Chapter 4 1 Notes Ionic compounds consist of












- Slides: 22

Chapter 4. 1 Notes Ionic compounds consist of ions held together in lattice structures by ionic bonds

Important terms for this section � Chemical bond � Valence electrons � Ions � Cations � Anions � Ionization � Ionic bond � Ionic compound � Lattice � Formula unit � Lattice energy � Volatility �Hydrated �Solvated �Binary �Electronegativity values �Bonding continuum �Note: only outer shell (valence) electrons take part in bonding

Ionic bonding �Atoms bonded chemically have different properties than the individual atoms �Ionic bonds are chemical bonds between oppositely charged particles � Ions are created by the gain or loss of valence electrons � Cations: metals – loss of electrons so positively charged (groups 1, 2, 13) � Anions: non-metals – gain of electrons so negatively charged (groups 15, 16, 17) �Stable transition metal ions to know: � Pb 2+, Sn 4+, Sn 2+, Ag+, H-, Cu+, Cu 2+, Fe 3+ �Polyatomic ions to know: see page 142

Ionic compounds �Ionic bonding: transfer of electrons from one atom to another �Ionic formula writing and naming: � Mg 2+ + F- � NH 4+ + PO 4 3 - � Al 3+ + S 2 - �Ionic formula writing: � Sodium sulfate � Magnesium cyanide � Mercury hydrogencarbonate

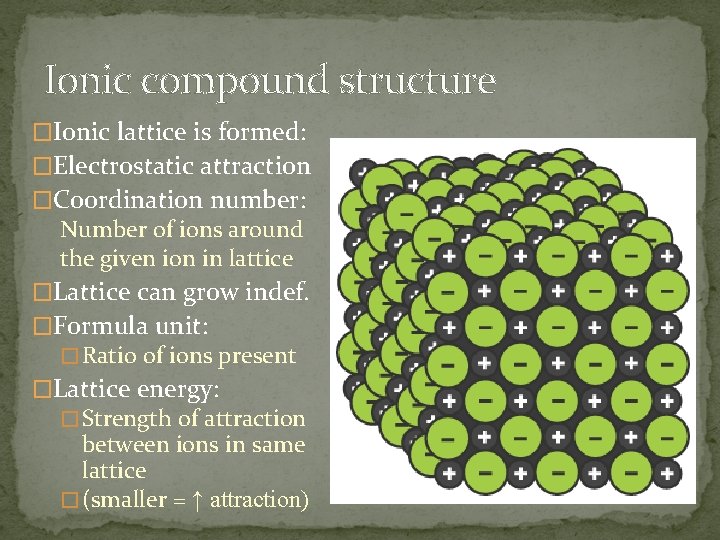
Ionic compound structure �Ionic lattice is formed: �Electrostatic attraction �Coordination number: Number of ions around the given ion in lattice �Lattice can grow indef. �Formula unit: � Ratio of ions present �Lattice energy: � Strength of attraction between ions in same lattice � (smaller = ↑ attraction)

Physical props. reflect lattice structure �Melting and boiling points are usually very high (strong electrostatic attraction means stronger bonds) �Industry tends to not extract metals from molten ionics due to the very high temps needed �Low tendency to vaporize = low volatility � Therefore, low odor (think of table salt)

Physical props. reflect lattice structure �Solubility: how easy will it dissolve into a solvent (become dispersed through a liquid) to form a solution �Like dissolves like! �Salt is ionic, so it will likely dissolve into…. ? � Polar solvent � Non-polar solvent � Ionic solvent �Ions dissociate in water and are surrounded by water molecules = hydrated �Ions dissociate in other liquid = solvated

Physical props. reflect lattice structure �Electrical conductivity: � Ionic compounds that are molten have moveable charges � Ionic compounds dissolved in solution have moveable charges �Brittleness: � Ionic compounds tend to shatter on impact (not malleable or ductile) � This occurs because the ions moving from impact will put like charges next to each other, so they repulse and split

