Advanced Chem Unit 2 Empirical Molecular Formulas Empirical






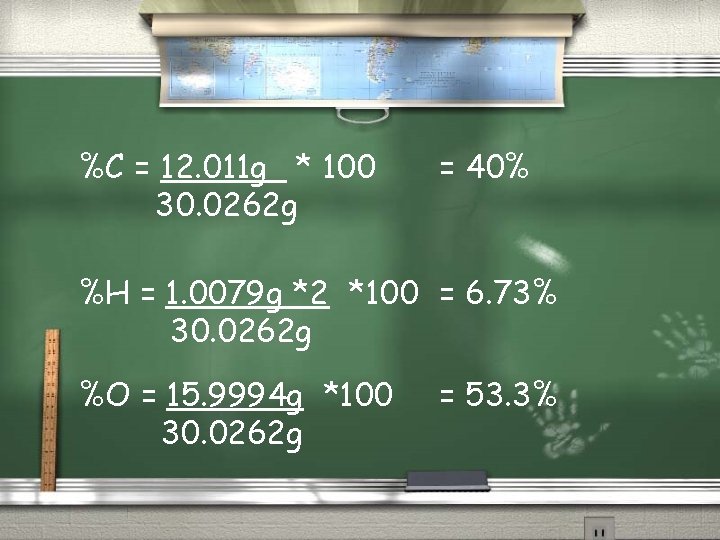























































































- Slides: 103

Advanced Chem. Unit #2 Empirical & Molecular Formulas

Empirical Formula giving the lowest whole number ratio of the atoms of the elements in a compound. Empirical formulas may represent either atoms or moles.

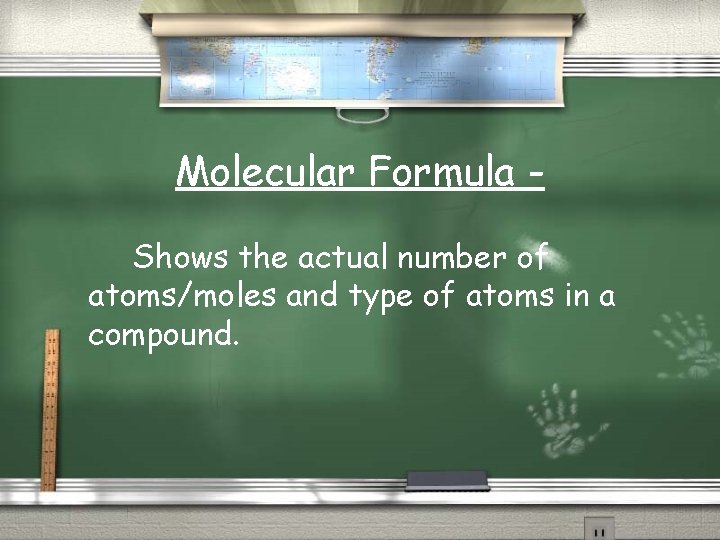
Molecular Formula Shows the actual number of atoms/moles and type of atoms in a compound.

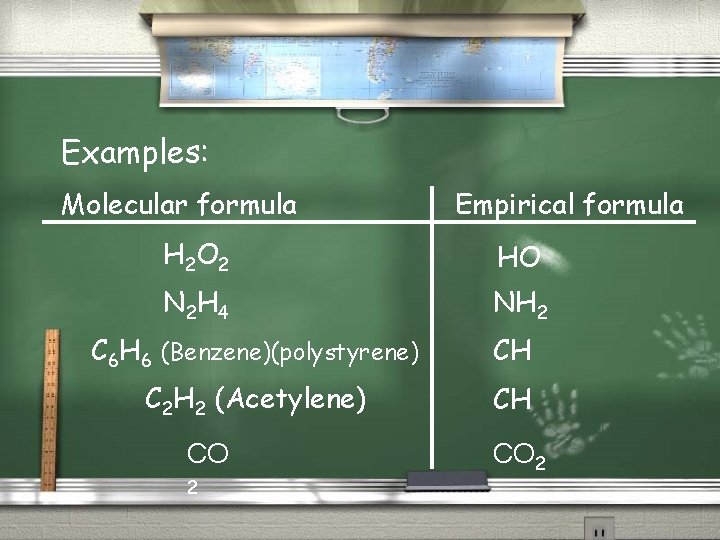
Examples: Molecular formula Empirical formula H 2 O 2 HO N 2 H 4 NH 2 C 6 H 6 (Benzene)(polystyrene) CH C 2 H 2 (Acetylene) CH CO 2

