Matter The Nature of Matter A Matter is












- Slides: 27

Matter

The Nature of Matter A. Matter is a term used to describe anything that has mass and takes up space. 1. All matter has its own properties that are used to classify them B. A substance is a type of matter with fixed composition. 1. It can either be an element or compound

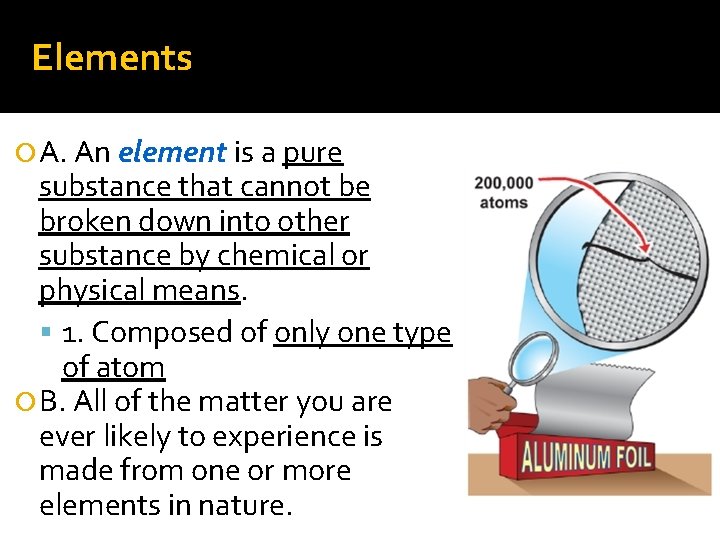
Elements A. An element is a pure substance that cannot be broken down into other substance by chemical or physical means. 1. Composed of only one type of atom B. All of the matter you are ever likely to experience is made from one or more elements in nature.

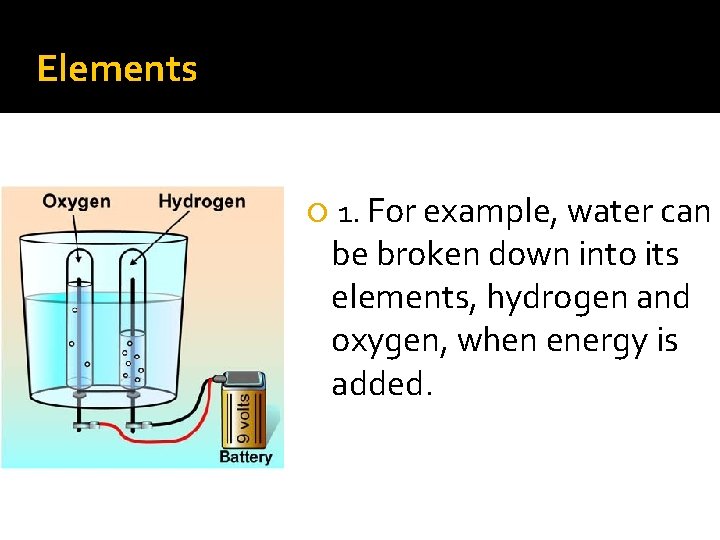
Elements 1. For example, water can be broken down into its elements, hydrogen and oxygen, when energy is added.

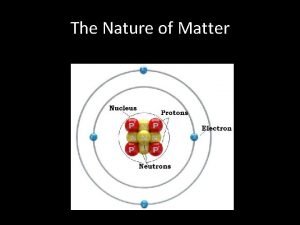
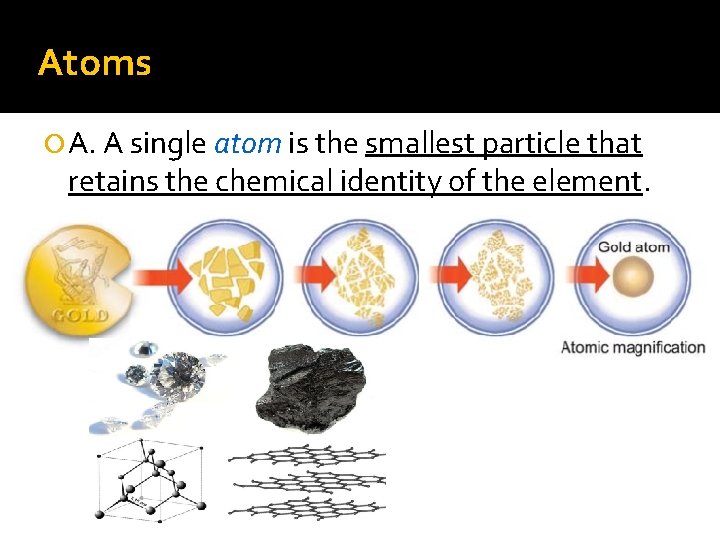
Atoms A. A single atom is the smallest particle that retains the chemical identity of the element.

