Empirical Formulas and Molecular Formulas ReviewPercent Composition Percent



- Slides: 8

Empirical Formulas and Molecular Formulas

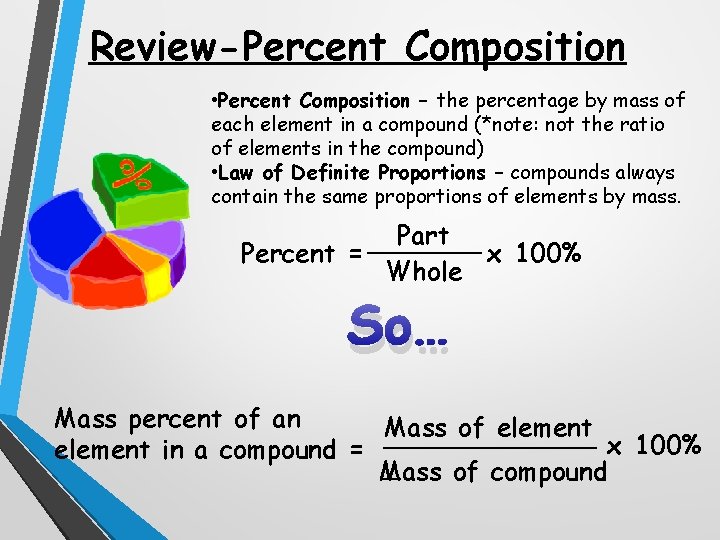
Review-Percent Composition • Percent Composition – the percentage by mass of each element in a compound (*note: not the ratio of elements in the compound) • Law of Definite Proportions – compounds always contain the same proportions of elements by mass. Part _______ Percent = x 100% Whole So… Mass percent of an Mass of element _______ x 100% element in a compound = _ Mass of compound

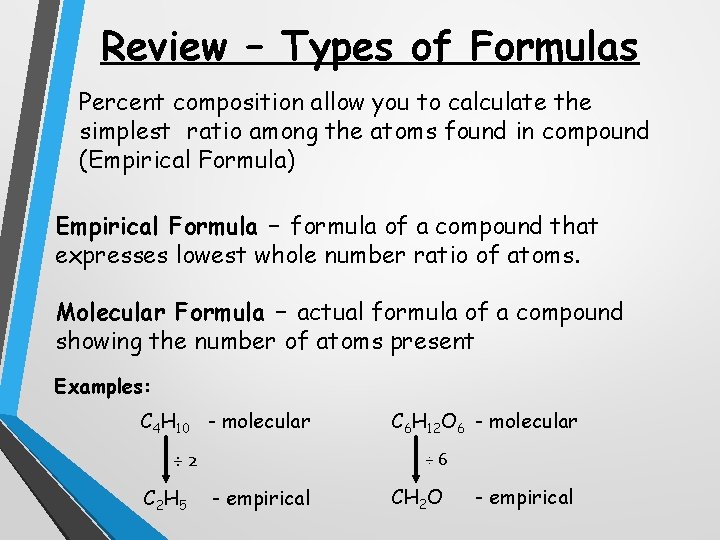
Review – Types of Formulas Percent composition allow you to calculate the simplest ratio among the atoms found in compound (Empirical Formula) Empirical Formula – formula of a compound that expresses lowest whole number ratio of atoms. Molecular Formula – actual formula of a compound showing the number of atoms present Examples: C 4 H 10 - molecular ÷ 2 C 2 H 5 C 6 H 12 O 6 - molecular ÷ 6 - empirical CH 2 O - empirical

