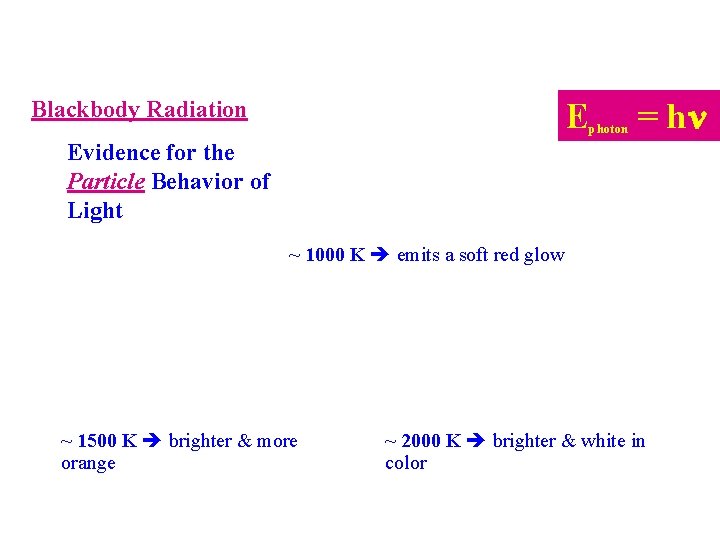
Blackbody Radiation E photon = hn Evidence for the Particle Behavior of Light ~ 1000 K emits a soft red glow ~ 1500 K brighter & more orange ~ 2000 K brighter & white in color

Blackbody Radiation Evidence for Particle Behavior of Light Only specific colors of light are emitted when blackbodies (heated solids) are heated 1. ~ 1000 K emits a soft red glow ~ 1500 K brighter and more orange ~ 2000 K brighter and white in color Max Planck’s (1900): Atoms can only absorb or give off specific packets or quanta of light energy. 2. • These packet of energy are called photons.

Particle Nature of Light Max Planck (1900) • EMR is emitted as weightless packets of energy called photons • Each photon has its own energy and frequency, n Ephoton = hn h = Planck’s constant = 6. 626 x 10 -34 J. s

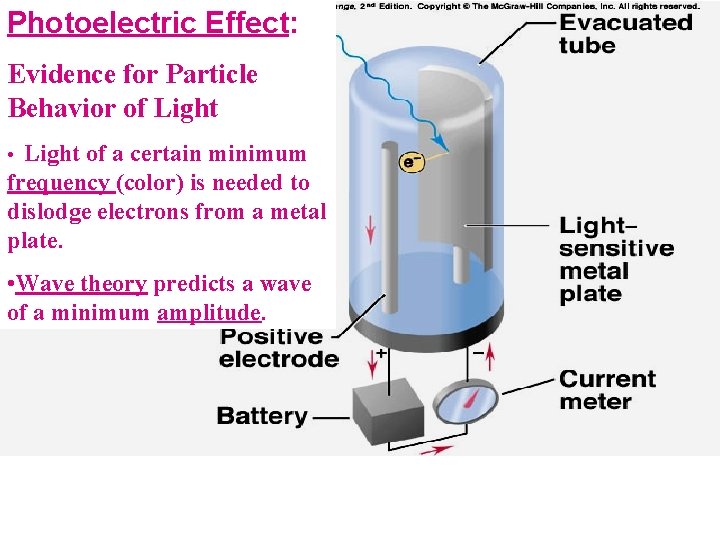
Photoelectric Effect: Evidence for Particle Behavior of Light • Light of a certain minimum frequency (color) is needed to dislodge electrons from a metal plate. • Wave theory predicts a wave of a minimum amplitude.

