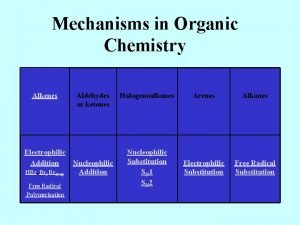
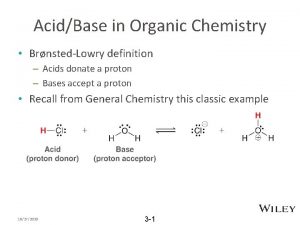
Organic Chemistry Sh javanshir Faculty of Chemistry Iran











- Slides: 93

Organic Chemistry Sh. javanshir Faculty of Chemistry Iran University of Science & Technology

Chapter 4 -2. Alkenes: continue

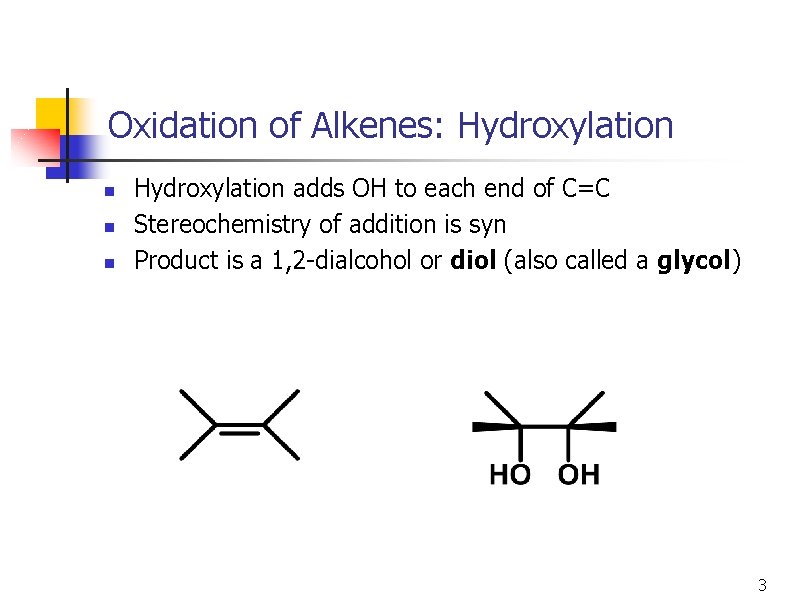
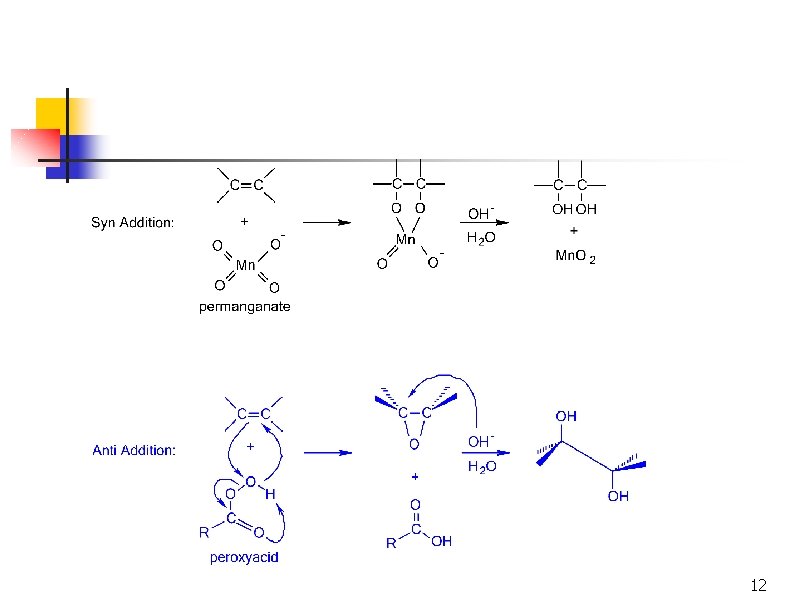
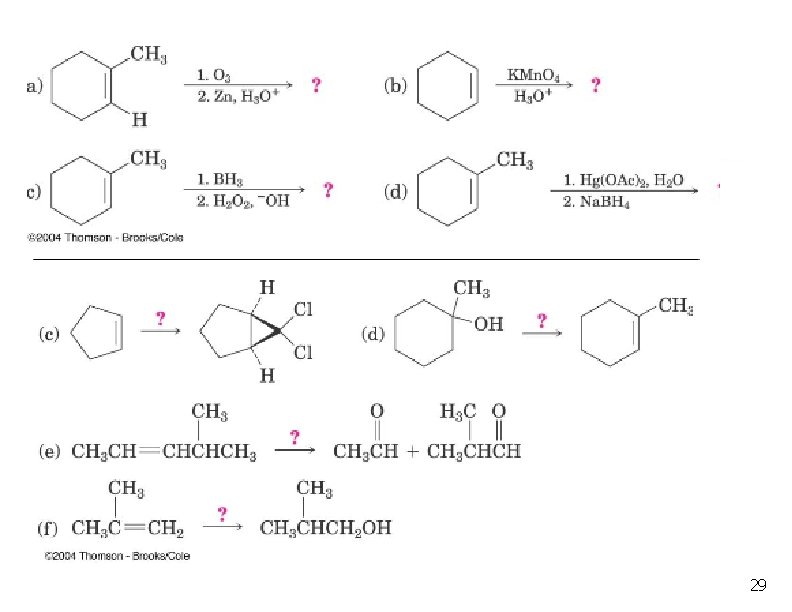
Oxidation of Alkenes: Hydroxylation n Hydroxylation adds OH to each end of C=C Stereochemistry of addition is syn Product is a 1, 2 -dialcohol or diol (also called a glycol) 3

Syn Hydroxylation of Alkenes n Two reagents: n n Osmium tetroxide (expensive!), followed by hydrogen peroxide or sodium bisulfate Cold, dilute aqueous potassium permanganate, followed by hydrolysis with base 4

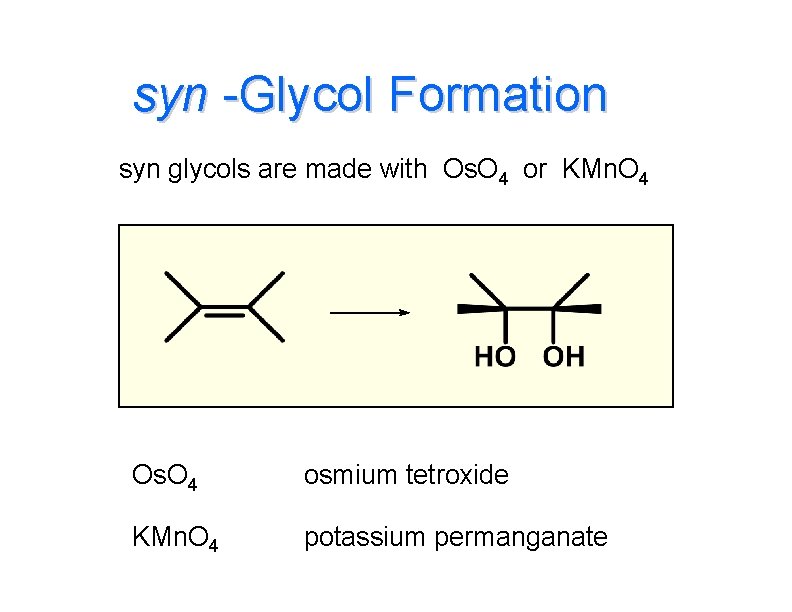
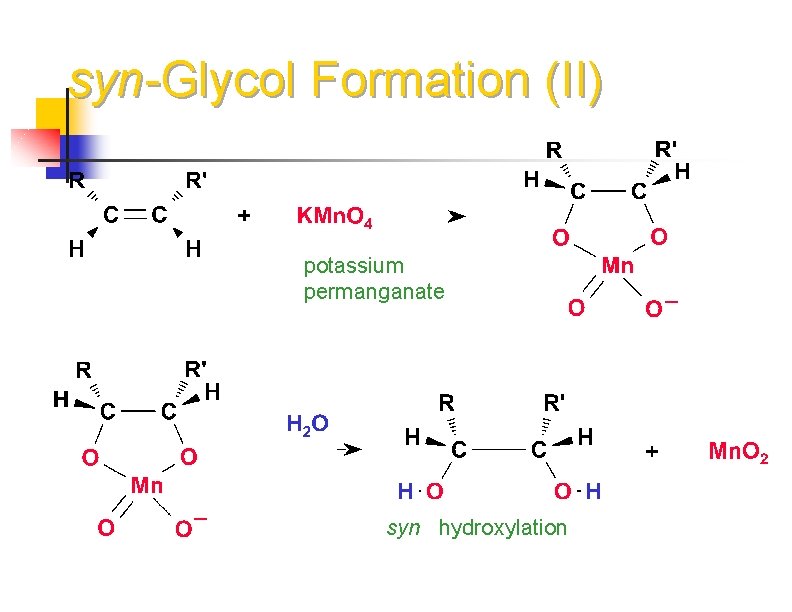
syn -Glycol Formation syn glycols are made with Os. O 4 or KMn. O 4 Os. O 4 osmium tetroxide KMn. O 4 potassium permanganate

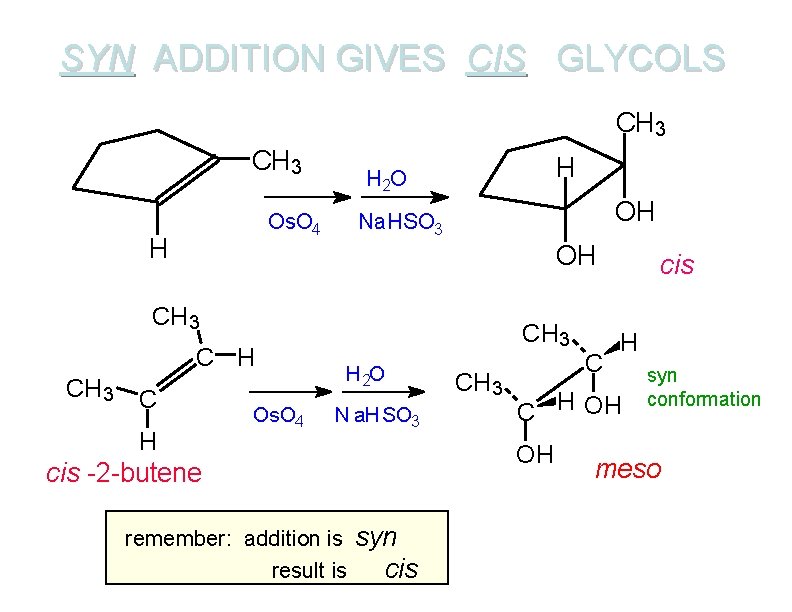
SYN ADDITION GIVES CIS GLYCOLS CH 3 Os. O 4 H H H 2 O OH Na. HSO 3 OH CH 3 C H Os. O 4 CH 3 H 2 O N a. HSO 3 syn cis H C H OH OH cis -2 -butene remember: addition is result is CH 3 C cis syn conformation meso

