The Flow of Energy Thermodynamics Thermodynamics the study



- Slides: 8

The Flow of Energy

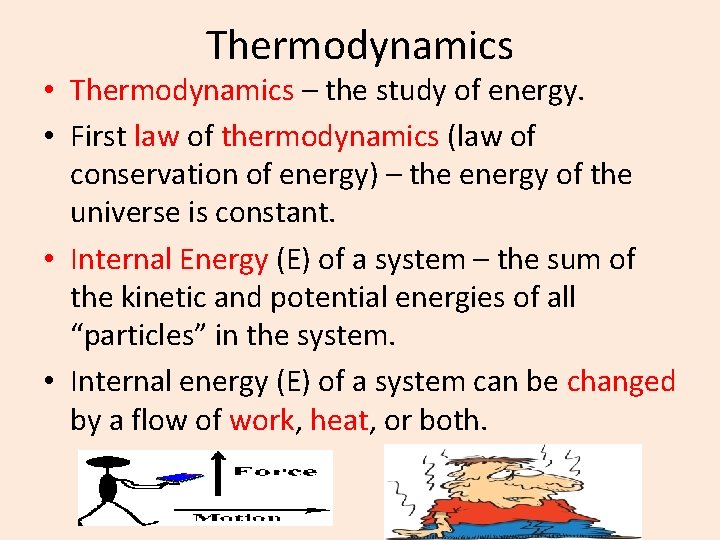
Thermodynamics • Thermodynamics – the study of energy. • First law of thermodynamics (law of conservation of energy) – the energy of the universe is constant. • Internal Energy (E) of a system – the sum of the kinetic and potential energies of all “particles” in the system. • Internal energy (E) of a system can be changed by a flow of work, heat, or both.

Internal Energy: ∆E = q + w Where ∆ (delta) means change in q represents heat w represents work • Thermodynamic quantities consist of 2 parts: Ø a number, giving the magnitude of the change Ø a sign, indicating the direction of the flow (from the system’s point of view)

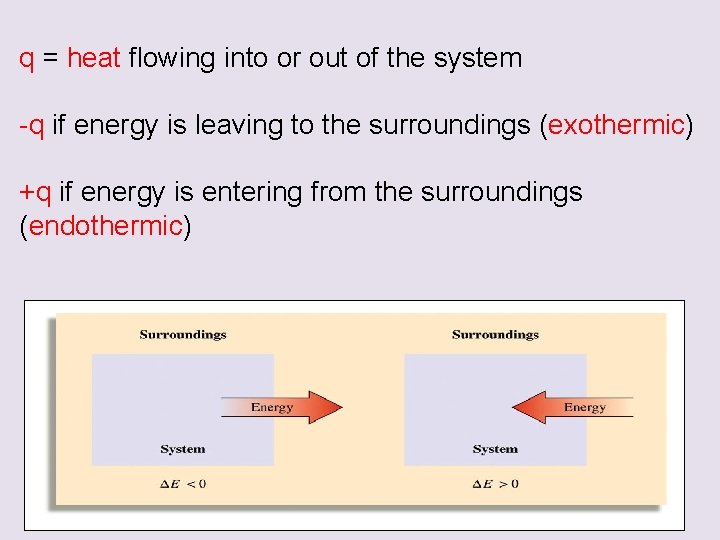
q = heat flowing into or out of the system -q if energy is leaving to the surroundings (exothermic) +q if energy is entering from the surroundings (endothermic)

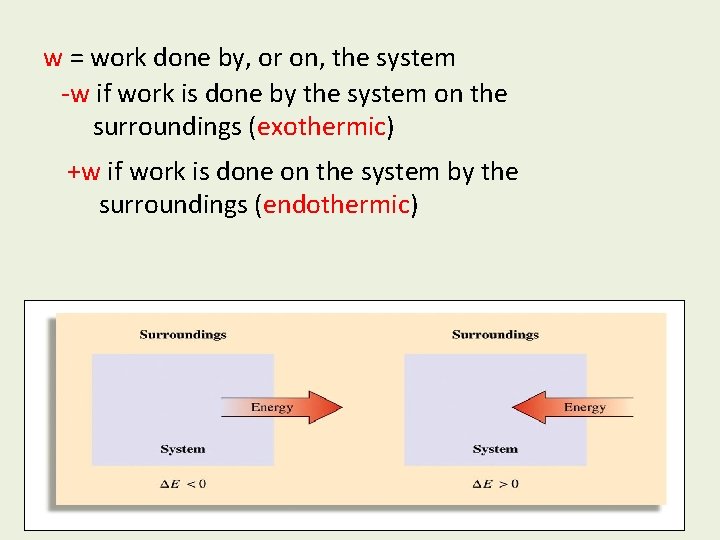
w = work done by, or on, the system -w if work is done by the system on the surroundings (exothermic) +w if work is done on the system by the surroundings (endothermic)

