CHEMISTRY The Molecular Nature of Matter and Change



- Slides: 23

CHEMISTRY The Molecular Nature of Matter and Change Third Edition Chapter 3 Lecture Outlines* *See Power. Point Image Slides for all figures and tables pre-inserted into Power. Point without notes. 3 -1 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Chapter 3 Stoichiometry 3 -2

Mole - Mass Relationships in Chemical Systems 3. 1 The Mole 3. 2 Determining the Formula of an Unknown Compound 3. 3 Writing and Balancing Chemical Equations 3. 4 Calculating the Amounts of Reactant and Product 3. 5 Fundamentals of Solution Stoichiometry 3 -3

mole - the amount of a substance that contains the same number of entities as there atoms in exactly 12 g of carbon-12. This amount is 6. 022 x 1023. The number is called Avogadro’s number and is abbrieviated as N. One mole (1 mol) contains 6. 022 x 1023 entities (to four significant figures) 3 -4

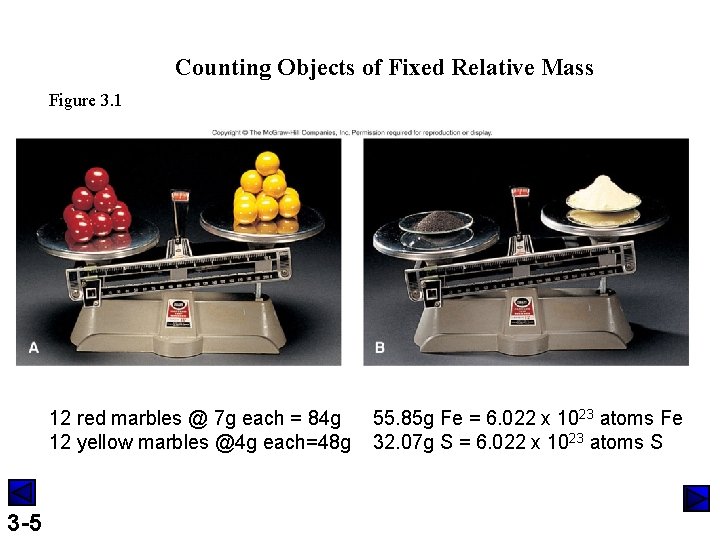
Counting Objects of Fixed Relative Mass Figure 3. 1 12 red marbles @ 7 g each = 84 g 12 yellow marbles @4 g each=48 g 3 -5 55. 85 g Fe = 6. 022 x 1023 atoms Fe 32. 07 g S = 6. 022 x 1023 atoms S

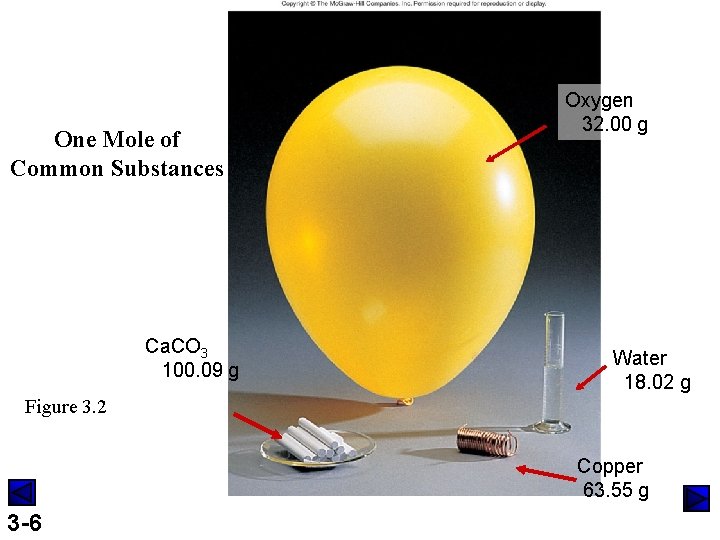
One Mole of Common Substances Ca. CO 3 100. 09 g Oxygen 32. 00 g Water 18. 02 g Figure 3. 2 Copper 63. 55 g 3 -6

