THERMODYNAMICS Thermodynamics is the study of energy relationships


- Slides: 71

THERMODYNAMICS Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating

Objectives: After finishing this unit, you should be able to: • State and apply the first and second laws of thermodynamics. • Demonstrate your understanding of adiabatic, isochoric, isothermal, and isobaric processes. • Write and apply a relationship for determining the ideal efficiency of a heat engine. • Write and apply a relationship for determining coefficient of performance for a refrigeratior.

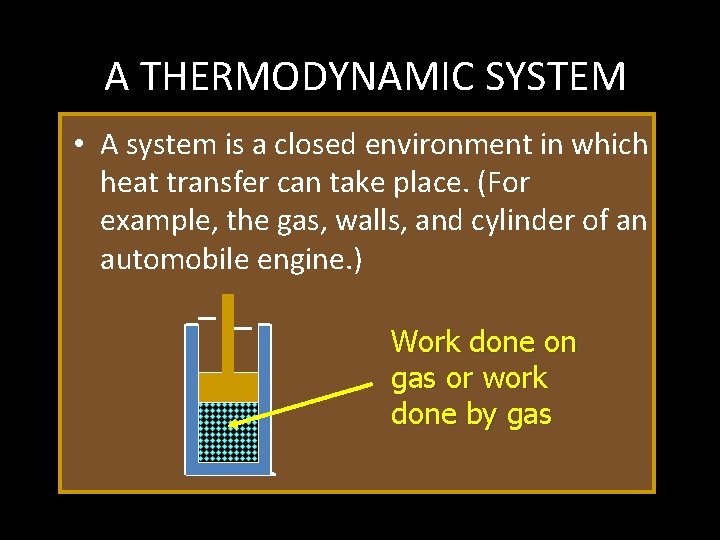
A THERMODYNAMIC SYSTEM • A system is a closed environment in which heat transfer can take place. (For example, the gas, walls, and cylinder of an automobile engine. ) Work done on gas or work done by gas

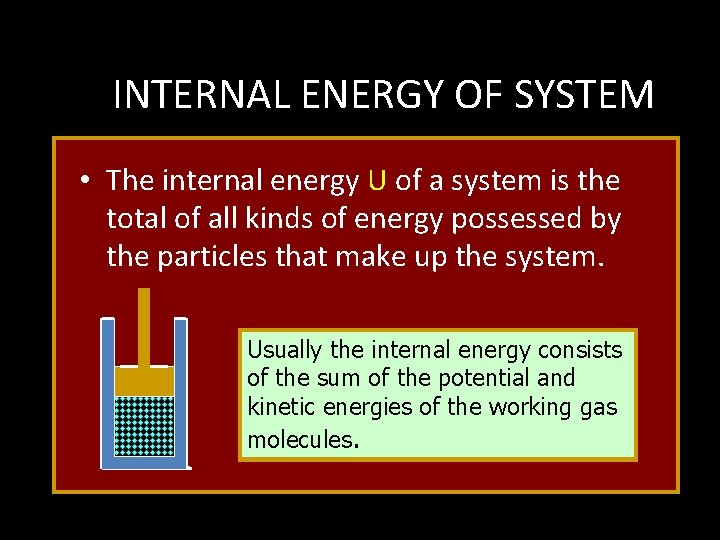
INTERNAL ENERGY OF SYSTEM • The internal energy U of a system is the total of all kinds of energy possessed by the particles that make up the system. Usually the internal energy consists of the sum of the potential and kinetic energies of the working gas molecules.

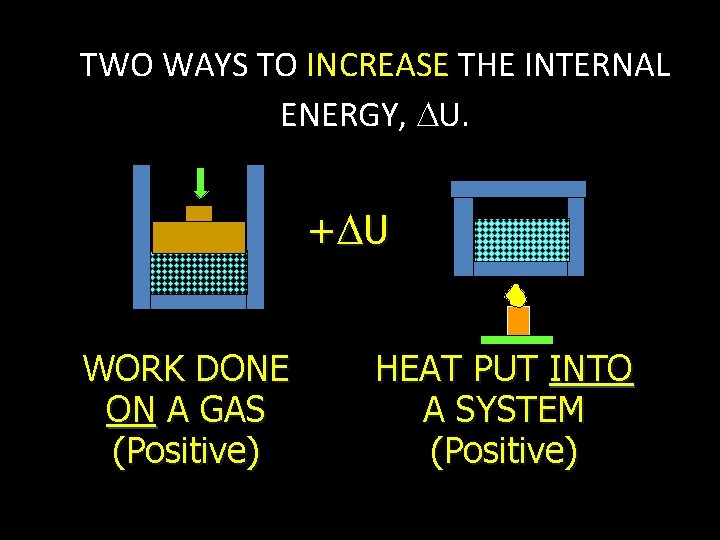
TWO WAYS TO INCREASE THE INTERNAL ENERGY, U. + U WORK DONE ON A GAS (Positive) HEAT PUT INTO A SYSTEM (Positive)

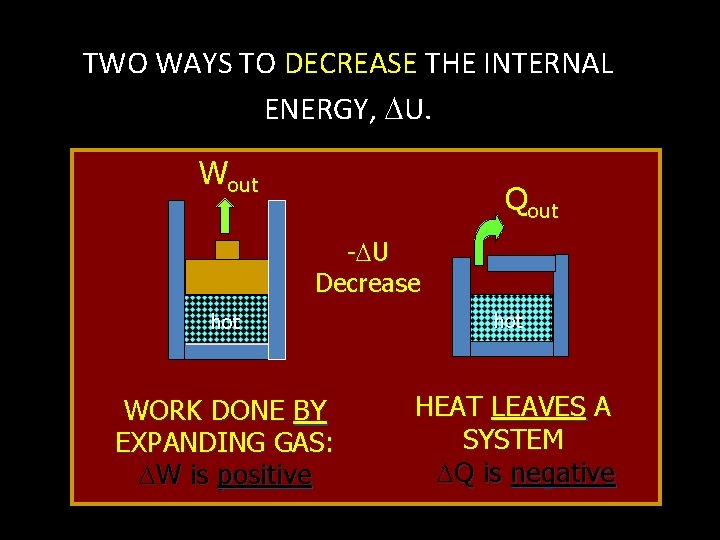
TWO WAYS TO DECREASE THE INTERNAL ENERGY, U. Wout Qout - U Decrease hot WORK DONE BY EXPANDING GAS: W is positive hot HEAT LEAVES A SYSTEM Q is negative

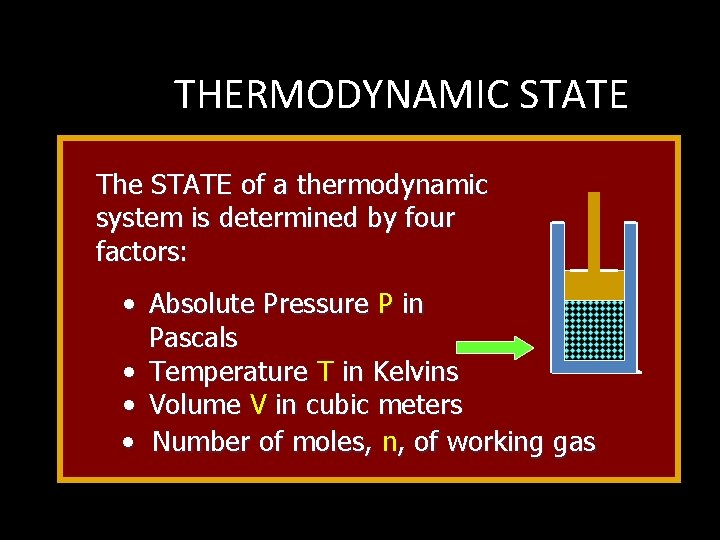
THERMODYNAMIC STATE The STATE of a thermodynamic system is determined by four factors: • Absolute Pressure P in Pascals • Temperature T in Kelvins • Volume V in cubic meters • Number of moles, n, of working gas

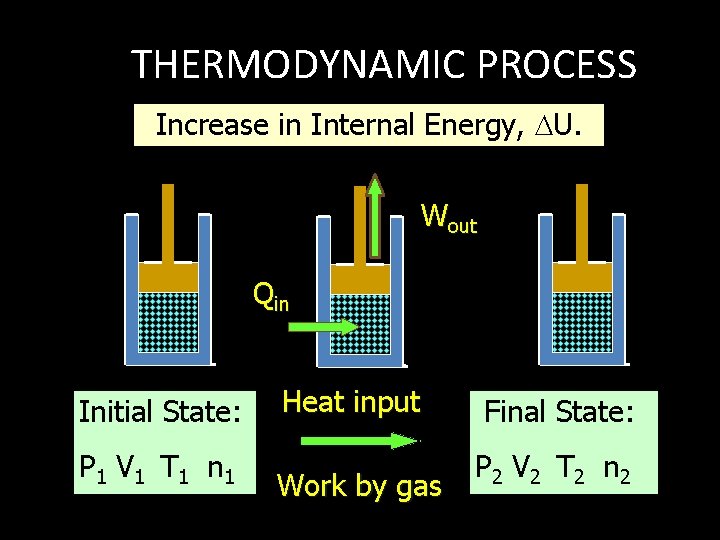
THERMODYNAMIC PROCESS Increase in Internal Energy, U. Wout Qin Initial State: P 1 V 1 T 1 n 1 Heat input Final State: Work by gas P 2 V 2 T 2 n 2

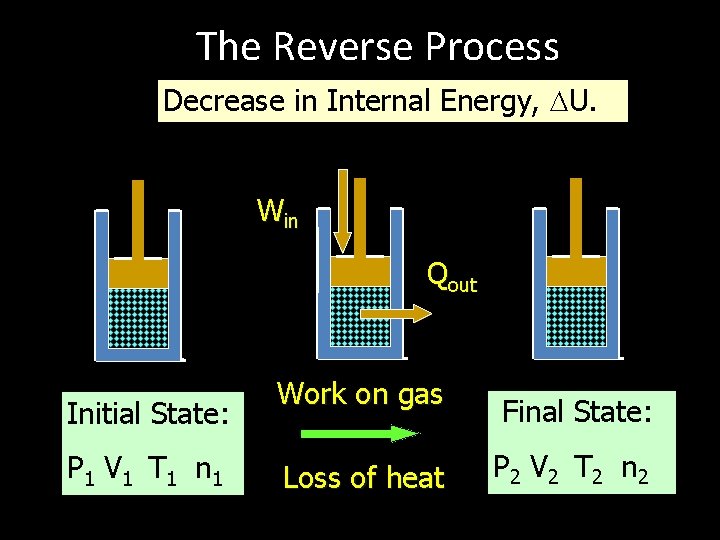
The Reverse Process Decrease in Internal Energy, U. Win Qout Initial State: P 1 V 1 T 1 n 1 Work on gas Loss of heat Final State: P 2 V 2 T 2 n 2

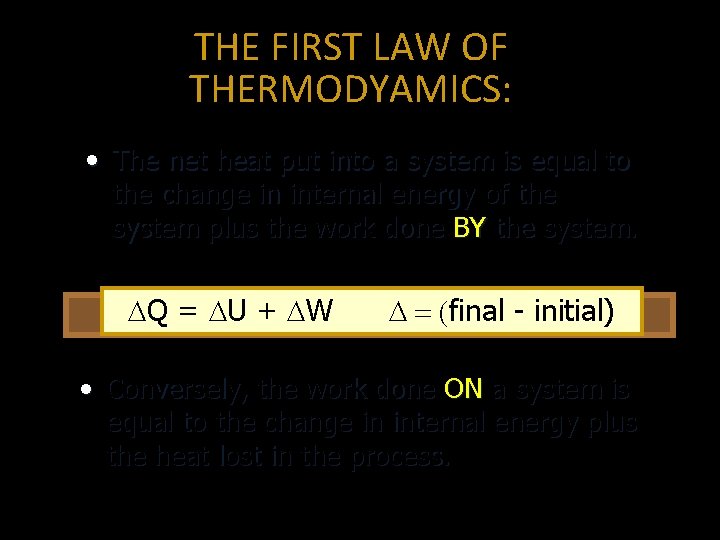
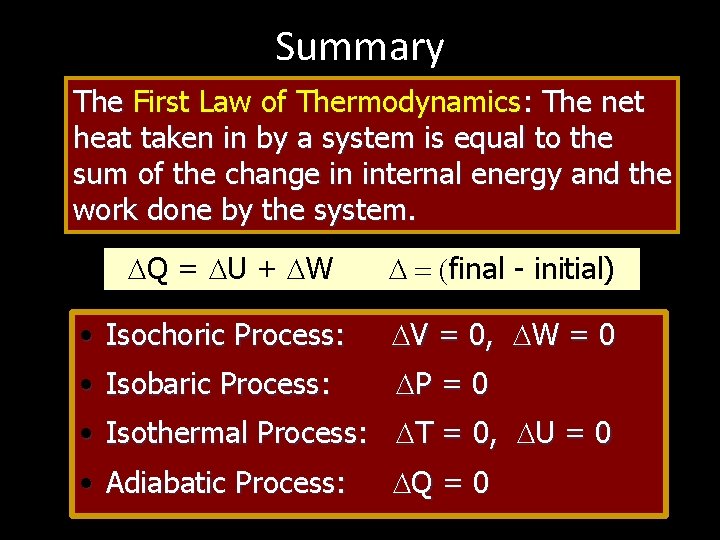
THE FIRST LAW OF THERMODYAMICS: • The net heat put into a system is equal to the change in internal energy of the system plus the work done BY the system. Q = U + W final - initial) • Conversely, the work done ON a system is equal to the change in internal energy plus the heat lost in the process.