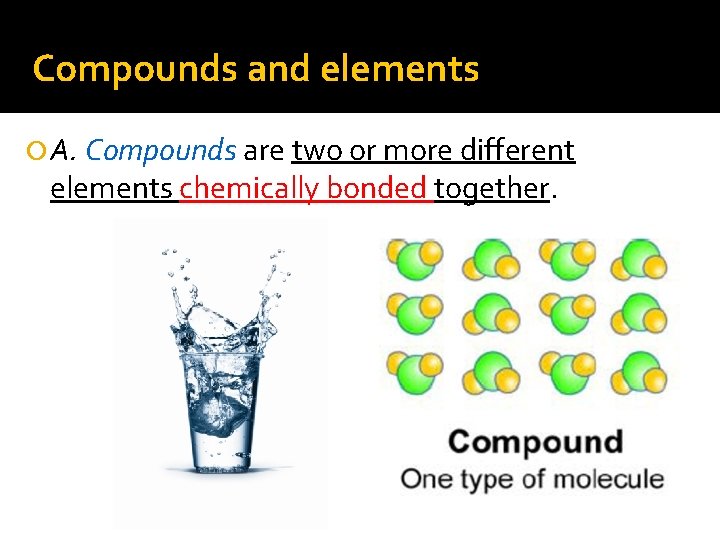
Compounds and elements A. Compounds are two or more different elements chemically bonded together.

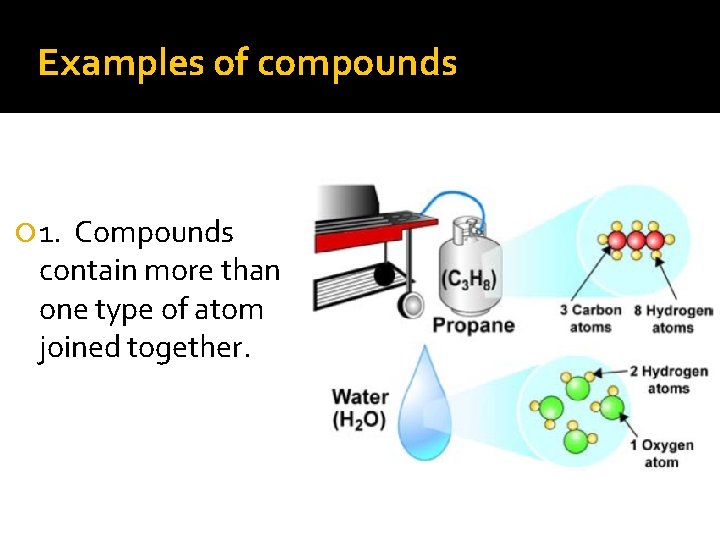
Examples of compounds 1. Compounds contain more than one type of atom joined together.

Molecules A. A molecule is a group of two or more atoms joined together chemically. 1. Everything from things you eat to items you wear all consist of molecules

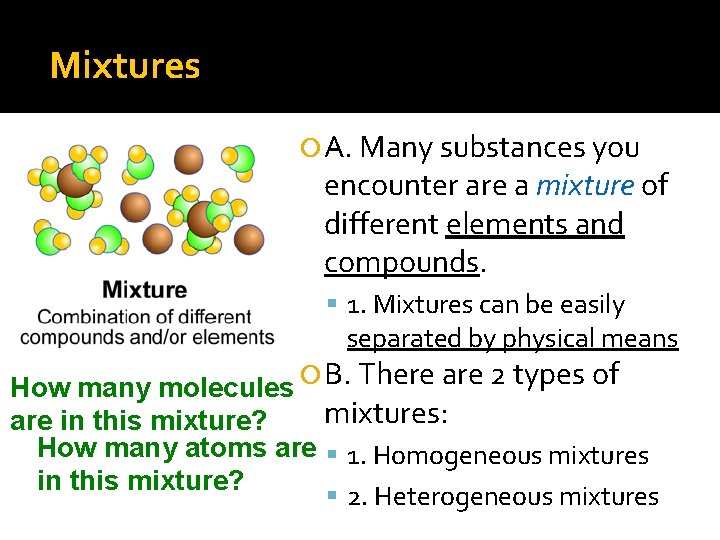
Mixtures A. Many substances you encounter are a mixture of different elements and compounds. 1. Mixtures can be easily separated by physical means How many molecules B. There are 2 types of mixtures: are in this mixture? How many atoms are 1. Homogeneous mixtures in this mixture? 2. Heterogeneous mixtures