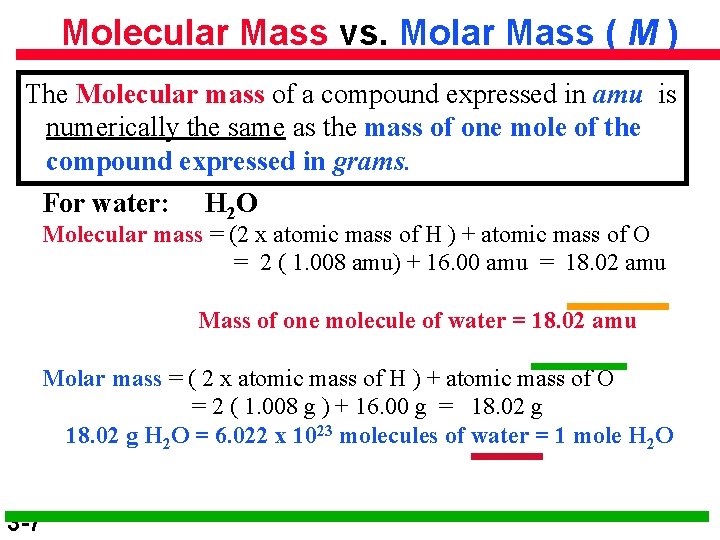
Molecular Mass vs. Molar Mass ( M ) The Molecular mass of a compound expressed in amu is numerically the same as the mass of one mole of the compound expressed in grams. For water: H 2 O Molecular mass = (2 x atomic mass of H ) + atomic mass of O = 2 ( 1. 008 amu) + 16. 00 amu = 18. 02 amu Mass of one molecule of water = 18. 02 amu Molar mass = ( 2 x atomic mass of H ) + atomic mass of O = 2 ( 1. 008 g ) + 16. 00 g = 18. 02 g H 2 O = 6. 022 x 1023 molecules of water = 1 mole H 2 O 3 -7

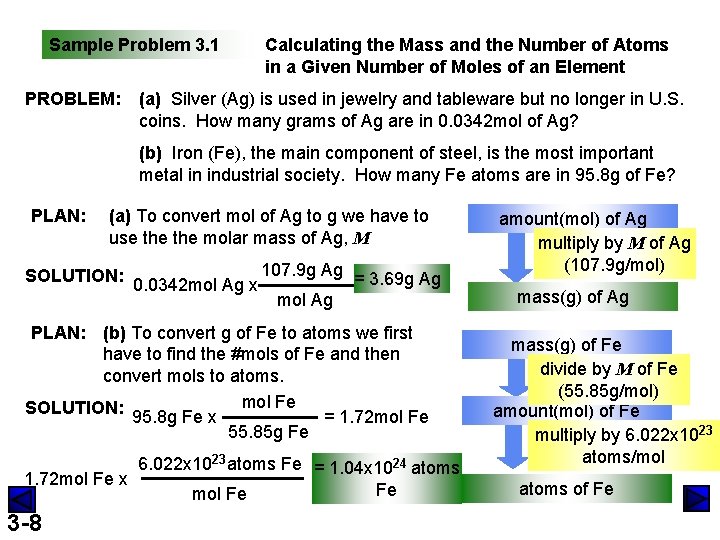
Sample Problem 3. 1 PROBLEM: Calculating the Mass and the Number of Atoms in a Given Number of Moles of an Element (a) Silver (Ag) is used in jewelry and tableware but no longer in U. S. coins. How many grams of Ag are in 0. 0342 mol of Ag? (b) Iron (Fe), the main component of steel, is the most important metal in industrial society. How many Fe atoms are in 95. 8 g of Fe? PLAN: (a) To convert mol of Ag to g we have to use the molar mass of Ag, M SOLUTION: 0. 0342 mol Ag x 107. 9 g Ag = 3. 69 g Ag mol Ag PLAN: (b) To convert g of Fe to atoms we first have to find the #mols of Fe and then convert mols to atoms. SOLUTION: 95. 8 g Fe x mol Fe = 1. 72 mol Fe 55. 85 g Fe 6. 022 x 1023 atoms Fe = 1. 04 x 1024 atoms 1. 72 mol Fe x Fe mol Fe 3 -8 amount(mol) of Ag multiply by M of Ag (107. 9 g/mol) mass(g) of Ag mass(g) of Fe divide by M of Fe (55. 85 g/mol) amount(mol) of Fe multiply by 6. 022 x 1023 atoms/mol atoms of Fe

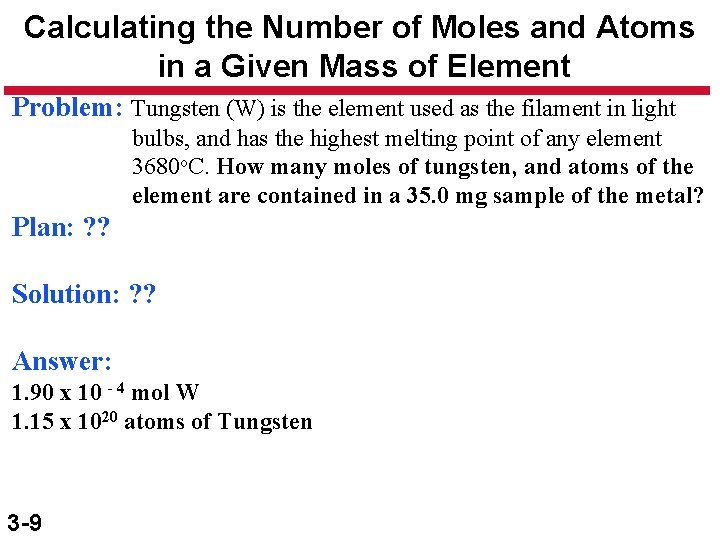
Calculating the Number of Moles and Atoms in a Given Mass of Element Problem: Tungsten (W) is the element used as the filament in light bulbs, and has the highest melting point of any element 3680 o. C. How many moles of tungsten, and atoms of the element are contained in a 35. 0 mg sample of the metal? Plan: ? ? Solution: ? ? Answer: 1. 90 x 10 - 4 mol W 1. 15 x 1020 atoms of Tungsten 3 -9