Examples: Molecular formula Empirical formula CH 2 O (formaldhyde) CH 2 O C 6 H 12 O 6 (glucose) CH 2 O C 2 H 4 O 2 (acetic acid) C 5 H 10 O 5 (ribose) N 2 O 5 CH 2 O N 2 O 5

Formula Weight Same as molecular weight, but of an ionic compound. A. K. A. Chart mass
Calculating mass percentages (percent composition) from the formula.

Mass percent of “A” = Mass of “A” in the whole Mass of the whole X 100

Example 1: Formaldehyde (CH 2 O) is a toxic gas with a pungent odor. (Uses include: (1) large amounts to make plastics. (2) a H 2 O solution of it is used to preserve biological specimens). Calculate the percent of each element in the whole.
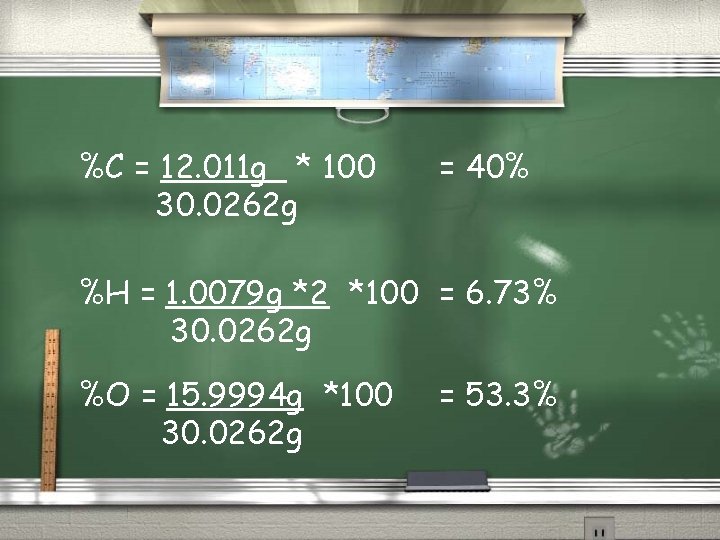
%C = 12. 011 g * 100 30. 0262 g = 40% %H = 1. 0079 g *2 *100 = 6. 73% 30. 0262 g %O = 15. 9994 g *100 30. 0262 g = 53. 3%

Example 2: Calculate percent of each in: KMn. O 4 158. 03395 g/mol %K = 39. 0983 158. 03395 *100 = 24. 7% %Mn = 54. 93805 158. 03395 *100 = 34. 8% (15. 9994 *4) 158. 03395 *100 = 40. 5% %O = 100%

Example 3: Copper(II)nitrate Cu(NO 3)2 = 187. 5558 g/mol %Cu = 63. 546 187. 5558 *100 %N = (14. 0067 *2) 187. 5558 *100 %O = (15. 9994*6) 187. 5558 *100 = 33. 9% = 14. 9% = 51. 2% 100%

Example 4: CF Halothane (an inhalation anesthetic) = 197. 3821096 g/mol 3 CHBr. Cl %C = (12. 011*2) * 100 197. 3821096 %F = (18. 9984032*3) *100 197. 3821096 %H = 1. 0079 *100 197. 3821096 %Br = 79. 904 197. 3821096 %Cl = 35. 453 197. 3821096 *100 = 12. 2% = 28. 9% = 0. 5% = 40. 5% = 18. 0% 100. 1%

Calculating the mass of an Element in a given mass of a compound. (% of Element “A”) x Mass of the compound = Grams of “A”

Example #1: Refer to previous example involving Formaldehyde (CH 2 O) %C = 40% %H = 6. 73% %O = 53. 3% Formula wt. = 30. 0262 g/mol