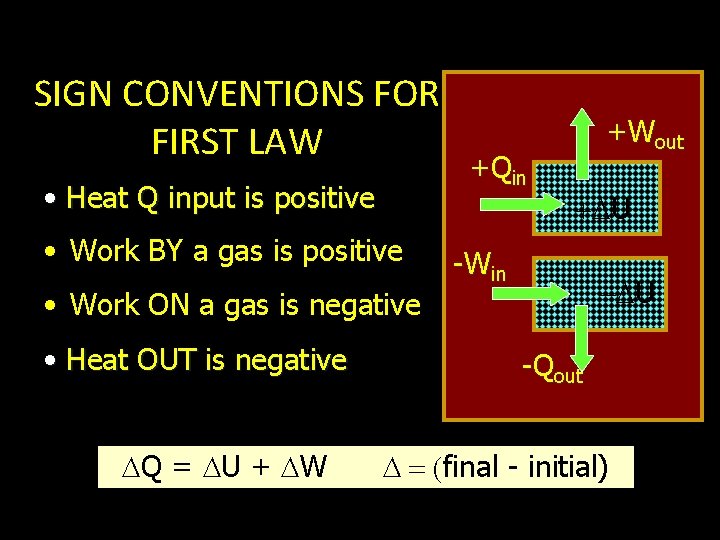
SIGN CONVENTIONS FOR FIRST LAW • Heat Q input is positive • Work BY a gas is positive • Work ON a gas is negative • Heat OUT is negative Q = U + W +Qin +Wout U -Win U -Qout final - initial)

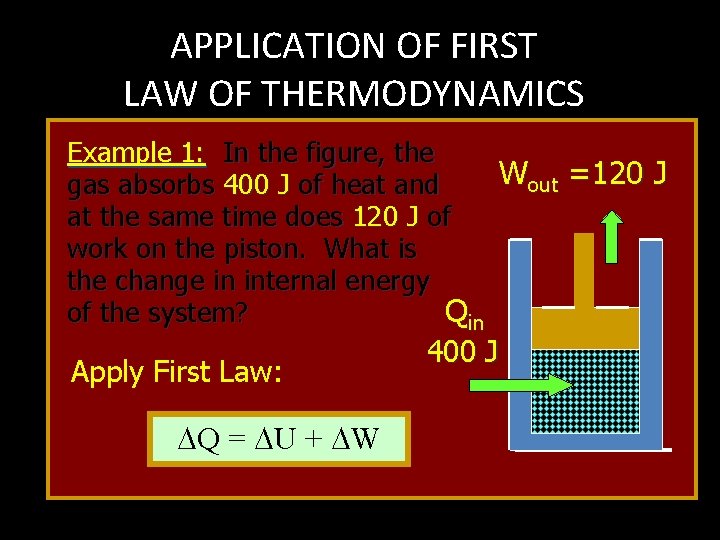
APPLICATION OF FIRST LAW OF THERMODYNAMICS Example 1: In the figure, the Wout =120 J gas absorbs 400 J of heat and at the same time does 120 J of work on the piston. What is the change in internal energy of the system? Qin Apply First Law: Q = U + W 400 J

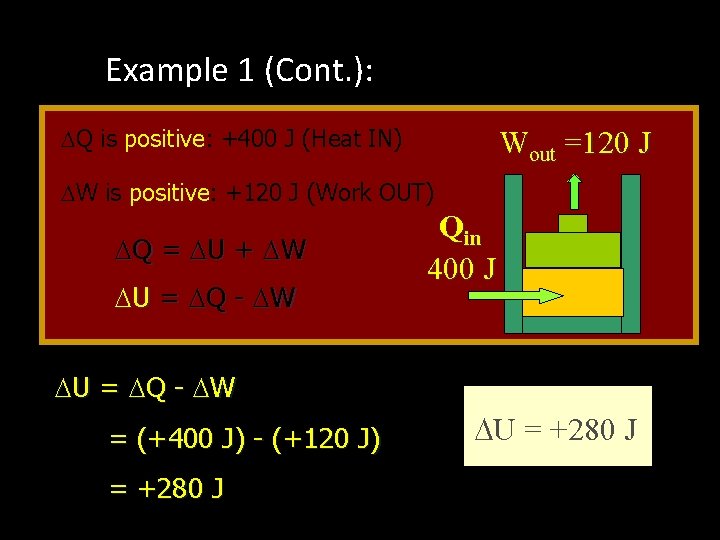
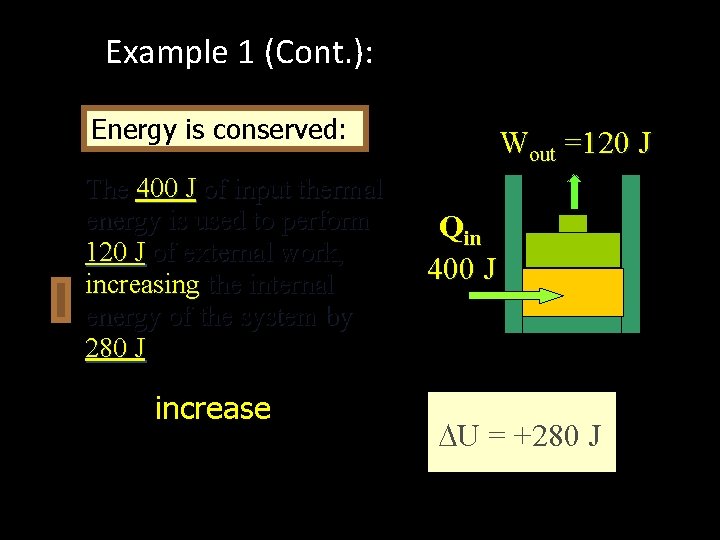
Example 1 (Cont. ): Apply First Law Q is positive: +400 J (Heat IN) Wout =120 J W is positive: +120 J (Work OUT) Q = U + W U = Q - W = (+400 J) - (+120 J) = +280 J Qin 400 J U = +280 J

Example 1 (Cont. ): Apply First Law Energy is conserved: The 400 J of input thermal energy is used to perform 120 J of external work, increasing the internal energy of the system by 280 J The increase in internal energy is: Wout =120 J Qin 400 J U = +280 J

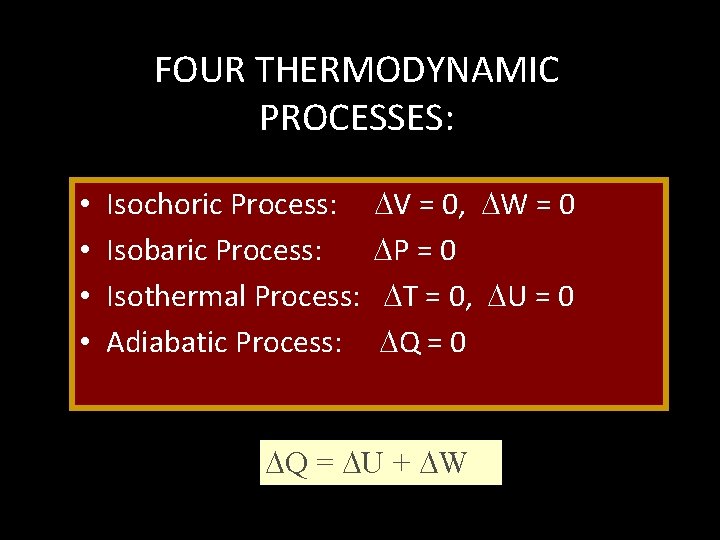
FOUR THERMODYNAMIC PROCESSES: • • Isochoric Process: Isobaric Process: Isothermal Process: Adiabatic Process: V = 0, W = 0 P = 0 T = 0, U = 0 Q = 0 Q = U + W

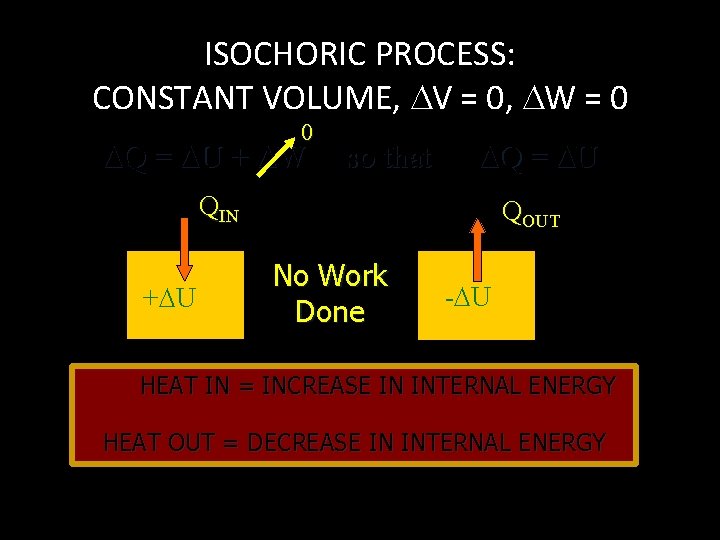
ISOCHORIC PROCESS: CONSTANT VOLUME, V = 0, W = 0 0 Q = U + W so that Q = U QIN + U QOUT No Work Done - U HEAT IN = INCREASE IN INTERNAL ENERGY HEAT OUT = DECREASE IN INTERNAL ENERGY

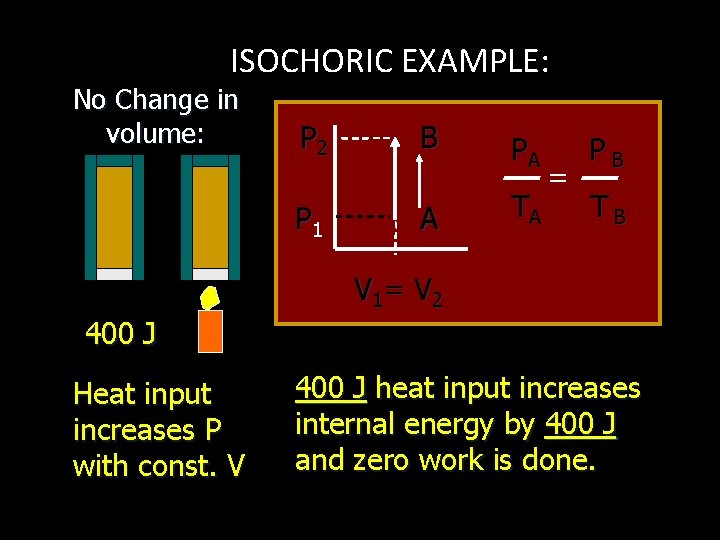
ISOCHORIC EXAMPLE: No Change in volume: P 2 B P 1 A PA TA = PB TB V 1= V 2 400 J Heat input increases P with const. V 400 J heat input increases internal energy by 400 J and zero work is done.