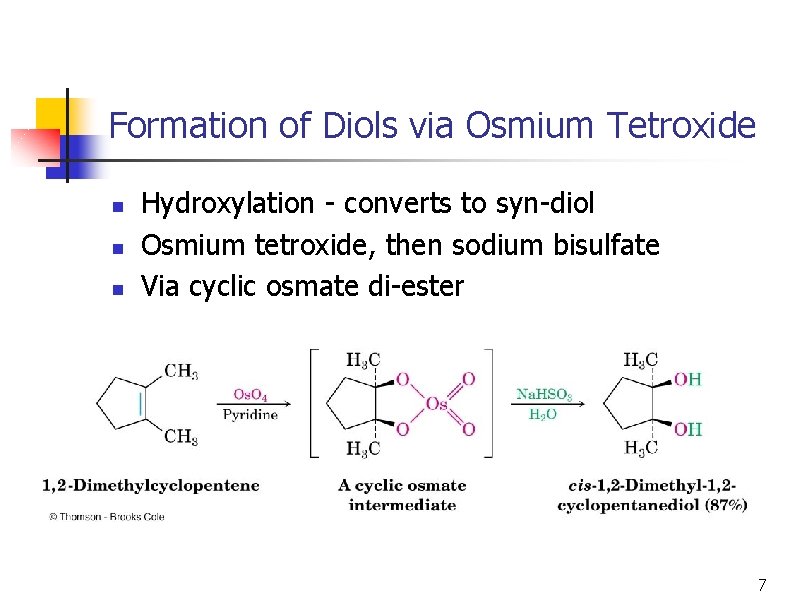
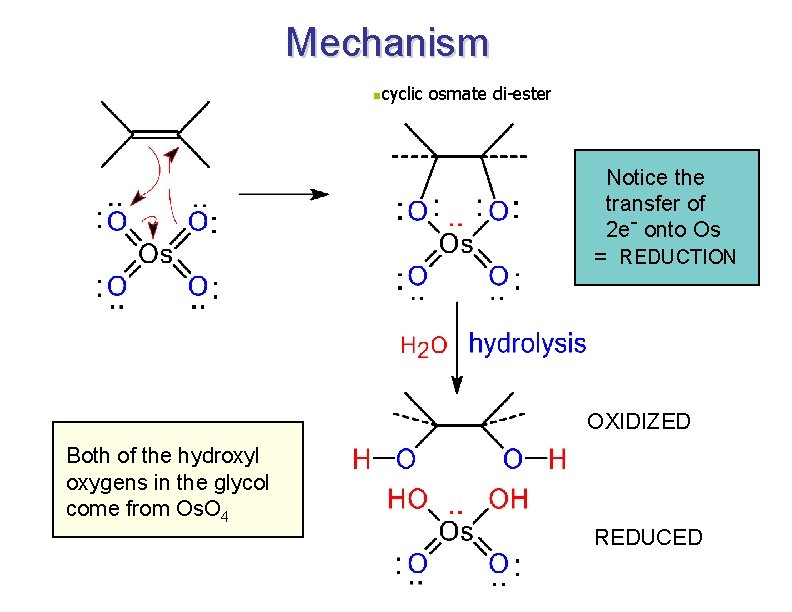
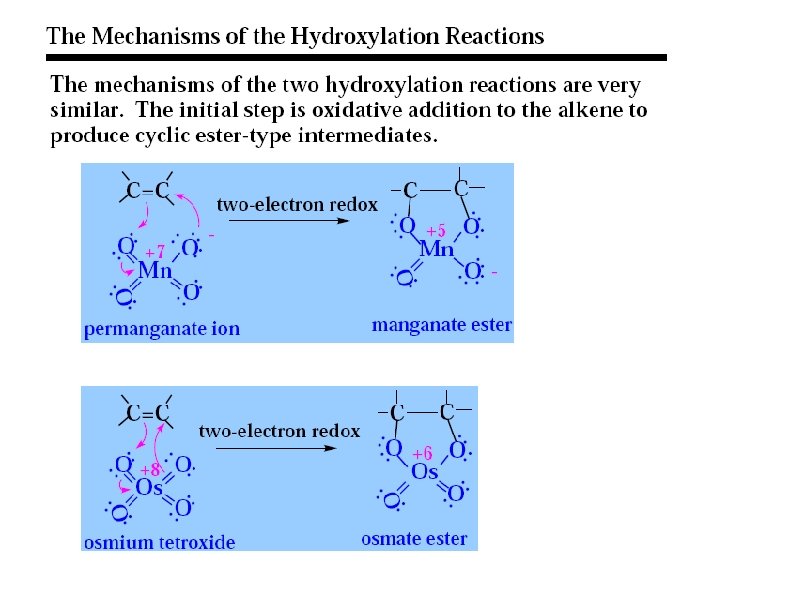
Formation of Diols via Osmium Tetroxide n n n Hydroxylation - converts to syn-diol Osmium tetroxide, then sodium bisulfate Via cyclic osmate di-ester 7

Mechanism ncyclic osmate di-ester Notice the transfer of 2 e- onto Os = REDUCTION OXIDIZED Both of the hydroxyl oxygens in the glycol come from Os. O 4 REDUCED

syn-Glycol Formation (II) potassium permanganate syn hydroxylation


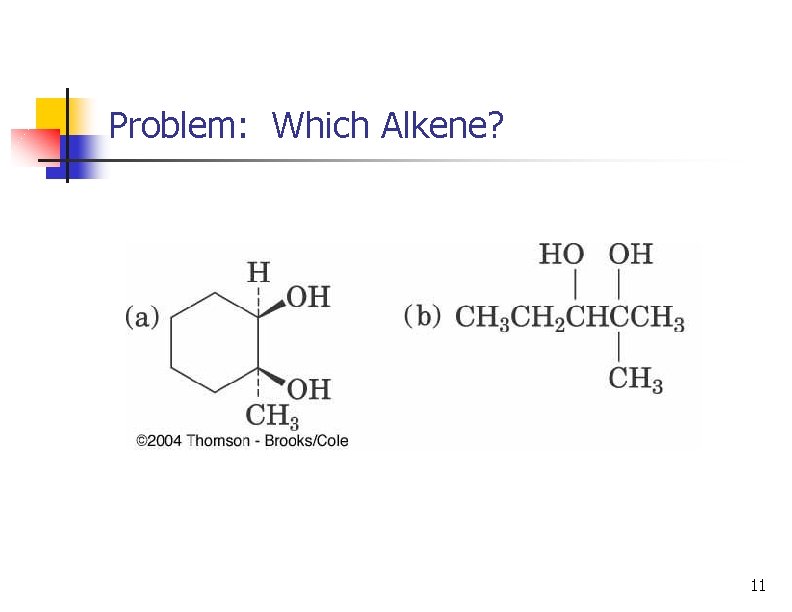
Problem: Which Alkene? 11

12

Cleavage Reactions of Alkenes Ozonolysis Hot Potassium Permanganate 13

Oxidative Cleavage n n n Both the pi and sigma bonds break. C=C becomes C=O. Two methods: n n n Warm or concentrated or acidic KMn. O 4. Ozonolysis Used to determine the position of a double bond in an unknown. 14

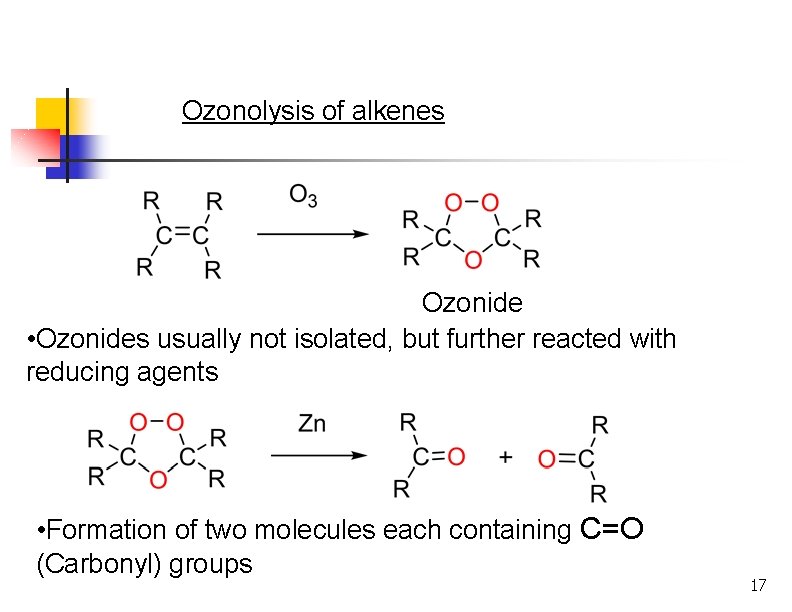
Ozonolysis n n Reaction with ozone forms an ozonide. Ozonides are not isolated, but are treated with a mild reducing agent like Zn or dimethyl sulfide. Milder oxidation than permanganate. Products formed are ketones or aldehydes. 15

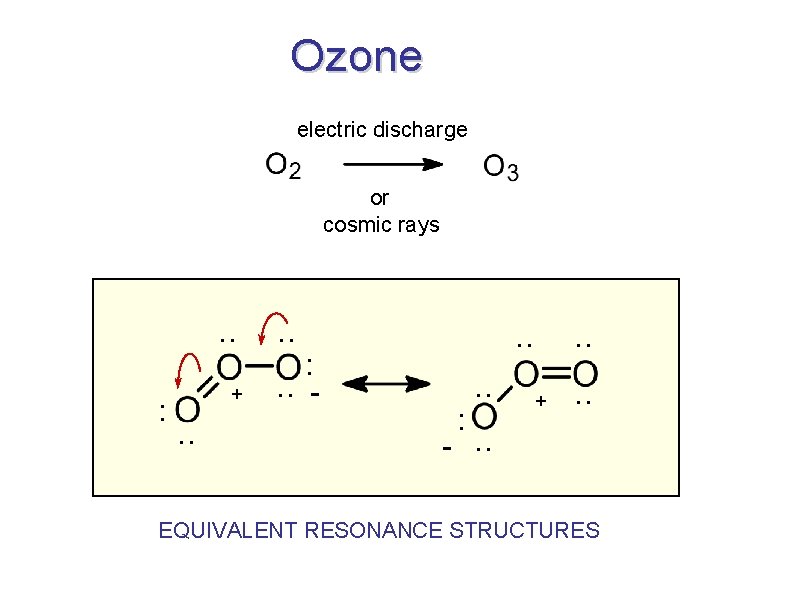
Ozone electric discharge or cosmic rays . . : + . . : . . - . . : . . + . . -. . EQUIVALENT RESONANCE STRUCTURES

Ozonolysis of alkenes Ozonide • Ozonides usually not isolated, but further reacted with reducing agents • Formation of two molecules each containing C=O (Carbonyl) groups 17

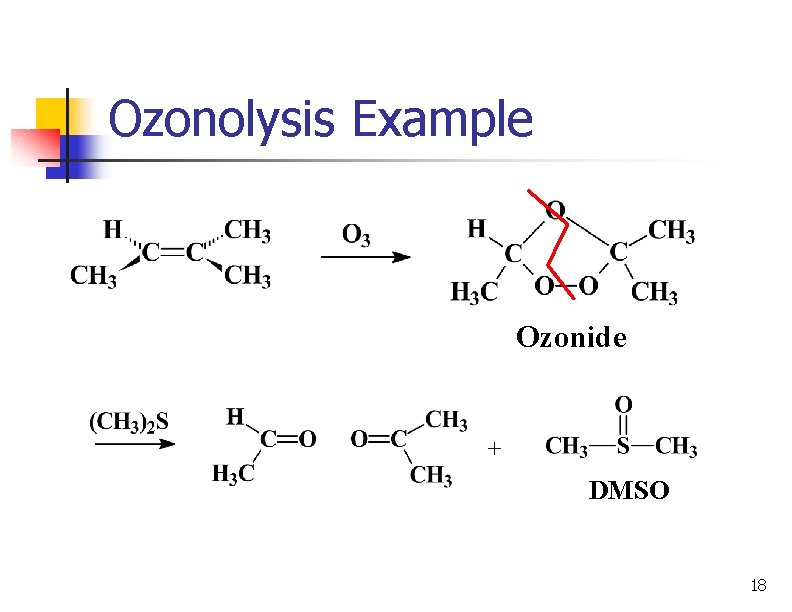
Ozonolysis Example Ozonide DMSO 18

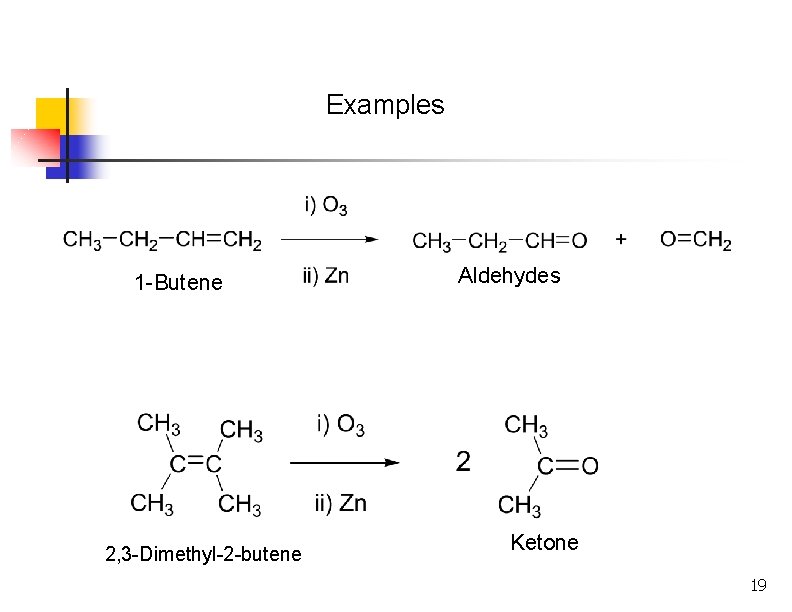
Examples 1 -Butene 2, 3 -Dimethyl-2 -butene Aldehydes Ketone 19

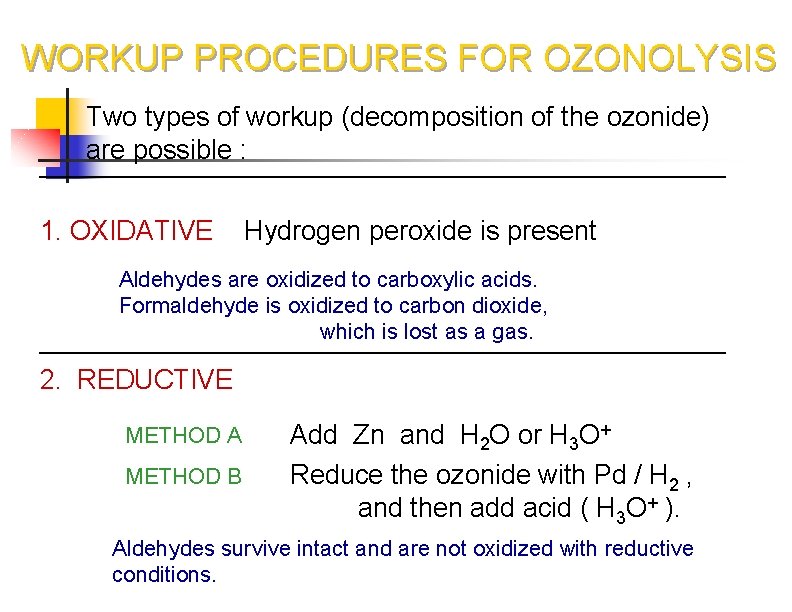
WORKUP PROCEDURES FOR OZONOLYSIS Two types of workup (decomposition of the ozonide) are possible : 1. OXIDATIVE Hydrogen peroxide is present Aldehydes are oxidized to carboxylic acids. Formaldehyde is oxidized to carbon dioxide, which is lost as a gas. 2. REDUCTIVE METHOD A Add Zn and H 2 O or H 3 O+ METHOD B Reduce the ozonide with Pd / H 2 , and then add acid ( H 3 O+ ). Aldehydes survive intact and are not oxidized with reductive conditions.

