6 8 NOTES Hydrates Hydrates An Ionic Crystal



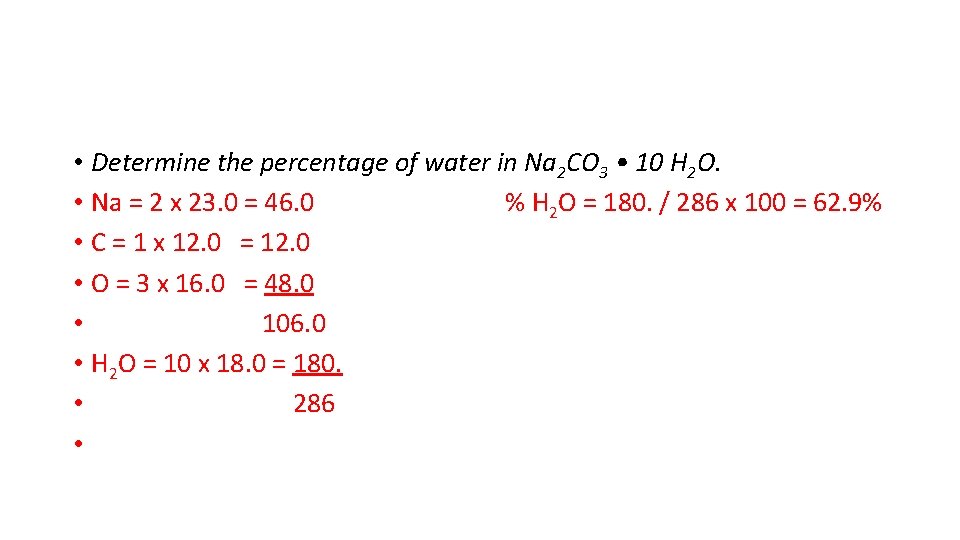







- Slides: 18

6. 8 – NOTES Hydrates

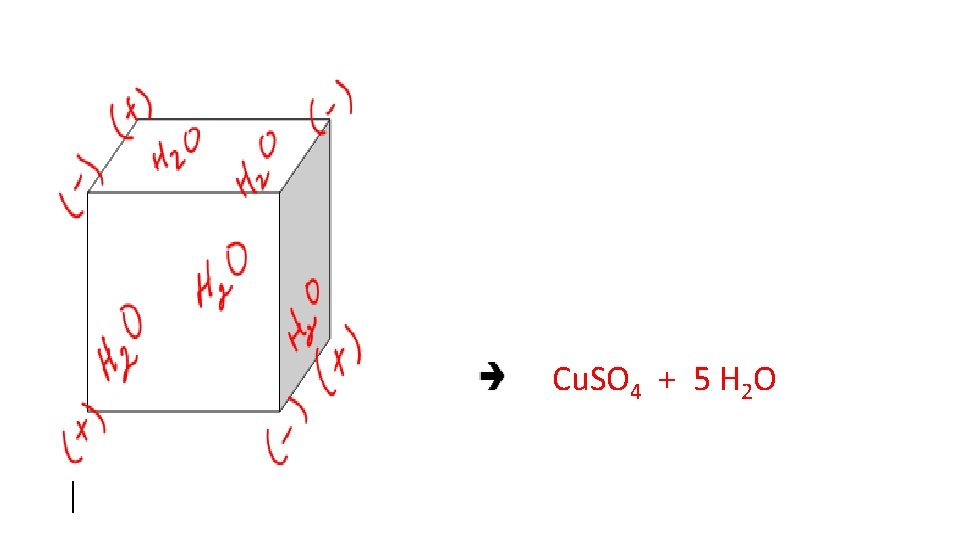
• Hydrates • An Ionic Crystal with water trapped within the crystal structure. • If water is removed it is called an anhydrous salt. • Cu. SO 4 5 H 2 O + heat Cu. SO 4 + 5 H 2 O