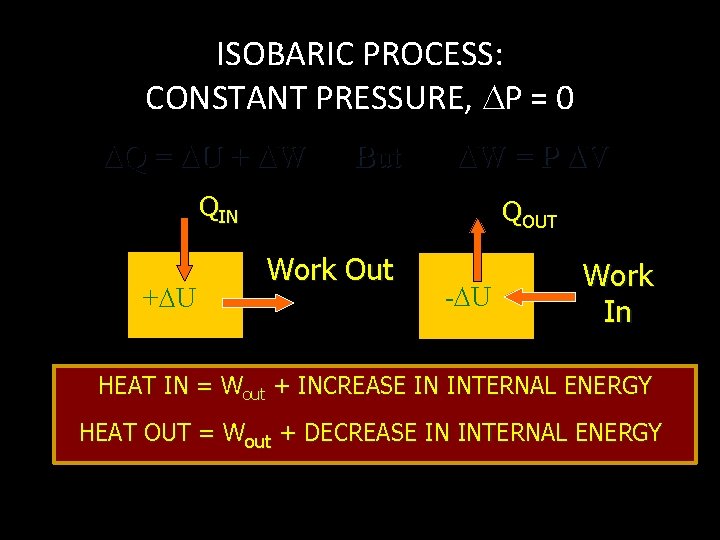
ISOBARIC PROCESS: CONSTANT PRESSURE, P = 0 Q = U + W But W = P V QIN + U QOUT Work Out - U Work In HEAT IN = Wout + INCREASE IN INTERNAL ENERGY HEAT OUT = Wout + DECREASE IN INTERNAL ENERGY

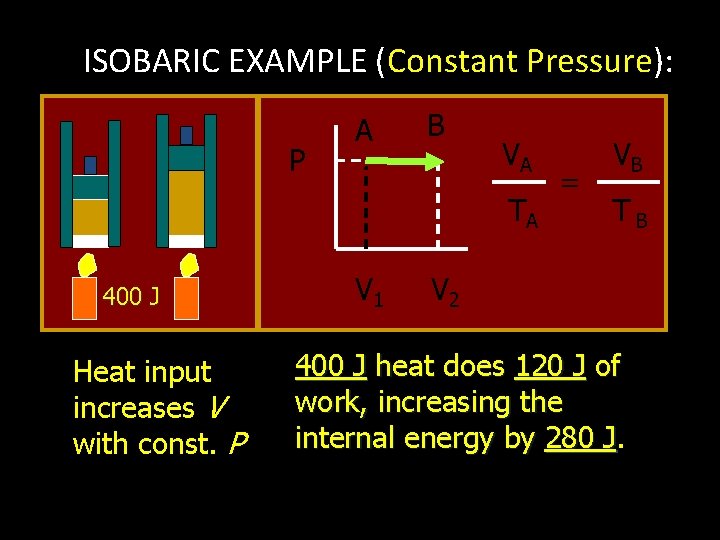
ISOBARIC EXAMPLE (Constant Pressure): P A B VA TA 400 J Heat input increases V with const. P V 1 = VB TB V 2 400 J heat does 120 J of work, increasing the internal energy by 280 J. J

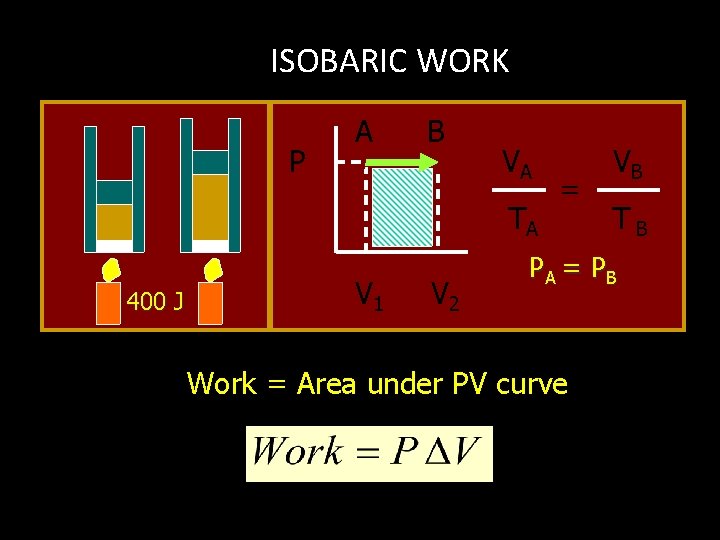
ISOBARIC WORK P A B VA TA 400 J V 1 V 2 = VB TB PA = P B Work = Area under PV curve

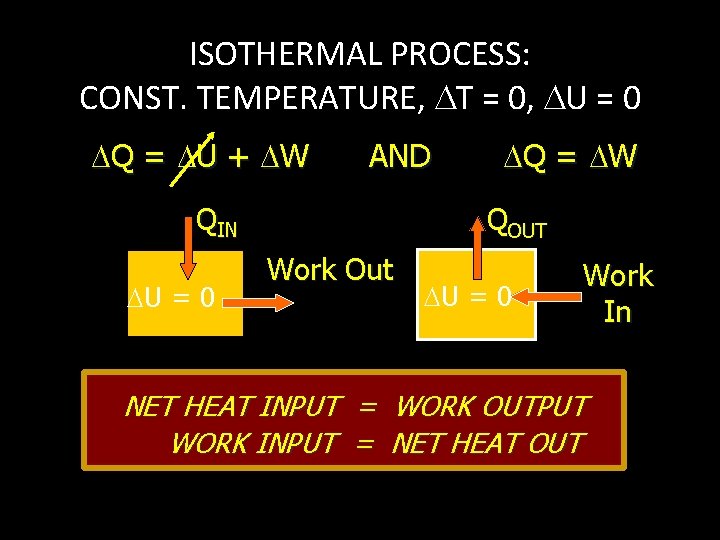
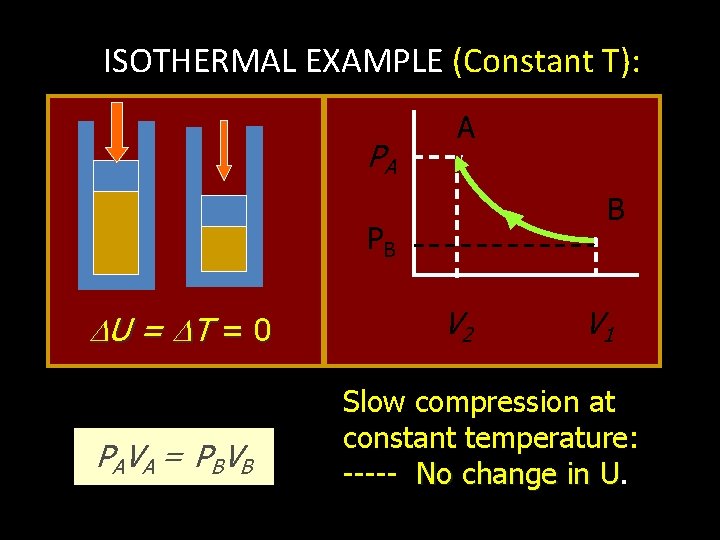
ISOTHERMAL PROCESS: CONST. TEMPERATURE, T = 0, U = 0 Q = U + W AND Q = W QIN U = 0 QOUT Work Out U = 0 Work In NET HEAT INPUT = WORK OUTPUT WORK INPUT = NET HEAT OUT

ISOTHERMAL EXAMPLE (Constant T): PA A B PB U = T = 0 P A V A = PBV B V 2 V 1 Slow compression at constant temperature: ----- No change in U. U

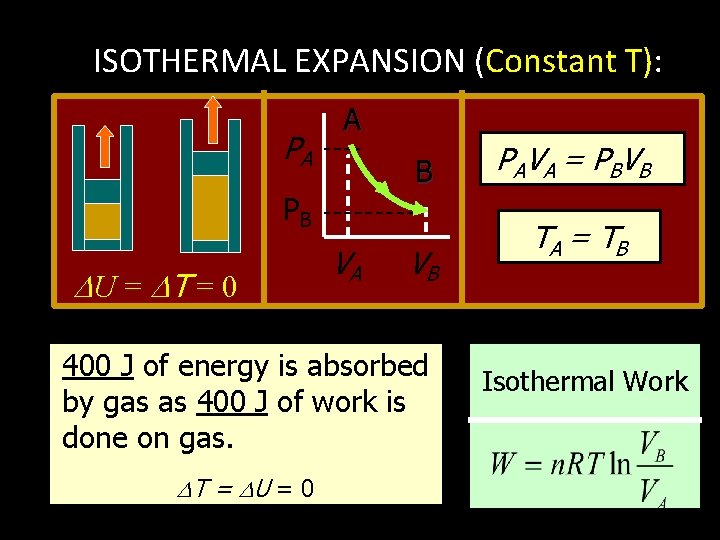
ISOTHERMAL EXPANSION (Constant T): PA A B PB U = T = 0 VA VB 400 J of energy is absorbed by gas as 400 J of work is done on gas. T = U = 0 PA V A = P BV B TA = T B Isothermal Work

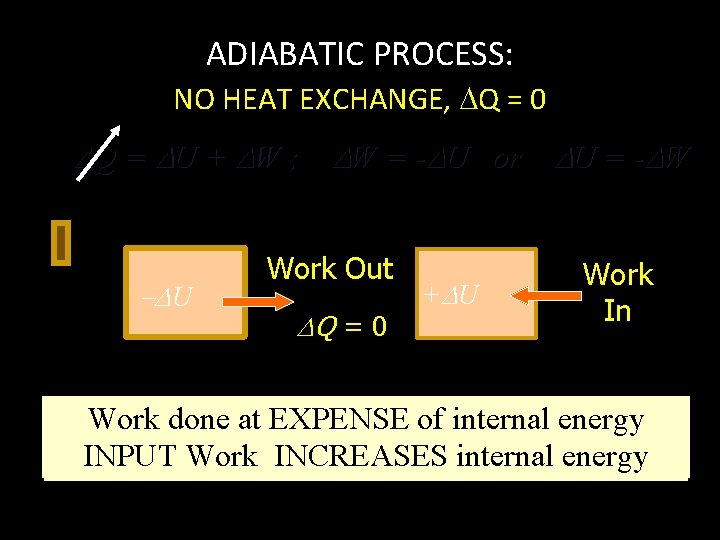
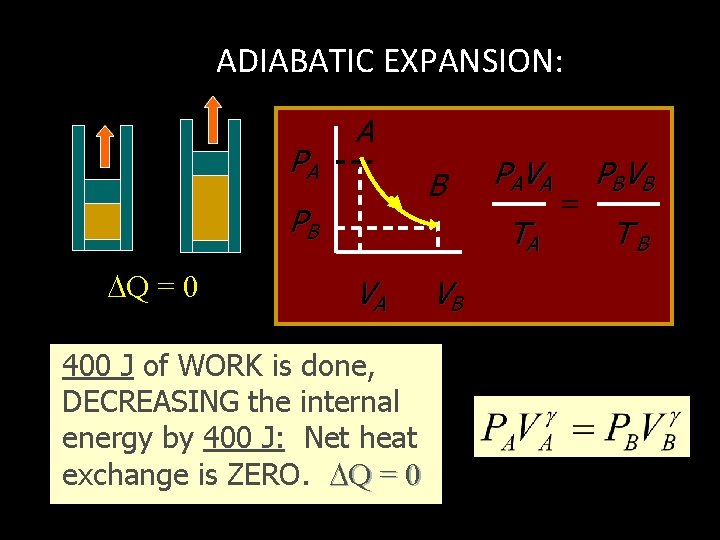
ADIABATIC PROCESS: NO HEAT EXCHANGE, Q = 0 Q = U + W ; W = - U or U = - W W = - U U Work Out Q = 0 + U Work In Work done at EXPENSE of internal energy INPUT Work INCREASES internal energy

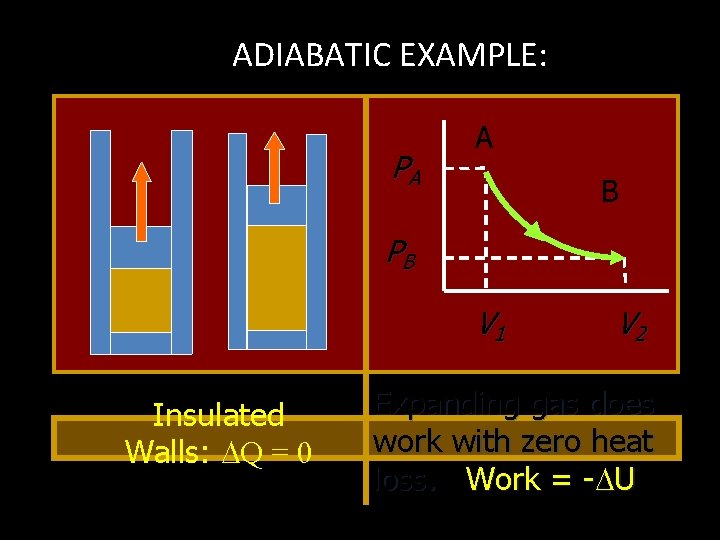
ADIABATIC EXAMPLE: PA A B PB V 1 Insulated Walls: Q = 0 V 2 Expanding gas does work with zero heat loss. Work = - U

ADIABATIC EXPANSION: PA A B PB Q = 0 PA V A TA VA 400 J of WORK is done, DECREASING the internal energy by 400 J: Net heat exchange is ZERO. Q = 0 VB = PBV B TB

MOLAR HEAT CAPACITY OPTIONAL TREATMENT The molar heat capacity C is defined as the heat per unit mole per Celsius degree. Check with your instructor to see if this more thorough treatment of thermodynamic processes is required.