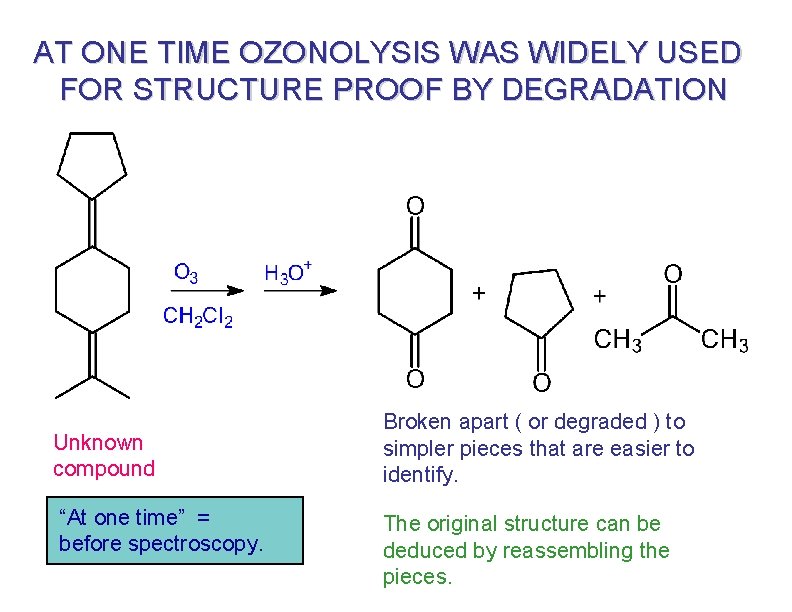
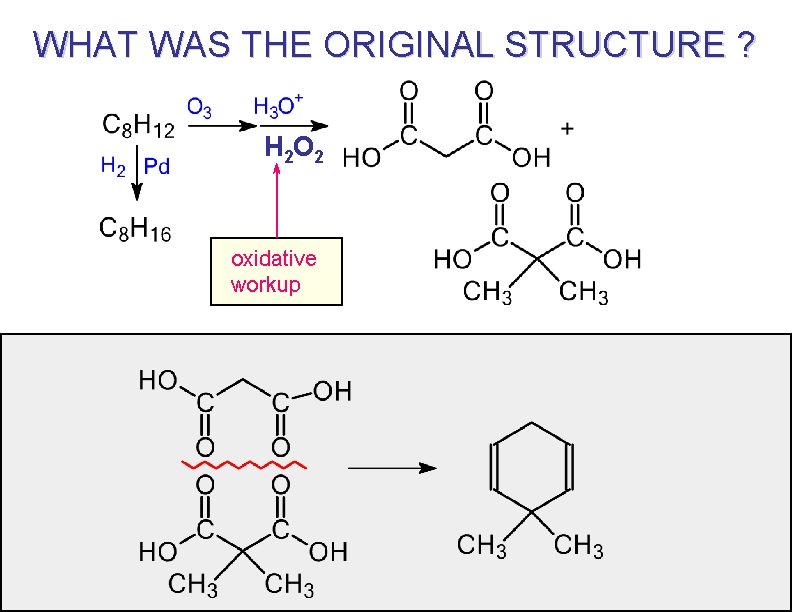
AT ONE TIME OZONOLYSIS WAS WIDELY USED FOR STRUCTURE PROOF BY DEGRADATION Unknown compound “At one time” = before spectroscopy. Broken apart ( or degraded ) to simpler pieces that are easier to identify. The original structure can be deduced by reassembling the pieces.

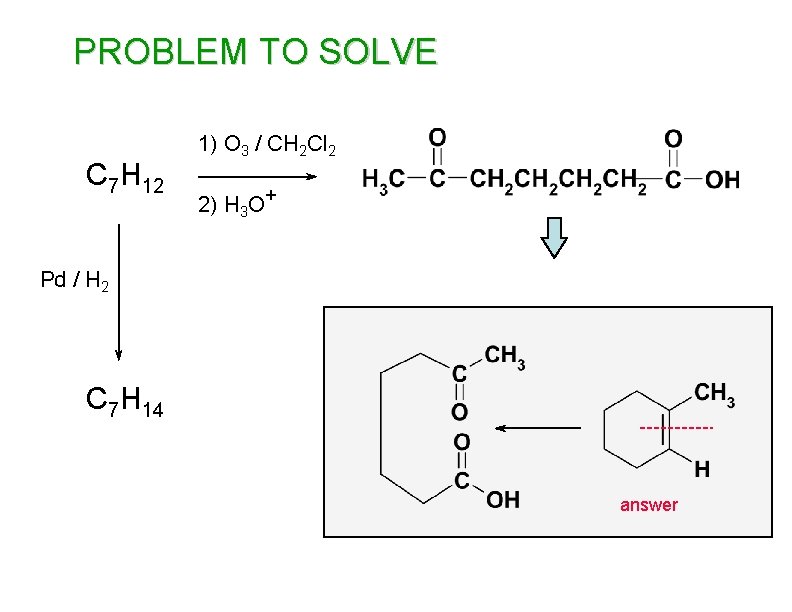
PROBLEM TO SOLVE C 7 H 12 1) O 3 / CH 2 Cl 2 2) H 3 O+ Pd / H 2 C 7 H 14 answer

WHAT WAS THE ORIGINAL STRUCTURE ? H 2 O 2 oxidative workup

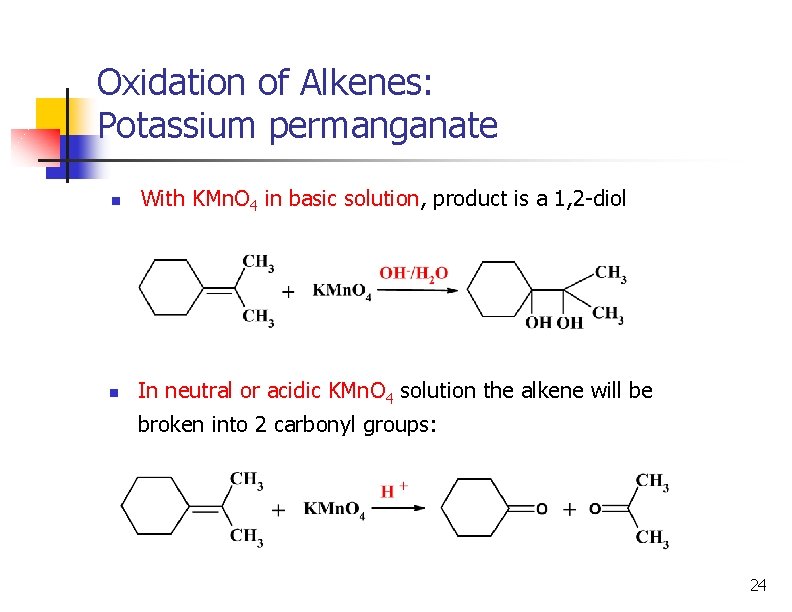
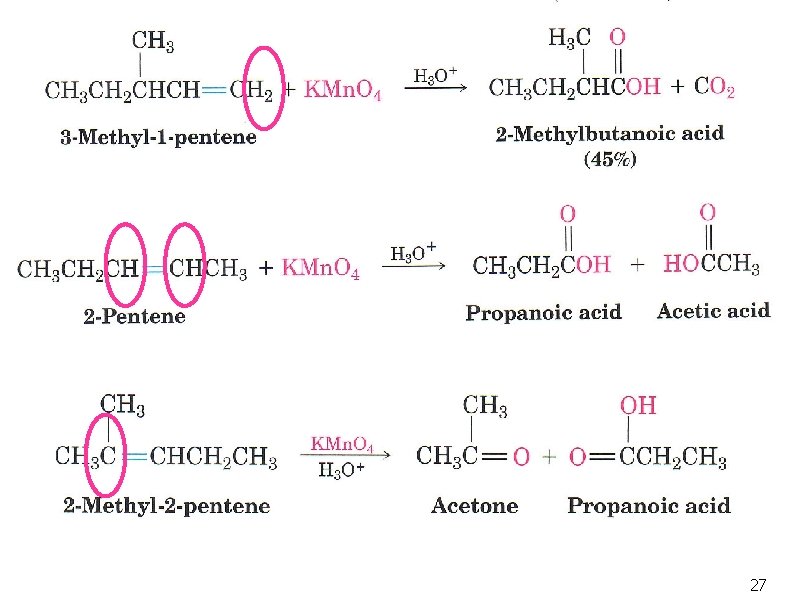
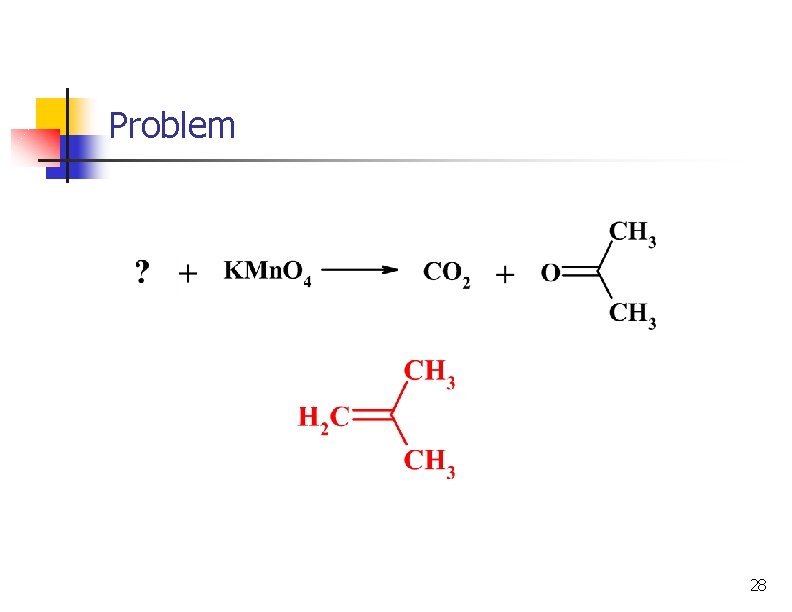
Oxidation of Alkenes: Potassium permanganate n With KMn. O 4 in basic solution, product is a 1, 2 -diol n In neutral or acidic KMn. O 4 solution the alkene will be broken into 2 carbonyl groups: 24