How many grams of each element are in 83. 5 grams of Formaldehyde? %C = 40% x 83. 5 g = 33. 4 g %H = 6. 73% x 83. 5 g = 5. 62 g %O = 53. 3% x 83. 5 g = 44. 5 g

How many grams of Nitrogen, Oxygen, and Hydrogen are in 48. 5 grams of Ammonium nitrate ? NH 4 NO 3 = 80. 0432 g/mol %N = %H = %O = (14. 0067 x 2) 80. 0432 (1. 0079 x 4) 80. 0432 x 100 = 35. 0% x 100 = 5. 04% (15. 9994 x 3) 80. 0432 x 100 = 60. 0%

How many grams of Nitrogen are in 48. 5 grams of Ammonium nitrate ? %N = 35. 0% = 0. 35 x 48. 5 g = 17. 0 g of Nitrogen

Urea is a fertilizer that is commonly purchased in a 46 -0 -0 formula (46% Nitrogen by mass (in the form of Ammonium nitrate), the remainder are inert ingredients). How many pounds of Nitrogen are in 3 ton of this fertilizer ? 46% of the total weight is Ammonium nitrate How many pounds of Nitrogen in this sample?

3 ton = 6000 lb that is 46% NH 4 NO 3 6000 lb x 0. 46 = 2760 lb of NH 4 NO 3 x 0. 35 = 966 lb of Nitrogen

Procaine hydrochloride (Novocain) (C 13 H 21 Cl. N 2 O 2) is a local anesthetic. How many grams of each element are in a 0. 23 cc dose of this substance ? (Assume Novocain = 1. 0 g/m. L)

1 st: Determine the mass of the sample. Mass of sample = 0. 23 grams

2 nd: Determine the molecular weight of the compound. Molecular weight = 272. 7741 g/mol

Which contains more carbon; 4. 71 grams of glucose, or 5. 85 grams of Ethyl alcohol (CH 3 CH 2 OH) ? 1 st: Find %Carbon in each compound. 2 nd: Calculate grams from percent in given masses.

Glucose Ethanol %C 40% 52. 1% Grams Of Carbon 1. 88 g 3. 05 g

Which contains more Sulfur; 37. 1 grams of Calcium sulfate, or 35. 2 grams of Sodium sulfite (Na 2 SO 3)? 1 st: Find %Sulfur in each compound. 2 nd: Calculate grams from percent in given masses.

%S Grams Of Sulfur Ca. SO 4 Na 2 SO 3 23. 6% 25. 4% 9. 63 g 8. 94 g

Calculating the percent of C and H by combustion. Suppose you have discovered a new compound composed of only C, H, and O, whose formula you wish to determine. What are the products of combustion if the reactants consist of only C, H, and O ? Answer: CO 2 and H 2 O

Every 1 mole of Carbon combusted will yield 1 mole of CO 2. Every 1 mole of Hydrogen combusted will yield 0. 5 mole of H 2 O.

Calculate the percent of Carbon, Hydrogen, and Oxygen by combustion. Acetic acid contains only C, H, & O. A 4. 24 mg sample is completely burned. It results in 6. 21 mg of CO 2, and 2. 54 mg of H 2 O. What is mass percentage of each element in acetic acid?

Step 1: Convert mass of CO 2 to moles of CO 2. 6. 21 mg = 0. 00621 g of CO 2 Use DIMO to convert g to moles. 0. 00621 g = 0. 000141 mol CO 2

Step 2: Relate moles of CO 2 to moles of C. Since 1 mol C produces 1 mol CO 2 Then: 0. 000141 mol CO 2 was produced by 0. 000141 mol of C.

Step 3: Convert moles of C to grams of C (use DIMO) 0. 000141 mol C = 0. 001695 g of C NOW DO THESE SAME THREE STEPS FOR HYDROGEN

Step 1: Convert mass of H 2 O to moles of H 2 O. 2. 54 mg = 0. 00254 g of H 2 O Use DIMO to convert g to moles. 0. 00254 g = 0. 000141 mol H 2 O

Step 2: Relate moles of H 2 O to moles of H. Since 1 mol H produces 0. 5 mol H 2 O Then: 0. 000141 mol H 2 O was produced by 0. 000282 mol of H.