Measuring Energy Changes • Common units of energy: the calorie and the joule. • Calorie – amount of energy (heat) required to raise the temperature of 1 gram of water by 1 o. C. • The “calorie” with which you are familiar is used to measure the energy content of food and is actually a kilocalorie (1000 calories), written with a capital C (Calorie) to distinguish it from the calorie used in chemistry. • Joule (an SI unit) – defined in terms of the calorie: 1 calorie = 4. 184 joules Abbreviated 1 cal = 4. 184 J • You will need to be able to convert between the two units.

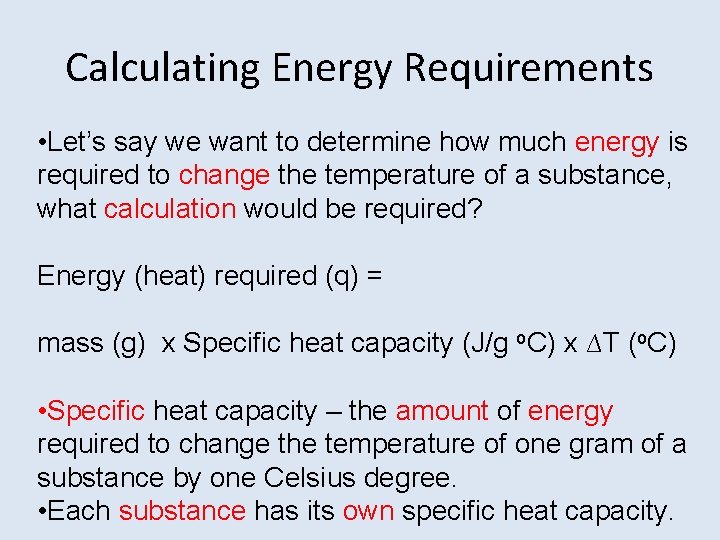
Calculating Energy Requirements • Let’s say we want to determine how much energy is required to change the temperature of a substance, what calculation would be required? Energy (heat) required (q) = mass (g) x Specific heat capacity (J/g o. C) x ∆T (o. C) • Specific heat capacity – the amount of energy required to change the temperature of one gram of a substance by one Celsius degree. • Each substance has its own specific heat capacity.

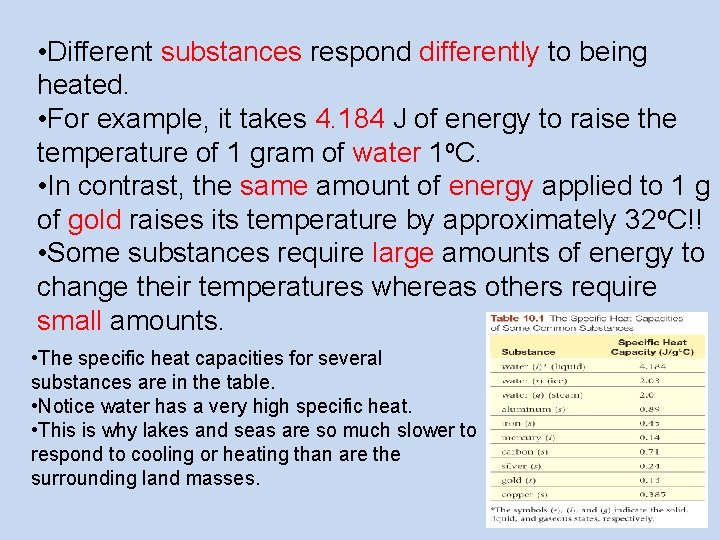
• Different substances respond differently to being heated. • For example, it takes 4. 184 J of energy to raise the temperature of 1 gram of water 1 o. C. • In contrast, the same amount of energy applied to 1 g of gold raises its temperature by approximately 32 o. C!! • Some substances require large amounts of energy to change their temperatures whereas others require small amounts. • The specific heat capacities for several substances are in the table. • Notice water has a very high specific heat. • This is why lakes and seas are so much slower to respond to cooling or heating than are the surrounding land masses.
 First law of thermodynamics in open system
First law of thermodynamics in open system Flow of energy vs flow of matter
Flow of energy vs flow of matter Oikos meaning
Oikos meaning First law of thermodynamics for steady state flow process
First law of thermodynamics for steady state flow process What is flow work in thermodynamics
What is flow work in thermodynamics Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Pressure is state function or path function
Pressure is state function or path function Statistical thermodynamics is a study of
Statistical thermodynamics is a study of