Cu. SO 4 + 5 H 2 O

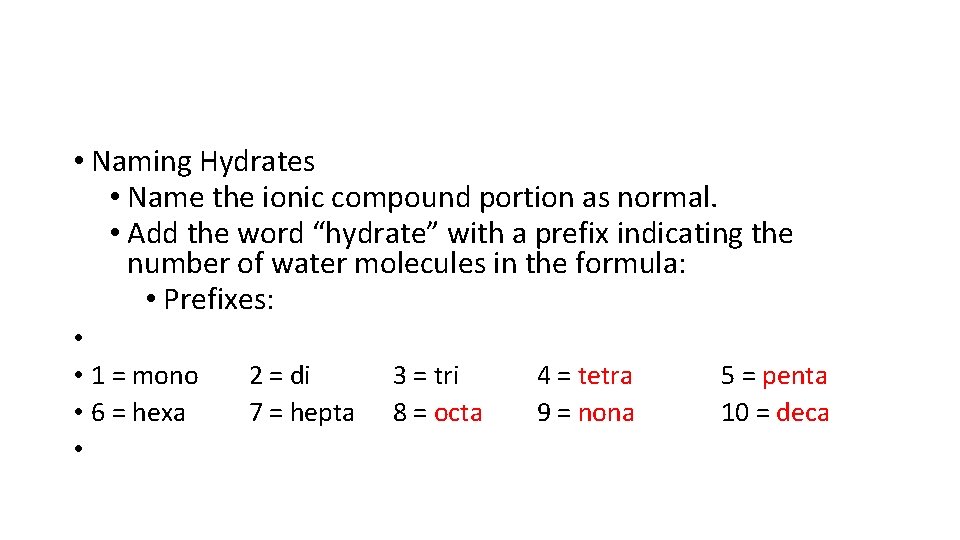
• Naming Hydrates • Name the ionic compound portion as normal. • Add the word “hydrate” with a prefix indicating the number of water molecules in the formula: • Prefixes: • • 1 = mono • 6 = hexa • 2 = di 7 = hepta 3 = tri 8 = octa 4 = tetra 9 = nona 5 = penta 10 = deca

• Examples: • Cu. SO 4 5 H 2 O • Na 2 CO 3 • 10 H 2 O • Ni. Cl 2 • 7 H 2 O

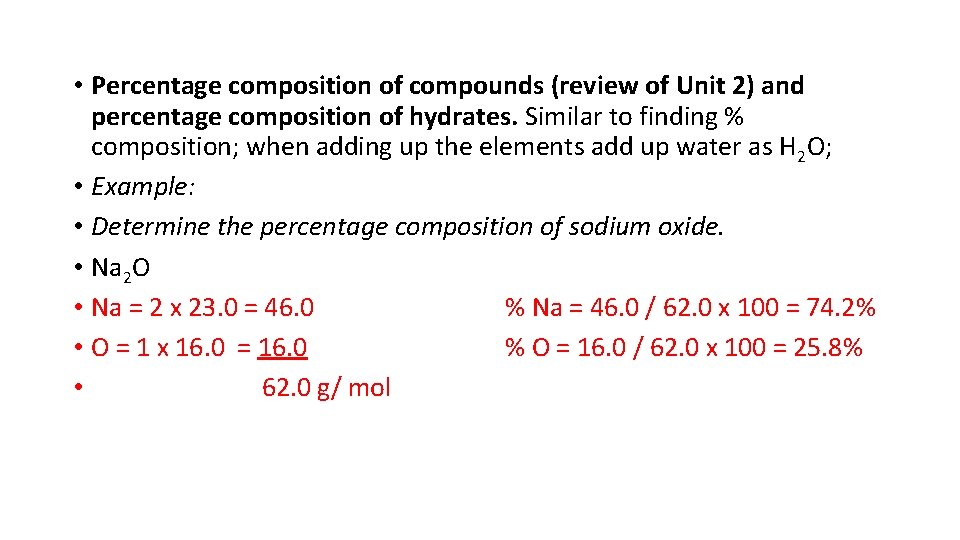
• Percentage composition of compounds (review of Unit 2) and percentage composition of hydrates. Similar to finding % composition; when adding up the elements add up water as H 2 O; • Example: • Determine the percentage composition of sodium oxide. • Na 2 O • Na = 2 x 23. 0 = 46. 0 % Na = 46. 0 / 62. 0 x 100 = 74. 2% • O = 1 x 16. 0 = 16. 0 % O = 16. 0 / 62. 0 x 100 = 25. 8% • 62. 0 g/ mol
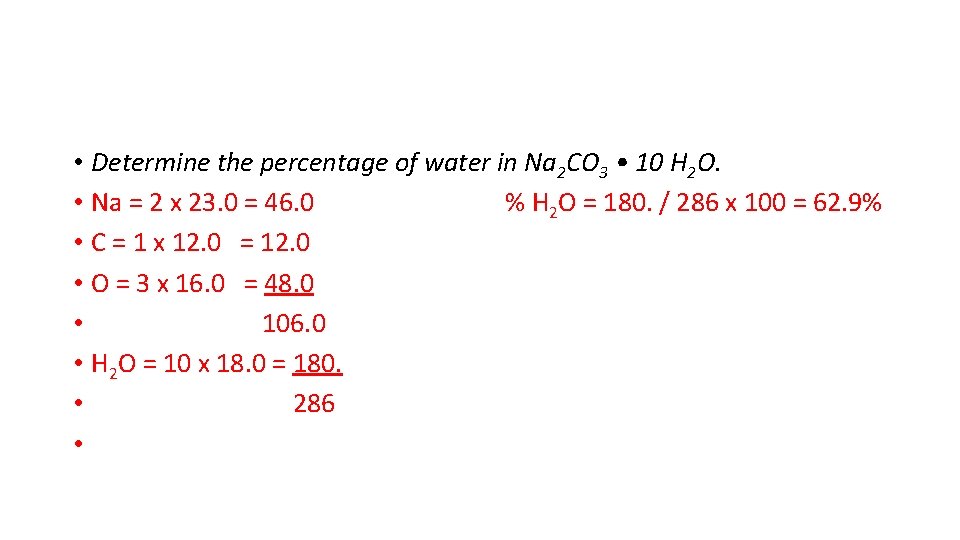
• Determine the percentage of water in Na 2 CO 3 • 10 H 2 O. • Na = 2 x 23. 0 = 46. 0 % H 2 O = 180. / 286 x 100 = 62. 9% • C = 1 x 12. 0 = 12. 0 • O = 3 x 16. 0 = 48. 0 • 106. 0 • H 2 O = 10 x 18. 0 = 180. • 286 •