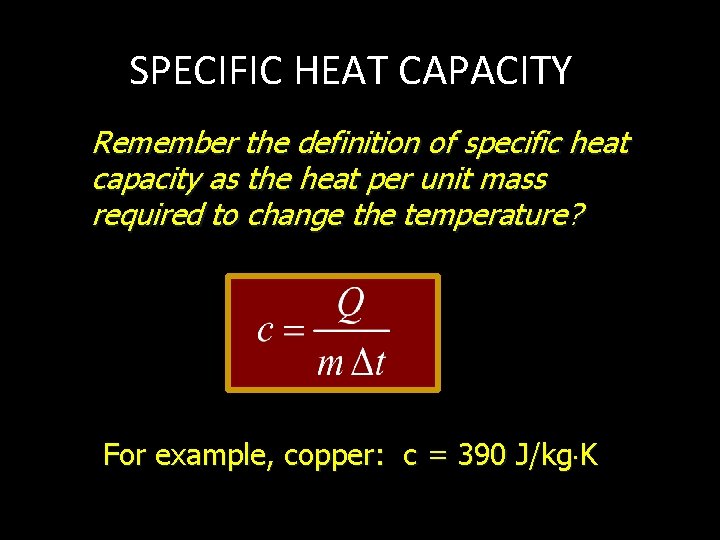
SPECIFIC HEAT CAPACITY Remember the definition of specific heat capacity as the heat per unit mass required to change the temperature? For example, copper: c = 390 J/kg K

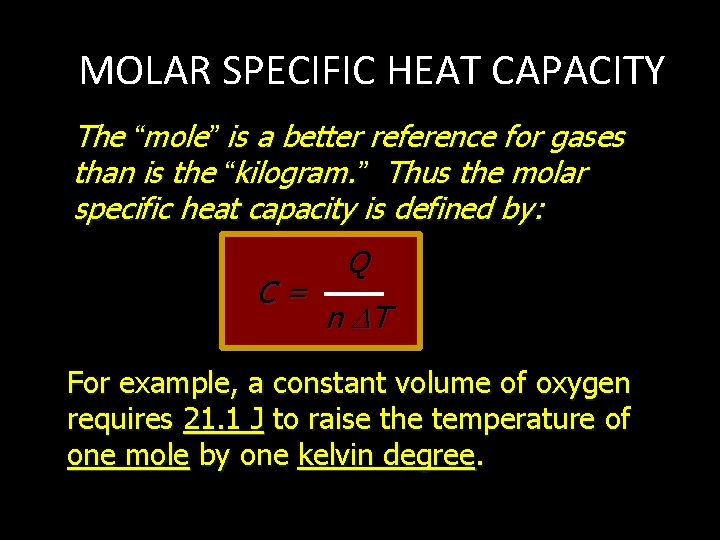
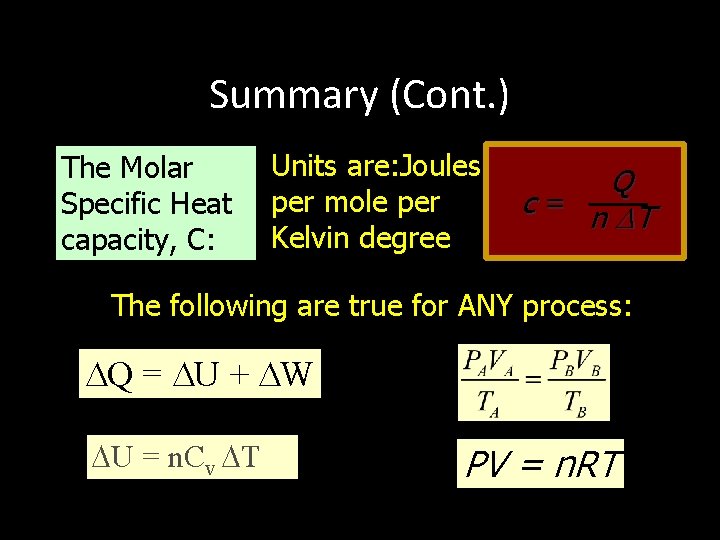
MOLAR SPECIFIC HEAT CAPACITY The “mole” is a better reference for gases than is the “kilogram. ” Thus the molar specific heat capacity is defined by: C= Q n T For example, a constant volume of oxygen requires 21. 1 J to raise the temperature of one mole by one kelvin degree.

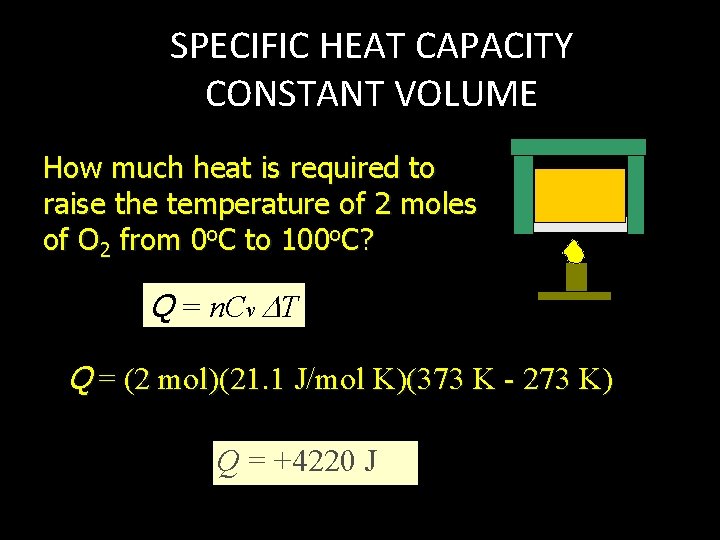
SPECIFIC HEAT CAPACITY CONSTANT VOLUME How much heat is required to raise the temperature of 2 moles of O 2 from 0 o. C to 100 o. C? Q = n. Cv T Q = (2 mol)(21. 1 J/mol K)(373 K - 273 K) Q = +4220 J

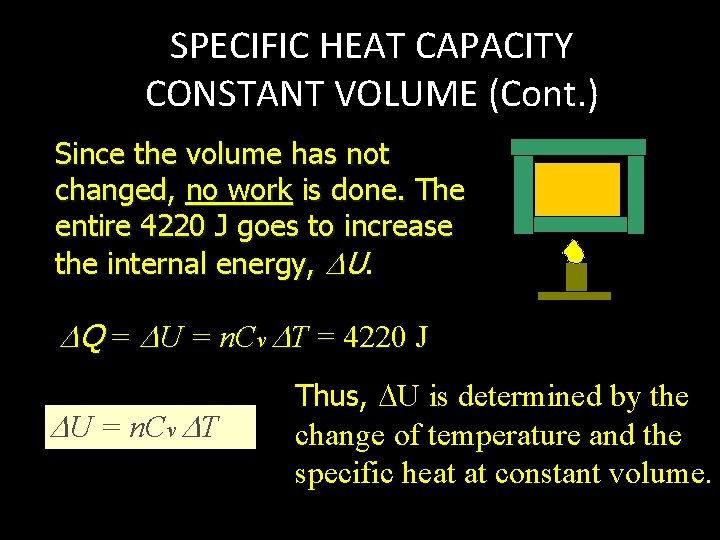
SPECIFIC HEAT CAPACITY CONSTANT VOLUME (Cont. ) Since the volume has not changed, no work is done. The entire 4220 J goes to increase the internal energy, U. Q = U = n. Cv T = 4220 J U = n. Cv T Thus, U is determined by the change of temperature and the specific heat at constant volume.

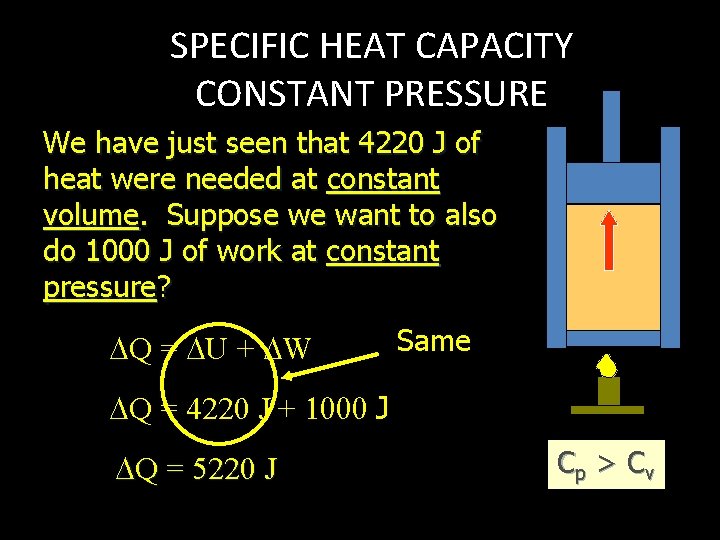
SPECIFIC HEAT CAPACITY CONSTANT PRESSURE We have just seen that 4220 J of heat were needed at constant volume. Suppose we want to also do 1000 J of work at constant pressure? Q = U + W Same Q = 4220 J + J Q = 5220 J Cp > C v

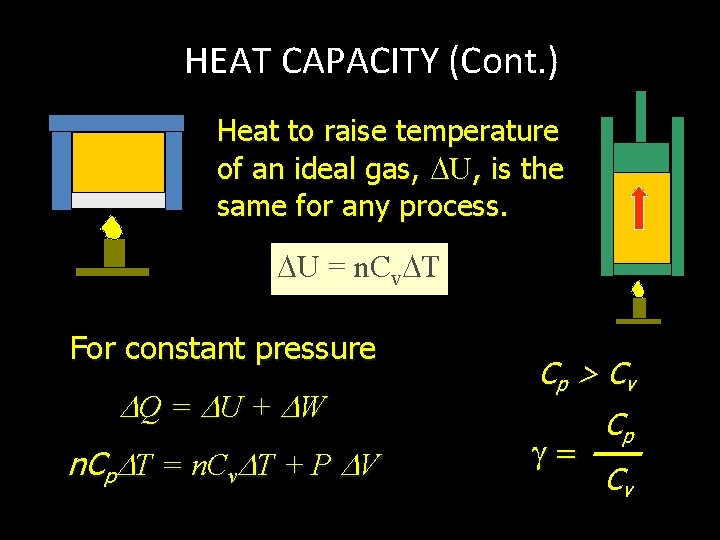
HEAT CAPACITY (Cont. ) Heat to raise temperature of an ideal gas, U, is the same for any process. U = n. Cv T For constant pressure Q = U + W n. Cp T = n. Cv T + P V Cp > C v Cp Cv

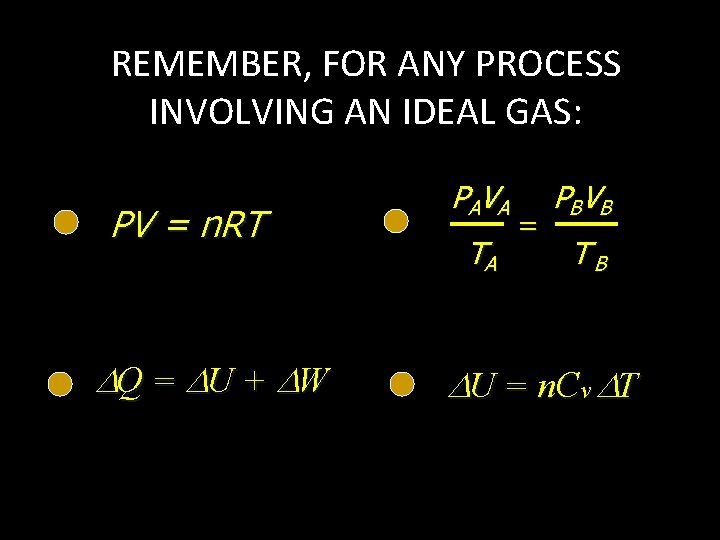
REMEMBER, FOR ANY PROCESS INVOLVING AN IDEAL GAS: PV = n. RT Q = U + W PA V A TA = PBV B TB U = n. Cv T

Example Problem: A 2 -L sample of Oxygen gas has an initial temperature and pressure of 200 K and 1 atm. The gas undergoes four processes: • AB: Heated at constant V to 400 K. • BC: Heated at constant P to 800 K. • CD: Cooled at constant V back to 1 atm. • DA: Cooled at constant P back to 200 K.