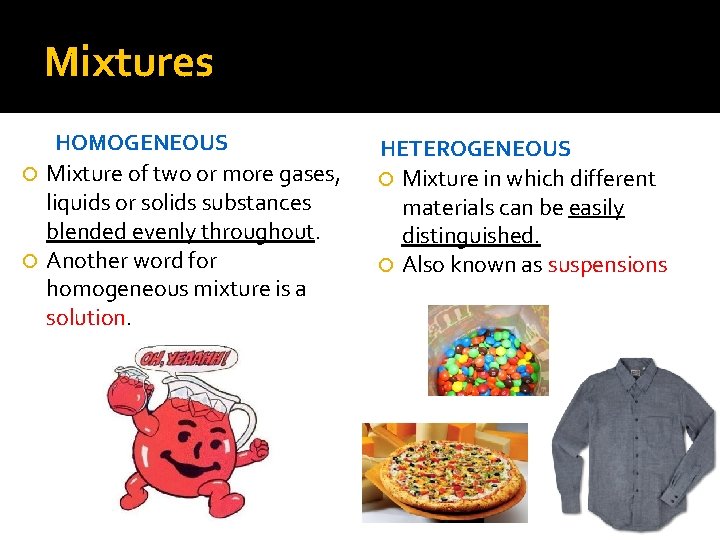
Mixtures HOMOGENEOUS Mixture of two or more gases, liquids or solids substances blended evenly throughout. Another word for homogeneous mixture is a solution. HETEROGENEOUS Mixture in which different materials can be easily distinguished. Also known as suspensions

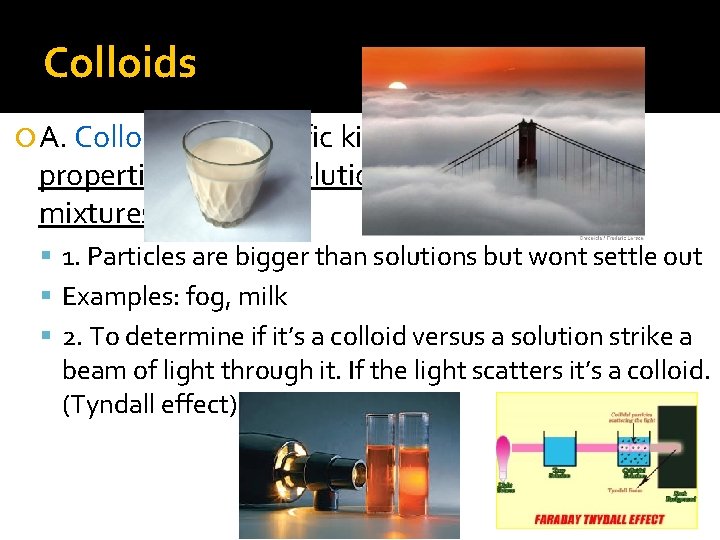
Colloids A. Colloids are specific kind of mixture. It has properties of both solutions & heterogeneous mixtures. 1. Particles are bigger than solutions but wont settle out Examples: fog, milk 2. To determine if it’s a colloid versus a solution strike a beam of light through it. If the light scatters it’s a colloid. (Tyndall effect)

Elements, compounds, and mixtures Can you distinguish between atoms and molecules in these images?


States of Matter

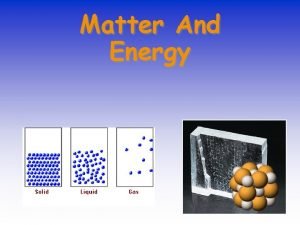
I. STATES OF MATTER A. Based upon particle arrangement B. Based upon energy of particles C. Based upon distance between particles

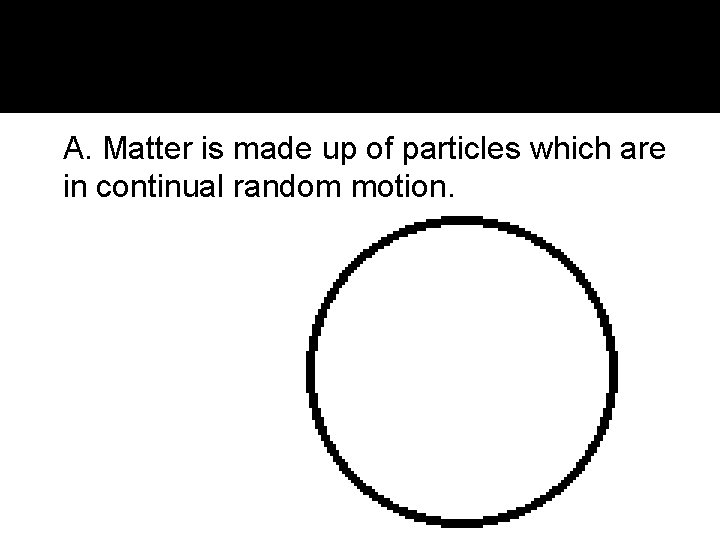
II. Kinetic Theory of Matter A. Matter is made up of particles which are in continual random motion.