Ionic character (how we know it’s ionic) �How do you know if it’s ionic or covalent? Sometimes the metalloids (for example) show some grey areas… �Si can have 4+ or 4 - and H can have 1 - or 1+ �Remember:

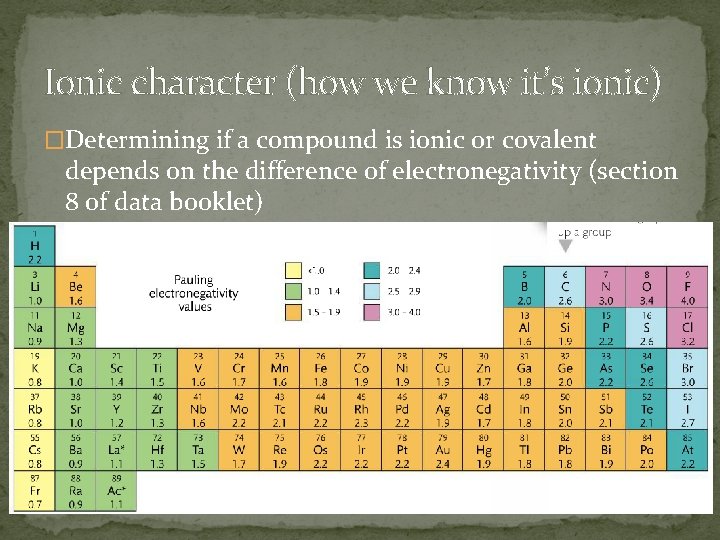
Ionic character (how we know it’s ionic) �Determining if a compound is ionic or covalent depends on the difference of electronegativity (section 8 of data booklet)

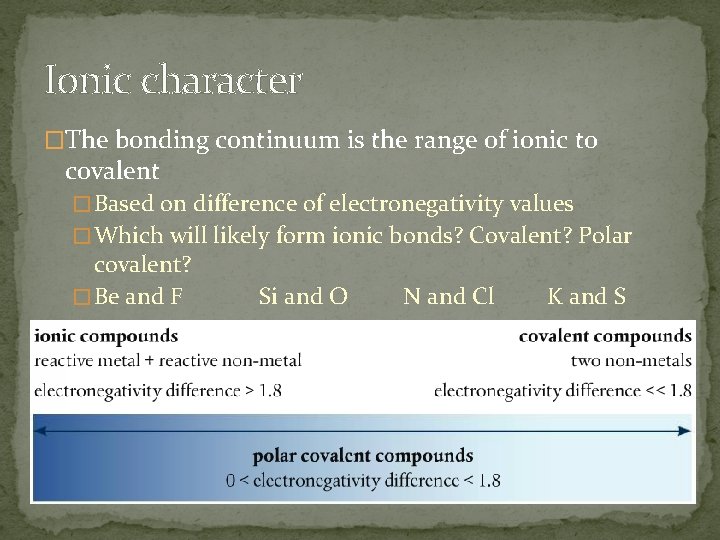
Ionic character �The bonding continuum is the range of ionic to covalent � Based on difference of electronegativity values � Which will likely form ionic bonds? Covalent? Polar covalent? � Be and F Si and O N and Cl K and S

Chapter 4. 2 Notes Covalent compounds form by the sharing of electrons

Important terms in this section �Molecule �Bond enthalpy �Diatomic �Polar �Triatomic �Directionality �Octet rule �Dipole �Non-bonding pairs �Partial charge �Double bond �Pure covalent �Triple bond �Bond length �Bond strength