Einstein’s Explanation of the Photoelectric Effect (1905) 1. 2. Light intensity is due to the number of photons striking the metal per second, not the amplitude A photon of some minimum energy must be absorbed by the metal E photon= hn

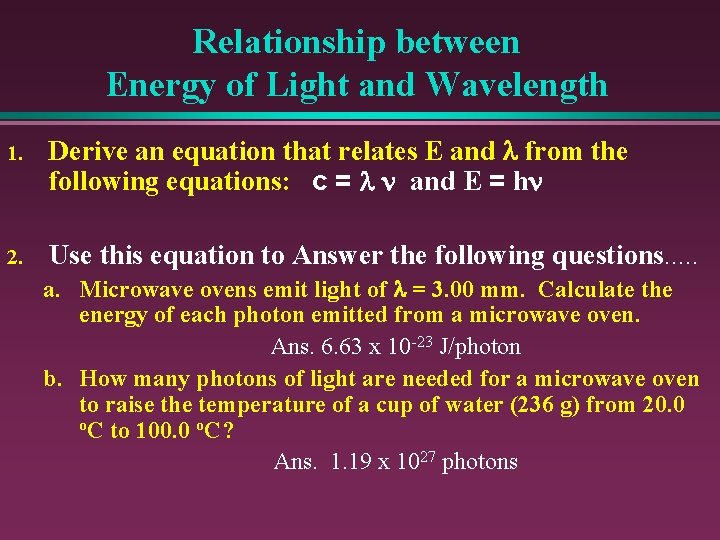
Relationship between Energy of Light and Wavelength 1. Derive an equation that relates E and l from the following equations: c = l n and E = hn 2. Use this equation to Answer the following questions. . . a. Microwave ovens emit light of l = 3. 00 mm. Calculate the energy of each photon emitted from a microwave oven. Ans. 6. 63 x 10 -23 J/photon b. How many photons of light are needed for a microwave oven to raise the temperature of a cup of water (236 g) from 20. 0 o. C to 100. 0 o. C? Ans. 1. 19 x 1027 photons

Section 7. 2 Atomic Spectra Continuous Spectrum 1. • Sunlight or from object heated to a very high temperature (e. g. light filament) Atomic Spectrum 2. • • Also called line, bright line or emission spectrum Due to an atom’s electron(s) excited by electricity or heat falling from a higher to a lower energy level— more about this later!!

Continuous Spectrum Line Spectra

Rydberg Equation Predicts the Hydrogen Spectrum Rydberg Equation • Empirically derived to fit hydrogen’s atomic spectrum • Predicts l’s of invisible line spectra e. g. Hydrogen’s Ultraviolet line spectrum (n. L = 1) L R = 1. 096776 x 107 m-1 H n = 1, 2, 3, 4, …


Using the Rydberg Equation Practice Exercise: Calculate the wavelength in nm and determine the color of the line in the visible spectrum of hydrogen for which n. L = 2 and n. H = 3. Ans. 656. 4 nm Color? ?