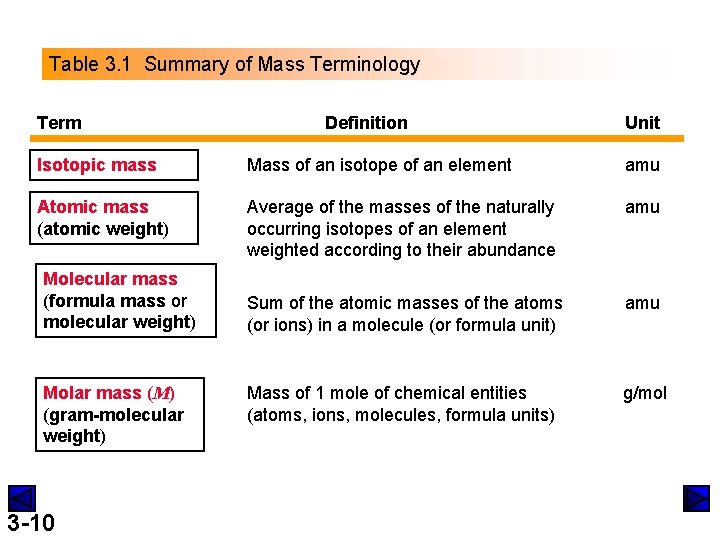
Table 3. 1 Summary of Mass Terminology Term Definition Unit Isotopic mass Mass of an isotope of an element amu Atomic mass (atomic weight) Average of the masses of the naturally occurring isotopes of an element weighted according to their abundance amu Sum of the atomic masses of the atoms (or ions) in a molecule (or formula unit) amu Mass of 1 mole of chemical entities (atoms, ions, molecules, formula units) g/mol Molecular mass (formula mass or molecular weight) Molar mass (M) (gram-molecular weight) 3 -10

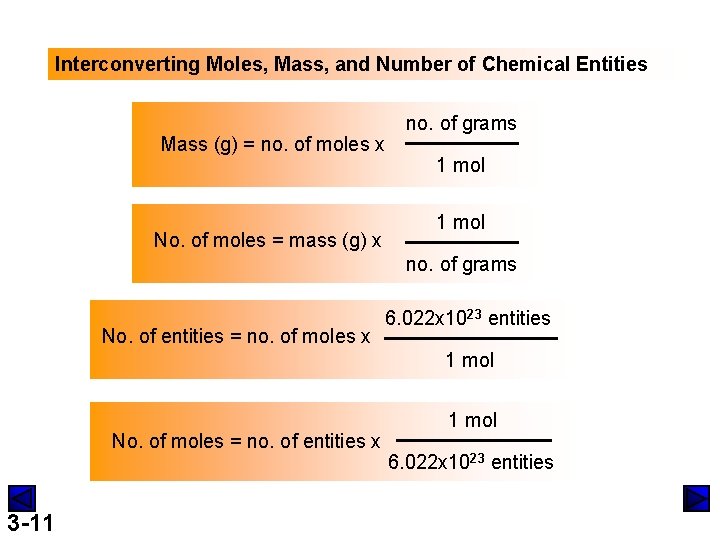
Interconverting Moles, Mass, and Number of Chemical Entities Mass (g) = no. of moles x No. of moles = mass (g) x no. of grams 1 mol no. of grams No. of entities = no. of moles x 6. 022 x 1023 entities 1 mol No. of moles = no. of entities x 3 -11 1 mol 6. 022 x 1023 entities