• Example: Cu. SO 4 • 5 H 2 O • Name the compound, then write the equation for the heating of this compound. • Cu. SO 4 • 5 H 2 O = copper (II) sulfate (or cupric sulfate) pentahydrate • • Cu. SO 4 • 5 H 2 O Cu. SO 4 + 5 H 2 O (anhydrous + water) water driven off as vapor

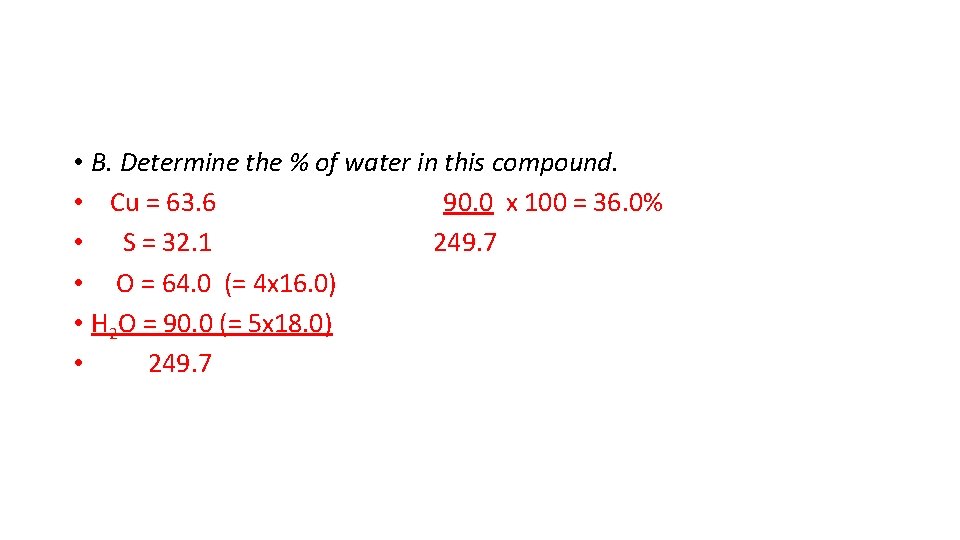
• B. Determine the % of water in this compound. • Cu = 63. 6 90. 0 x 100 = 36. 0% • S = 32. 1 249. 7 • O = 64. 0 (= 4 x 16. 0) • H 2 O = 90. 0 (= 5 x 18. 0) • 249. 7

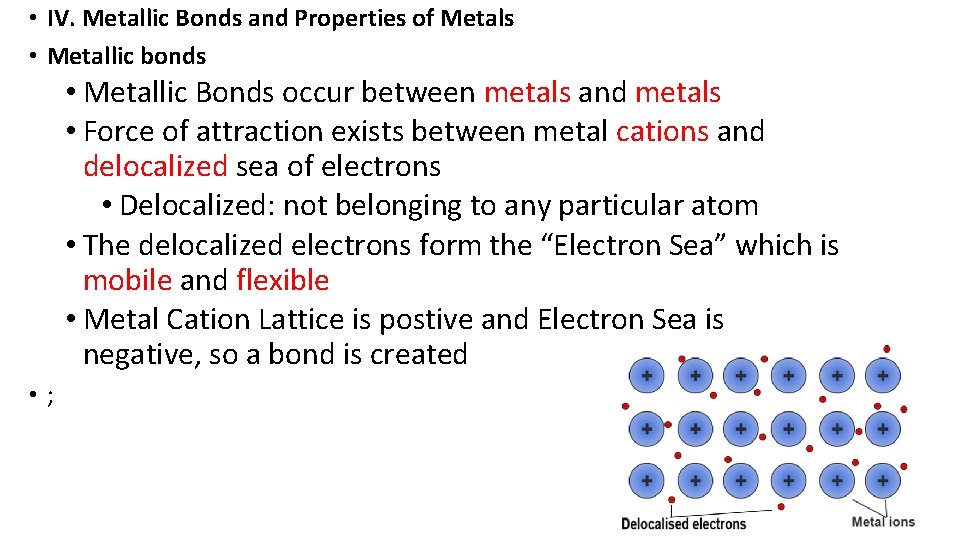
• IV. Metallic Bonds and Properties of Metals • Metallic bonds • Metallic Bonds occur between metals and metals • Force of attraction exists between metal cations and delocalized sea of electrons • Delocalized: not belonging to any particular atom • The delocalized electrons form the “Electron Sea” which is mobile and flexible • Metal Cation Lattice is postive and Electron Sea is negative, so a bond is created • ;