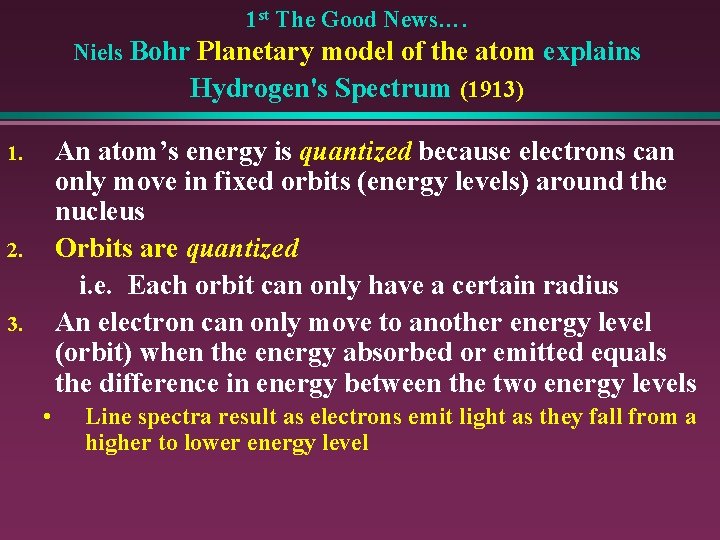
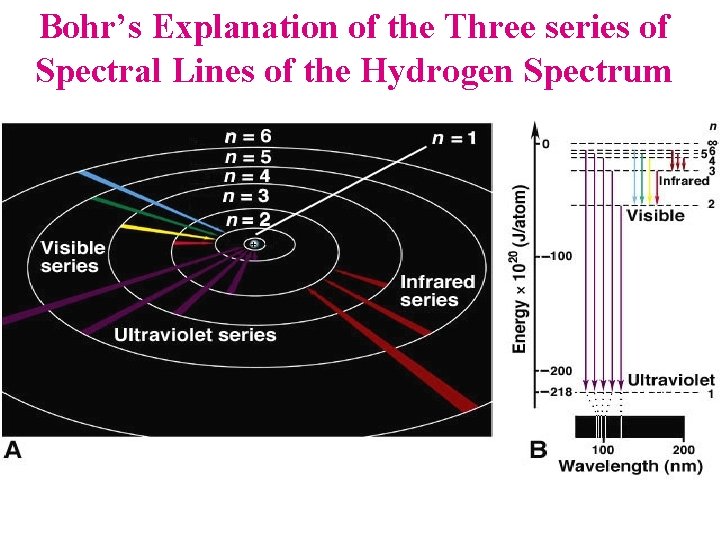
1 st The Good News…. Niels Bohr Planetary model of the atom explains Hydrogen's Spectrum (1913) An atom’s energy is quantized because electrons can only move in fixed orbits (energy levels) around the nucleus Orbits are quantized i. e. Each orbit can only have a certain radius An electron can only move to another energy level (orbit) when the energy absorbed or emitted equals the difference in energy between the two energy levels 1. 2. 3. • Line spectra result as electrons emit light as they fall from a higher to lower energy level

Bohr’s Explanation of the Three series of Spectral Lines of the Hydrogen Spectrum


Animation of Bohr’s Planetary Model 1. 2. Animation (Flash) Animation (Quick. Time)

Bohr’s Equation Derived from the Ideas of Planck, Einstein & Classical Physics 1. DEelectron = ELower - EHigher or DEelectron = Efinal - Einitial 2. DEelectron = -2. 18 x 10 -19 J (1/n 2 Lower - 1/n 2 higher) But…… DE = hc/ l, substitution yields… 3. 1/l = 1. 10 x 107 m-1 (1/n 2 Lower - 1/n 2 higher) • • Bohr’s Constant is within 0. 05 % of the Rydberg Constant Equation provides a theoretical explanation of Hydrogen’s Atomic Spectrum

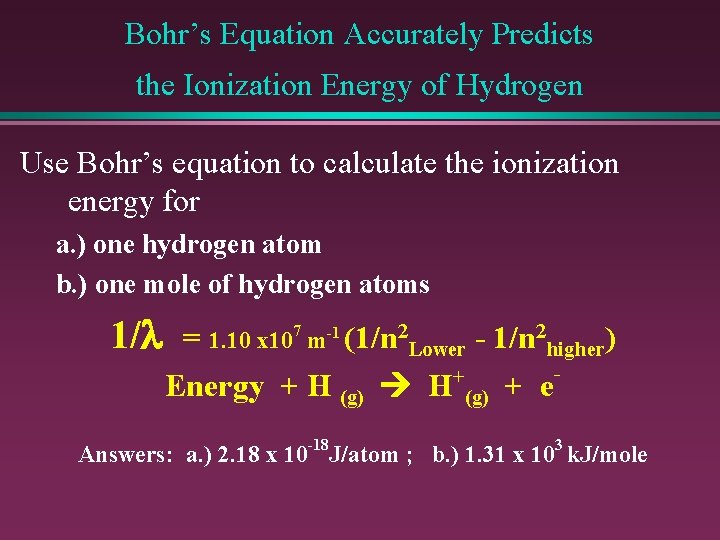
Bohr’s Equation Accurately Predicts the Ionization Energy of Hydrogen Use Bohr’s equation to calculate the ionization energy for a. ) one hydrogen atom b. ) one mole of hydrogen atoms 1/l = 1. 10 x 107 m-1 (1/n 2 Lower - 1/n 2 higher) + Energy + H (g) + e Answers: a. ) 2. 18 x 10 -18 J/atom ; b. ) 1. 31 x 103 k. J/mole