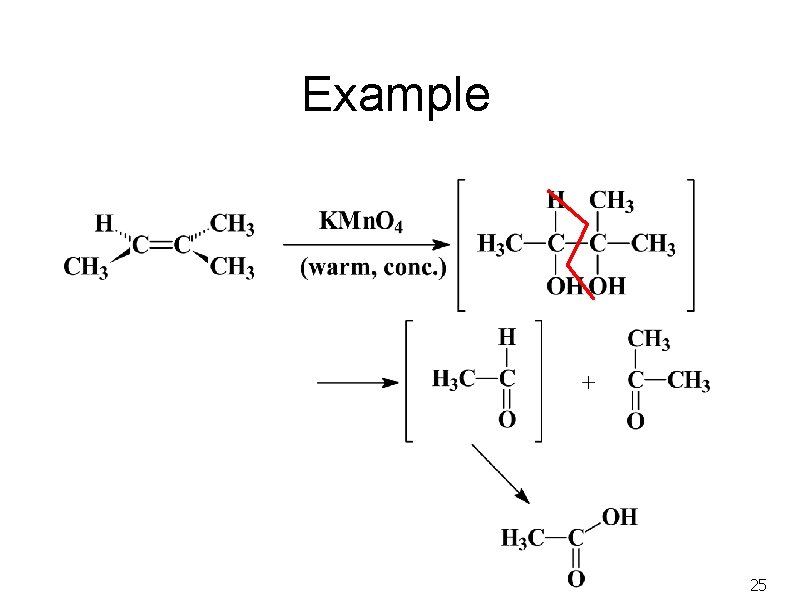
Example 25

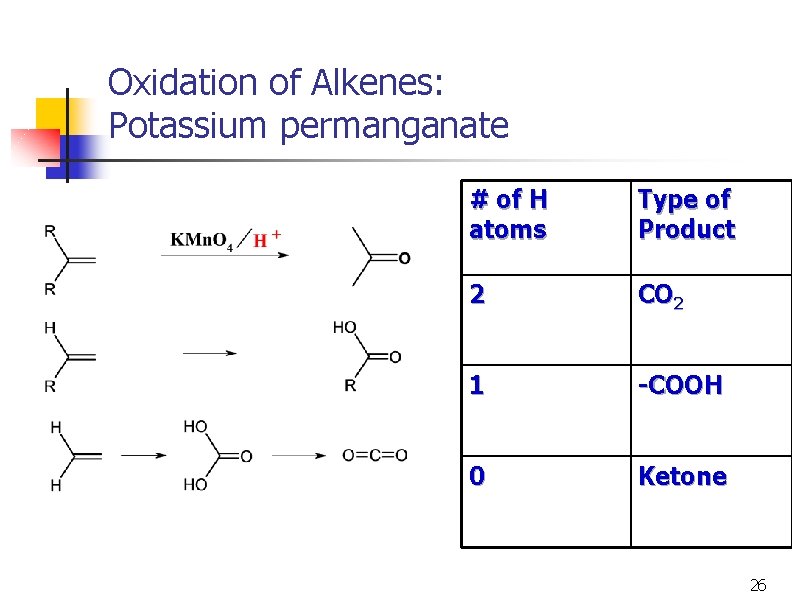
Oxidation of Alkenes: Potassium permanganate # of H atoms Type of Product 2 CO 2 1 -COOH 0 Ketone 26

27

Problem 28

29

Preparation of Alkenes: A Preview of Elimination Reactions 30

Alkene Synthesis Overview n n E 2 dehydrohalogenation (-HX) E 1 dehydrohalogenation (-HX) Dehalogenation of vicinal dibromides (-X 2) Dehydration of alcohols (-H 2 O)

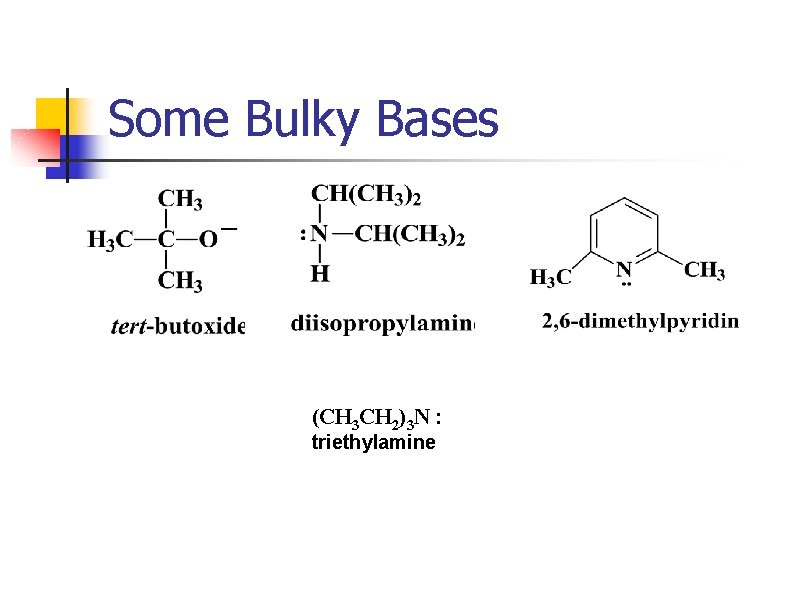
Removing HX via E 2 n n n Strong base abstracts H+ as X- leaves from the adjacent carbon. Tertiary and hindered secondary alkyl halides give good yields. Use a bulky base if the alkyl halide usually forms substitution products.

Some Bulky Bases (CH 3 CH 2)3 N : triethylamine

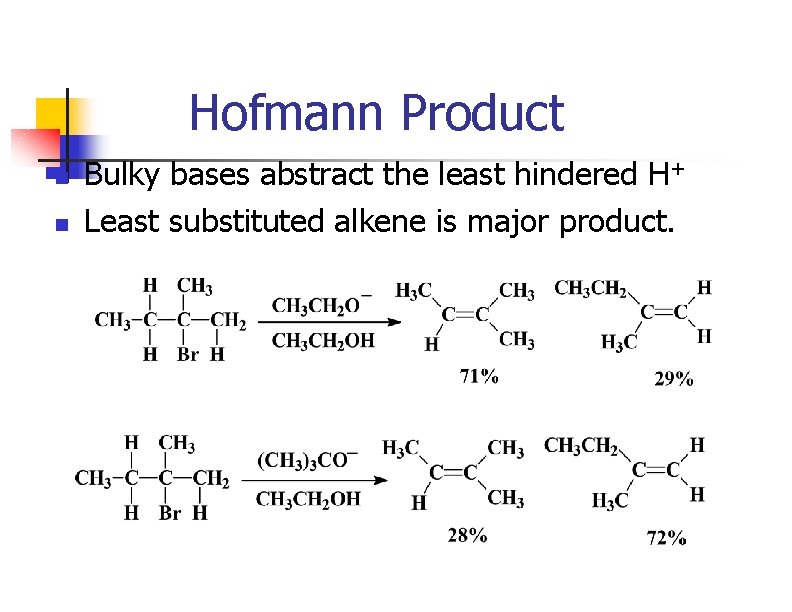
Hofmann Product n n Bulky bases abstract the least hindered H+ Least substituted alkene is major product.

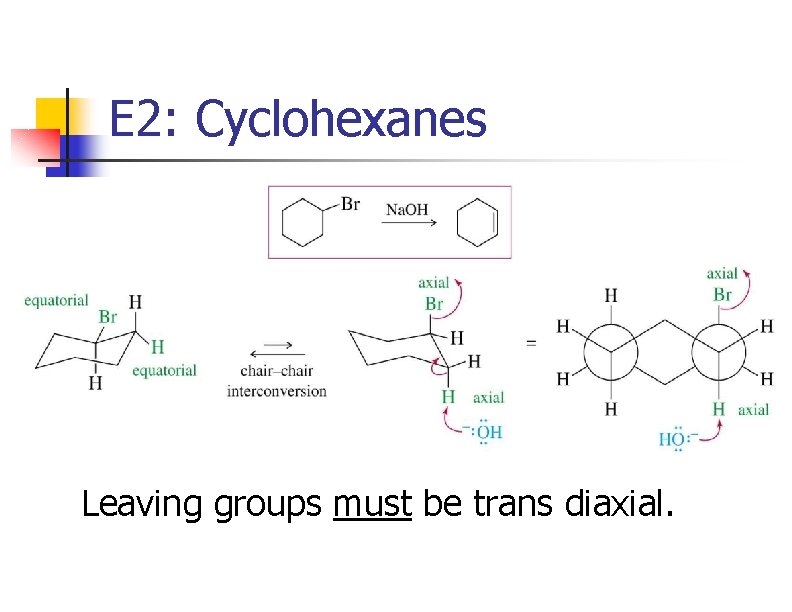
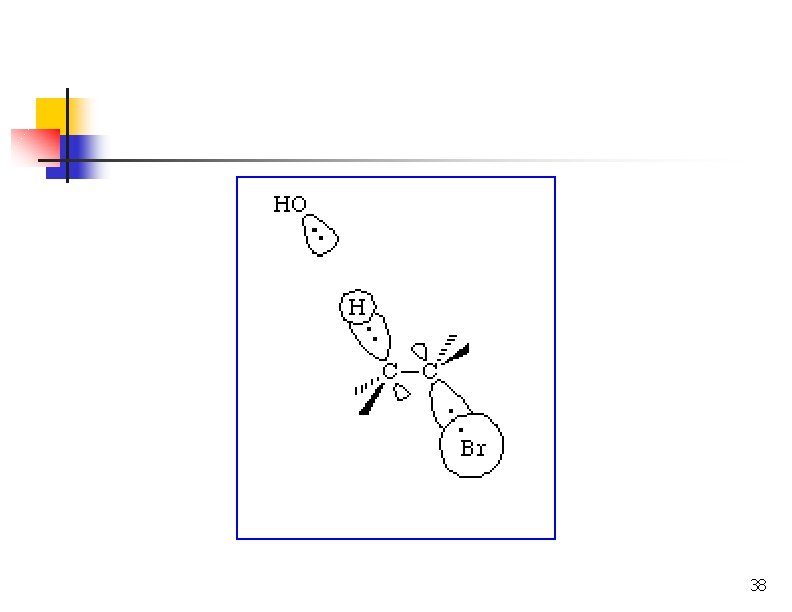
E 2: Cyclohexanes Leaving groups must be trans diaxial.

Removing HX via E 1 n n Secondary or tertiary halides Formation of carbocation intermediate Weak nucleophile Usually have substitution products too

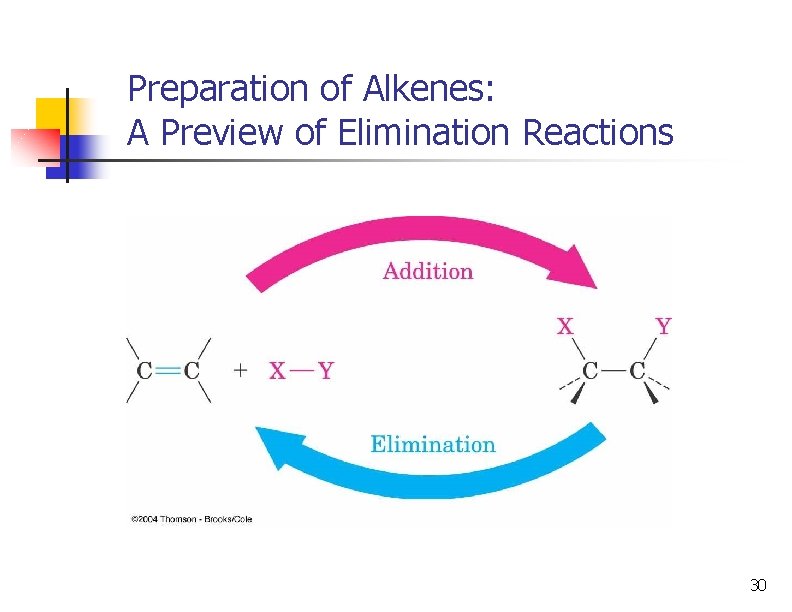
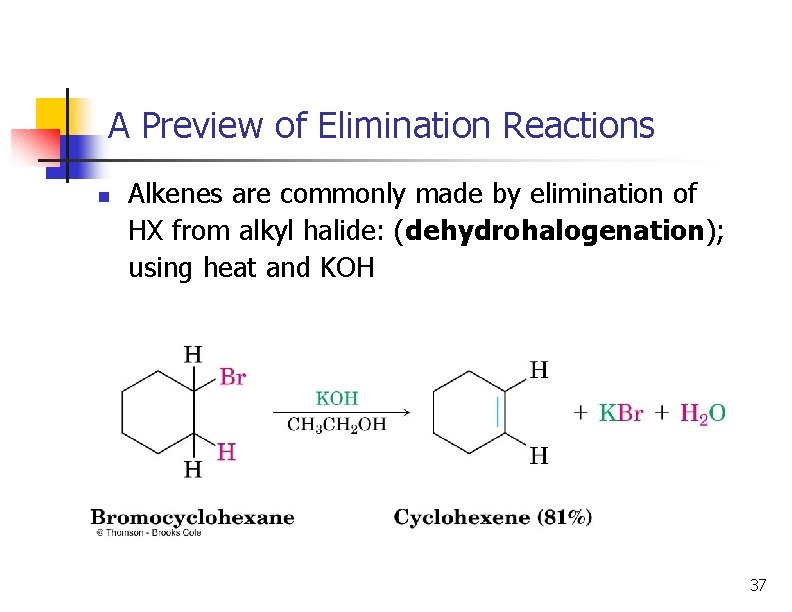
A Preview of Elimination Reactions n Alkenes are commonly made by elimination of HX from alkyl halide: (dehydrohalogenation); using heat and KOH 37

