Molecular formulas Molecular Mass properties and hydrates Unit







- Slides: 14

Molecular formulas, Molecular Mass, properties and hydrates Unit 5 Notes #5

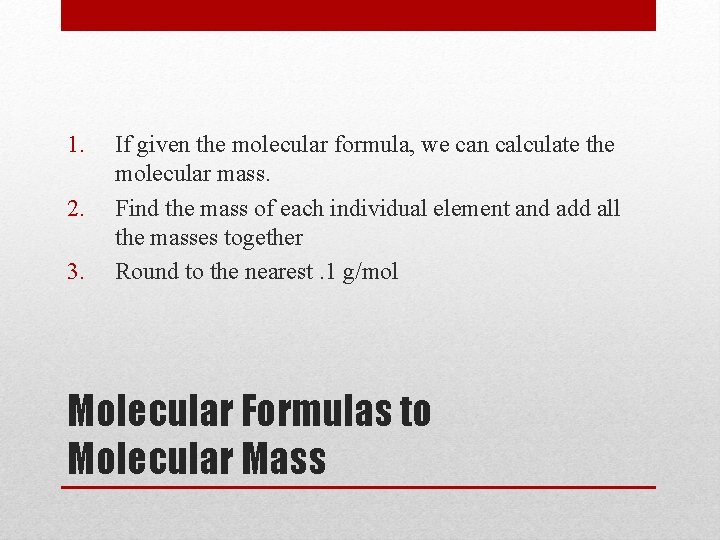
1. 2. 3. If given the molecular formula, we can calculate the molecular mass. Find the mass of each individual element and add all the masses together Round to the nearest. 1 g/mol Molecular Formulas to Molecular Mass

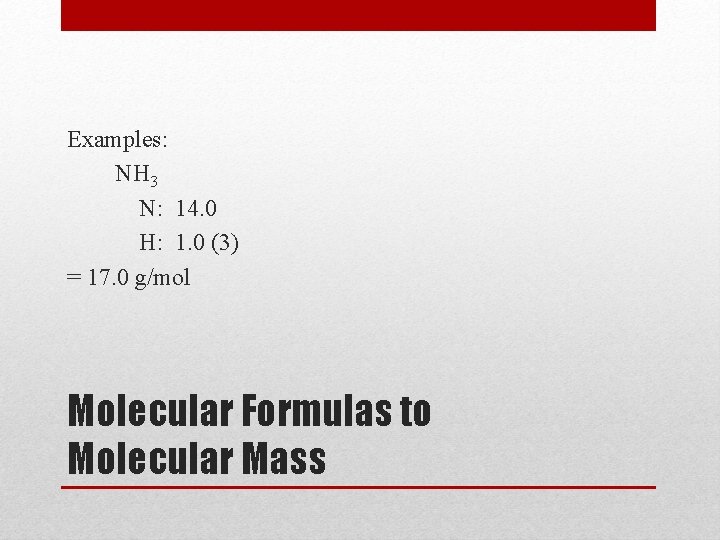
Examples: NH 3 N: 14. 0 H: 1. 0 (3) = 17. 0 g/mol Molecular Formulas to Molecular Mass

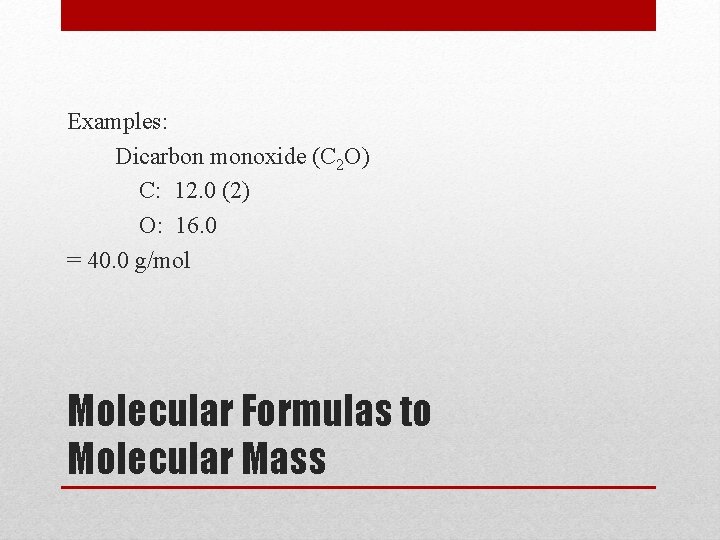
Examples: Dicarbon monoxide (C 2 O) C: 12. 0 (2) O: 16. 0 = 40. 0 g/mol Molecular Formulas to Molecular Mass