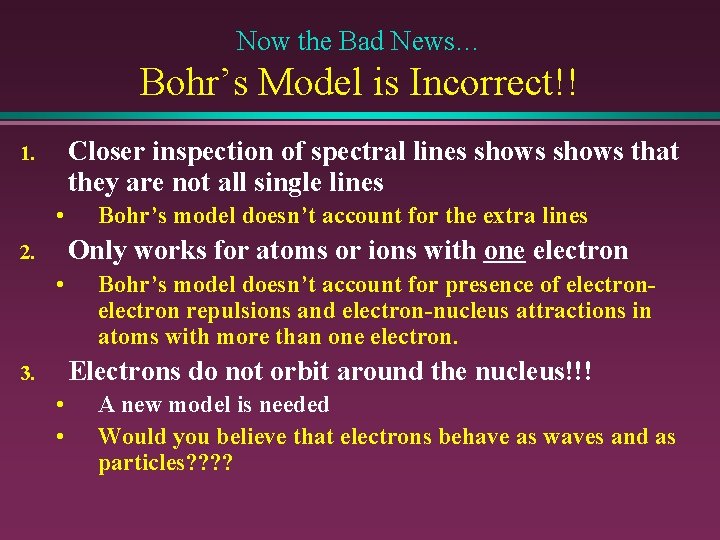
Now the Bad News… Bohr’s Model is Incorrect!! Closer inspection of spectral lines shows that they are not all single lines 1. • Bohr’s model doesn’t account for the extra lines Only works for atoms or ions with one electron 2. • Bohr’s model doesn’t account for presence of electron repulsions and electron-nucleus attractions in atoms with more than one electron. Electrons do not orbit around the nucleus!!! 3. • • A new model is needed Would you believe that electrons behave as waves and as particles? ?

Section 7. 3 The Wave-Particle Duality of Matter Electron Diffraction: Evidence that electrons behave as waves! Davisson & Germer (1927) Electrons are diffracted by solids just like Xrays! Hence, electrons behave as waves! X-Ray tube Source of electrons Aluminum X-Ray diffraction pattern of Aluminum Electron diffraction pattern of Aluminum


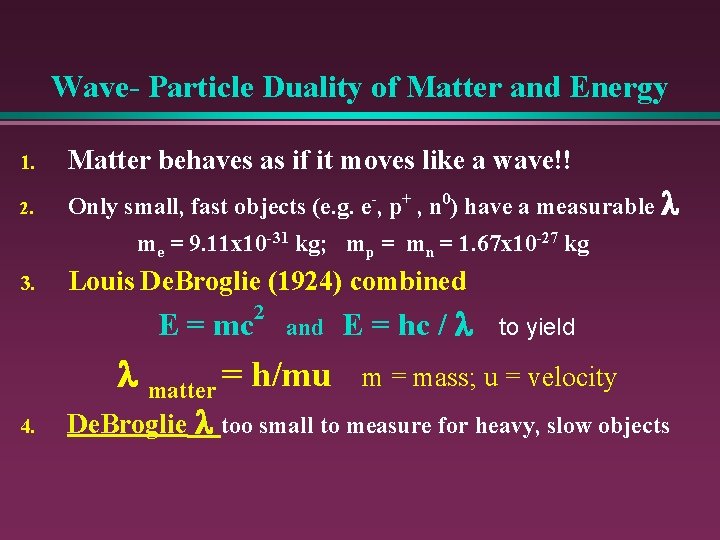
Wave- Particle Duality of Matter and Energy 1. Matter behaves as if it moves like a wave!! 2. Only small, fast objects (e. g. e-, p+ , n 0) have a measurable l me = 9. 11 x 10 -31 kg; mp = mn = 1. 67 x 10 -27 kg 3. Louis De. Broglie (1924) combined E = mc 2 and l matter = h/mu 4. E = hc / l to yield m = mass; u = velocity De. Broglie l too small to measure for heavy, slow objects