Step 3: Convert moles of H to grams of H (use DIMO) 0. 000282 mol H = 0. 000284 g of H TO FINISH: CALCULATE MASS PERCENTAGES OF C & H IN ACETIC ACID

%C = 0. 001695 g of C 0. 00424 g of Acetic acid = 39. 98% Carbon in Acetic acid x 100

%H = 0. 000284 g of H 0. 00424 g of Acetic acid x 100 = 6. 698% Hydrogen in Acetic acid TO FIND %OXYGEN: SUBTRACT %C and %H FROM 100%

100% - (39. 98% C + 6. 698% H) = TOTAL = 99. 998% 53. 32% O

A 3. 87 mg sample of Ascorbic acid (Vitamin C) gives 5. 80 mg CO 2 and 1. 58 mg of H 2 O when combusted. What is the percent composition of this substance ? (contains only C, H, and O)

Step 1: Convert mass of CO 2 to moles of CO 2. 5. 80 mg = 0. 00580 g of CO 2 Use DIMO to convert g to moles. 0. 00580 g = 0. 000132 mol CO 2

Step 2: Relate moles of CO 2 to moles of C. Since 1 mol C produces 1 mol CO 2 Then: 0. 000132 mol CO 2 was produced by 0. 000132 mol of C.

Step 3: Convert moles of C to grams of C (use DIMO) 0. 000132 mol C = 0. 00158 g of C NOW DO THESE SAME THREE STEPS FOR HYDROGEN

Step 1: Convert mass of H 2 O to moles of H 2 O. 1. 58 mg = 0. 00158 g of H 2 O Use DIMO to convert g to moles. 0. 00158 g = 0. 0000877 mol H 2 O

Step 2: Relate moles of H 2 O to moles of H. Since 1 mol H produces 0. 5 mol H 2 O Then: 0. 0000877 mol H 2 O was produced by 0. 000175 mol of H.

Step 3: Convert moles of H to grams of H (use DIMO) 0. 000175 mol H = 0. 000177 g of H TO FINISH: CALCULATE MASS PERCENTAGES OF C & H IN ACETIC ACID

%C = 0. 00158 g of C 0. 00387 g of Ascorbic acid = 40. 8% Carbon in Ascorbic acid x 100

%H = 0. 000177 g of H 0. 00387 g of Ascorbic acid x 100 = 4. 57% Hydrogen in Ascorbic acid TO FIND %OXYGEN: SUBTRACT %C and %H FROM 100%

100% - (40. 8% C + 4. 57% H) = TOTAL = 100% 54. 63% O

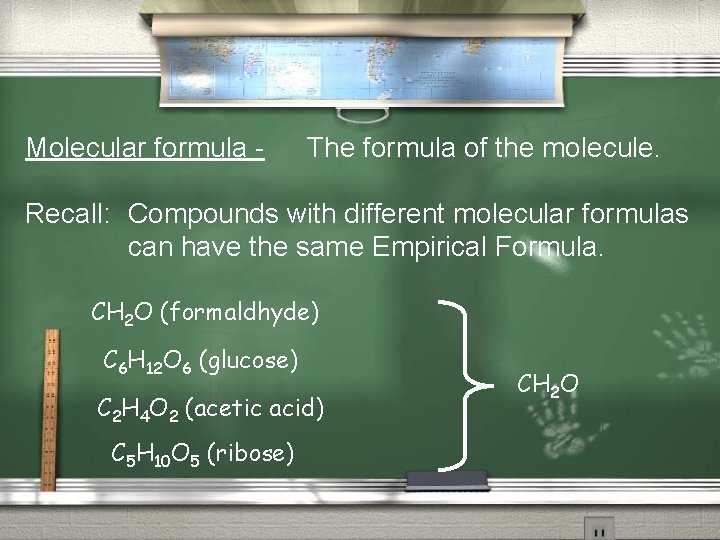
Determining Empirical Formula From Mass Percent Remember: Empirical Formula - “Formula in lowest terms”

Molecular formula - The formula of the molecule. Recall: Compounds with different molecular formulas can have the same Empirical Formula. CH 2 O (formaldhyde) C 6 H 12 O 6 (glucose) C 2 H 4 O 2 (acetic acid) C 5 H 10 O 5 (ribose) CH 2 O

Example 1: How to calculate empirical formulas from percent composition. What is the empirical formula of a compound that is 94. 1% oxygen and 5. 9% Hydrogen ?