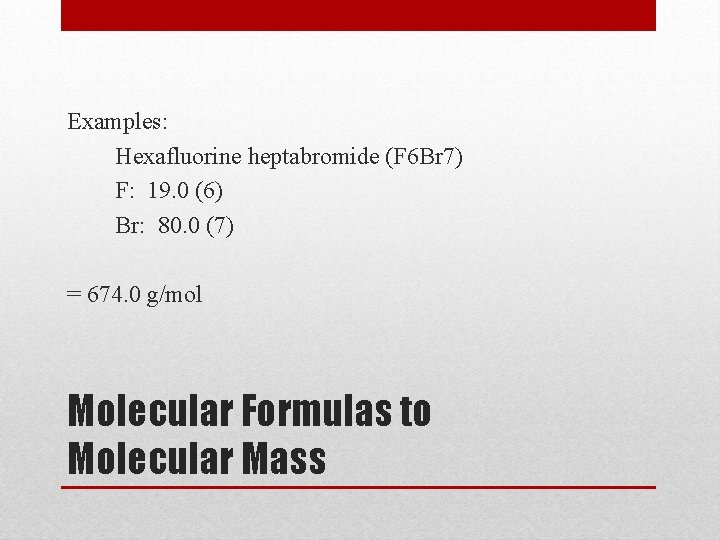
Examples: Hexafluorine heptabromide (F 6 Br 7) F: 19. 0 (6) Br: 80. 0 (7) = 674. 0 g/mol Molecular Formulas to Molecular Mass

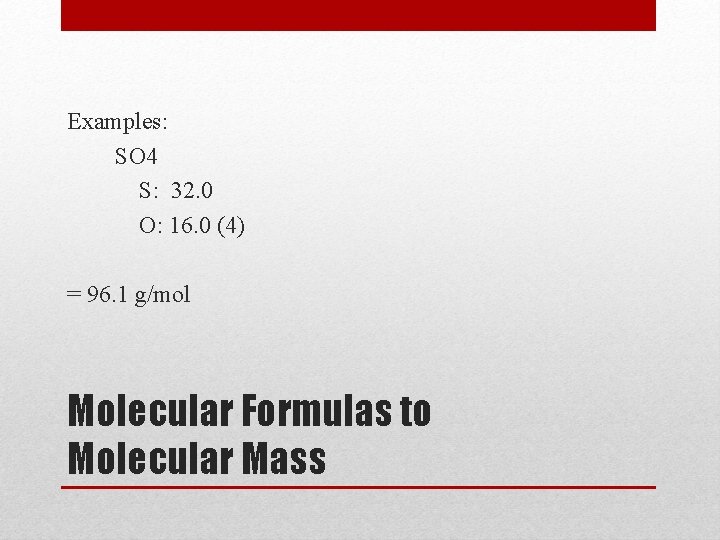
Examples: SO 4 S: 32. 0 O: 16. 0 (4) = 96. 1 g/mol Molecular Formulas to Molecular Mass