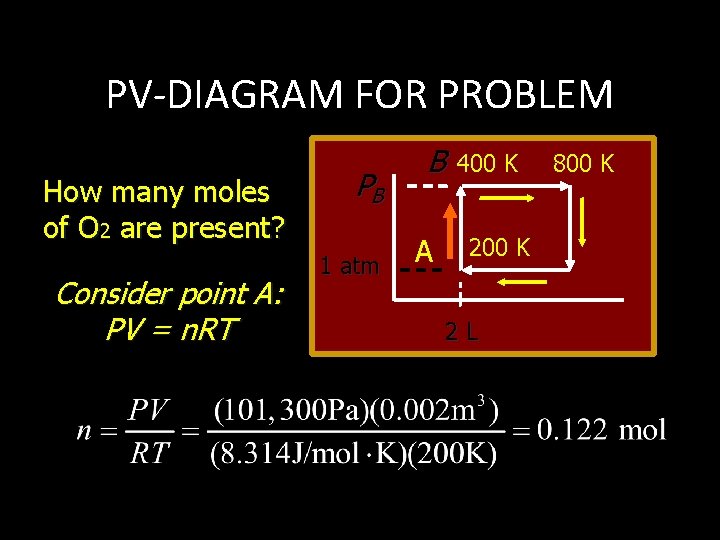
PV-DIAGRAM FOR PROBLEM How many moles of O 2 are present? Consider point A: PV = n. RT PB 1 atm B A 400 K 2 L 800 K

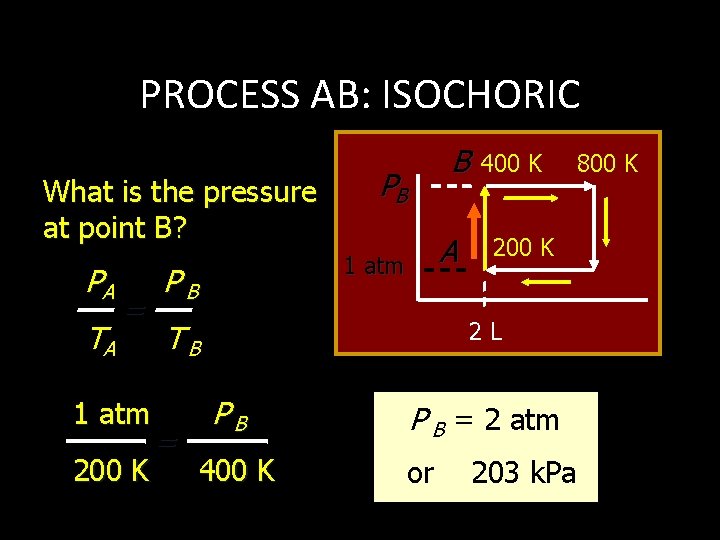
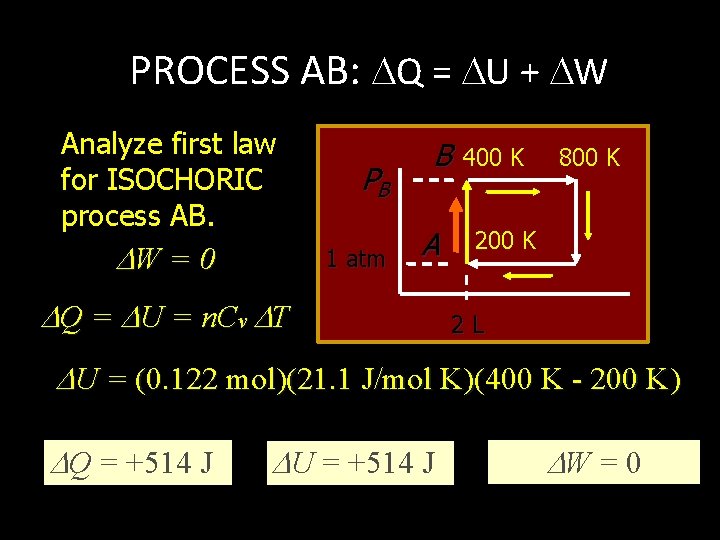
PROCESS AB: ISOCHORIC What is the pressure at point B? PA TA = 1 atm 200 K B PB A 1 atm PB 200 K 2 L TB = 400 K PB 400 K P B = 2 atm or 203 k. Pa 800 K

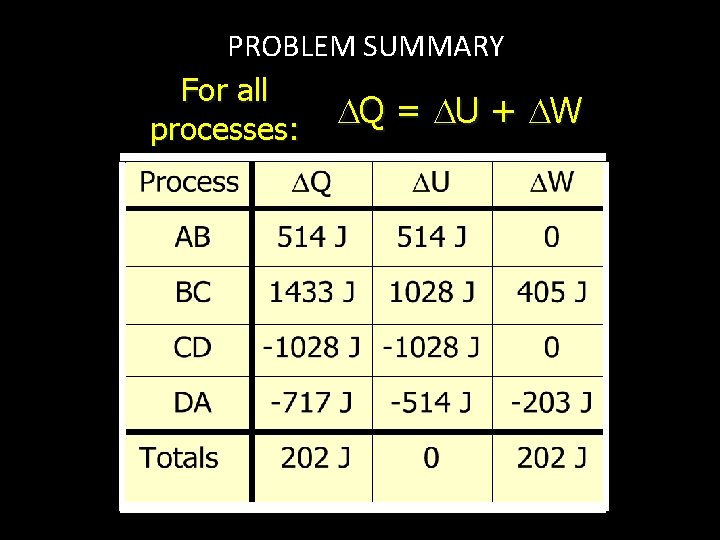
PROCESS AB: Q = U + W Analyze first law for ISOCHORIC process AB. W = 0 PB 1 atm B A Q = U = n. Cv T 400 K 800 K 2 L U = (0. 122 mol)(21. 1 J/mol K)(400 K - 200 K) Q = +514 J U = +514 J W = 0

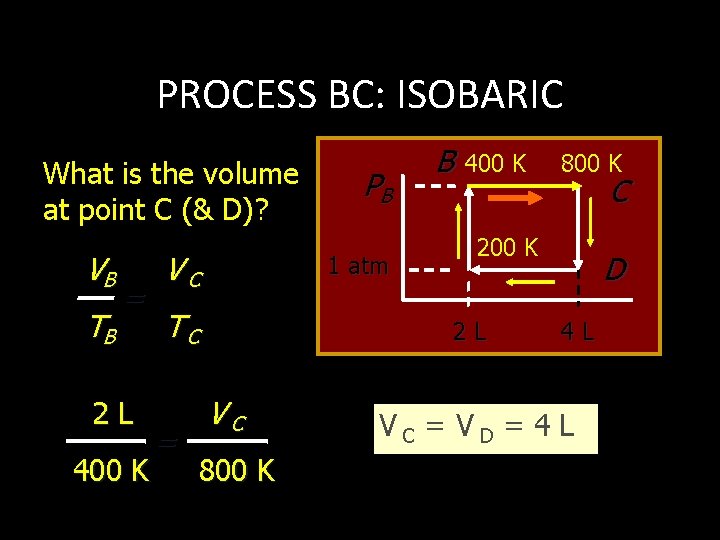
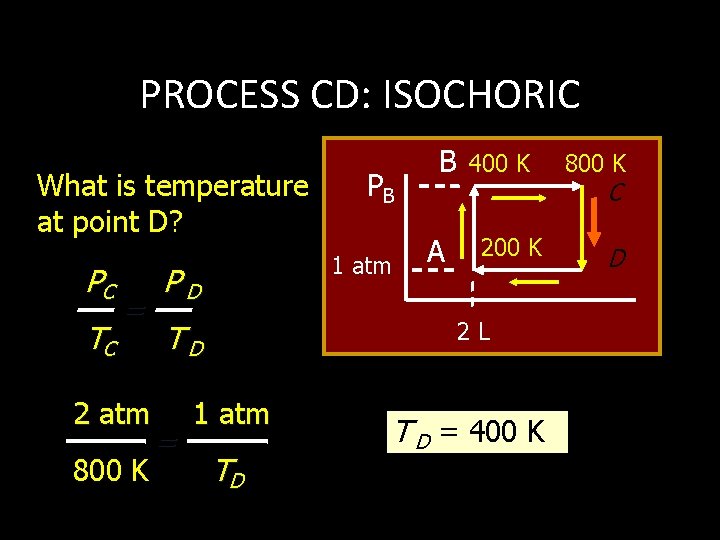
PROCESS BC: ISOBARIC What is the volume at point C (& D)? VB TB = 2 L 400 K VC 1 atm TC = PB B 400 K 800 K C 200 K 2 L VC 800 K D 4 L VC = VD = 4 L

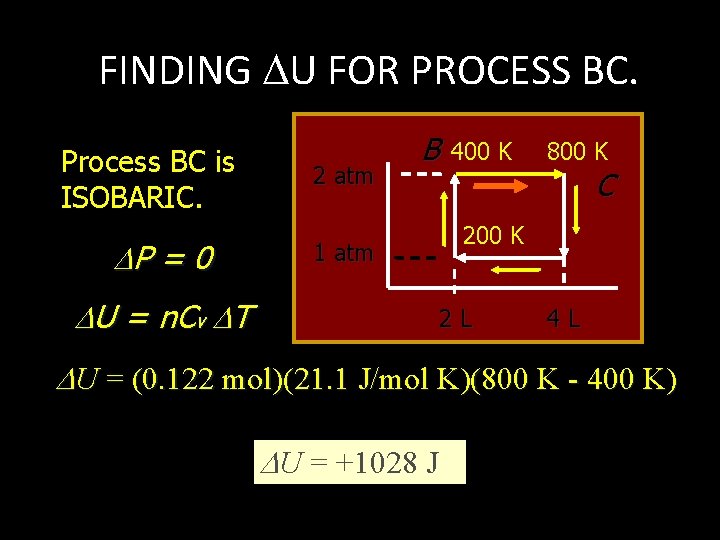
FINDING U FOR PROCESS BC. Process BC is ISOBARIC. P = 0 2 atm B 1 atm U = n. Cv T 400 K 800 K C 200 K 2 L 4 L U = (0. 122 mol)(21. 1 J/mol K)(800 K - 400 K) U = +1028 J

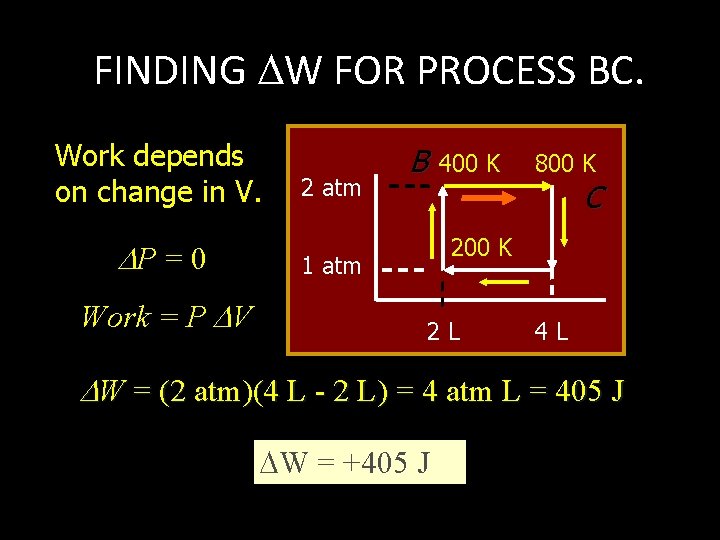
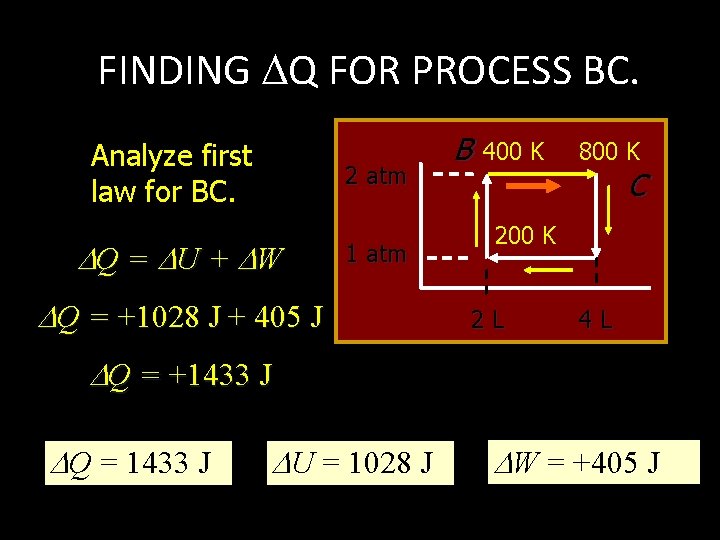
FINDING W FOR PROCESS BC. Work depends on change in V. P = 0 Work = P V 2 atm B 400 K 800 K C 200 K 1 atm 2 L 4 L W = (2 atm)(4 L - 2 L) = 4 atm L = 405 J W = +405 J

FINDING Q FOR PROCESS BC. Analyze first law for BC. 2 atm Q = U + W 1 atm Q = +1028 J + 405 J B 400 K 800 K C 200 K 2 L 4 L Q = +1433 J Q = 1433 J U = 1028 J W = +405 J

PROCESS CD: ISOCHORIC What is temperature at point D? PC TC = 2 atm 800 K 1 atm PD A 400 K 2 L TD = PB B 1 atm TD T D = 400 K 800 K C D

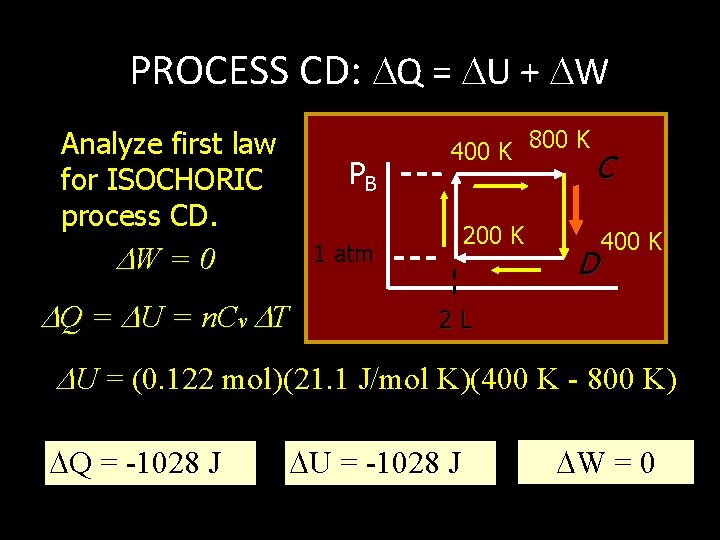
PROCESS CD: Q = U + W Analyze first law for ISOCHORIC process CD. PB W = 0 400 K 200 K 1 atm Q = U = n. Cv T 800 K C D 400 K 2 L U = (0. 122 mol)(21. 1 J/mol K)(400 K - 800 K) Q = -1028 J U = -1028 J W = 0

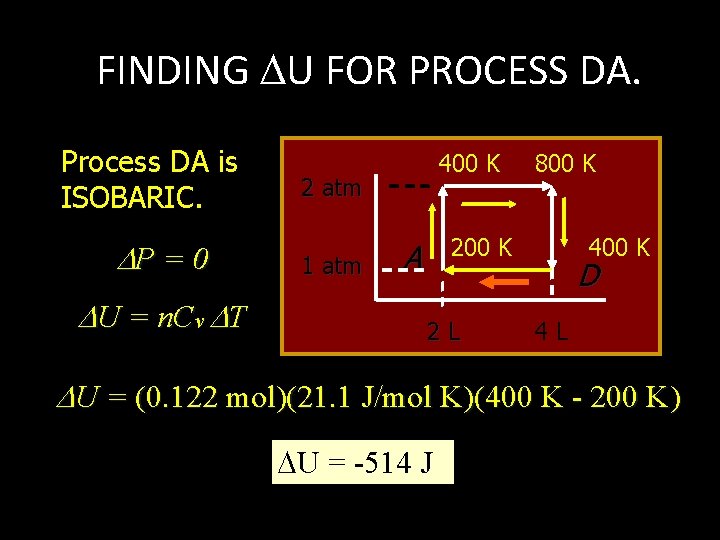
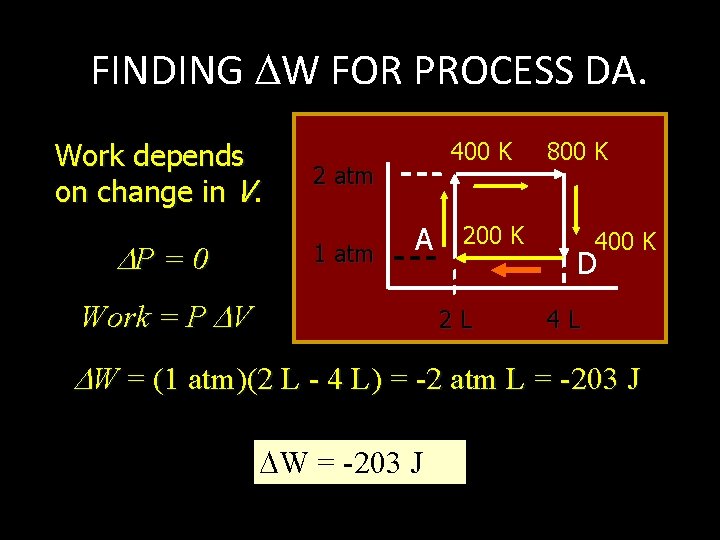
FINDING U FOR PROCESS DA. Process DA is ISOBARIC. P = 0 U = n. Cv T 400 K 2 atm 1 atm 800 K 200 K A 2 L 400 K D 4 L U = (0. 122 mol)(21. 1 J/mol K)(400 K - 200 K) U = -514 J

FINDING W FOR PROCESS DA. Work depends on change in V. P = 0 400 K 2 atm 1 atm A Work = P V 200 K 2 L 800 K 400 K D 4 L W = (1 atm)(2 L - 4 L) = -2 atm L = -203 J W = -203 J

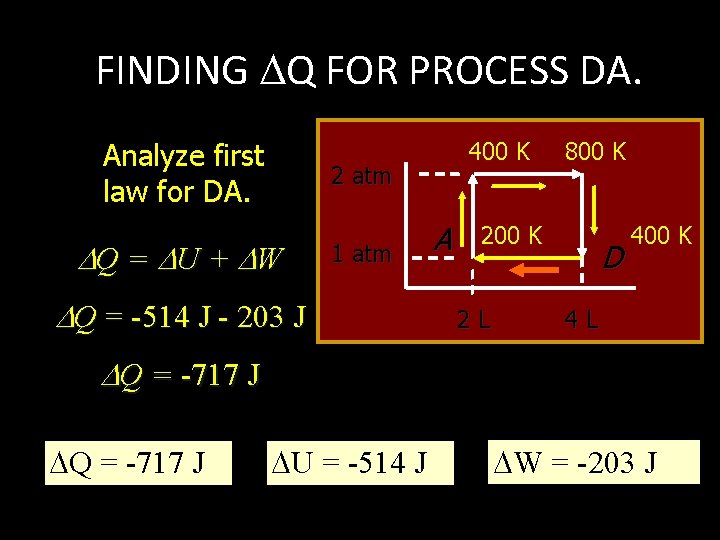
FINDING Q FOR PROCESS DA. Analyze first law for DA. 400 K 2 atm Q = U + W 1 atm Q = -514 J - 203 J A 800 K 2 L D 400 K 4 L Q = -717 J U = -514 J W = -203 J

PROBLEM SUMMARY For all Q = U + W processes:

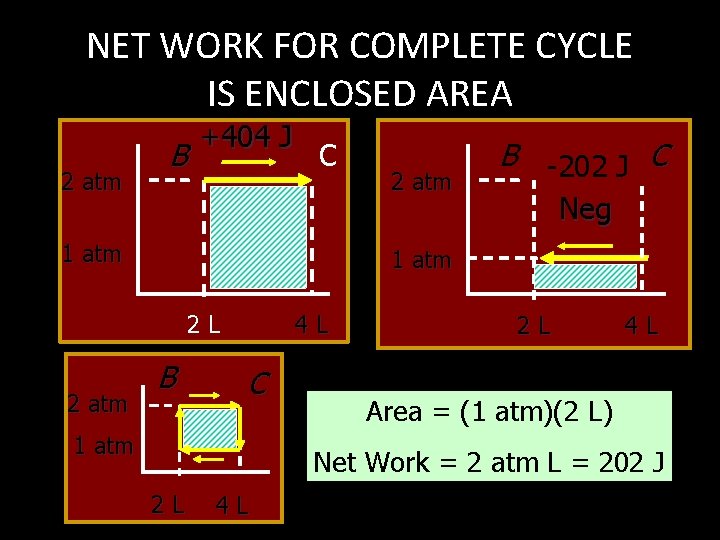
NET WORK FOR COMPLETE CYCLE IS ENCLOSED AREA 2 atm B +404 J C 1 atm Neg 1 atm 2 L 2 atm B -202 J C B 4 L C 1 atm 2 L 4 L Area = (1 atm)(2 L) Net Work = 2 atm L = 202 J 2 L 4 L

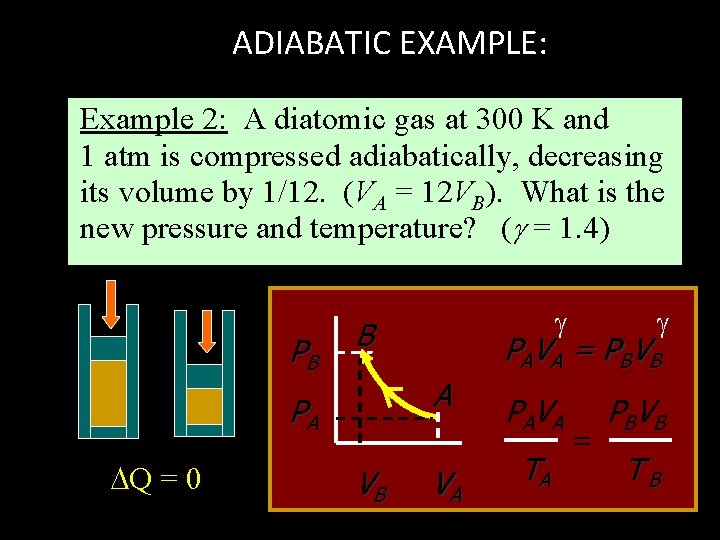
ADIABATIC EXAMPLE: Example 2: A diatomic gas at 300 K and 1 atm is compressed adiabatically, decreasing its volume by 1/12. (VA = 12 VB). What is the new pressure and temperature? ( = 1. 4) PB B Q = 0 VB PA V A PBV B PA V A = P BV B A PA VA TA = TB

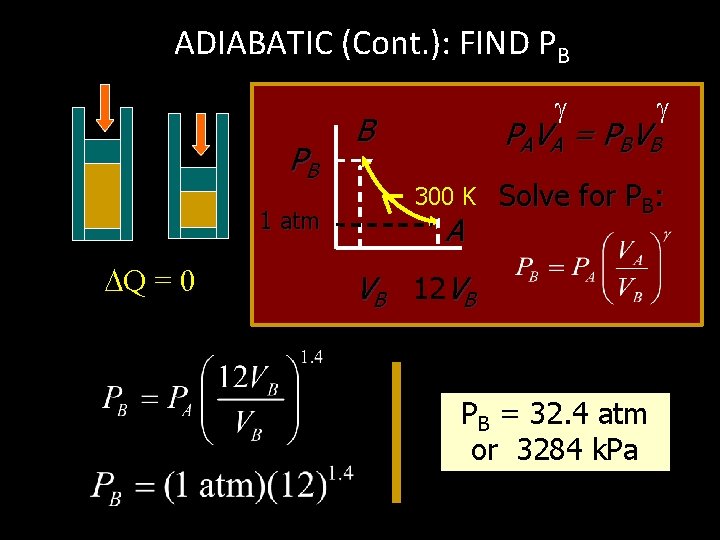
ADIABATIC (Cont. ): FIND PB PB 1 atm Q = 0 B PA V A = P BV B 300 K A Solve for PB: VB 12 VB PB = 32. 4 atm or 3284 k. Pa

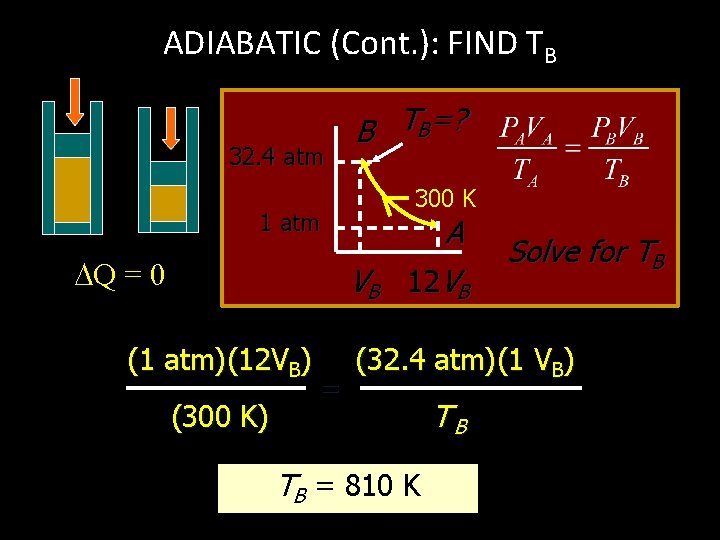
ADIABATIC (Cont. ): FIND TB 32. 4 atm B TB=? 300 K 1 atm Q = 0 VB (1 atm)(12 VB) (300 K) = A 12 VB Solve for TB (32. 4 atm)(1 VB) TB = 810 K TB

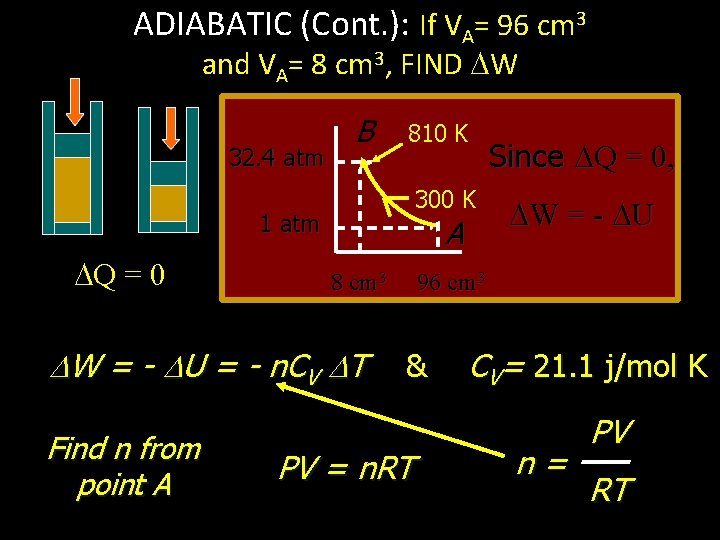
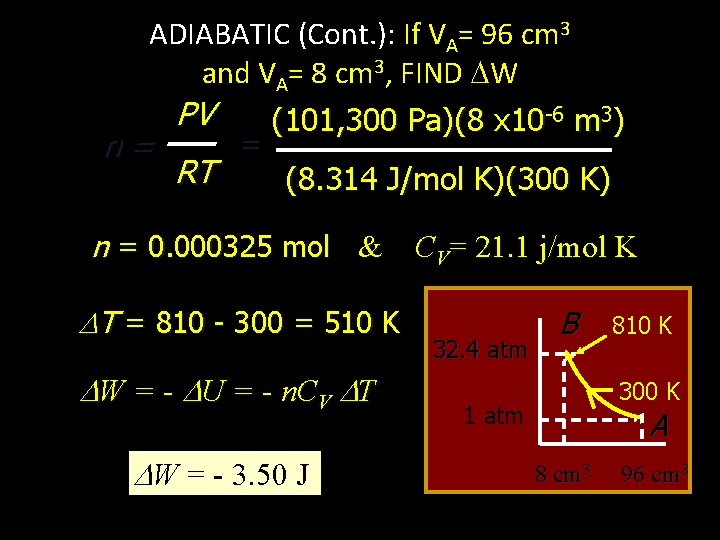
ADIABATIC (Cont. ): If VA= 96 cm 3 and VA= 8 cm 3, FIND W 32. 4 atm B 810 K 300 K 1 atm Q = 0 A 8 cm 3 W = - U = - n. CV T Find n from point A Since Q = 0, W = - U 96 cm 3 & PV = n. RT CV= 21. 1 j/mol K n= PV RT

ADIABATIC (Cont. ): If VA= 96 cm 3 and VA= 8 cm 3, FIND W n= PV RT = (101, 300 Pa)(8 x 10 -6 m 3) (8. 314 J/mol K)(300 K) n = 0. 000325 mol & CV= 21. 1 j/mol K T = 810 - 300 = 510 K W = - U = - n. CV T W = - 3. 50 J 32. 4 atm B 810 K 300 K 1 atm A 8 cm 3 96 cm 3

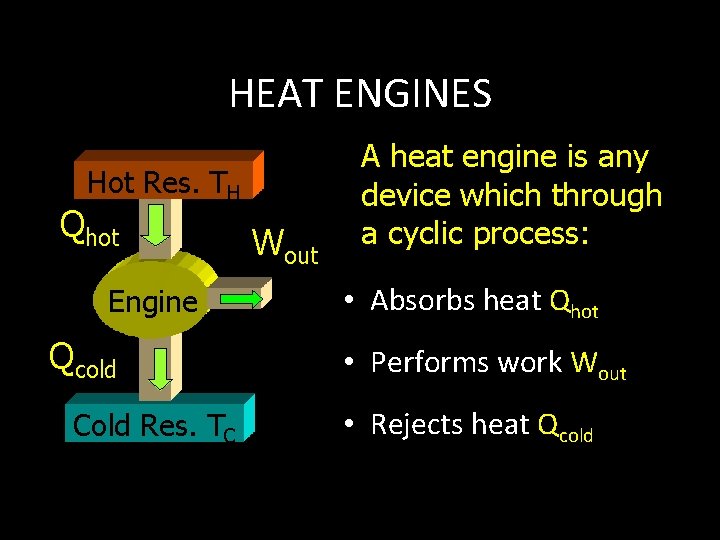
HEAT ENGINES Hot Res. TH Qhot Engine Qcold Cold Res. TC Wout A heat engine is any device which through a cyclic process: • Absorbs heat Qhot • Performs work Wout • Rejects heat Qcold

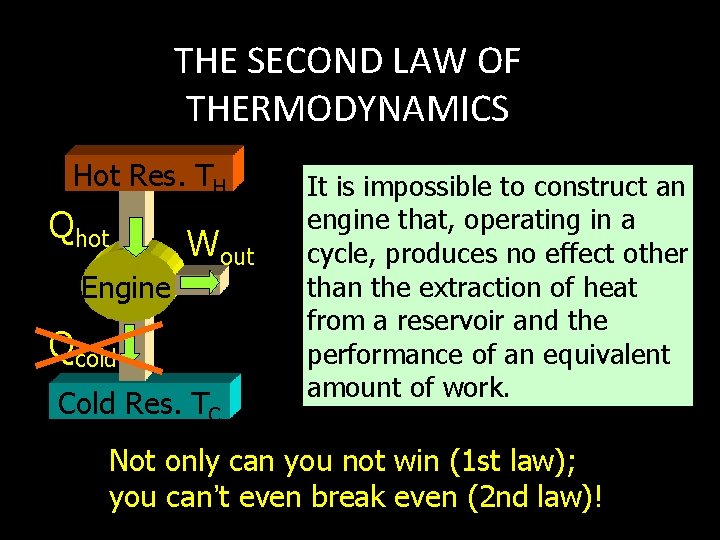
THE SECOND LAW OF THERMODYNAMICS Hot Res. TH Qhot Engine Wout Qcold Cold Res. TC It is impossible to construct an engine that, operating in a cycle, produces no effect other than the extraction of heat from a reservoir and the performance of an equivalent amount of work. Not only can you not win (1 st law); you can’t even break even (2 nd law)!

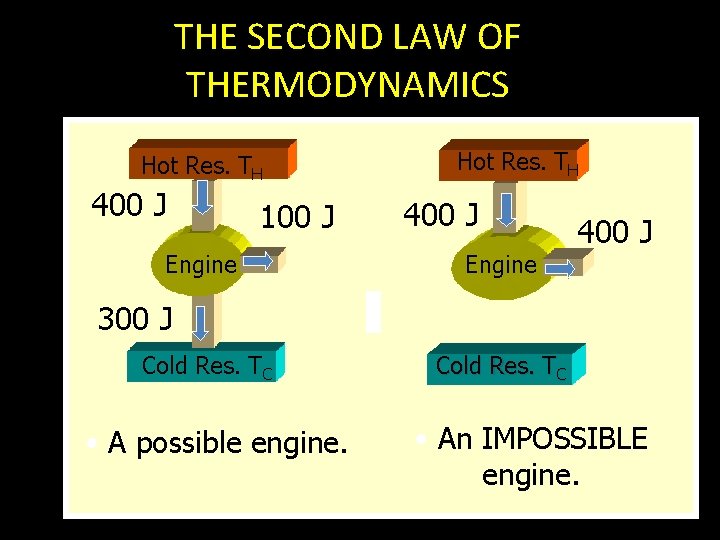
THE SECOND LAW OF THERMODYNAMICS Hot Res. TH 400 J 100 J Engine Hot Res. TH 400 J Engine 400 J 300 J Cold Res. TC • A possible engine. Cold Res. TC • An IMPOSSIBLE engine.

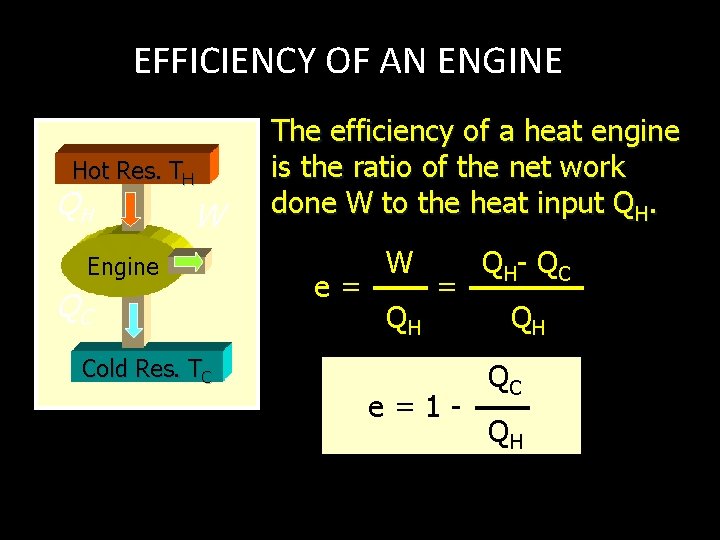
EFFICIENCY OF AN ENGINE Hot Res. TH QH W Engine QC The efficiency of a heat engine is the ratio of the net work done W to the heat input QH. e= W QH = Cold Res. TC e=1 - Q H- Q C QH QC QH

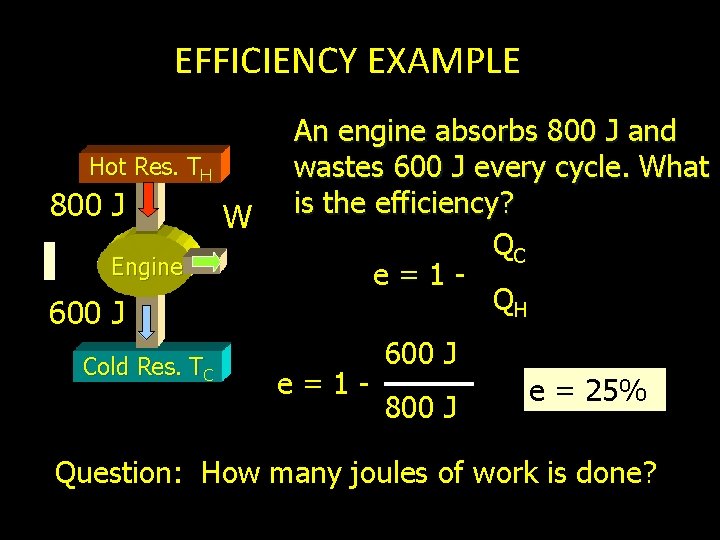
EFFICIENCY EXAMPLE Hot Res. TH 800 J Engine 600 J Cold Res. TC W An engine absorbs 800 J and wastes 600 J every cycle. What is the efficiency? QC e=1 QH e=1 - 600 J 800 J e = 25% Question: How many joules of work is done?

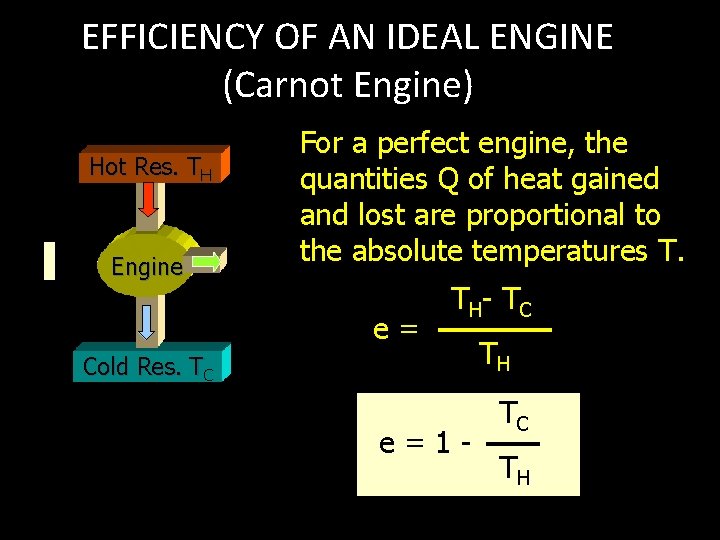
EFFICIENCY OF AN IDEAL ENGINE (Carnot Engine) Hot Res. TH QH Engine QC W For a perfect engine, the quantities Q of heat gained and lost are proportional to the absolute temperatures T. e= T H- T C Cold Res. TC e=1 - TH TC TH

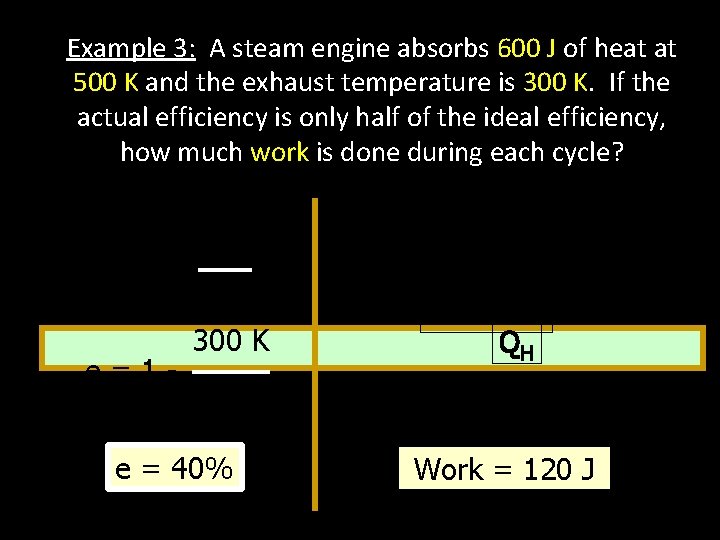
Example 3: A steam engine absorbs 600 J of heat at 500 K and the exhaust temperature is 300 K. If the actual efficiency is only half of the ideal efficiency, how much work is done during each cycle? e=1 e=1 - TC Actual e = 0. 5 ei = 20% TH W 300 K 500 K e = 40% e= QH W = e. QH = 0. 20 (600 J) Work = 120 J