Determining Molecular Formula From Empirical Formula – Tyler De. Witt

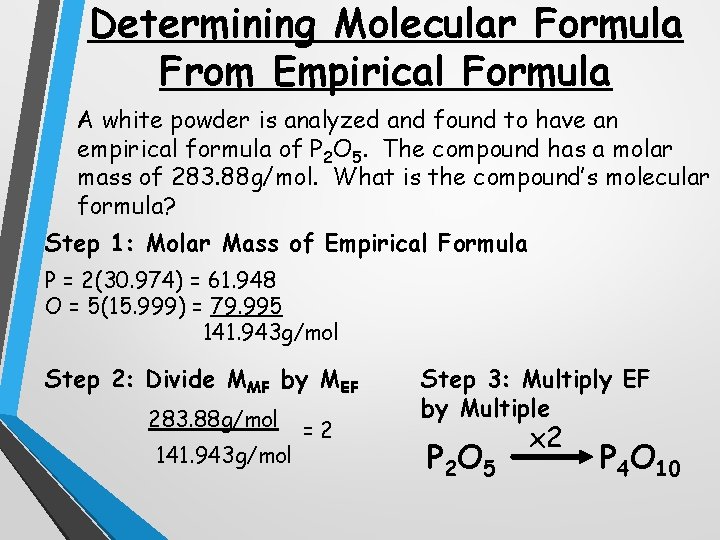
Determining Molecular Formula From Empirical Formula A white powder is analyzed and found to have an empirical formula of P 2 O 5. The compound has a molar mass of 283. 88 g/mol. What is the compound’s molecular formula? Step 1: Molar Mass of Empirical Formula P = 2(30. 974) = 61. 948 O = 5(15. 999) = 79. 995 141. 943 g/mol Step 2: Divide MMF by MEF 283. 88 g/mol 141. 943 g/mol =2 Step 3: Multiply EF by Multiple P 2 O 5 x 2 P 4 O 10

Empirical and Molecular Formula From % Composition – Step-by-Step Science

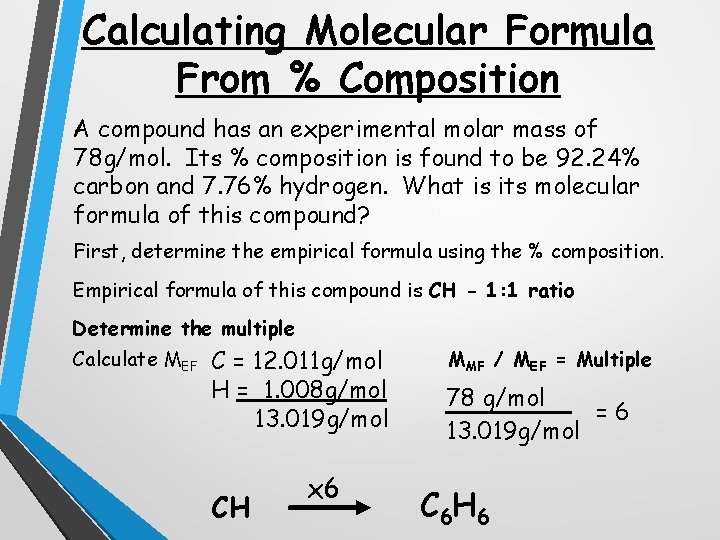
Calculating Molecular Formula From % Composition A compound has an experimental molar mass of 78 g/mol. Its % composition is found to be 92. 24% carbon and 7. 76% hydrogen. What is its molecular formula of this compound? First, determine the empirical formula using the % composition. Empirical formula of this compound is CH - 1: 1 ratio Determine the multiple Calculate MEF C = 12. 011 g/mol H = 1. 008 g/mol 13. 019 g/mol CH x 6 MMF / MEF = Multiple 78 g/mol =6 13. 019 g/mol C 6 H 6

Homework Read Section 6. 9 in the text Page 298 #1 Page 300 #2 Take the Check Your Understanding Quiz
 How to find molecular formula
How to find molecular formula Calculating percentage composition by mass
Calculating percentage composition by mass How to calculate empirical formula using percentages
How to calculate empirical formula using percentages Potassium permanganate percent composition
Potassium permanganate percent composition Empirical formula to percent composition
Empirical formula to percent composition How to get empirical formula from percentages
How to get empirical formula from percentages How to find molecular formula
How to find molecular formula How to get empirical formula from percentages
How to get empirical formula from percentages Empirical and molecular formulas worksheet
Empirical and molecular formulas worksheet