38

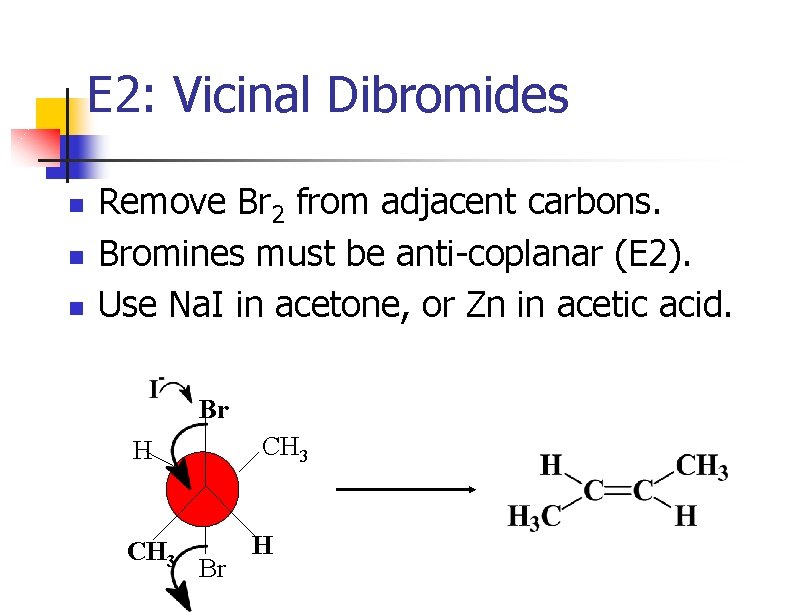
E 2: Vicinal Dibromides n n n Remove Br 2 from adjacent carbons. Bromines must be anti-coplanar (E 2). Use Na. I in acetone, or Zn in acetic acid. Br CH 3 H CH 3 Br H

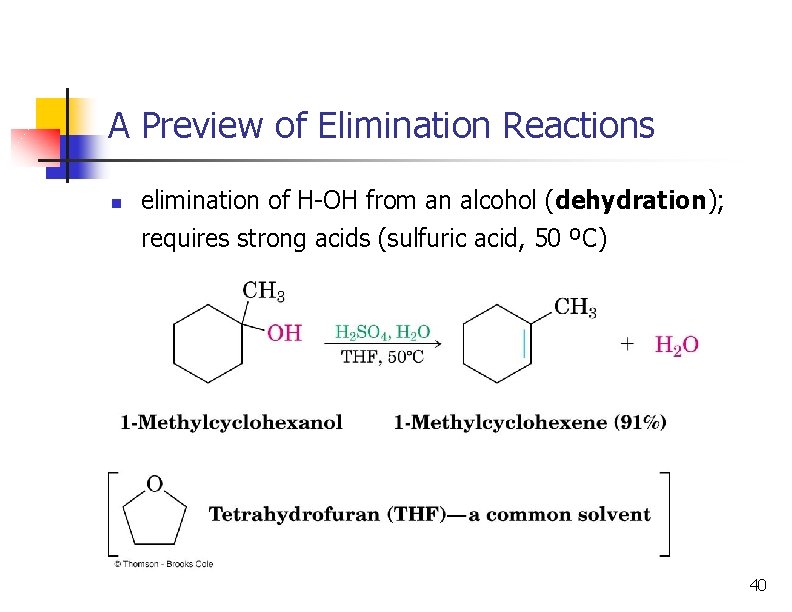
A Preview of Elimination Reactions n elimination of H-OH from an alcohol (dehydration); requires strong acids (sulfuric acid, 50 ºC) 40

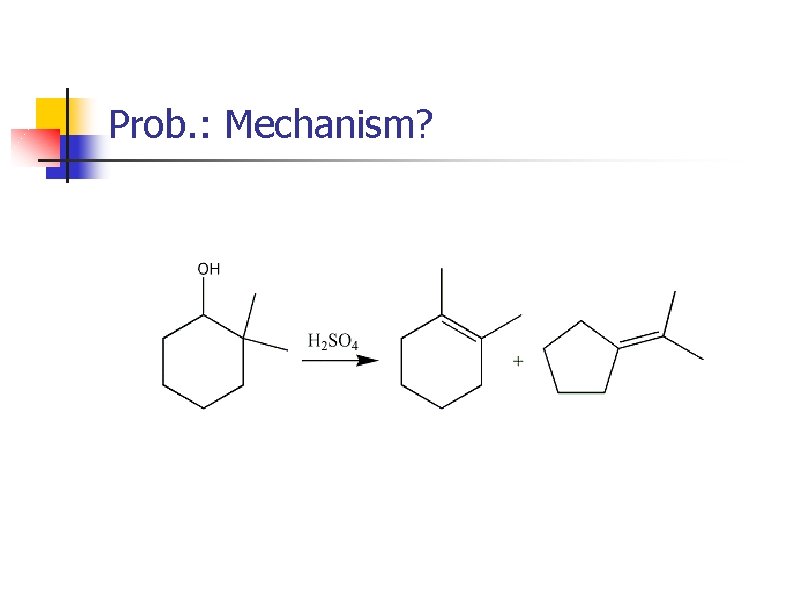
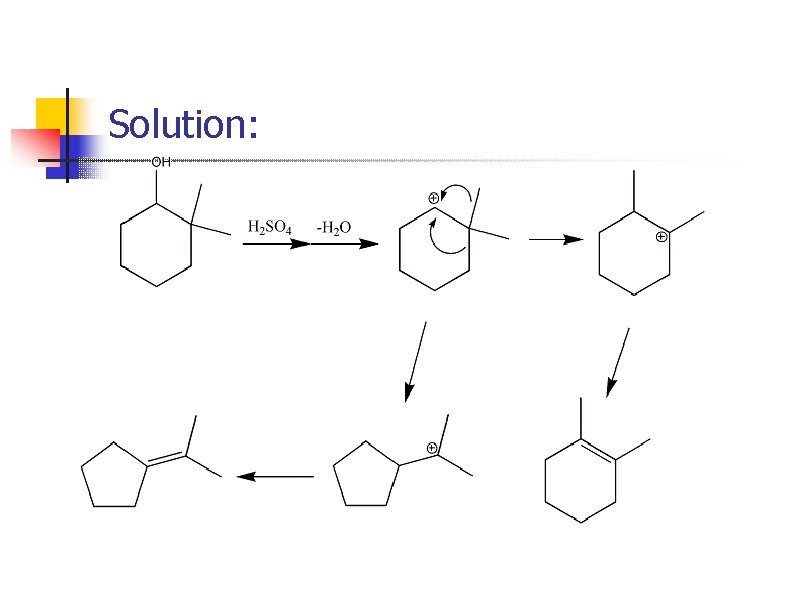
Prob. : Mechanism?

Solution:

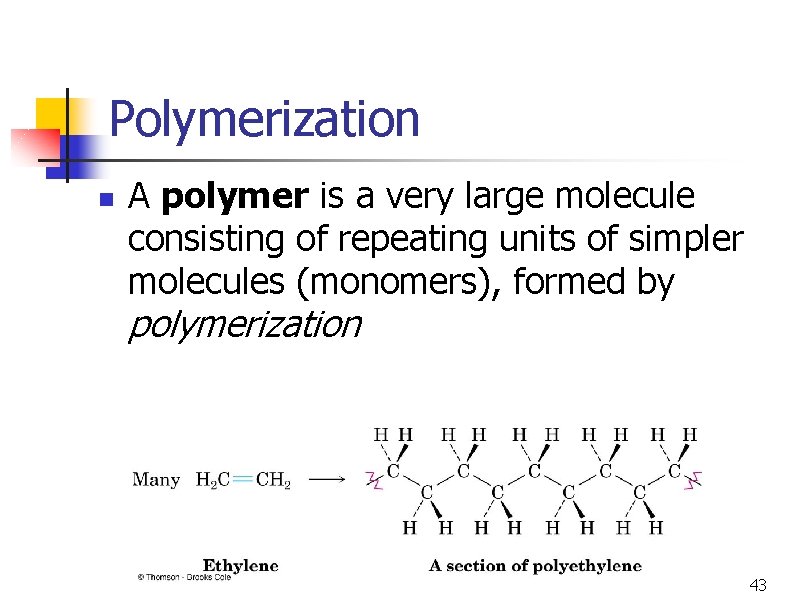
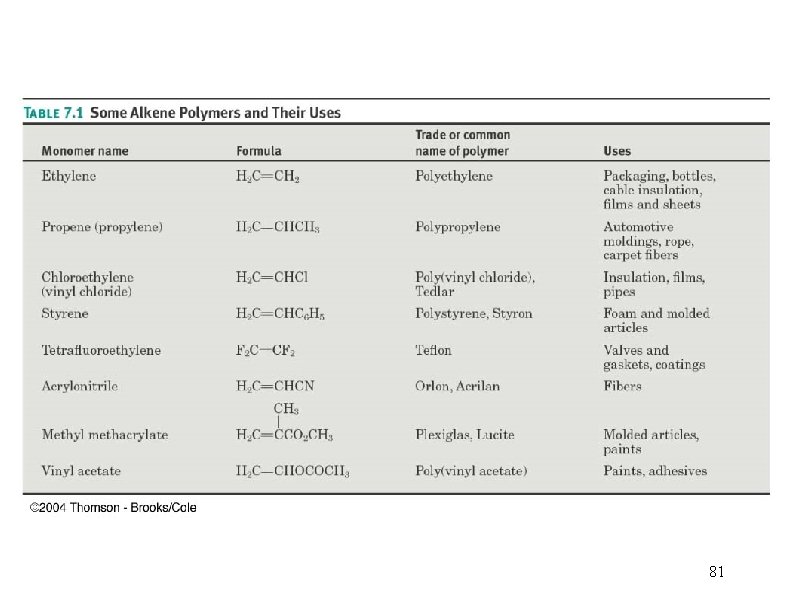
Polymerization n A polymer is a very large molecule consisting of repeating units of simpler molecules (monomers), formed by polymerization 43

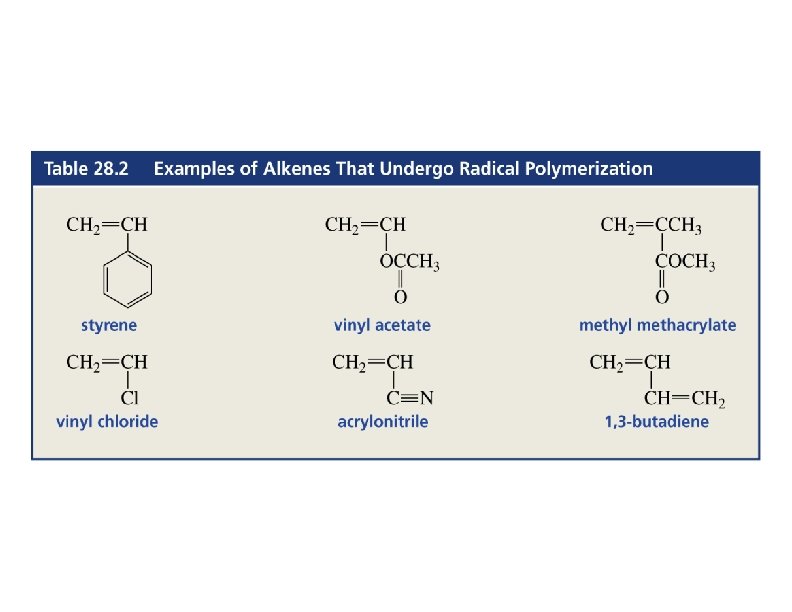
Polymerization • An alkene (monomer) can add to another molecule like itself to form a chain (polymer). • Three methods: ØCationic, a carbocation intermediate ØFree radical ØAnionic, a carbanion intermediate (rare) 44