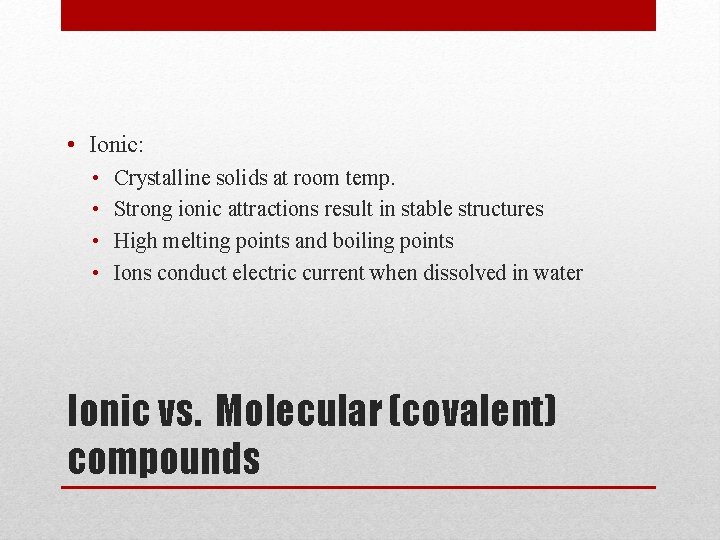
• Ionic: • • Crystalline solids at room temp. Strong ionic attractions result in stable structures High melting points and boiling points Ions conduct electric current when dissolved in water Ionic vs. Molecular (covalent) compounds

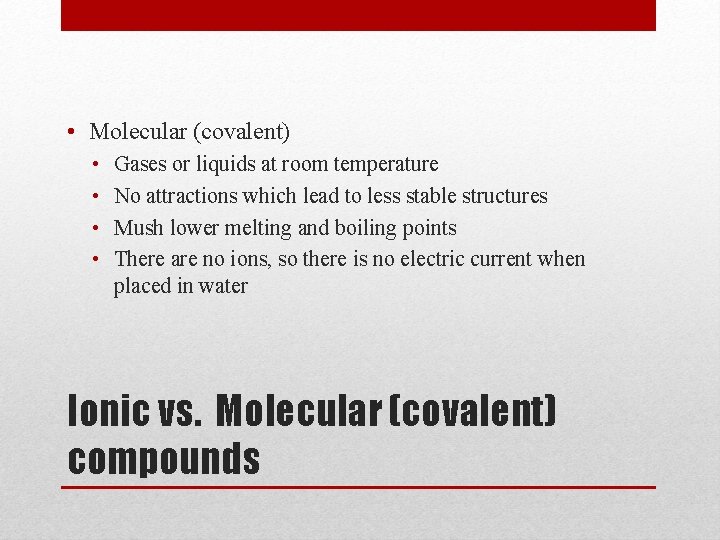
• Molecular (covalent) • • Gases or liquids at room temperature No attractions which lead to less stable structures Mush lower melting and boiling points There are no ions, so there is no electric current when placed in water Ionic vs. Molecular (covalent) compounds

• Compounds that absorb water into their solid structures form substances called hydrates • These substances combine with water in different ratios • Hydrates typically have different properties than anhydrous substances (no water) • These properties depend on whether or not the compound is hydrated, so we must include the degree of hydration Hydrates

1. 2. 3. 4. Write the compound as you normally would (just like any binary molecular, ionic, or polyatomic compound) Add a dot (·) and the number of water molecules that you need. When naming, use the correct prefix for the number of water molecules needed Add the word hydrate for the actual water Lets name hydrates

• Cu. SO 4· 5 H 20 The first part is copper (II) sulfate Add the water and it is Copper (II) sulfate pentahydrate Examples

• Mg. SO 4· 8 H 2 O Magnesium sulfate octahydrate • Barium chloride dyhydrate Ba. Cl 2· 2 H 2 O Examples

• • Ca. SO 4· 6 H 2 O N 4 O 3· 3 H 2 O Calcium chloride dihydrate Copper (II) sulfate decahydrate Try these on your own

• • Ca. SO 4· 6 H 2 O – calcium sulfate hexahydrate N 4 O 3· 3 H 2 O – tetranitrogen trioxide trihydrate Calcium chloride dihydrate – Ca. Cl 2· 2 H 2 O Copper (II) sulfate decahydrate- Cu. SO 4· 10 H 2 O Try these on your own