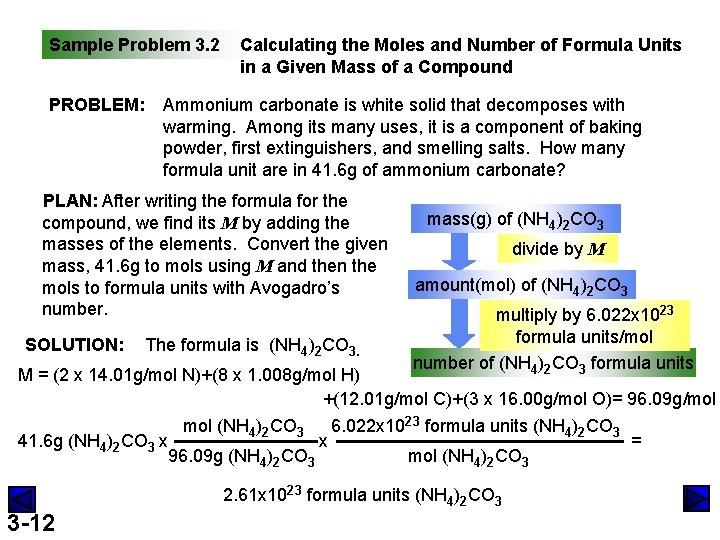
Sample Problem 3. 2 PROBLEM: Calculating the Moles and Number of Formula Units in a Given Mass of a Compound Ammonium carbonate is white solid that decomposes with warming. Among its many uses, it is a component of baking powder, first extinguishers, and smelling salts. How many formula unit are in 41. 6 g of ammonium carbonate? PLAN: After writing the formula for the compound, we find its M by adding the masses of the elements. Convert the given mass, 41. 6 g to mols using M and then the mols to formula units with Avogadro’s number. SOLUTION: The formula is (NH 4)2 CO 3. mass(g) of (NH 4)2 CO 3 divide by M amount(mol) of (NH 4)2 CO 3 multiply by 6. 022 x 1023 formula units/mol number of (NH 4)2 CO 3 formula units M = (2 x 14. 01 g/mol N)+(8 x 1. 008 g/mol H) +(12. 01 g/mol C)+(3 x 16. 00 g/mol O)= 96. 09 g/mol 41. 6 g (NH 4)2 CO 3 x mol (NH 4)2 CO 3 96. 09 g (NH 4)2 CO 3 x 6. 022 x 1023 formula units (NH 4)2 CO 3 mol (NH 4)2 CO 3 2. 61 x 1023 formula units (NH 4)2 CO 3 3 -12 =

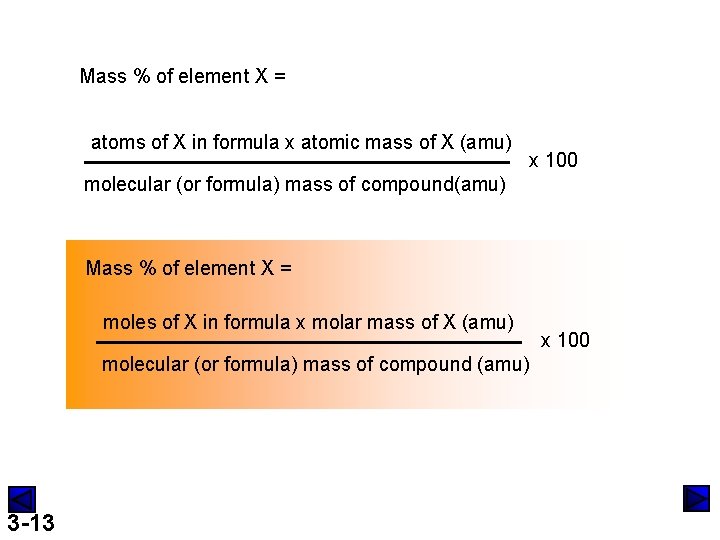
Mass % of element X = atoms of X in formula x atomic mass of X (amu) x 100 molecular (or formula) mass of compound(amu) Mass % of element X = moles of X in formula x molar mass of X (amu) molecular (or formula) mass of compound (amu) 3 -13 x 100

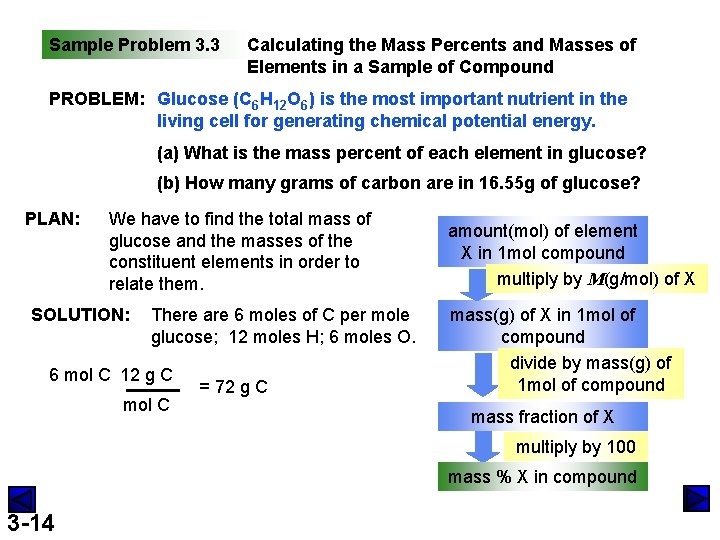
Sample Problem 3. 3 Calculating the Mass Percents and Masses of Elements in a Sample of Compound PROBLEM: Glucose (C 6 H 12 O 6) is the most important nutrient in the living cell for generating chemical potential energy. (a) What is the mass percent of each element in glucose? (b) How many grams of carbon are in 16. 55 g of glucose? PLAN: We have to find the total mass of glucose and the masses of the constituent elements in order to relate them. SOLUTION: There are 6 moles of C per mole glucose; 12 moles H; 6 moles O. 6 mol C 12 g C mol C = 72 g C amount(mol) of element X in 1 mol compound multiply by M(g/mol) of X mass(g) of X in 1 mol of compound divide by mass(g) of 1 mol of compound mass fraction of X multiply by 100 mass % X in compound 3 -14