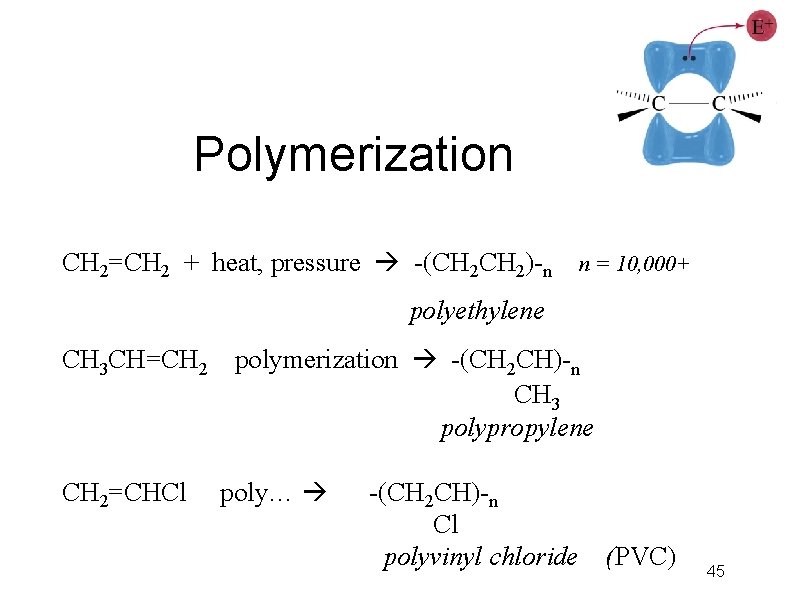
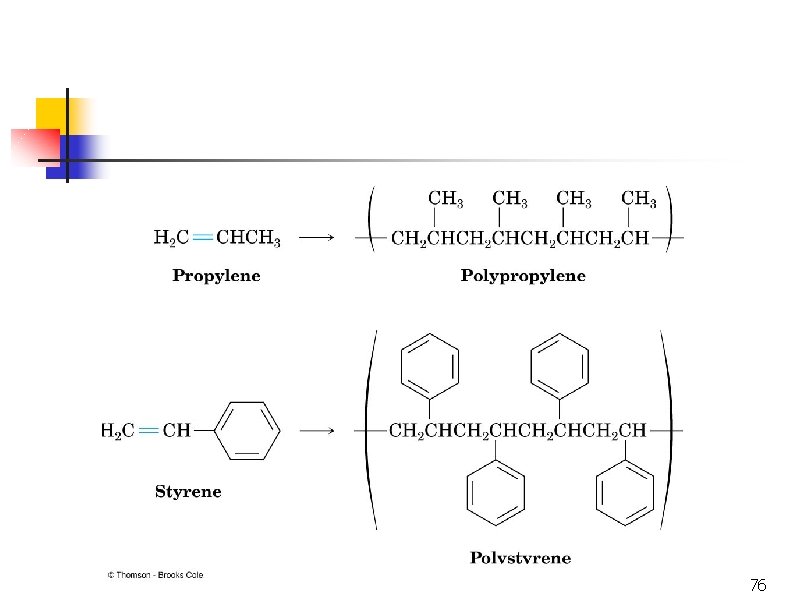
Polymerization CH 2=CH 2 + heat, pressure -(CH 2)-n n = 10, 000+ polyethylene CH 3 CH=CH 2=CHCl polymerization -(CH 2 CH)-n CH 3 polypropylene poly… -(CH 2 CH)-n Cl polyvinyl chloride (PVC) 45

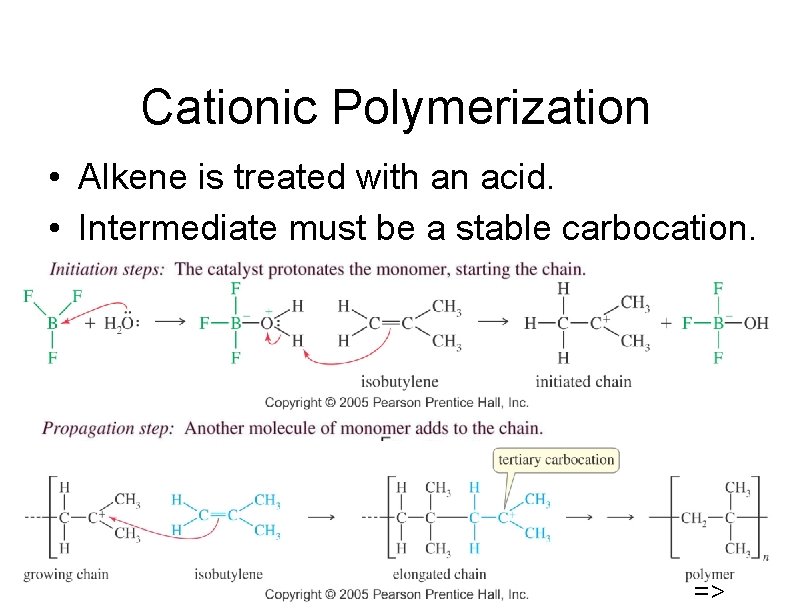
Cationic Polymerization • Alkene is treated with an acid. • Intermediate must be a stable carbocation. 46 =>

47

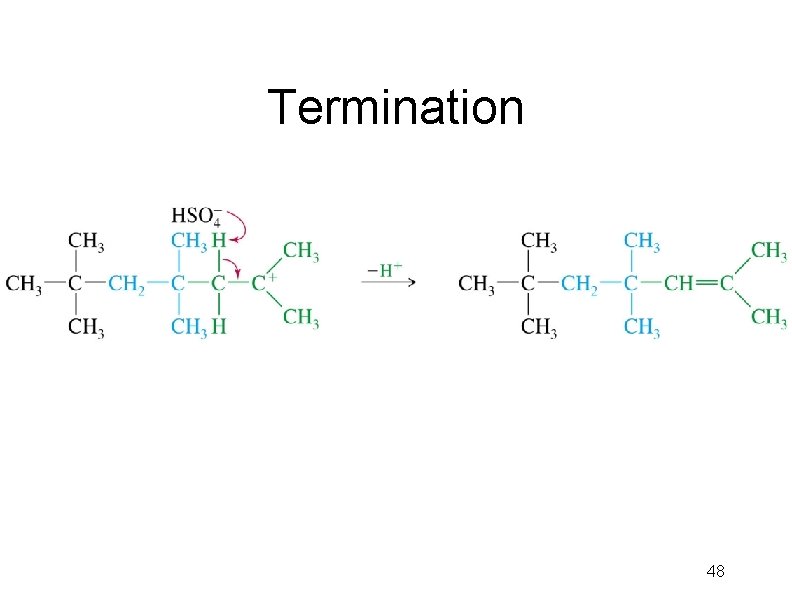
Termination 48

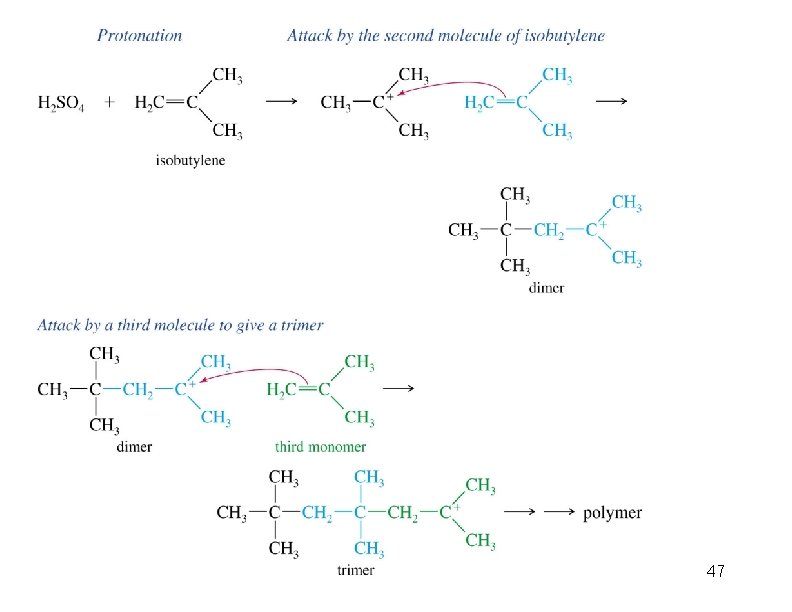
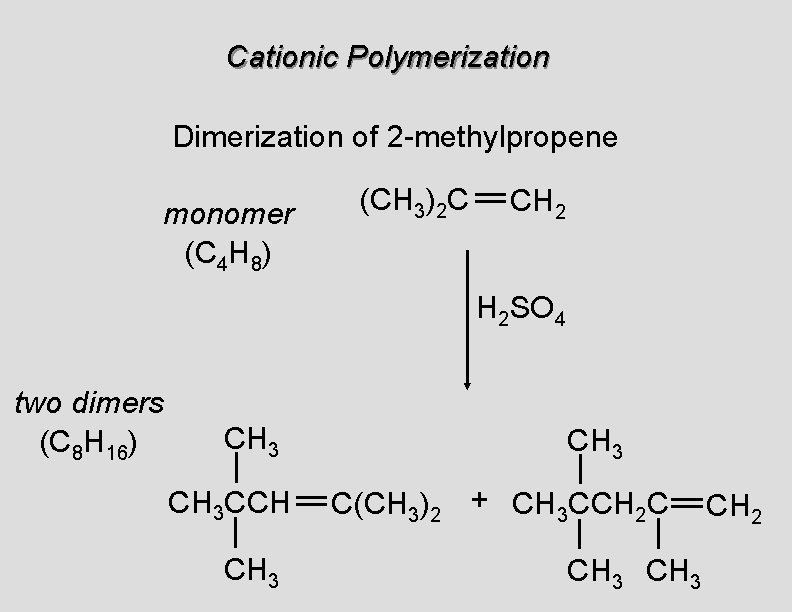
Cationic Polymerization Dimerization of 2 -methylpropene monomer (C 4 H 8) (CH 3)2 C CH 2 H 2 SO 4 two dimers (C 8 H 16) CH 3 CCH CH 3 C(CH 3)2 + CH 3 CCH 2 C CH 3 CH 2

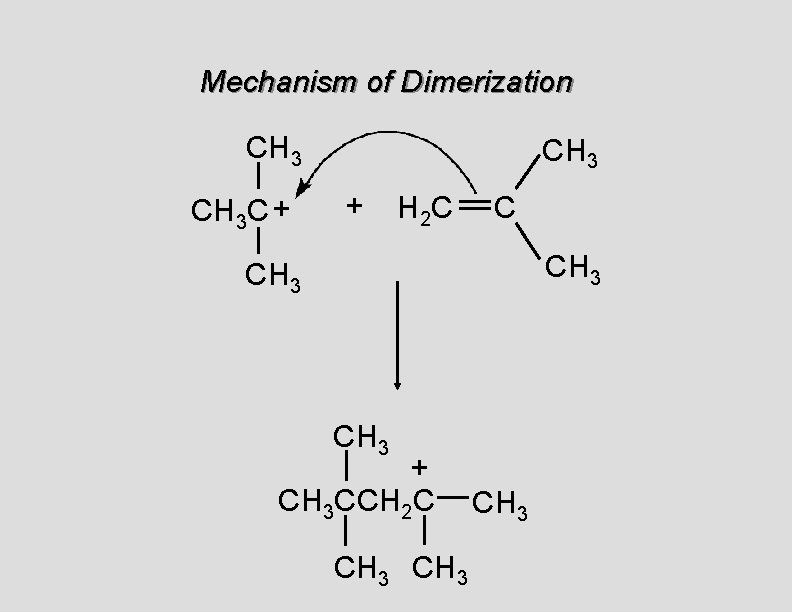
Mechanism of Dimerization CH 3 C + CH 3 + H 2 C C CH 3 + CH 3 CCH 2 C CH 3

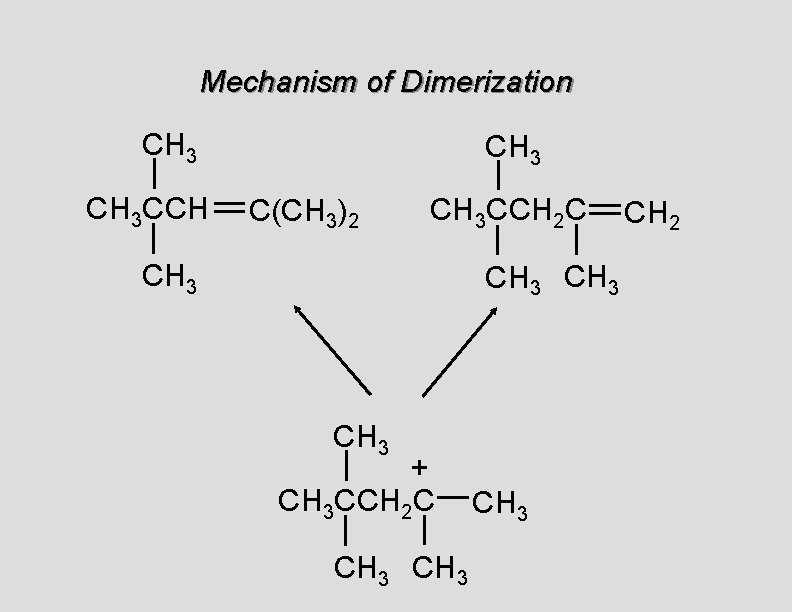
Mechanism of Dimerization CH 3 CCH CH 3 C(CH 3)2 CH 3 CCH 2 C CH 3 + CH 3 CCH 2 C CH 3 CH 2

Free Radical Polymerization of Alkenes n Alkenes combine many times to give polymer n Reactivity induced by formation of free radicals