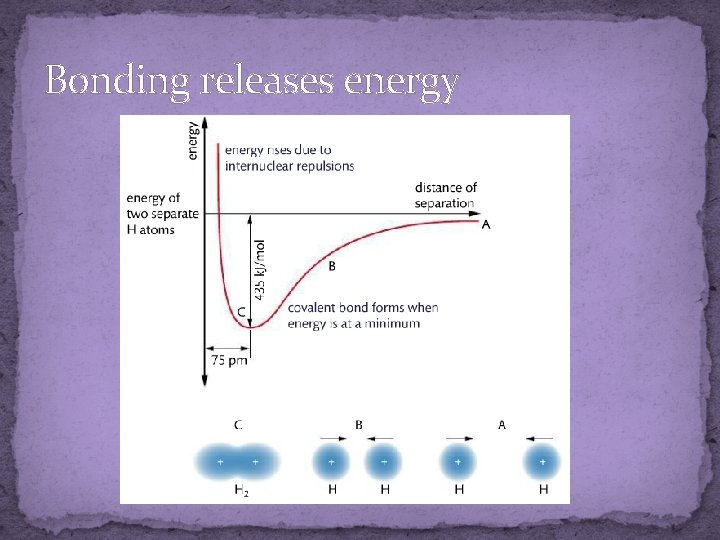
Covalent bonding �Atoms in covalent molecules share electrons �Covalent bond is an electrostatic attraction between a pair of e- and the positive nuclei �Atoms with covalent bonds are called molecules (as opposed to the ionic called formula units) �Hₓ • H H – H �Two hydrogen atoms bond covalently to form a hydrogen molecule (these are diatomic molecules) ex. of triatomic? �Covalent bonding is favorable (stabilizes the atoms) so forming the bonds releases energy

Bonding releases energy

Covalent bonding �Octet rule: tendency for atoms to form stable arrangements of 8 e- in outer shell (like Ar) � This is not really a “rule” but a common occurrence as this “rule” has exceptions �Non-bonding pairs or lone pairs: pairs of electrons on an atom that do not participate in the covalent bond

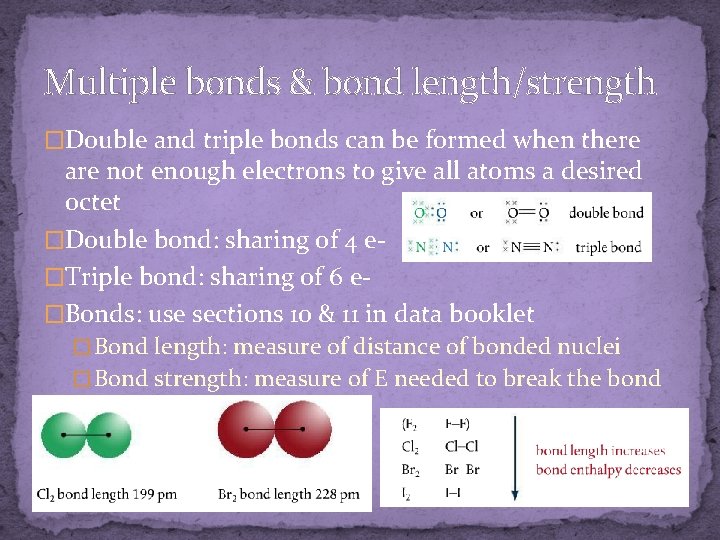
Multiple bonds & bond length/strength �Double and triple bonds can be formed when there are not enough electrons to give all atoms a desired octet �Double bond: sharing of 4 e�Triple bond: sharing of 6 e�Bonds: use sections 10 & 11 in data booklet � Bond length: measure of distance of bonded nuclei � Bond strength: measure of E needed to break the bond (bond enthalpy)