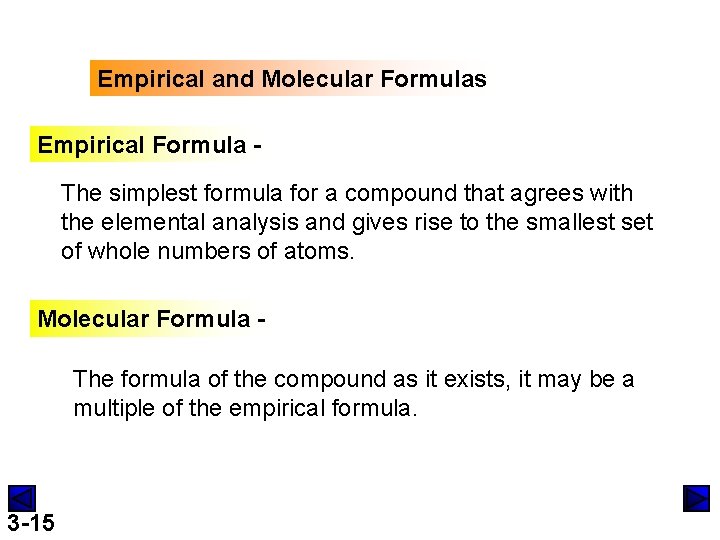
Empirical and Molecular Formulas Empirical Formula The simplest formula for a compound that agrees with the elemental analysis and gives rise to the smallest set of whole numbers of atoms. Molecular Formula The formula of the compound as it exists, it may be a multiple of the empirical formula. 3 -15

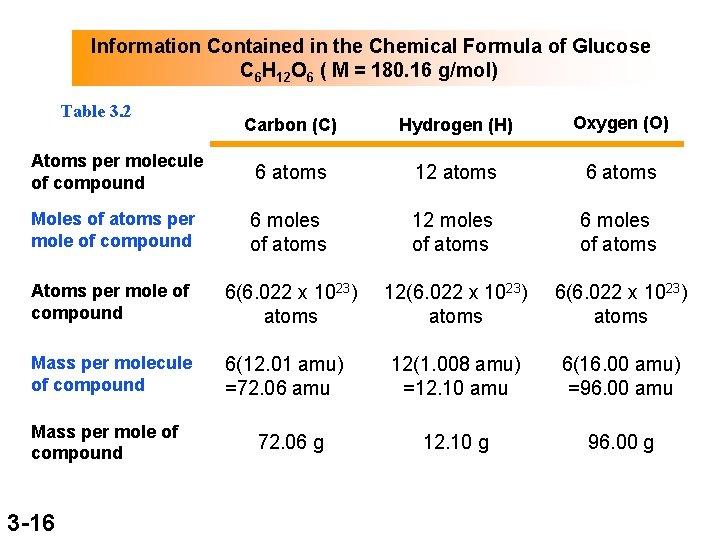
Information Contained in the Chemical Formula of Glucose C 6 H 12 O 6 ( M = 180. 16 g/mol) Table 3. 2 Carbon (C) Hydrogen (H) Oxygen (O) Atoms per molecule of compound 6 atoms 12 atoms 6 atoms Moles of atoms per mole of compound 6 moles of atoms 12 moles of atoms 6 moles of atoms Atoms per mole of compound 6(6. 022 x 1023) atoms 12(6. 022 x 1023) atoms 6(6. 022 x 1023) atoms Mass per molecule of compound 6(12. 01 amu) =72. 06 amu 12(1. 008 amu) =12. 10 amu 6(16. 00 amu) =96. 00 amu 12. 10 g 96. 00 g Mass per mole of compound 3 -16 72. 06 g