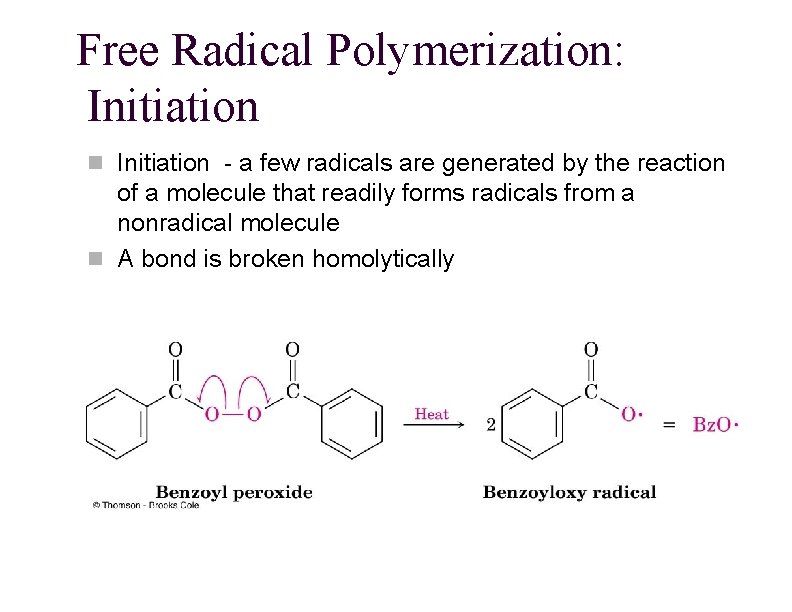
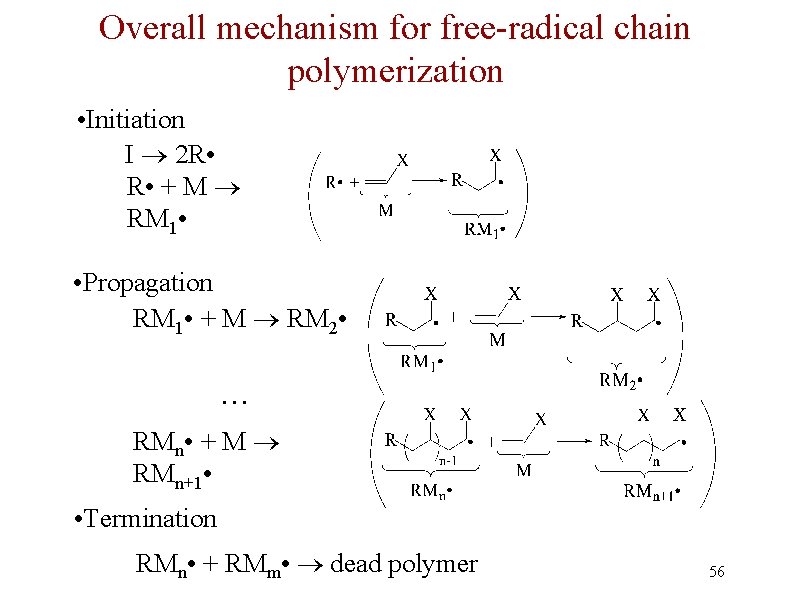
Free Radical Polymerization: Initiation n Initiation - a few radicals are generated by the reaction of a molecule that readily forms radicals from a nonradical molecule n A bond is broken homolytically

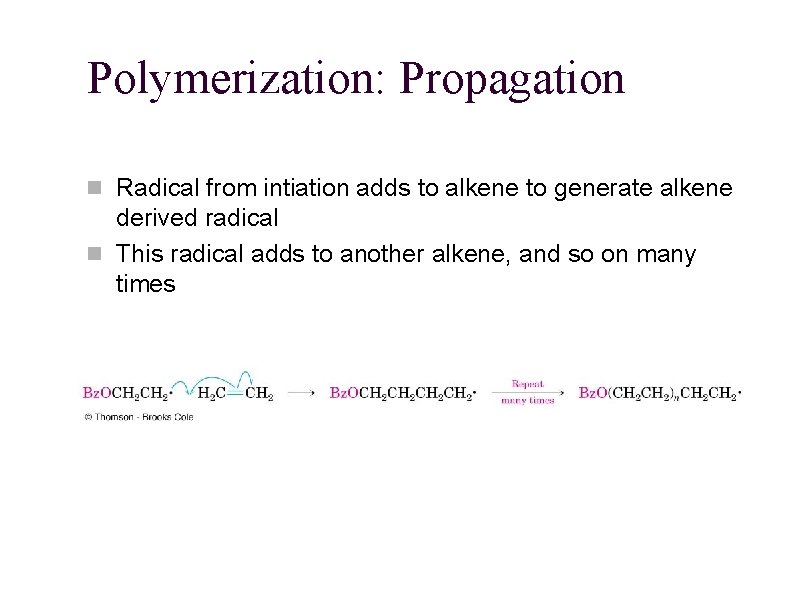
Polymerization: Propagation n Radical from intiation adds to alkene to generate alkene derived radical n This radical adds to another alkene, and so on many times

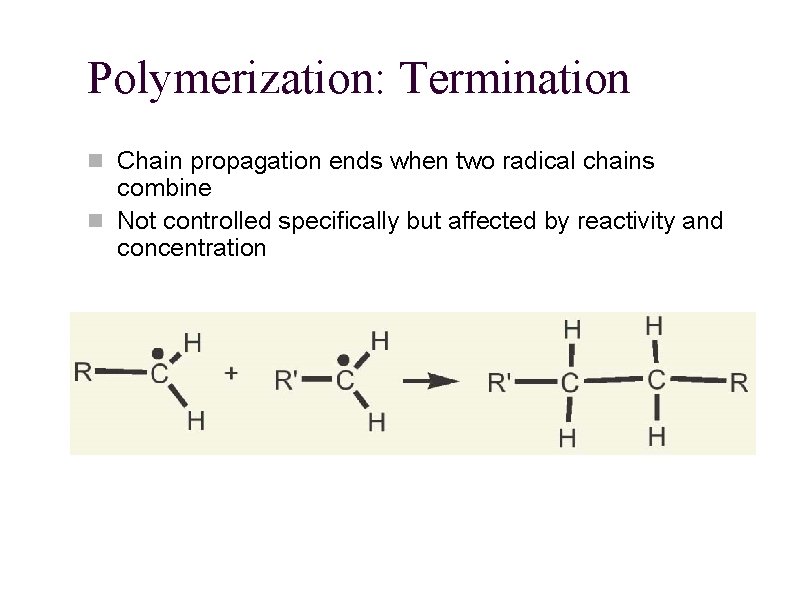
Polymerization: Termination n Chain propagation ends when two radical chains combine n Not controlled specifically but affected by reactivity and concentration

Overall mechanism for free-radical chain polymerization • Initiation I 2 R • + M RM 1 • • Propagation RM 1 • + M RM 2 • … RMn • + M RMn+1 • • Termination RMn • + RMm • dead polymer 56

57

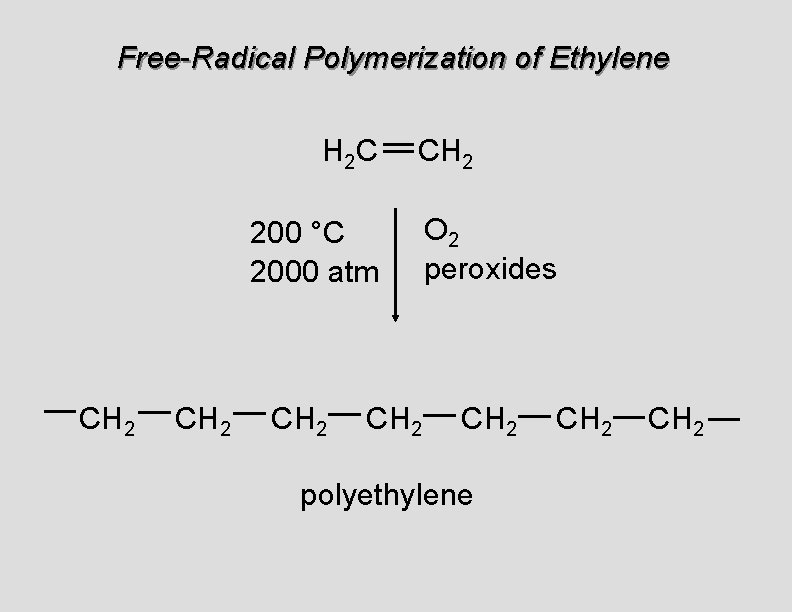
Free-Radical Polymerization of Ethylene H 2 C CH 2 200 °C 2000 atm CH 2 O 2 peroxides CH 2 polyethylene CH 2

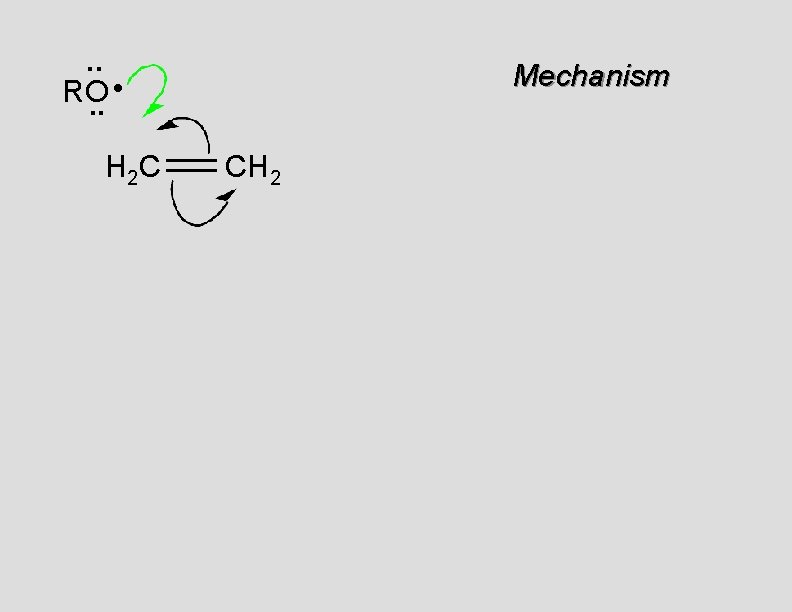
. . • RO. . H 2 C Mechanism CH 2

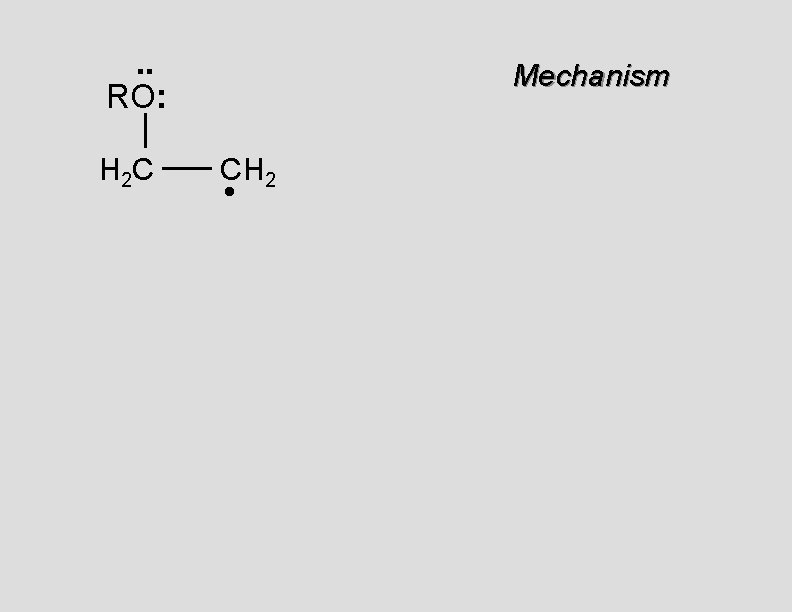
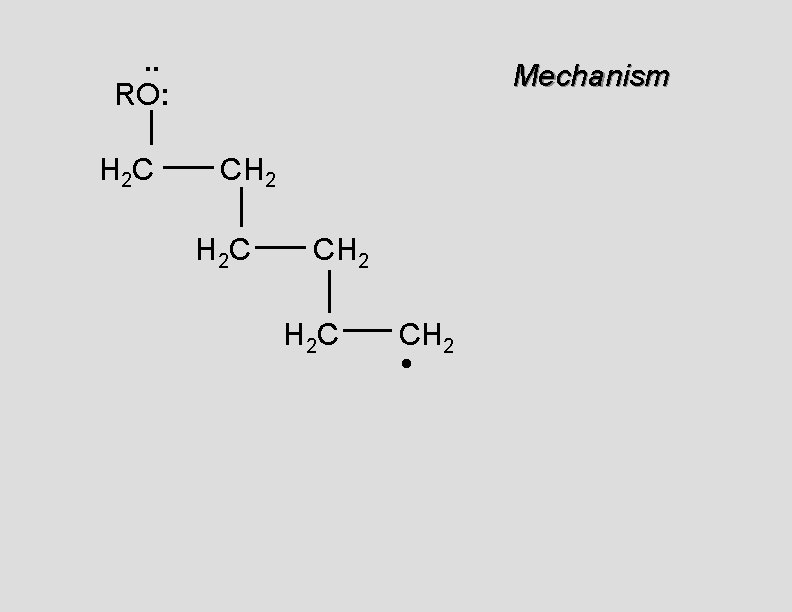
. . RO: H 2 C Mechanism CH 2 •