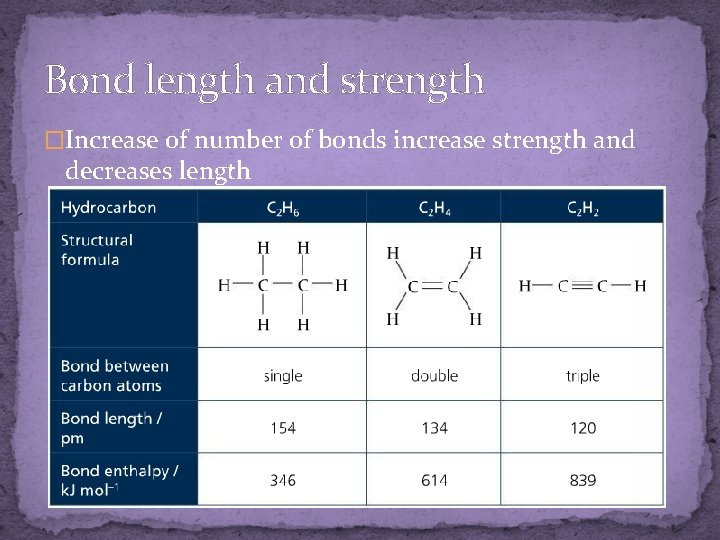
Bond length and strength �Increase of number of bonds increase strength and decreases length

Polar covalent bonds �These bonds do not share electrons evenly �There is DIRECTIONALITY to the bond and/or the molecule �Dipole: having one end being slightly more positive and one end being slightly more negative

Polar covalent bonds �Again, the difference in electronegativity will tell you the extent of how polar the bond

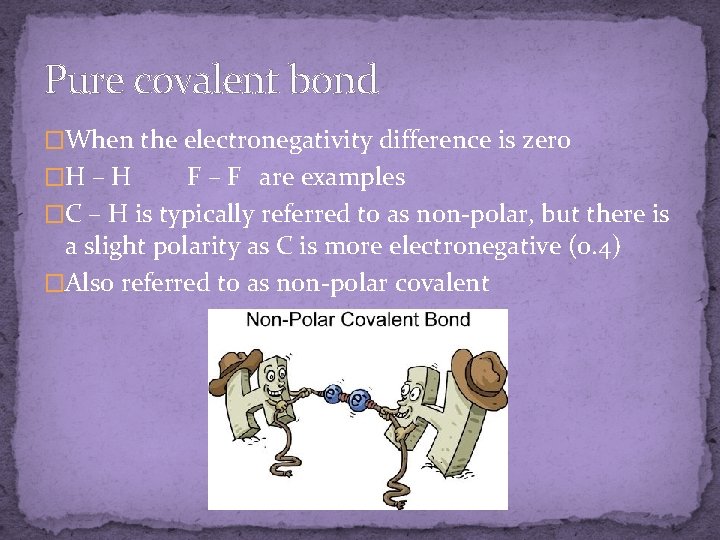
Pure covalent bond �When the electronegativity difference is zero �H – H F – F are examples �C – H is typically referred to as non-polar, but there is a slight polarity as C is more electronegative (0. 4) �Also referred to as non-polar covalent

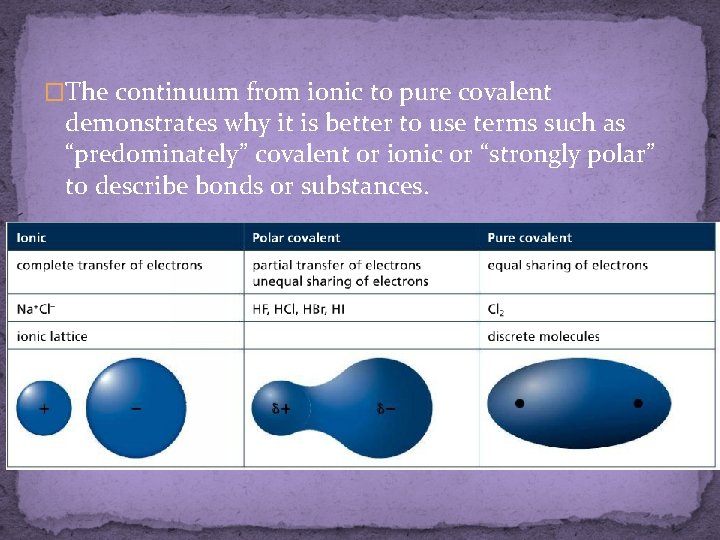
�The continuum from ionic to pure covalent demonstrates why it is better to use terms such as “predominately” covalent or ionic or “strongly polar” to describe bonds or substances.