Realize that percent composition is a ratio of masses of the elements in the problem. Calculate the lowest whole number ratio of the moles of the elements.

Step 1: Assume 100 grams of the sample. Therefore you have how many grams of: Oxygen (94. 1%) = 94. 1 grams Hydrogen (5. 9%) = 5. 9 grams

Step 2: Turn grams into moles (use DIMO). Oxygen = 94. 1 g = Hydrogen = 5. 9 g = 5. 88 mol of Oxygen 5. 85 mol of Hydrogen

Step 3: Set up mole ratio, and divide by the smallest number of moles. H 5. 85 mol O 5. 88 mol 5. 85 mol H 1 O 1. 01 = HO

Example 2: How to calculate empirical formulas from percent composition. What is the empirical formula of a compound that is 67. 6% Mercury, 10. 8% Sulfur, and 21. 6% Oxygen ?

Step 1: Assume 100 grams of the sample. Therefore you have how many grams of: Hg (67. 6%) = 67. 6 grams S (10. 8%) = 10. 8 grams O (21. 6%) = 21. 6 grams

Step 2: Turn grams into moles (use DIMO). Hg = 67. 6 g = 0. 337 mol of Hg S = 10. 8 g = 0. 337 mol of S O = 21. 6 g = 1. 35 mol of O

Step 3: Set up mole ratio, and divide by the smallest number of moles. Hg 0. 337 mol S 0. 337 mol O 1. 35 mol 0. 337 mol Hg 1 S 1 O 4. 01 = 0. 337 mol Hg. SO 4

Example 3: How to calculate empirical formulas from percent composition. What is the empirical formula of a compound that is 17. 6% Sodium, 39. 7% Chromium, and 42. 7% Oxygen ?

Step 1: Assume 100 grams of the sample. Therefore you have how many grams of: Na (17. 6%) = 17. 6 grams Cr (39. 7%) = 39. 7 grams O (42. 7%) = 42. 7 grams

Step 2: Turn grams into moles (use DIMO). Na = 17. 6 g = 0. 766 mol of Na Cr = 39. 7 g = 0. 764 mol of Cr O = 42. 7 g = 2. 67 mol of O

Step 3: Set up mole ratio, and divide by the smallest number of moles. Na 0. 766 mol Cr 0. 764 mol O 2. 67 mol 0. 764 mol Na 1 Cr 1 O 3. 49= 0. 764 mol Na 2 Cr 2 O 7

So far in this unit, you have learned: 1. How to calculate percent composition from the molecular formula. 2. How determine grams of an element in a given mass of a compound from it’s percent composition.

So far in this unit, you have learned (con’t): 3. How to calculate grams of Carbon, Hydrogen, and Oxygen by combustion. 4. How determine the empirical formula of a compound from it’s percent composition.

The last topic of this unit: How to determine the Molecular formula of a compound from its percent composition and molecular weight.

This process requires you to calculate the “factor, n”. “n” = Molecular weight of the compound Empirical weight of the compound Multiplying the ratios of the empirical formula by “n” will result in the Molecular formula.

Example 1: A 15 gram sample of a substance that has a molecular weight of 60 AMU was determined to have a composition consisting of: 5. 99 g of Carbon 1. 01 g of Hydrogen 8. 01 g of Oxygen

1 st: Find the empirical formula. 2 nd: Using “n”, determine the molecular formula.