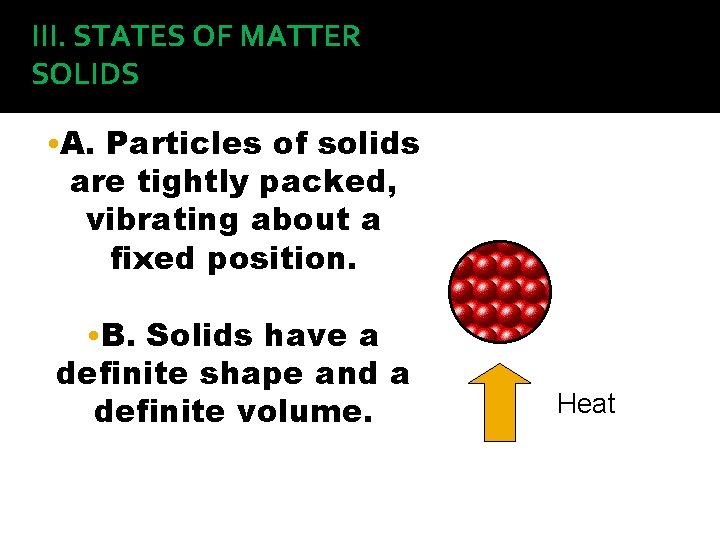
III. STATES OF MATTER SOLIDS • A. Particles of solids are tightly packed, vibrating about a fixed position. • B. Solids have a definite shape and a definite volume. Heat

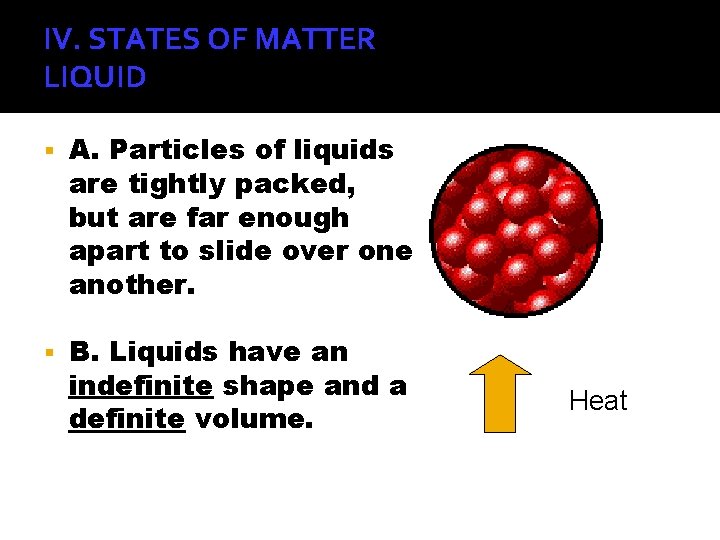
IV. STATES OF MATTER LIQUID A. Particles of liquids are tightly packed, but are far enough apart to slide over one another. B. Liquids have an indefinite shape and a definite volume. Heat

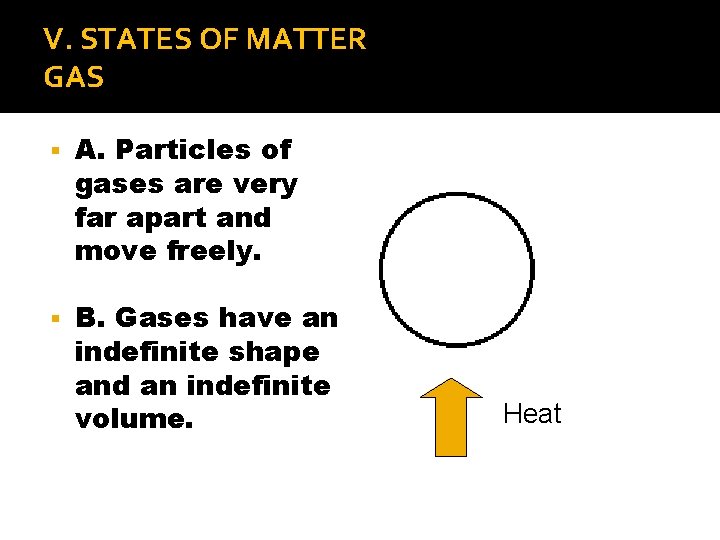
V. STATES OF MATTER GAS A. Particles of gases are very far apart and move freely. B. Gases have an indefinite shape and an indefinite volume. Heat