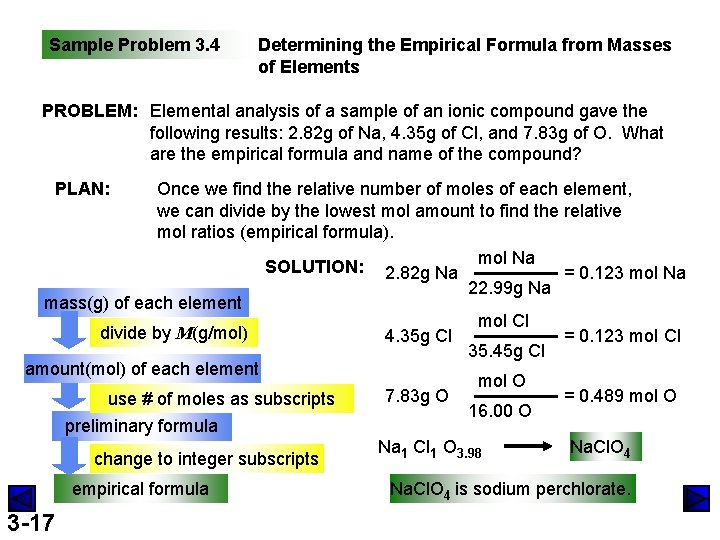
Sample Problem 3. 4 Determining the Empirical Formula from Masses of Elements PROBLEM: Elemental analysis of a sample of an ionic compound gave the following results: 2. 82 g of Na, 4. 35 g of Cl, and 7. 83 g of O. What are the empirical formula and name of the compound? PLAN: Once we find the relative number of moles of each element, we can divide by the lowest mol amount to find the relative mol ratios (empirical formula). SOLUTION: 2. 82 g Na mol Na = 0. 123 mol Na 22. 99 g Na mass(g) of each element mol Cl divide by M(g/mol) 4. 35 g Cl = 0. 123 mol Cl 35. 45 g Cl amount(mol) of each element mol O 7. 83 g O = 0. 489 mol O use # of moles as subscripts 16. 00 O preliminary formula Na 1 Cl 1 O 3. 98 Na. Cl. O 4 change to integer subscripts empirical formula 3 -17 Na. Cl. O 4 is sodium perchlorate.

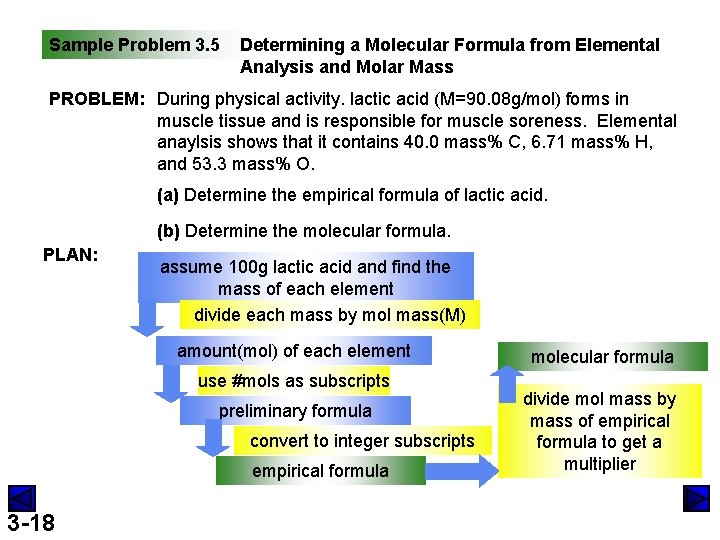
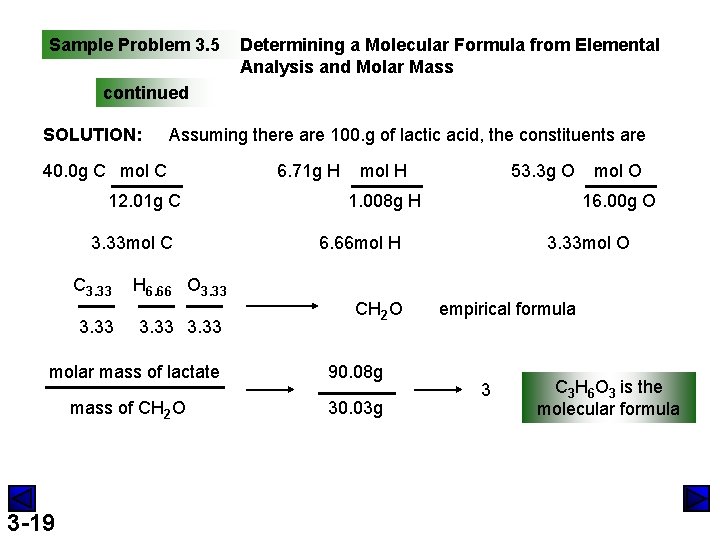
Sample Problem 3. 5 Determining a Molecular Formula from Elemental Analysis and Molar Mass PROBLEM: During physical activity. lactic acid (M=90. 08 g/mol) forms in muscle tissue and is responsible for muscle soreness. Elemental anaylsis shows that it contains 40. 0 mass% C, 6. 71 mass% H, and 53. 3 mass% O. (a) Determine the empirical formula of lactic acid. (b) Determine the molecular formula. PLAN: assume 100 g lactic acid and find the mass of each element divide each mass by mol mass(M) amount(mol) of each element use #mols as subscripts preliminary formula convert to integer subscripts empirical formula 3 -18 molecular formula divide mol mass by mass of empirical formula to get a multiplier

