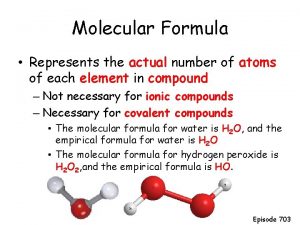
Jeopardy Nomenclature Empirical Formulas Molecular Formulas Hydrates Percent


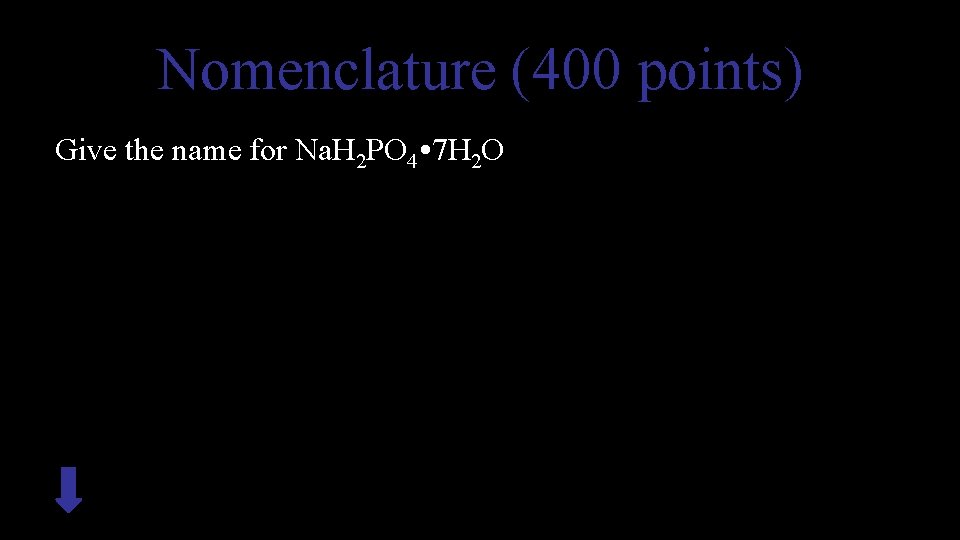























- Slides: 26

Jeopardy Nomenclature Empirical Formulas Molecular Formulas Hydrates Percent Composition 200 200 200 400 400 400 600 600 600 800 800 800 1000 1000

Nomenclature (200 points) Give the formula for zinc (II) sulfate heptahydrate
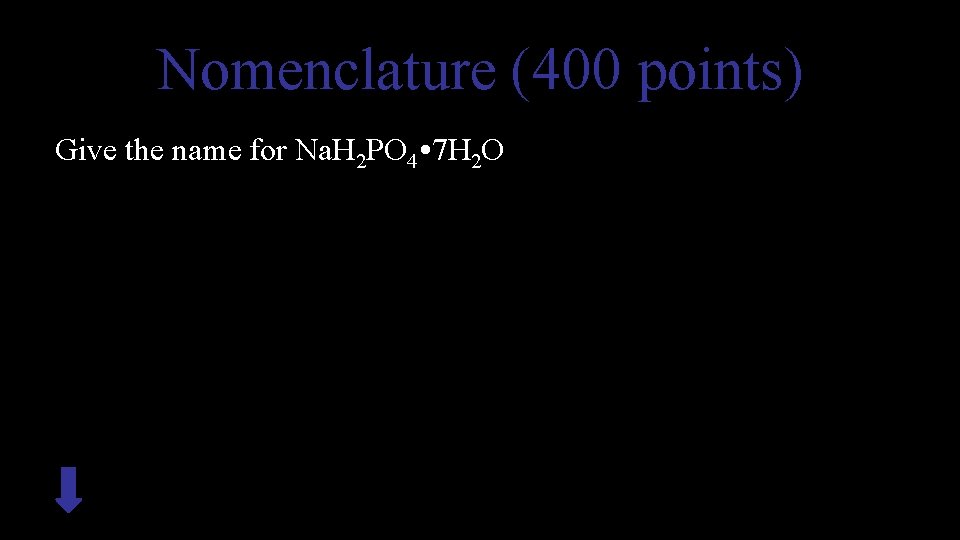
Nomenclature (400 points) Give the name for Na. H 2 PO 4 • 7 H 2 O

Nomenclature (600 points) Give the name for Na 2 Cr 2 O 7 • 2 H 2 O

Nomenclature (800 points) Give the formula for Tin (IV) Chloride Pentahydrate.

Nomenclature (1000 points) Give the formula for sodium acetate trihydrate.

Empirical Formulas (200 points) • What is the empirical formula for a compound which contains 0. 0134 g of iron, 0. 00769 g of sulfur and 0. 0115 g of oxygen?

Empirical Formulas (400 points) • . Find the empirical formula for a compound which contains 32. 8% chromium and 67. 2% chlorine.

Empirical Formulas (600 points) • What is the empirical formula for a compound which contains 67. 1% zinc and the rest is oxygen?

Empirical Formulas (800 points) • Barry Um has a sample of a compound which weighs 200 grams and contains only carbon, hydrogen, oxygen and nitrogen. By analysis, he finds that it contains 97. 56 grams of carbon, 4. 878 g of hydrogen, 52. 03 g of oxygen and 45. 53 g of nitrogen. Find its empirical formula.

Empirical Formulas (1000 points) • 300 grams of a compound which contains only carbon, hydrogen and oxygen is analyzed and found to contain the exact same percentage of carbon as it has oxygen. The percentage of hydrogen is known to be 5. 98823%. Find the empirical formula of the compound. •

Molecular Formulas (200 points) Determine the molecular formula of a compound with an empirical formula of NH 2 and a formula mass of 32. 06 g/mol.

Molecular Formulas (400 points) The empirical formula of a compound of phosphorus and oxygen was found to be P 2 O 5. Experimentation shows that the molar mass of this compound is 283. 89 g/mol. What is the compounds molecular formula?

Molecular Formulas (600 points) A compound with an empirical formula of CFBr. O and a molar mass of 254. 7 grams per mole.

Molecular Formulas (800 points) If 4. 04 g of N combine with 11. 46 g O to produce a compound with a molar mass of 108. 0 g/mol, what is the molecular formula of this compound?

Molecular Formulas (1000 points) • A component of protein called serine has an approximate molar mass of 100 g/mole. If the percent composition is as follows, what is the molecular formula of serine? • C = 34. 95 % H= 6. 844 % O = 46. 56 % N= 13. 59 %

Hydrates (200 points) Write the formula for the hydrate that contains 89. 2% barium bromide and 10. 8 % water. Write the name of the hydrate. .

Hydrates (400 points) A 14. 5 g sample of hydrated lithium bromide is heated and the water is driven off. The anhydrous salt has a mass of 12. 0 g. Write the formula of the hydrate and name it.

Hydrates (600 points) Determine the formula for a hydrate that contains 76. 9 % Ca. SO 3 and 23. 1 % H 2 O

Hydrates (800 points) A hydrate of magnesium sulfate has a mass of 13. 52 g. This sample is heated until no water remains. The anhydrous sample has a mass of 6. 60 g. Find the formula.

Hydrates (1000 points) A sample of hydrated copper(II) sulfate has a mass of 3. 97 g. After heating, the Cu. SO 4 that remains has a mass of 2. 54 g. Determine the correct formula.

Percent Composition (200 points) Calculate the mass percentage of copper in Cu. Cl 2

Percent Composition (400 points) Calculate the mass percentage of water in sodium sulfate pentahydrate.

Percent Composition (600 points) What mass of water can be obtained from 5. 00 g of nickel (II) sulfate hexahydrate?

Percent Composition (800 points) What is the mass of the anhydrous sample if you dehydrate a 4. 52 g sample of magnesium sulfate heptahydrate?

Percent Composition (1000 points) Vanillin contains 63. 15% carbon and 5. 30% hydrogen, and the rest oxygen. How many oxygen atoms are in a 100 g sample of Vanillin?
 Empirical and molecular formulas worksheet
Empirical and molecular formulas worksheet Empirical and molecular formulas worksheet
Empirical and molecular formulas worksheet Writing formulas and naming compounds section 3
Writing formulas and naming compounds section 3 Naming hydrates
Naming hydrates Hydrates de carbone
Hydrates de carbone How to name acids
How to name acids Formila
Formila Percentage composition of ch3cooh
Percentage composition of ch3cooh Courtesy formulas
Courtesy formulas Nylon 6 empirical formula
Nylon 6 empirical formula How to find empirical formula with percentages
How to find empirical formula with percentages Potassium permanganate percent composition
Potassium permanganate percent composition Percent by mass formula
Percent by mass formula Molecular formula
Molecular formula Empirical formula to percent composition
Empirical formula to percent composition Valid percent and cumulative percent
Valid percent and cumulative percent Empirical and molecular formula worksheet doc
Empirical and molecular formula worksheet doc Molecular vs empirical formula
Molecular vs empirical formula Molar mass quiz
Molar mass quiz Molecular formula
Molecular formula How to find molecular formula
How to find molecular formula Emprical formula
Emprical formula Empirical formula vs
Empirical formula vs Empirical formula poem
Empirical formula poem Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Compound ratio
Compound ratio Cho empirical formula
Cho empirical formula