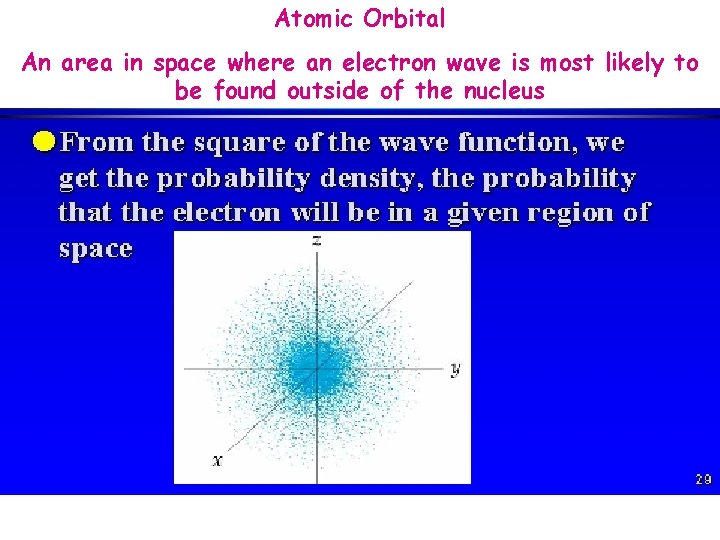
Locating an Electron. . an uncertain affair!! Orbital 1. • Region in space where an electron wave is most likely to be found Exact location of an electron can’t be determined Can only determine the probability of finding an electron. . why? 2. 3. • • Electrons behave as waves!! In order to “see” the position of an electron we must probe it with radiation which changes its position and/or velocity




Section 7. 4 Quantum Mechanical Model of the Atom: Electron Waves in Atoms Electrons are standing waves 1. • • Peaks and troughs only move up and down Similar to how guitar strings move Orbitals 2. • • Are areas in space where electron waves are most likely to be found Orbitals are made of electron waves


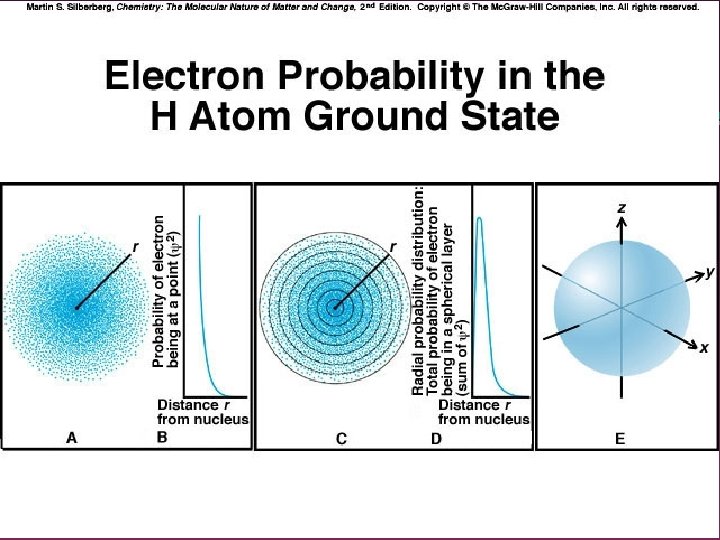
Quantum Mechanics and Atomic Orbitals • • Erin Schrodinger (1926) developed a mathematical equation called a wave function to describe the energy of electrons The square of the wave function gives the probability of finding an electron at any point in space, thus producing a map of an orbital


Atomic Orbital An area in space where an electron wave is most likely to be found outside of the nucleus