Sample Problem 3. 5 Determining a Molecular Formula from Elemental Analysis and Molar Mass continued SOLUTION: Assuming there are 100. g of lactic acid, the constituents are 40. 0 g C mol C 12. 01 g C 3. 33 mol C C 3. 33 H 6. 66 O 3. 33 molar mass of lactate mass of CH 2 O 3 -19 6. 71 g H 53. 3 g O mol H 1. 008 g H 16. 00 g O 6. 66 mol H CH 2 O 90. 08 g 30. 03 g mol O 3. 33 mol O empirical formula 3 C 3 H 6 O 3 is the molecular formula

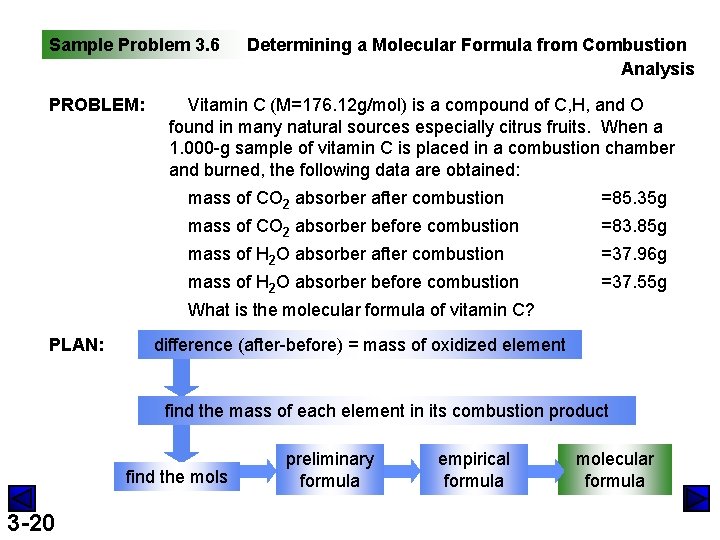
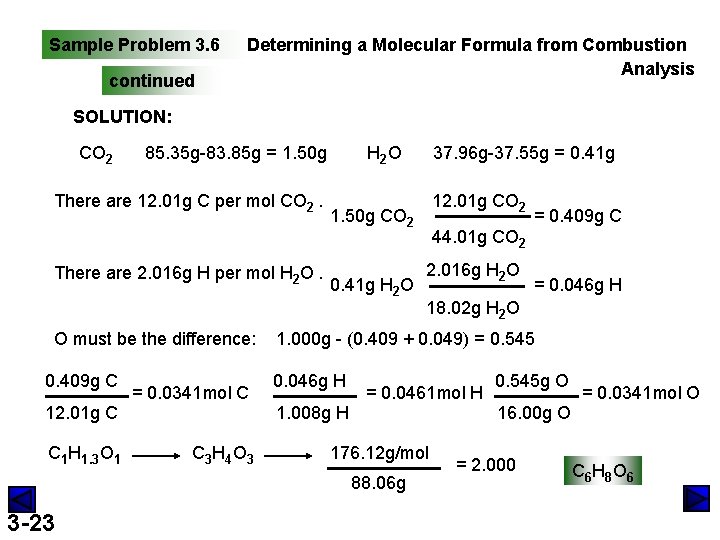
Sample Problem 3. 6 PROBLEM: Determining a Molecular Formula from Combustion Analysis Vitamin C (M=176. 12 g/mol) is a compound of C, H, and O found in many natural sources especially citrus fruits. When a 1. 000 -g sample of vitamin C is placed in a combustion chamber and burned, the following data are obtained: mass of CO 2 absorber after combustion =85. 35 g mass of CO 2 absorber before combustion =83. 85 g mass of H 2 O absorber after combustion =37. 96 g mass of H 2 O absorber before combustion =37. 55 g What is the molecular formula of vitamin C? PLAN: difference (after-before) = mass of oxidized element find the mass of each element in its combustion product find the mols 3 -20 preliminary formula empirical formula molecular formula