VI. PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

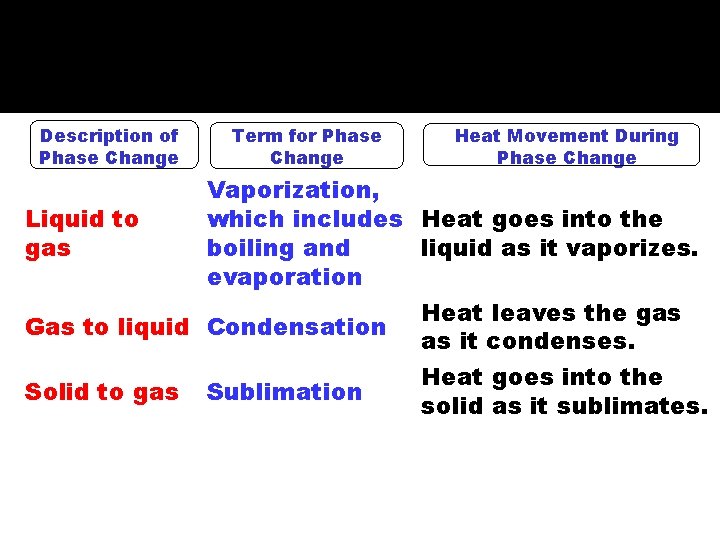
VI. PHASE CHANGES Description of Phase Change Term for Phase Change Heat Movement During Phase Change Vaporization, which includes Heat goes into the Liquid to gas boiling and liquid as it vaporizes. evaporation Heat leaves the gas Gas to liquid Condensation as it condenses. Heat goes into the Solid to gas Sublimation solid as it sublimates.

But what happens if you raise the temperature to super-high levels… between 1000°C and 1, 000, 000°C ? Will everything just be a gas?

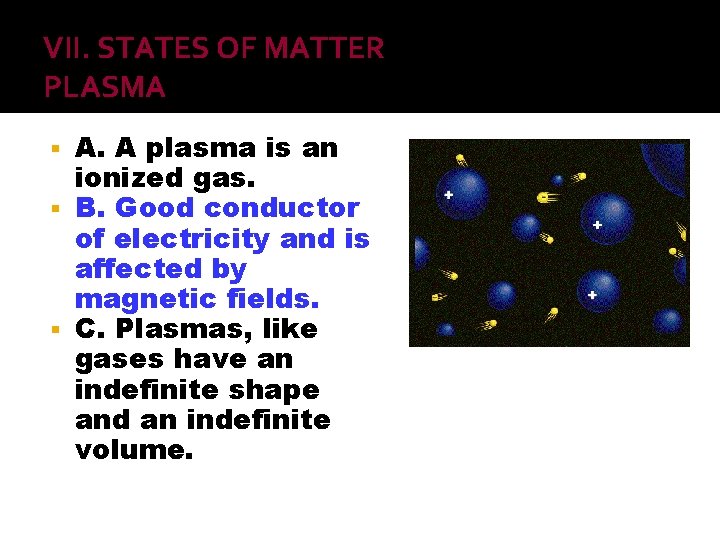
VII. STATES OF MATTER PLASMA A. A plasma is an ionized gas. B. Good conductor of electricity and is affected by magnetic fields. C. Plasmas, like gases have an indefinite shape and an indefinite volume.

Some places where plasmas are found… 1. Flames

2. Lightning

3. Aurora (Northern Lights)

The Sun is an example of a star in its plasma state
 Nature and nature's laws lay hid in night meaning
Nature and nature's laws lay hid in night meaning Determinace lidské psychiky
Determinace lidské psychiky Chó sói
Chó sói V cc
V cc Thể thơ truyền thống
Thể thơ truyền thống Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ đại từ thay thế
đại từ thay thế Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Diễn thế sinh thái là
Diễn thế sinh thái là Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau 101012 bằng
101012 bằng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Lời thề hippocrates
Lời thề hippocrates Chụp tư thế worms-breton
Chụp tư thế worms-breton Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Bổ thể
Bổ thể Phản ứng thế ankan
Phản ứng thế ankan Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Hát lên người ơi
Hát lên người ơi điện thế nghỉ
điện thế nghỉ