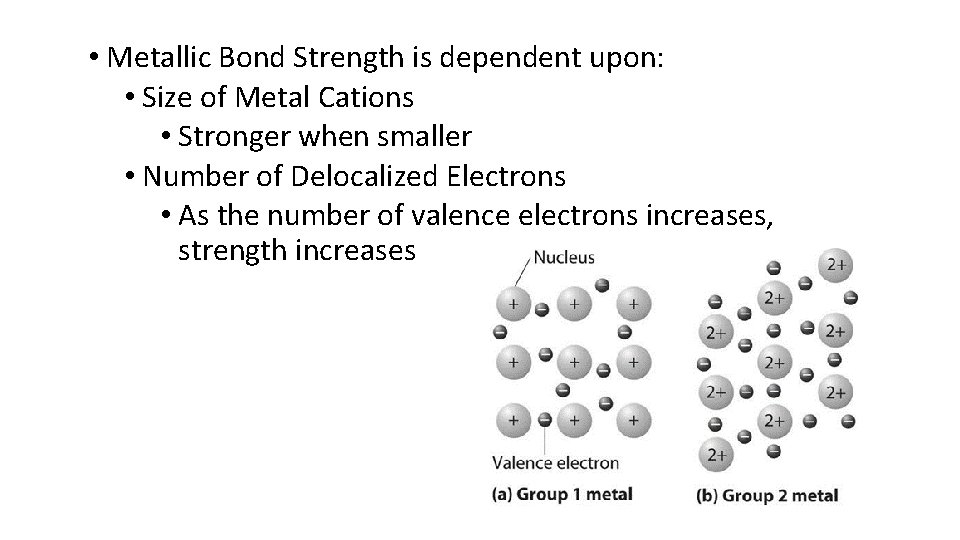
• Metallic Bond Strength is dependent upon: • Size of Metal Cations • Stronger when smaller • Number of Delocalized Electrons • As the number of valence electrons increases, strength increases

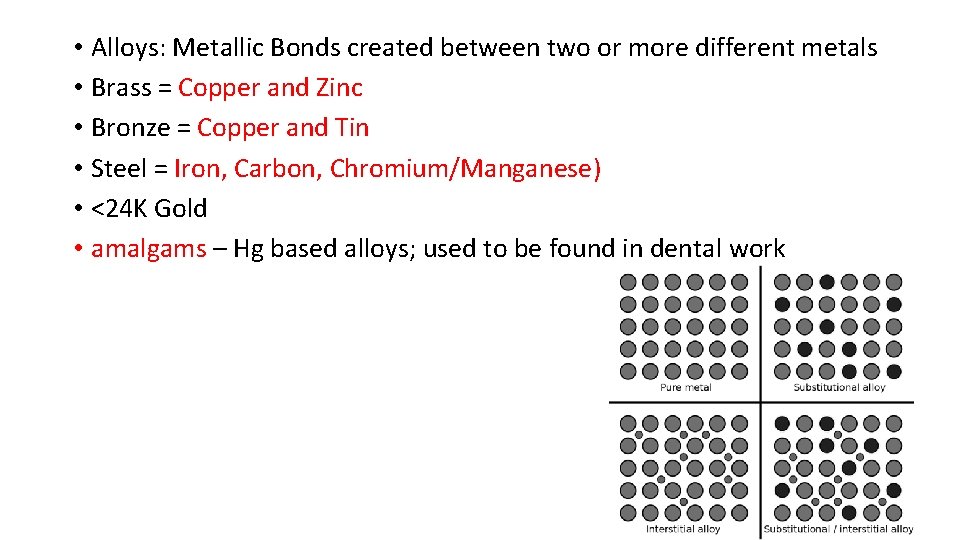
• Alloys: Metallic Bonds created between two or more different metals • Brass = Copper and Zinc • Bronze = Copper and Tin • Steel = Iron, Carbon, Chromium/Manganese) • <24 K Gold • amalgams – Hg based alloys; used to be found in dental work

2. Properties of metals • Malleable and Ductile • Conductive • Lustrous • High MP • Very High BP • Hardness - increases with stronger bonding