1 st: The Empirical formula: 5. 99 g of Carbon = 0. 500 mol of C 1. 01 g of Hydrogen = 1. 00 mol of H 8. 01 g of Oxygen = 0. 500 mol of O C 0. 500 H 1. 00 O 0. 500 = CH 2 O 0. 500

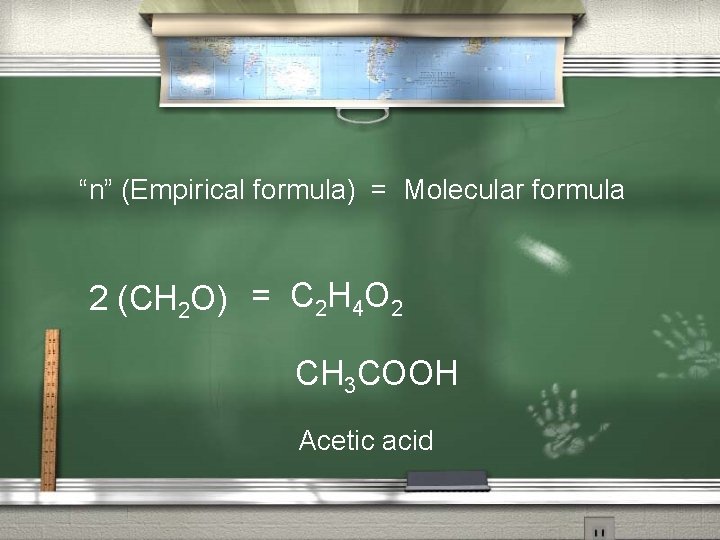
2 nd: Find “n”, then determine the Molecular formula. “n” = Molecular weight of the compound Empirical weight of the compound “n” = 60. 0 AMU 30. 0 AMU = 2

“n” (Empirical formula) = Molecular formula 2 (CH 2 O) = C 2 H 4 O 2 CH 3 COOH Acetic acid

Example 2: 7. 36 grams of compound “X” decomposed to give 6. 93 grams of Oxygen and the rest is Hydrogen. If “X” has an atomic weight of 34. 0 g/mol, what is its formula ? : 7. 36 grams total of “X” 6. 93 g of Oxygen

1 st: Find the empirical formula. 2 nd: Using “n”, determine the molecular formula.

1 st: The Empirical formula: 7. 36 g total of “X” 6. 93 g of Oxygen = 0. 433 mol of O 0. 43 g of Hydrogen = 0. 427 mol of H H 0. 427 O 0. 433 = HO 0. 427

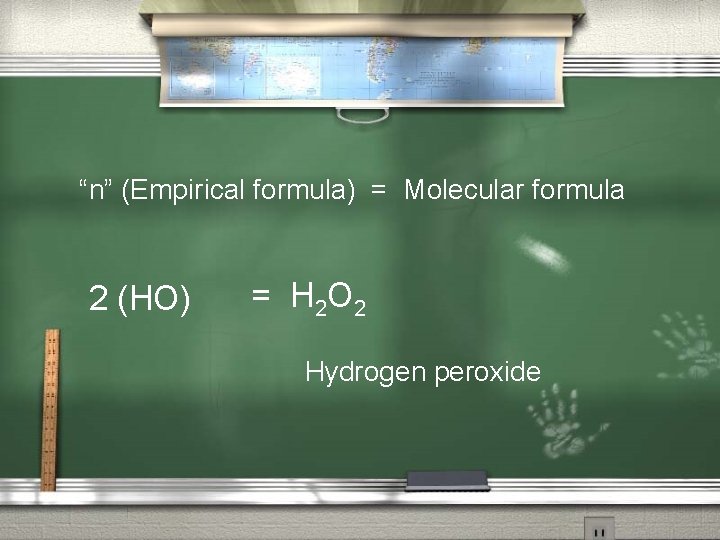
2 nd: Find “n”, then determine the Molecular formula. “n” = Molecular weight of the compound Empirical weight of the compound “n” = 34. 0 AMU 17. 0 AMU = 2

“n” (Empirical formula) = Molecular formula 2 (HO) = H 2 O 2 Hydrogen peroxide

Example 3: Methyl butanoate smells like apples. It’s molecular weight is 102 AMU. It was determined to have a composition consisting of: 58. 8% 9. 8% 31. 4% Carbon Hydrogen Oxygen What is it’s molecular formula ?