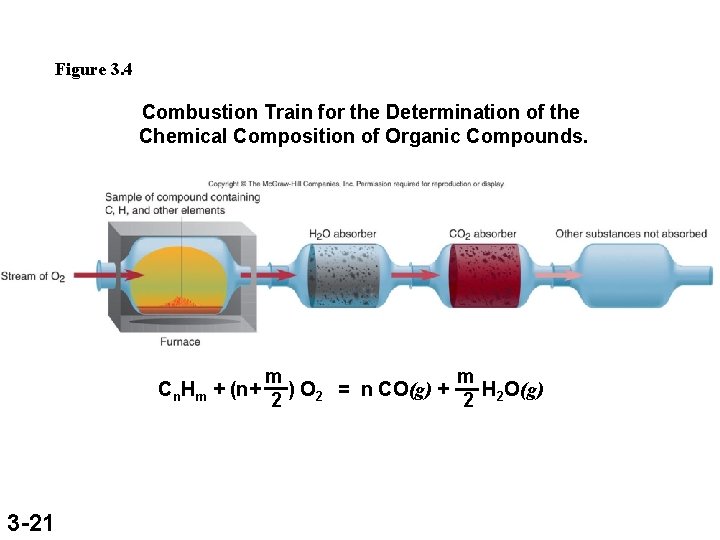
Figure 3. 4 Combustion Train for the Determination of the Chemical Composition of Organic Compounds. Cn. Hm + (n+ 3 -21 m m ) O 2 = n CO(g) + H O(g) 2 2 2

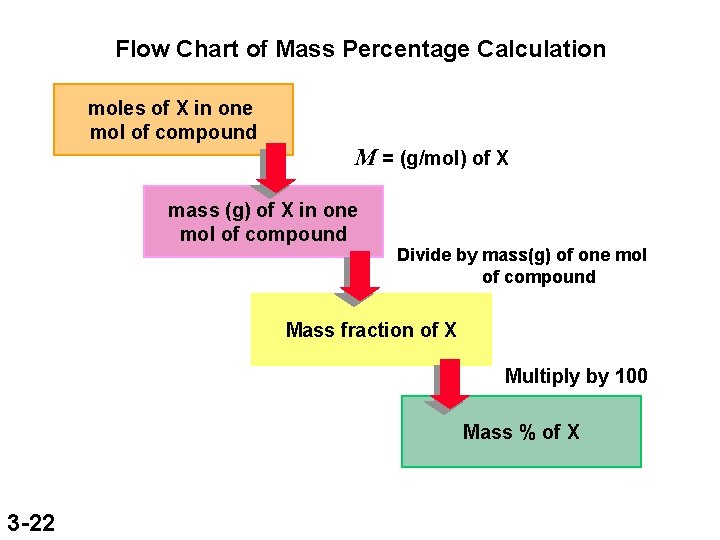
Flow Chart of Mass Percentage Calculation moles of X in one mol of compound M = (g/mol) of X mass (g) of X in one mol of compound Divide by mass(g) of one mol of compound Mass fraction of X Multiply by 100 Mass % of X 3 -22

Sample Problem 3. 6 continued Determining a Molecular Formula from Combustion Analysis SOLUTION: CO 2 85. 35 g-83. 85 g = 1. 50 g There are 12. 01 g C per mol CO 2. There are 2. 016 g H per mol H 2 O. O must be the difference: 0. 409 g C 12. 01 g C C 1 H 1. 3 O 1 = 0. 0341 mol C C 3 H 4 O 3 H 2 O 12. 01 g CO 2 1. 50 g CO 2 0. 41 g H 2 O = 0. 409 g C 44. 01 g CO 2 2. 016 g H 2 O = 0. 046 g H 18. 02 g H 2 O 1. 000 g - (0. 409 + 0. 049) = 0. 545 0. 046 g H 1. 008 g H = 0. 0461 mol H 176. 12 g/mol 88. 06 g 3 -23 37. 96 g-37. 55 g = 0. 41 g 0. 545 g O 16. 00 g O = 2. 000 = 0. 0341 mol O C 6 H 8 O 6
 Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chapter 10 study guide the mole
Chapter 10 study guide the mole 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 10 study guide the mole
Chapter 10 study guide the mole 2 matter and change answer key
2 matter and change answer key Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Foods that are heterogeneous mixtures
Foods that are heterogeneous mixtures Covalent bond boiling point
Covalent bond boiling point Ionic covalent metallic
Ionic covalent metallic Giant molecular structure vs simple molecular structure
Giant molecular structure vs simple molecular structure Gray and white matter
Gray and white matter Gray matter and white matter
Gray matter and white matter Gray matter and white matter
Gray matter and white matter Gray matter
Gray matter Chemistry matter and its changes
Chemistry matter and its changes Democritus atomic model diagram
Democritus atomic model diagram Ap chemistry unit 9 notes
Ap chemistry unit 9 notes Nature and nature's law lay hid in night
Nature and nature's law lay hid in night Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter