CALCULATING PERCENT COMPOSITION EMPIRICAL FORMULAS AND MOLECULAR FORMULAS



















- Slides: 34

CALCULATING: PERCENT COMPOSITION EMPIRICAL FORMULAS AND MOLECULAR FORMULAS

Law of Constant Composition A compound contains elements in a certain fixed proportions (ratios) and in no other combinations, regardless of how the compound is prepared or where it is found in nature.

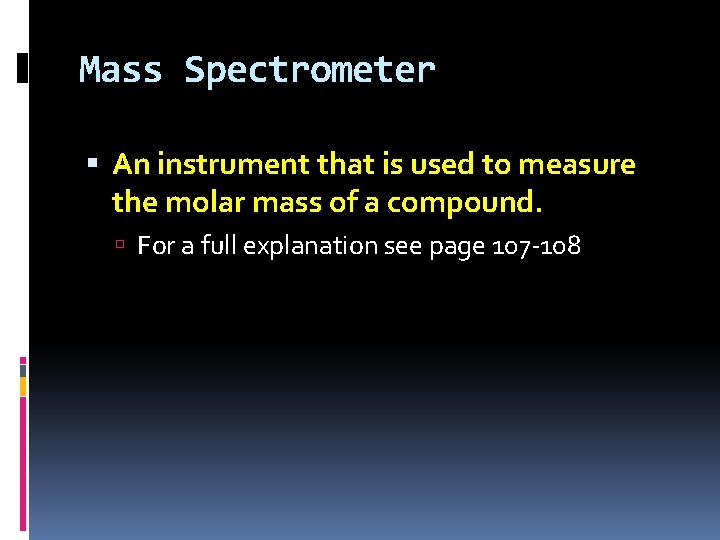
Mass Spectrometer An instrument that is used to measure the molar mass of a compound. For a full explanation see page 107 -108

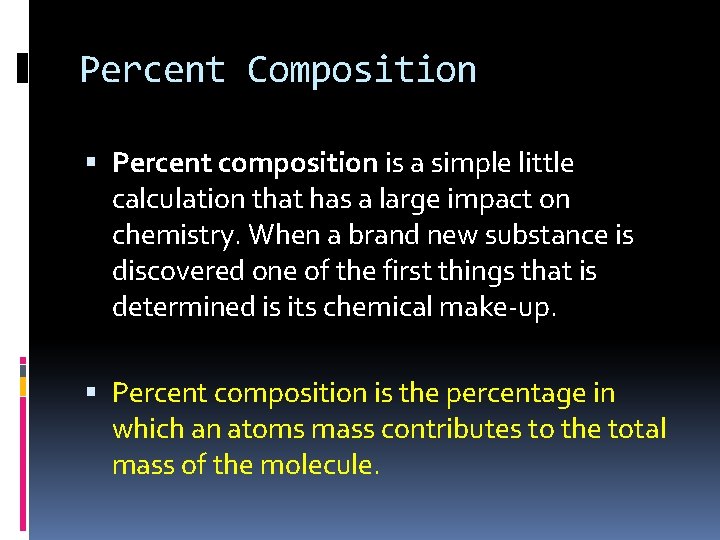
Percent Composition Percent composition is a simple little calculation that has a large impact on chemistry. When a brand new substance is discovered one of the first things that is determined is its chemical make-up. Percent composition is the percentage in which an atoms mass contributes to the total mass of the molecule.

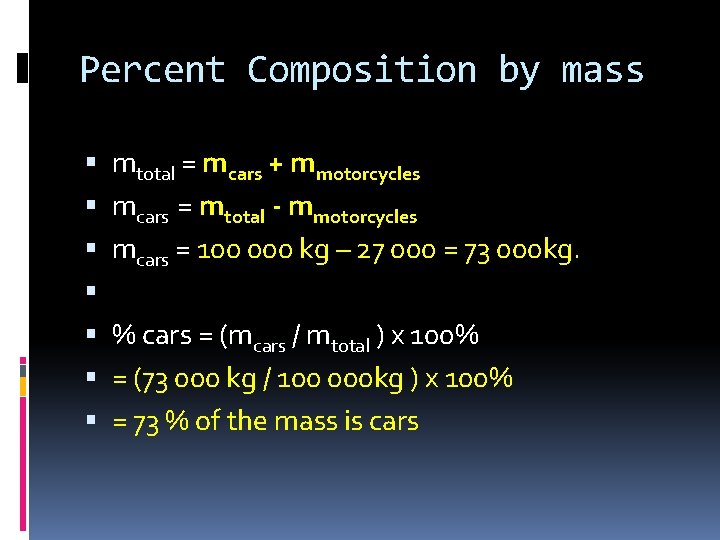
Percent Composition by mass Calculating by mass- this is done with lab results Let’s say we have a parking lot full of cars and motorcycles. The lot contains 100 000 kg of steel. 27 000 of those kilograms are motorcycles. What is percentage mass of the cars?

Percent Composition by mass mtotal = mcars + mmotorcycles mcars = mtotal - mmotorcycles mcars = 100 000 kg – 27 000 = 73 000 kg. % cars = (mcars / mtotal ) x 100% = (73 000 kg / 100 000 kg ) x 100% = 73 % of the mass is cars

% Composition by mass example 2 Chemistry example 3. 75 g of Hydrogen gas reacts with Oxygen to produce 33. 75 g of water. What is the mass and percent composition of the Oxygen. ?

% Composition by mass example 2 m. H 2 = 3. 75 g m. H 2 O = 33. 75 g m. O 2 = ? m. H 2 O - m. H 2 = m. O 2 = 33. 75 g - 3. 75 g = 30. 00 g

% Composition by mass example 2 %H = (m. H / m. H 2 O ) x 100% = = (3. 75 g / 33. 75 g ) x 100% = 11. 1 % % O = (m. O / m. H 2 O ) x 100% = = (30. 00 g / 33. 75 g ) x 100% = 88. 9 %

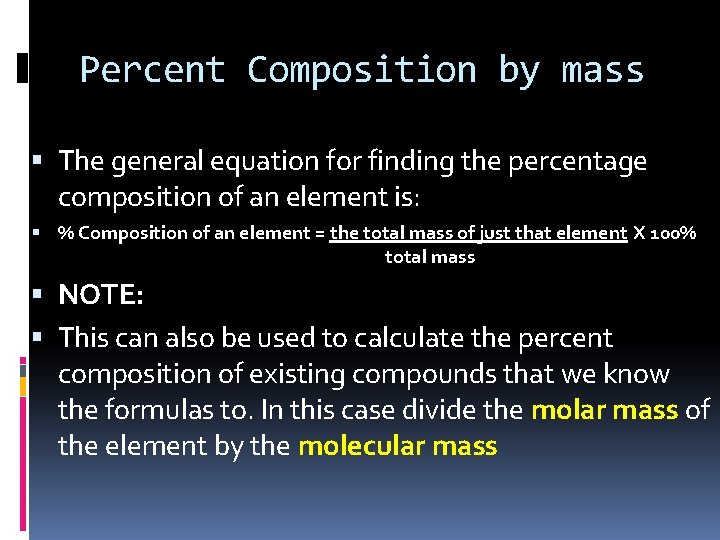
Percent Composition by mass The general equation for finding the percentage composition of an element is: % Composition of an element = the total mass of just that element X 100% total mass NOTE: This can also be used to calculate the percent composition of existing compounds that we know the formulas to. In this case divide the molar mass of the element by the molecular mass

% Composition by Formula Calculating by formula- we use this method if we want to see what we will get theoretically from a reaction. If you know the chemical formula it is relatively easy. Find the molar mass of each element and divide it by the molar mass of the molecule.

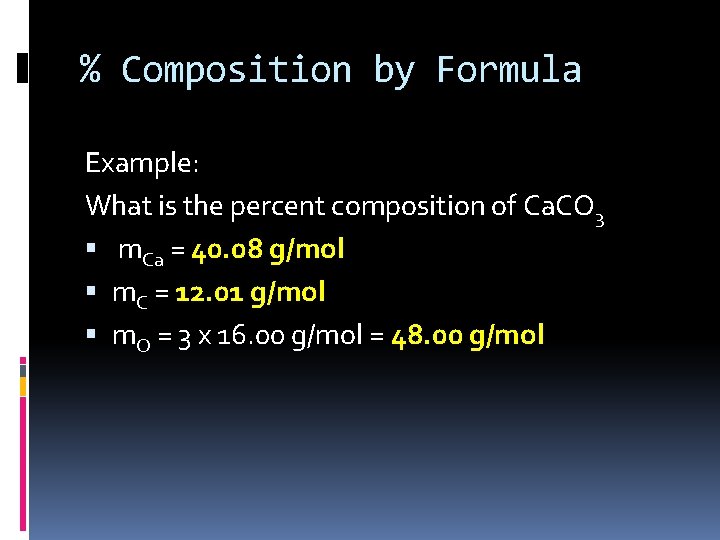
% Composition by Formula Example: What is the percent composition of Ca. CO 3 m. Ca = 40. 08 g/mol m. C = 12. 01 g/mol m. O = 3 x 16. 00 g/mol = 48. 00 g/mol

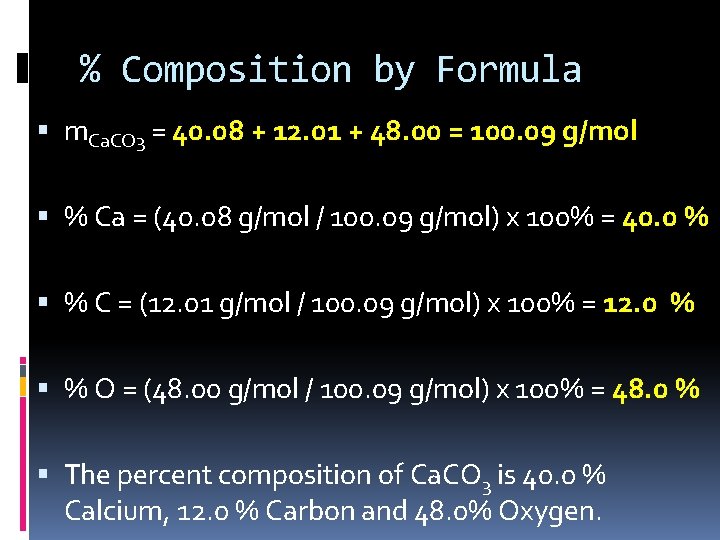
% Composition by Formula m. Ca. CO 3 = 40. 08 + 12. 01 + 48. 00 = 100. 09 g/mol % Ca = (40. 08 g/mol / 100. 09 g/mol) x 100% = 40. 0 % % C = (12. 01 g/mol / 100. 09 g/mol) x 100% = 12. 0 % % O = (48. 00 g/mol / 100. 09 g/mol) x 100% = 48. 0 % The percent composition of Ca. CO 3 is 40. 0 % Calcium, 12. 0 % Carbon and 48. 0% Oxygen.

Review: % composition Two ways to calculate it 1. By Mass. (Normally found by lab results) Normally given the mass of the final product and the mass of one of the reactants. The mass of the element is divided by the total mass of the compound. 2. By Formula (Normally theoretical) Given the Chemical formula. Molar masses must be calculated for each element and divided by the total molar mass of the compound.

Questions 1. What is the percentage composition of Ca. O? 2. A sample of a liquid with a mass of 8. 657 grams was decomposed into its elements and gave 5. 217 grams of carbon, 0. 9620 grams of hydrogen, and 2. 478 grams of oxygen. What is the percentage composition of this compound? 3. The drug known as LSD has the formula C 20 H 25 N 3 O. One suspected sample contained 74. 07%C, 7. 95%H, and 9. 99% N. Are the percentages given in the question consistent for LSD? 4. Calculate the percentage of nitrogen in the two important nitrogen fertilizers, ammonia, NH 3 and urea, CO(NH 2)2

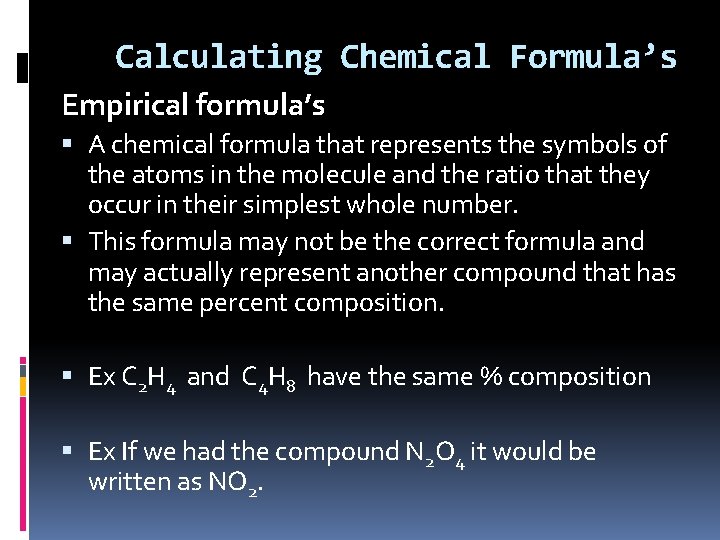
Calculating Chemical Formula’s Empirical formula’s A chemical formula that represents the symbols of the atoms in the molecule and the ratio that they occur in their simplest whole number. This formula may not be the correct formula and may actually represent another compound that has the same percent composition. Ex C 2 H 4 and C 4 H 8 have the same % composition Ex If we had the compound N 2 O 4 it would be written as NO 2.

Calculating Chemical Formula’s Empirical formula’s Found by calculating the percent composition by mass.

Calculating Empirical formula’s Steps (3) 1. Find the mass of each element in 100 g of the compound by using percent composition. 2. Find the amount of moles of each element by converting the mass in 100. 0 g to moles, using the molar mass of the element. 3. Find the whole-number ratio of atoms in 100 g to determine the empirical formula. Reduce to lowest terms.

Calculating Empirical formula’s Ex: Sodium undergoes a combustion reaction with pure Oxygen gas. % Na = 74. 00% , % O = 26. 00 % Find the empirical formula

Empirical Example Step 1 m. Na =. 7400 x 100. 0 g = 74. 00 g m. O =. 2600 x 100. 0 g = 26. 00 g

Empirical Example Step 2 n=? n= m/M n. Na = 74. 00 g / 22. 94 g/mol = 3. 219 mol n. O = 26. 00 g / 16. 00 g/mol = 1. 625 mol

Empirical Example Step 3 The ratio of Na : O is 3. 219 : 1. 625 (3. 219 / 1. 625) = 2 (1. 625 / 1. 625) = 1 The ratio is 2 : 1 Therefore the empirical formula would be Na 2 O

Questions: page 114 # 1, 2

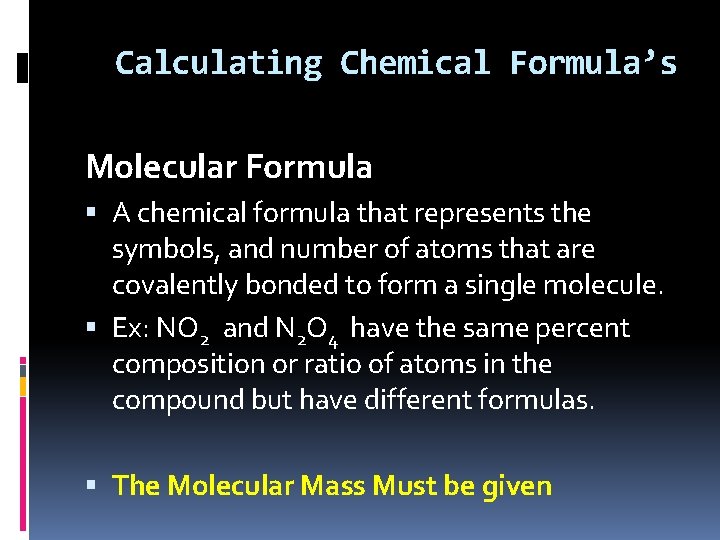
Calculating Chemical Formula’s Molecular Formula A chemical formula that represents the symbols, and number of atoms that are covalently bonded to form a single molecule. Ex: NO 2 and N 2 O 4 have the same percent composition or ratio of atoms in the compound but have different formulas. The Molecular Mass Must be given

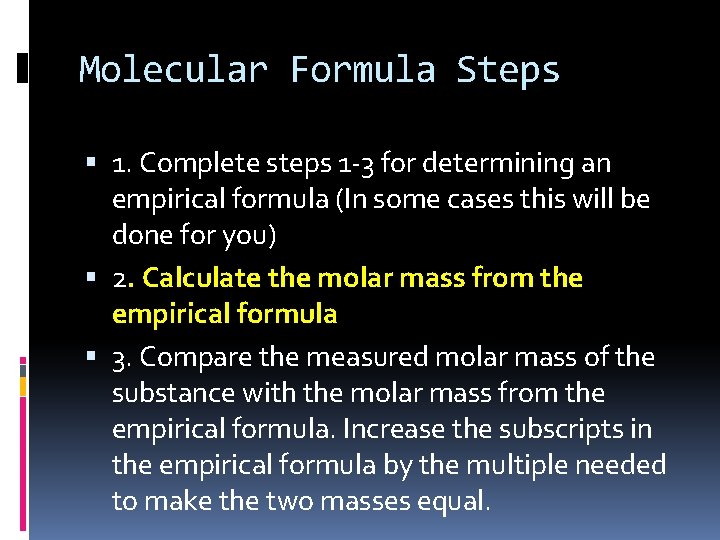
Molecular Formula Steps 1. Complete steps 1 -3 for determining an empirical formula (In some cases this will be done for you) 2. Calculate the molar mass from the empirical formula 3. Compare the measured molar mass of the substance with the molar mass from the empirical formula. Increase the subscripts in the empirical formula by the multiple needed to make the two masses equal.

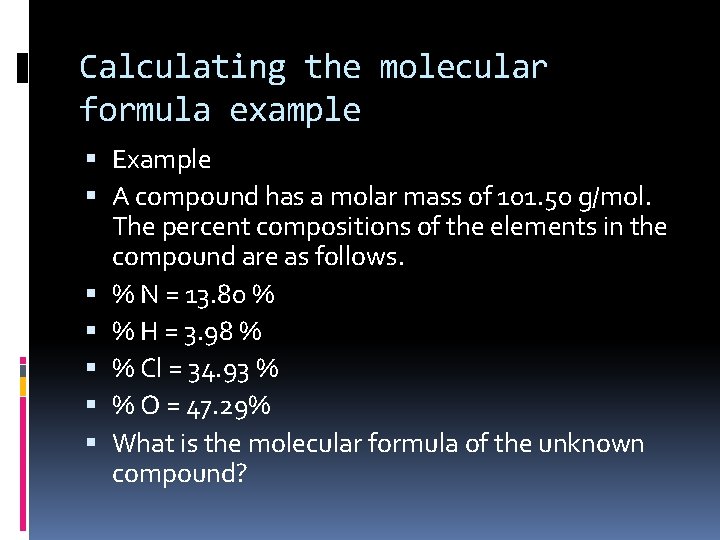
Calculating the molecular formula example Example A compound has a molar mass of 101. 50 g/mol. The percent compositions of the elements in the compound are as follows. % N = 13. 80 % % H = 3. 98 % % Cl = 34. 93 % % O = 47. 29% What is the molecular formula of the unknown compound?

Calculating the molecular formula example Step 1 m. N = 13. 80 % x 100. 0 g = 13. 80 g m. H = 3. 98 % x 100. 0 g = 3. 980 g m. Cl = 34. 93 % x 100. 0 g = 34. 93 g m. O = 47. 29% x 100. 0 g = 47. 29 g

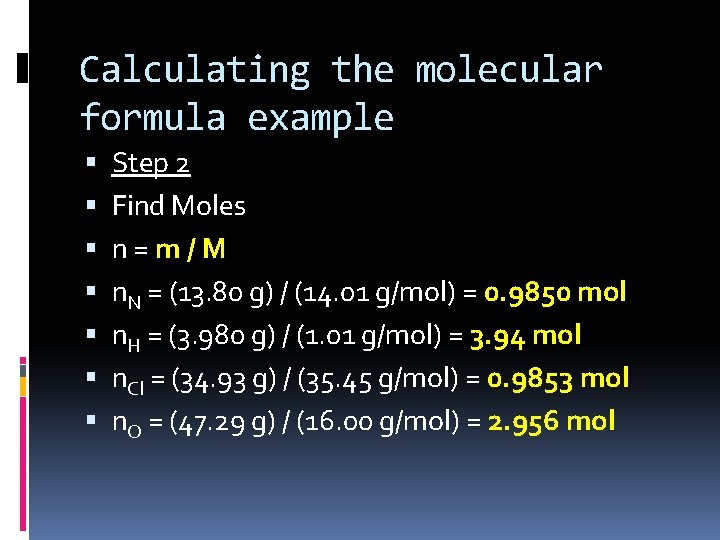
Calculating the molecular formula example Step 2 Find Moles n=m/M n. N = (13. 80 g) / (14. 01 g/mol) = 0. 9850 mol n. H = (3. 980 g) / (1. 01 g/mol) = 3. 94 mol n. Cl = (34. 93 g) / (35. 45 g/mol) = 0. 9853 mol n. O = (47. 29 g) / (16. 00 g/mol) = 2. 956 mol

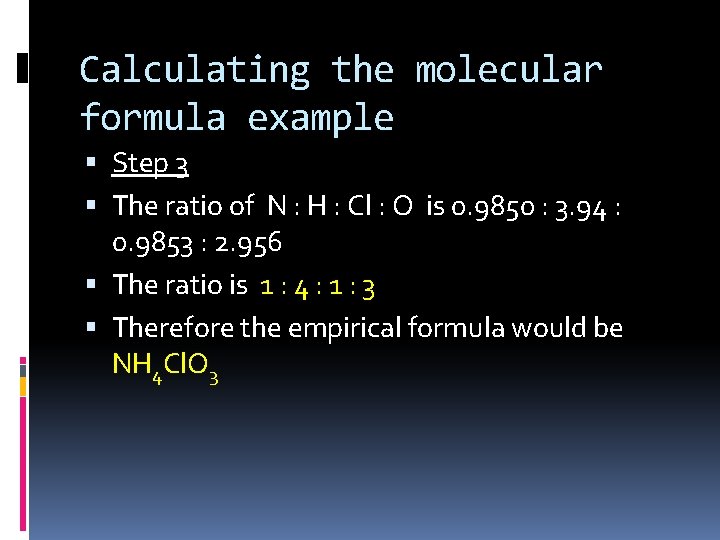
Calculating the molecular formula example Step 3 The ratio of N : H : Cl : O is 0. 9850 : 3. 94 : 0. 9853 : 2. 956 The ratio is 1 : 4 : 1 : 3 Therefore the empirical formula would be NH 4 Cl. O 3

Calculating the molecular formula example Step 4 M = (14. 01 g/mol) + 4 x (1. 01 g/mol) + (35. 45 g/mol) + 3 x (16. 00 g/mol) = 101. 50 g /mol

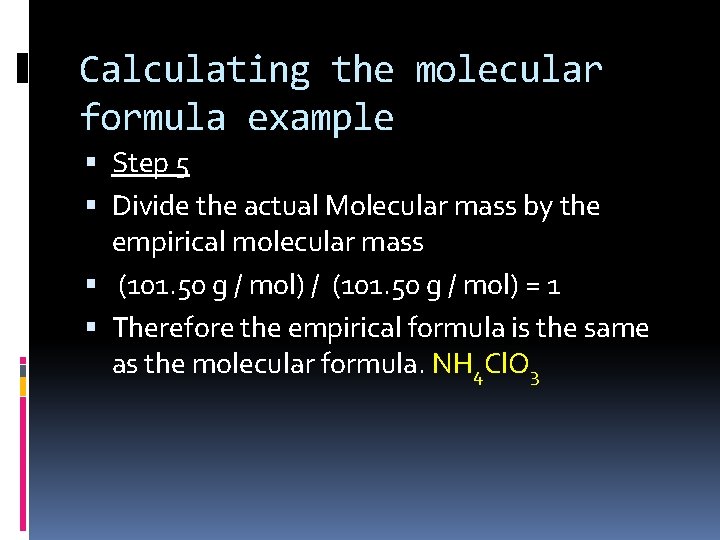
Calculating the molecular formula example Step 5 Divide the actual Molecular mass by the empirical molecular mass (101. 50 g / mol) / (101. 50 g / mol) = 1 Therefore the empirical formula is the same as the molecular formula. NH 4 Cl. O 3

NOTE Had the answer in step 5 been a number such as a 2 or 3, the empirical formula would have been multiplied by that number.

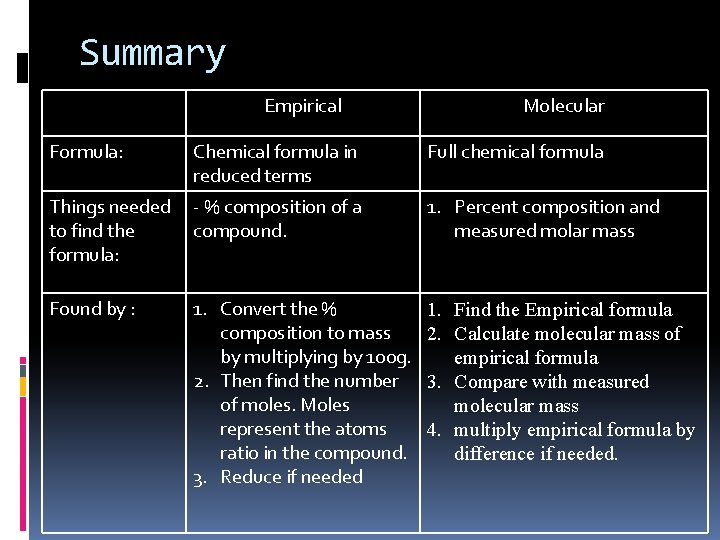
Summary Empirical Molecular Formula: Chemical formula in reduced terms Full chemical formula Things needed to find the formula: - % composition of a compound. 1. Percent composition and measured molar mass Found by : 1. Convert the % composition to mass by multiplying by 100 g. 2. Then find the number of moles. Moles represent the atoms ratio in the compound. 3. Reduce if needed 1. Find the Empirical formula 2. Calculate molecular mass of empirical formula 3. Compare with measured molecular mass 4. multiply empirical formula by difference if needed.

Questions: Page 118 # 3 -6, page 120 # 1, 3, 4 - 8.