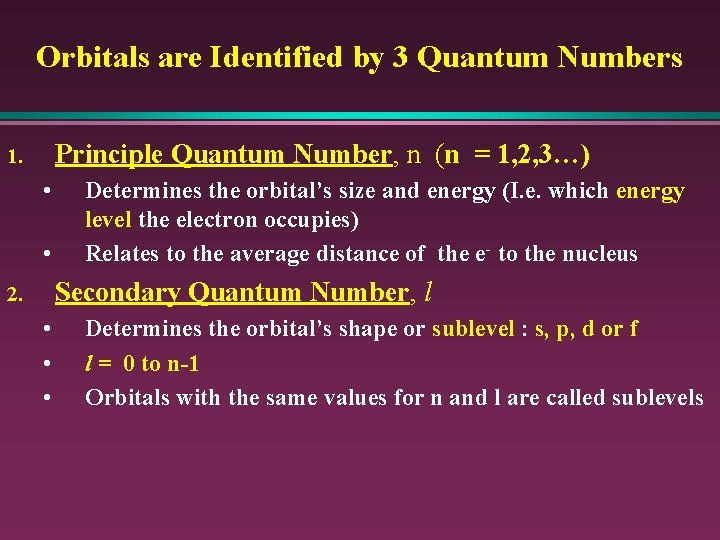
Orbitals are Identified by 3 Quantum Numbers Principle Quantum Number, n (n = 1, 2, 3…) 1. • • Determines the orbital’s size and energy (I. e. which energy level the electron occupies) Relates to the average distance of the e- to the nucleus Secondary Quantum Number, l 2. • • • Determines the orbital’s shape or sublevel : s, p, d or f l = 0 to n-1 Orbitals with the same values for n and l are called sublevels

Orbitals are Identified by 3 Quantum Numbers Magnetic Quantum Number, ml 3. • • • Determines the orbital’s orientation in space ml = -l, …, 0 , …+l ml represents the orbital within the sublevel. S - sublevel has 1 orbital p - sublevel has 3 orbitals d - sublevel has 5 orbitals F - sublevel has 7 orbitals


n = Principal quantum Number (size and energy of orbital) l = Angular momentum Q. N. (shape of orbital) ml = magnetic Q. N. (orientation of orbital)

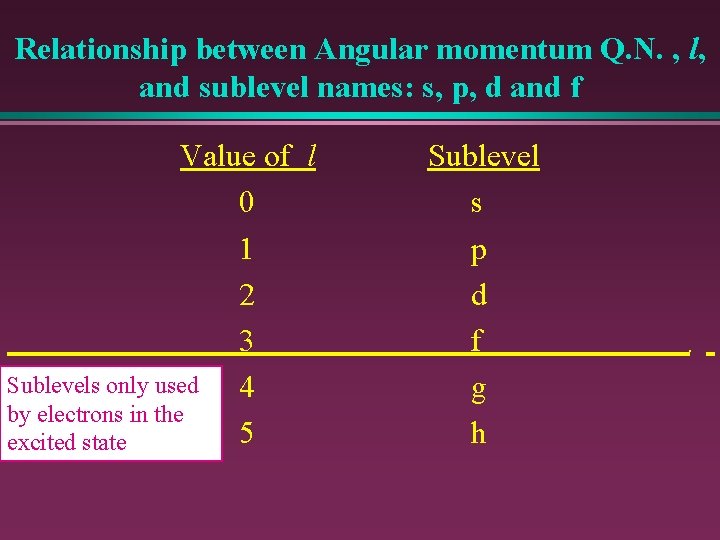
Relationship between Angular momentum Q. N. , l, and sublevel names: s, p, d and f Value of l Sublevel 0 s 1 p 2 d 3 f Sublevels only used 4 g by electrons in the 5 h excited state f

Summary of Relationships Between n, l and ml



Practice Makes Perfect? 1. What is the subshell (e. g. 1 s, 2 p, etc. ) corresponding to the following values for n and l? a. b. c. d. e. n = 2, l = 1 n = 4, l = 0 n = 3, l = 2 n = 5, l = 3 n = 3, l =3