1 st: Find the empirical formula. 2 nd: Using “n”, determine the molecular formula.

1 st: The Empirical formula: 58. 8 g of Carbon = 5. 00 mol of H 9. 8 g of Hydrogen= 31. 4 g of Oxygen 2(C 2. 5 H 5. 00 O)1. 00 2. 50 mol of C = = 1. 00 mol of O C 5 H 10 O 2

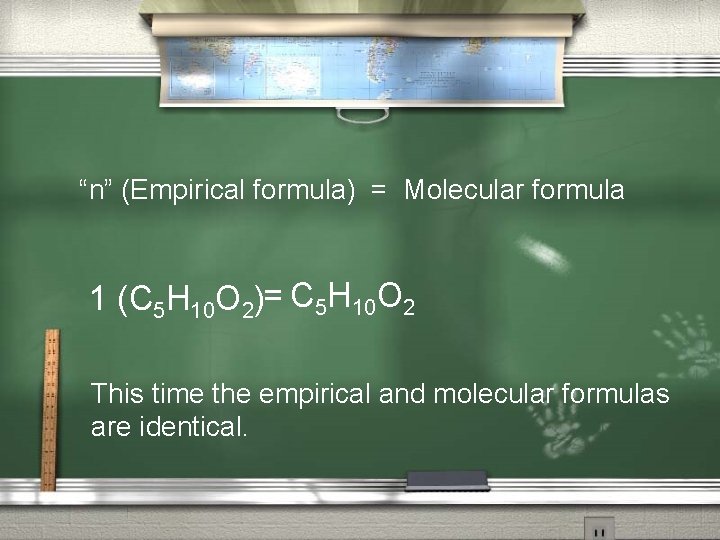
2 nd: Find “n”, then determine the Molecular formula. “n” = Molecular weight of the compound Empirical weight of the compound “n” = 102 AMU = 1

“n” (Empirical formula) = Molecular formula 1 (C 5 H 10 O 2)= C 5 H 10 O 2 This time the empirical and molecular formulas are identical.

Example 4: Putrescine, a substance produced by decaying animal flesh, is 54. 5% C, 13. 7% H, and 31. 8% N. Several determinations of molecular weight gave values in the range of 87 -90 g/mol. What is it’s molecular formula ? : 54. 5 g of Carbon 13. 7 g of Hydrogen 31. 8 g of Nitrogen

1 st: Find the empirical formula. 2 nd: Using “n”, determine the molecular formula.

1 st: The Empirical formula: 54. 5 g of Carbon = 4. 54 mol of C 13. 7 g of Hydrogen = 13. 6 mol of H 31. 8 g of Nitrogen= C 4. 54 H 13. 6 N 2. 27 mol of N = C 2 H 6 N

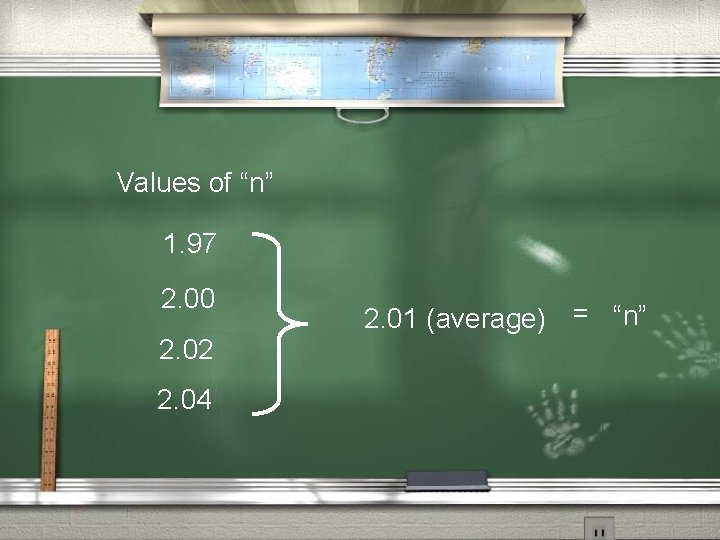
2 nd: Find “n”, then determine the Molecular formula. “n” = Molecular weight of the compound Empirical weight of the compound “n” = 87 AMU 44. 0761 AMU = 1. 97