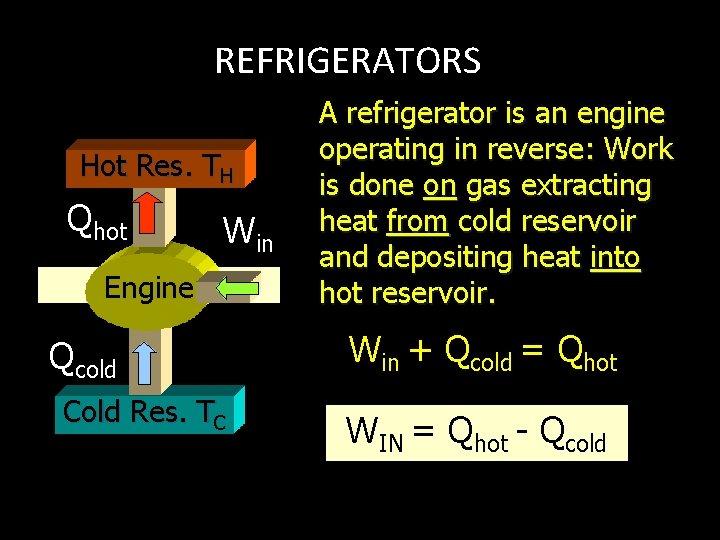
REFRIGERATORS Hot Res. TH Qhot Win Engine Qcold Cold Res. TC A refrigerator is an engine operating in reverse: Work is done on gas extracting heat from cold reservoir and depositing heat into hot reservoir. Win + Qcold = Qhot WIN = Qhot - Qcold

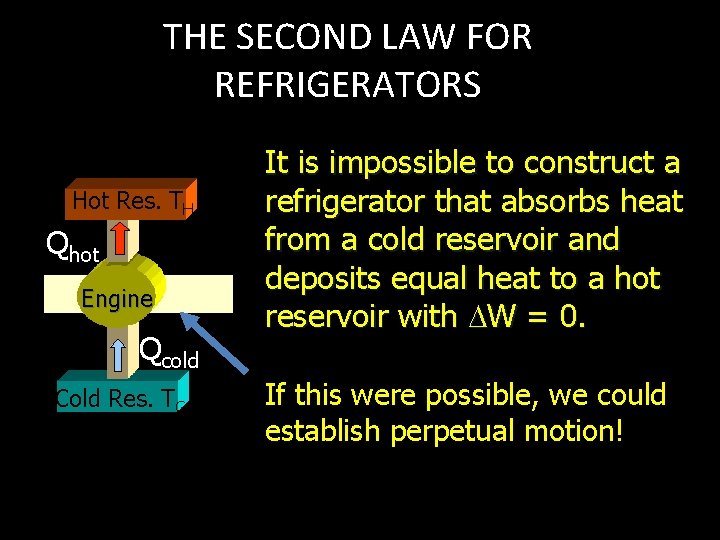
THE SECOND LAW FOR REFRIGERATORS Hot Res. TH Qhot Engine Qcold Cold Res. TC It is impossible to construct a refrigerator that absorbs heat from a cold reservoir and deposits equal heat to a hot reservoir with W = 0. If this were possible, we could establish perpetual motion!

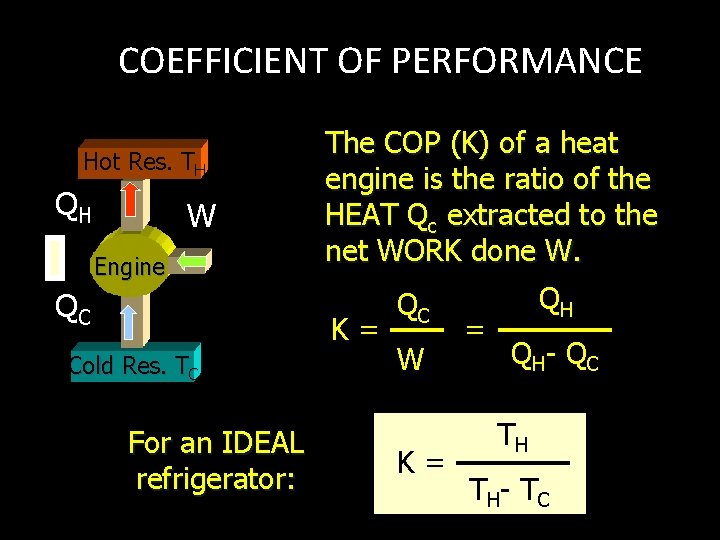
COEFFICIENT OF PERFORMANCE Hot Res. TH QH W Engine QC The COP (K) of a heat engine is the ratio of the HEAT Qc extracted to the net WORK done W. K= Cold Res. TC For an IDEAL refrigerator: QC W K= = QH Q H- Q C TH T H- T C

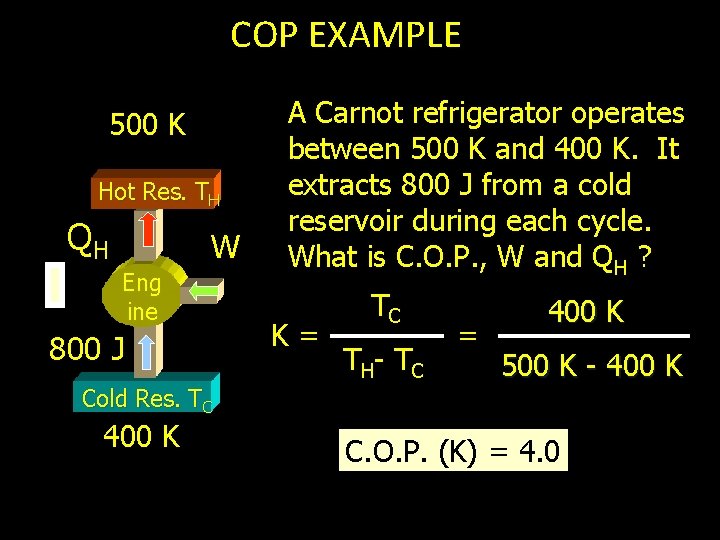
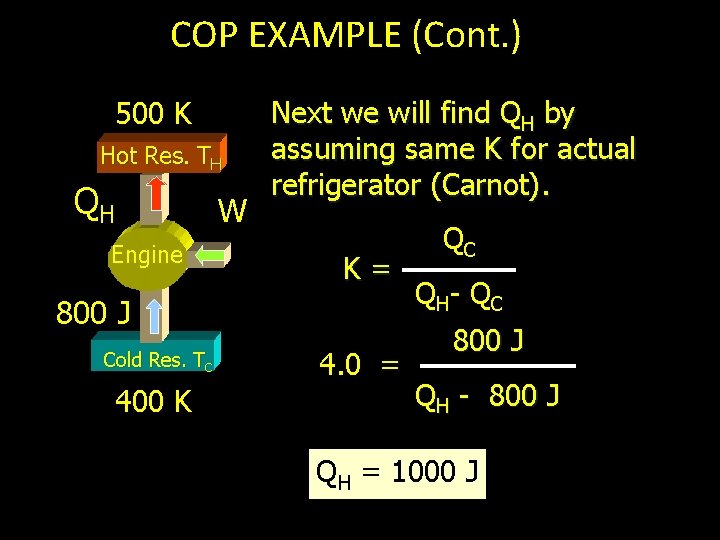
COP EXAMPLE 500 K Hot Res. TH QH W Eng ine 800 J Cold Res. TC 400 K A Carnot refrigerator operates between 500 K and 400 K. It extracts 800 J from a cold reservoir during each cycle. What is C. O. P. , W and QH ? K= TC T H- T C = 400 K 500 K - 400 K C. O. P. (K) = 4. 0

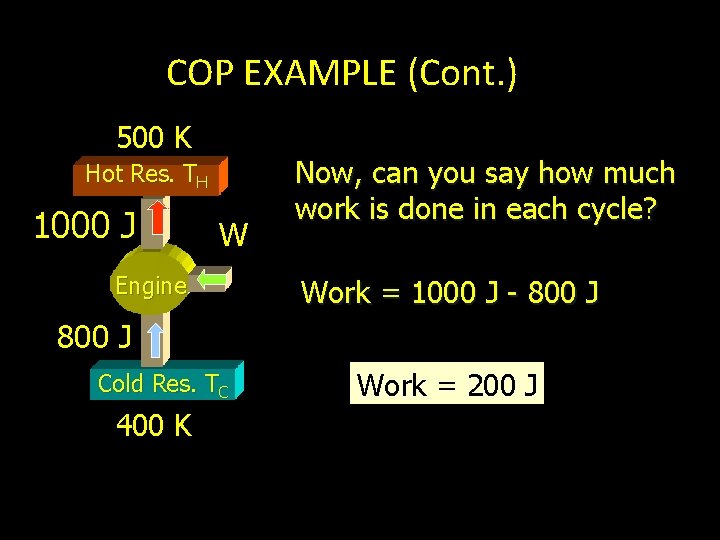
COP EXAMPLE (Cont. ) 500 K Hot Res. TH QH W Engine Next we will find QH by assuming same K for actual refrigerator (Carnot). K= 800 J Cold Res. TC 400 K 4. 0 = QC Q H- Q C 800 J QH - 800 J QH = 1000 J

COP EXAMPLE (Cont. ) 500 K Hot Res. TH 1000 J W Engine Now, can you say how much work is done in each cycle? Work = 1000 J - 800 J Cold Res. TC 400 K Work = 200 J

Summary The First Law of Thermodynamics: The net heat taken in by a system is equal to the sum of the change in internal energy and the work done by the system. Q = U + W final - initial) • Isochoric Process: V = 0, W = 0 • Isobaric Process: P = 0 • Isothermal Process: T = 0, U = 0 • Adiabatic Process: Q = 0

Summary (Cont. ) The Molar Specific Heat capacity, C: Units are: Joules per mole per Kelvin degree Q c = n T The following are true for ANY process: Q = U + W U = n. Cv T PV = n. RT

Summary (Cont. ) Hot Res. TH Qhot Wout Engine Qcold Cold Res. TC The Second Law of Thermo: It is impossible to construct an engine that, operating in a cycle, produces no effect other than the extraction of heat from a reservoir and the performance of an equivalent amount of work. Not only can you not win (1 st law); you can’t even break even (2 nd law)!

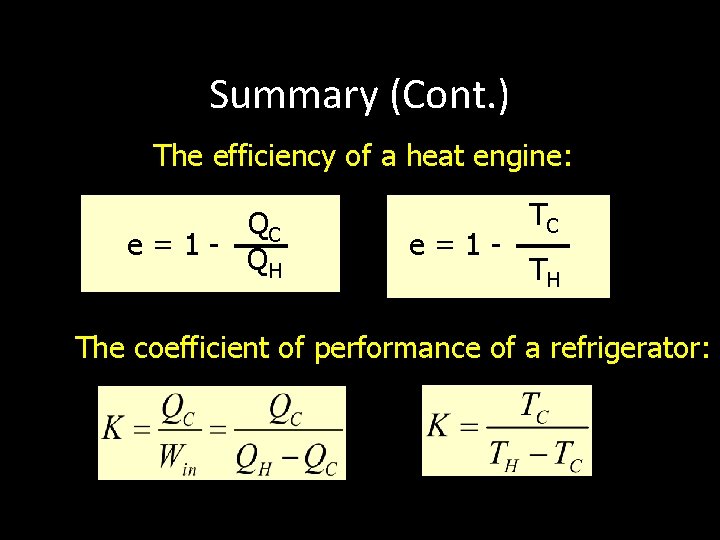
Summary (Cont. ) The efficiency of a heat engine: QC e=1 - Q H e=1 - TC TH The coefficient of performance of a refrigerator:
 Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Pressure is state function or path function
Pressure is state function or path function Statistical thermodynamics is a study of
Statistical thermodynamics is a study of Thermodynamics study guide
Thermodynamics study guide An iron bar of mass 869 g cools from
An iron bar of mass 869 g cools from Internal energy formula thermodynamics
Internal energy formula thermodynamics Nozzle and diffuser
Nozzle and diffuser Internal energy in thermodynamics definition
Internal energy in thermodynamics definition Internal energy in thermodynamics definition
Internal energy in thermodynamics definition Conservation of energy thermodynamics
Conservation of energy thermodynamics Internal energy formula thermodynamics
Internal energy formula thermodynamics Thermal energy calculation formula
Thermal energy calculation formula Sociology the study of human relationships textbook
Sociology the study of human relationships textbook Sociology chapter 14
Sociology chapter 14 Conflict theory
Conflict theory Studying a virtual ecosystem on a computer is an example of
Studying a virtual ecosystem on a computer is an example of Sociology the study of human relationships
Sociology the study of human relationships What is the most inclusive level of organization
What is the most inclusive level of organization Simple ecosystem
Simple ecosystem Energy relationships and life 2
Energy relationships and life 2 Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton là gì
Tư thế worm breton là gì Chúa sống lại
Chúa sống lại Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Giọng cùng tên là
Giọng cùng tên là Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Số nguyên là gì
Số nguyên là gì Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Sơ đồ cơ thể người
Sơ đồ cơ thể người Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi What is case series
What is case series Retrospective cohort study
Retrospective cohort study What is work study in management
What is work study in management Marty lobdel
Marty lobdel Phytogeographical regions of india
Phytogeographical regions of india Objectives of work study
Objectives of work study Time and motion study example ppt
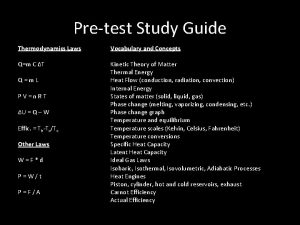
Time and motion study example ppt Chapter 12 study guide thermal energy
Chapter 12 study guide thermal energy Chapter 11 study guide physics
Chapter 11 study guide physics