Practice Makes Perfect? 2. Which of the following sets of quantum numbers are not possible? a. b. c. d. e. n = 2, l = 1, m l = 0 n = 2, l = 2, m l = 1 n = 2, l = 1, m l = -2 n = 3, l = 2, m l = -2 n = 0, l = 0, m l = 0

The Relationship between the 4 Quantum Numbers, Energy Levels, Sublevels and Orbitals See figure 6. 15, page 239 in Brady (Transp. )

Practice Makes Perfect? 1. 2. What subshells are found in the 4 th shell? Which subshell is higher in energy? a. 3 s or 3 p b. 4 p or 4 d c. 3 p or 4 p

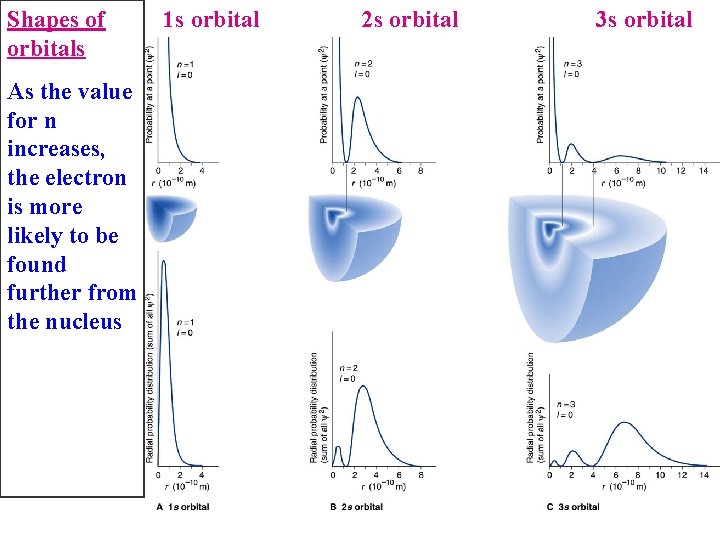
Shapes of orbitals As the value for n increases, the electron is more likely to be found further from the nucleus 1 s orbital 2 s orbital 3 s orbital

Fig. 7. 18 Shapes of the three orbitals in the 2 p sublevel: 2 px 2 py 2 pz Note that the three orbitals are mutually perpendicular to each other (fig. D), thus contributing to an atoms overall spherical shape An accurate representation of the 2 pz orbital Stylized shape of 2 pz used in most texts

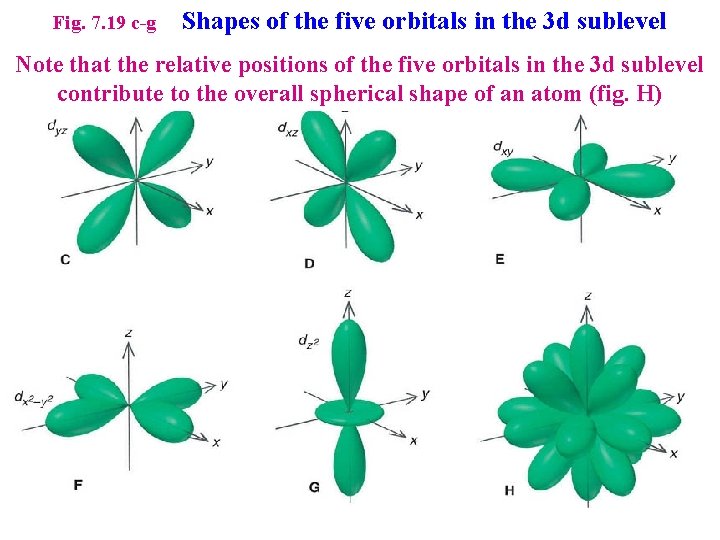
Fig. 7. 19 c-g Shapes of the five orbitals in the 3 d sublevel Note that the relative positions of the five orbitals in the 3 d sublevel contribute to the overall spherical shape of an atom (fig. H)