2 nd: Find “n”, then determine the Molecular formula. “n” = Molecular weight of the compound Empirical weight of the compound “n” = 88 AMU 44. 0761 AMU = 2. 00

2 nd: Find “n”, then determine the Molecular formula. “n” = Molecular weight of the compound Empirical weight of the compound “n” = 89 AMU 44. 0761 AMU = 2. 02

2 nd: Find “n”, then determine the Molecular formula. “n” = Molecular weight of the compound Empirical weight of the compound “n” = 90 AMU 44. 0761 AMU = 2. 04

Values of “n” 1. 97 2. 00 2. 02 2. 04 2. 01 (average) = “n”

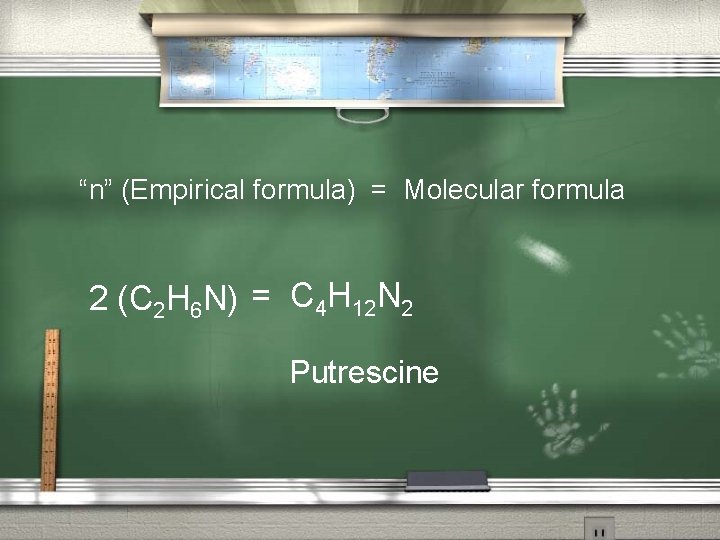
“n” (Empirical formula) = Molecular formula 2 (C 2 H 6 N) = C 4 H 12 N 2 Putrescine

Example 5: Adipic acid is used to manufacture nylon. It is 49. 3% C, 6. 9% H, and 43. 8% O by mass; with a molecular weight of 146 g/mol. What is it’s molecular formula ?

Combustion of 354 mg of a particular toxic compound composed of only Carbon, Hydrogen, and Oxygen; yielded 500 mg of CO 2, and 308 mg of H 2 O. How many grams of Oxygen were required to combust this compound ?

Clues to solving previous problem: 1 st: Determine the percent composition of the “Toxic” compound.

2 nd: Using the percent composition, determine the mass in grams of the Oxygen in the “Toxic” compound.

3 rd: Using percent composition of the products determine the total grams of Oxygen in these compounds.

4 th: Calculate the answer to this question using the answers from clues #2 and #3.

Hydrate - A compound that contains water molecules weakly bonded in its crystals. Examples: Fe. Cl 2 4 H 2 O Fe. SO 4 7 H 2 O 63. 8% 36. 2% 54. 6% 45. 4%

The weight on a hydrated crystal (sample) Contains the water. Heating the crystal (sample) Will drive off the water leaving an Anhydrous sample. The difference in mass between the hydrated And dehydrated sample is the now missing Hydrate (water).

To calculate the ratio of anhydrous sample to water in the hydrated form, determine the molar ratio of anhydrous sample to water.

Example 1) A 5. 00 gram sample of Aluminum nitrate, a hydrated sample, was heated long enough to remove any moisture from the sample. After it cooled, it was weighed again. This time its mass was 2. 84 grams. Find the molecular formula of the hydrated sample.

Clues: 1. Find the mass of the water. 2. Determine how many moles of anhydrous sample and the moles of water that you have. 3. Divide by the smallest number of moles to get the ratio.