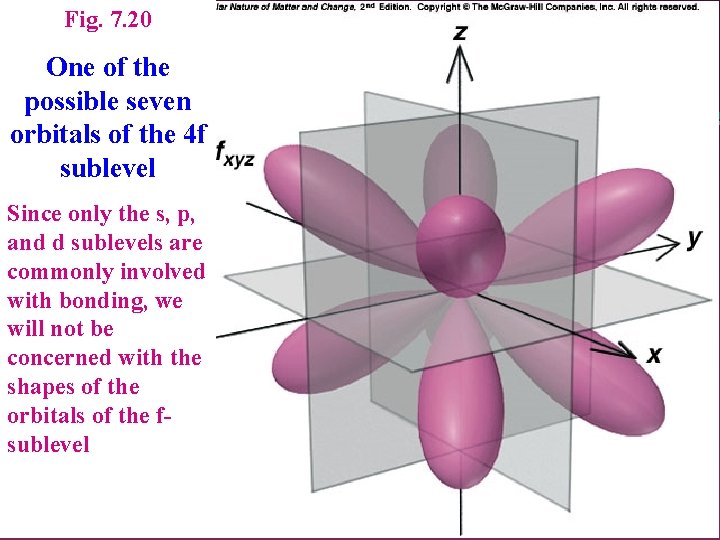
Fig. 7. 20 One of the possible seven orbitals of the 4 f sublevel Since only the s, p, and d sublevels are commonly involved with bonding, we will not be concerned with the shapes of the orbitals of the fsublevel
 Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 2 matter and change answer key
2 matter and change answer key Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
Basic food chemistry the nature of matter Physical state of covalent compounds
Physical state of covalent compounds Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Zinc oxide + nitric acid → zinc nitrate + water
Zinc oxide + nitric acid → zinc nitrate + water Grey matter
Grey matter Primary taste cortex
Primary taste cortex Gray matter and white matter
Gray matter and white matter What is grey and white matter
What is grey and white matter Chemistry matter and its changes
Chemistry matter and its changes Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Ap chemistry unit 9 notes
Ap chemistry unit 9 notes Nature and nature's laws lay hid in night meaning
Nature and nature's laws lay hid in night meaning Section 1 composition of matter
Section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter Chapter 1 review matter and change
Chapter 1 review matter and change Big idea 9 changes in matter
Big idea 9 changes in matter Unit 2 matter and change
Unit 2 matter and change Chapter 1 matter and change worksheet answers
Chapter 1 matter and change worksheet answers Chapter 8 lesson 1
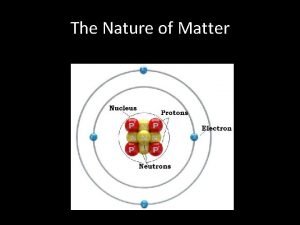
Chapter 8 lesson 1 The nature of matter chapter 2
The nature of matter chapter 2 Examples of matter in chemistry
Examples of matter in chemistry Mixture flow chart
Mixture flow chart Matter flowchart chemistry
Matter flowchart chemistry Graphic organizer of matter
Graphic organizer of matter Determinace lidské psychiky
Determinace lidské psychiky Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Change of state of matter
Change of state of matter Two types of changes
Two types of changes Physicl change
Physicl change Absolute change and relative change formula
Absolute change and relative change formula Differences between physical and chemical changes
Differences between physical and chemical changes Supply and demand curve shifts
Supply and demand curve shifts What is example of physical change
What is example of physical change Rocks change due to temperature and pressure change
Rocks change due to temperature and pressure change Whats the difference between a physical and chemical change
Whats the difference between a physical and chemical change First order change examples
First order change examples Factor of social change
Factor of social change Nature of planned change
Nature of planned change Heating and cooling curves
Heating and cooling curves Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worms-breton
Tư thế worms-breton Chúa sống lại
Chúa sống lại Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công của trọng lực
Công của trọng lực Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