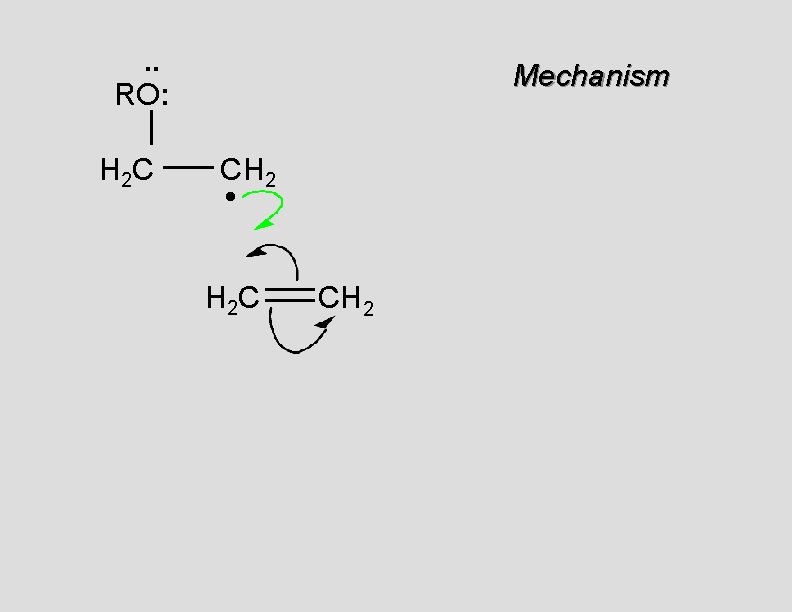
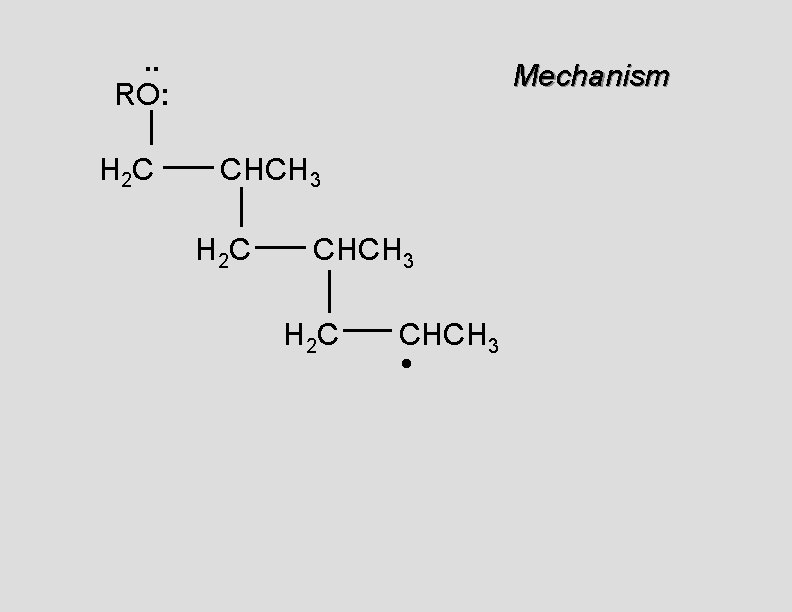
. . RO: H 2 C Mechanism CH 2 • H 2 C CH 2

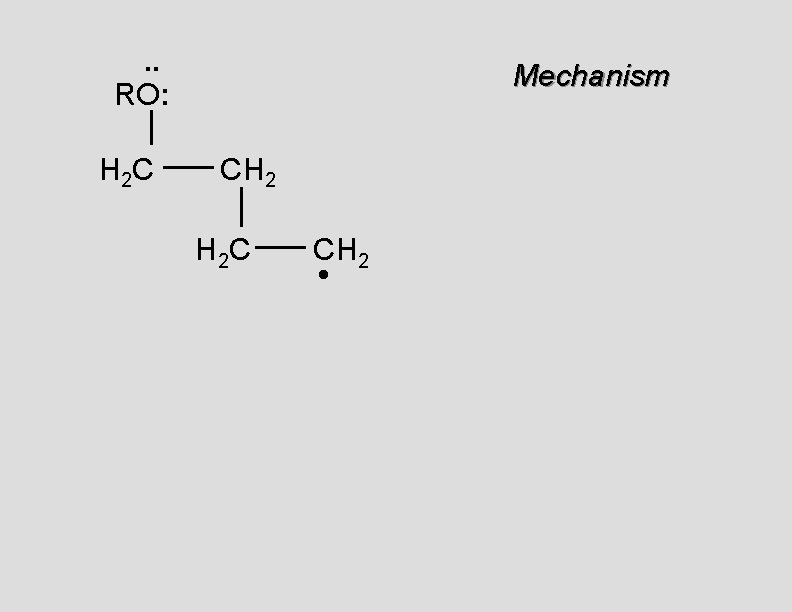
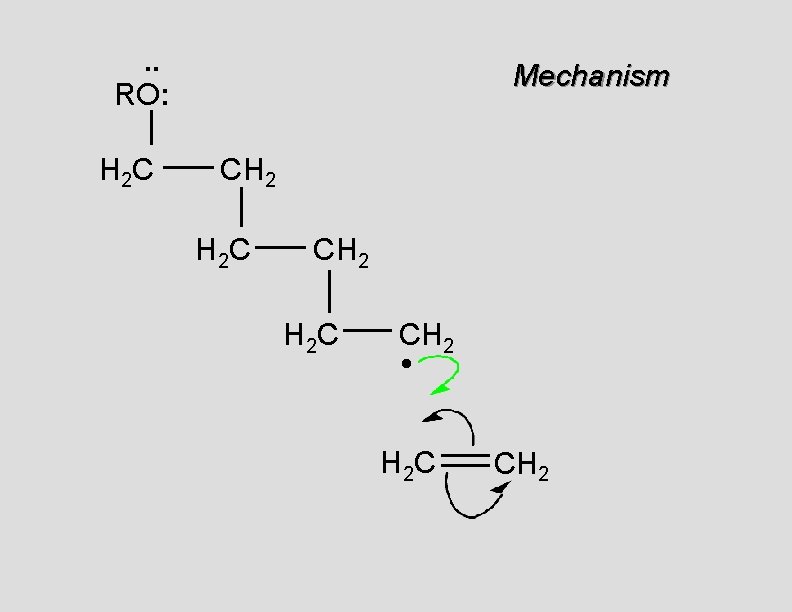
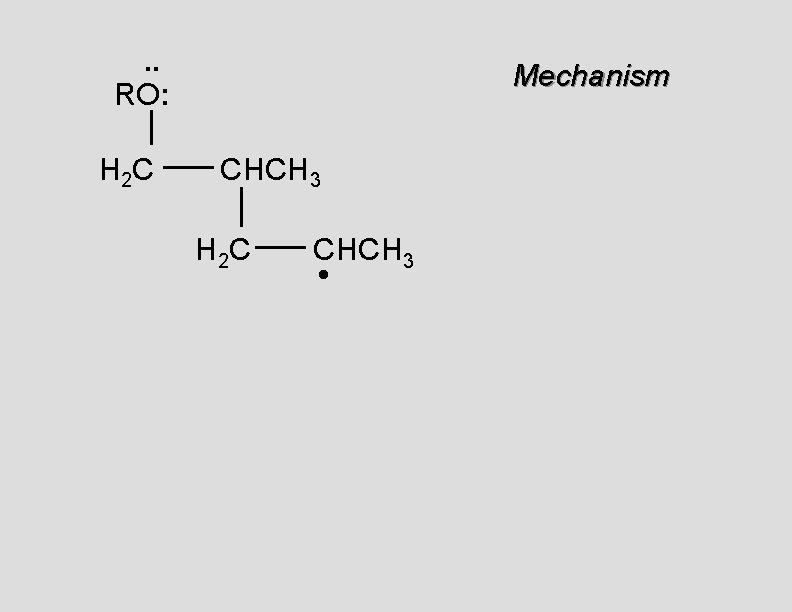
. . RO: H 2 C Mechanism CH 2 H 2 C CH 2 •

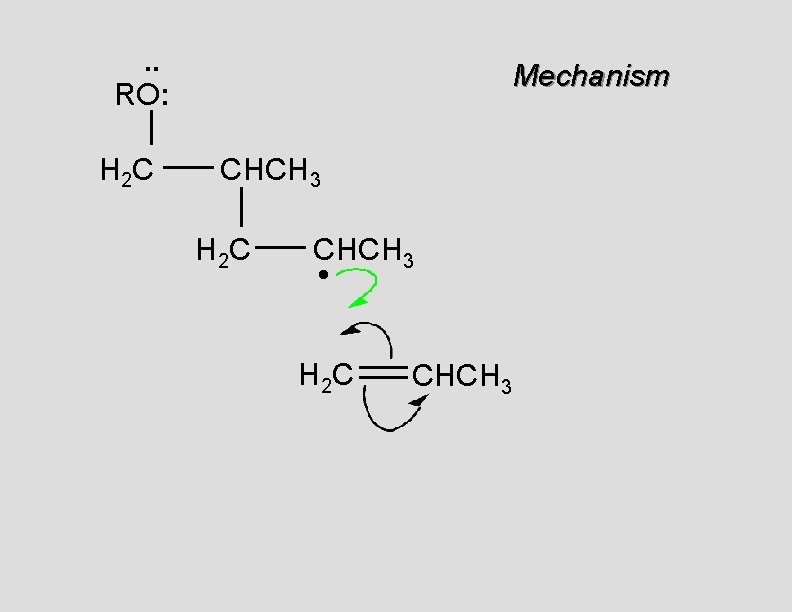
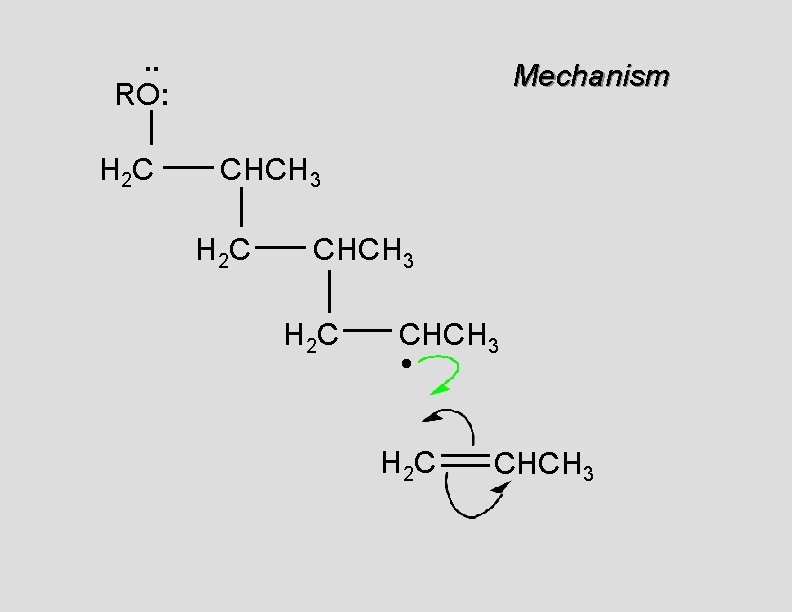
. . RO: H 2 C Mechanism CH 2 H 2 C CH 2 • H 2 C CH 2

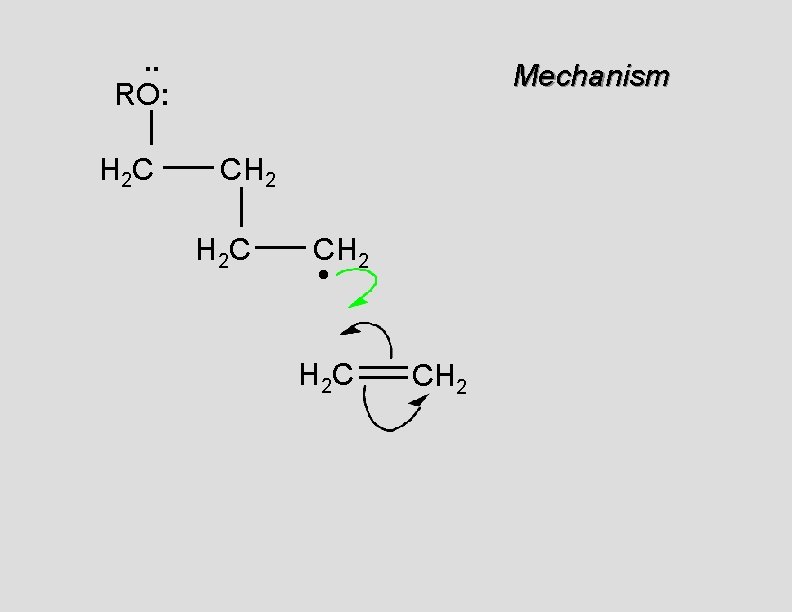
. . RO: H 2 C Mechanism CH 2 H 2 C CH 2 •

. . RO: H 2 C Mechanism CH 2 H 2 C CH 2 • H 2 C CH 2

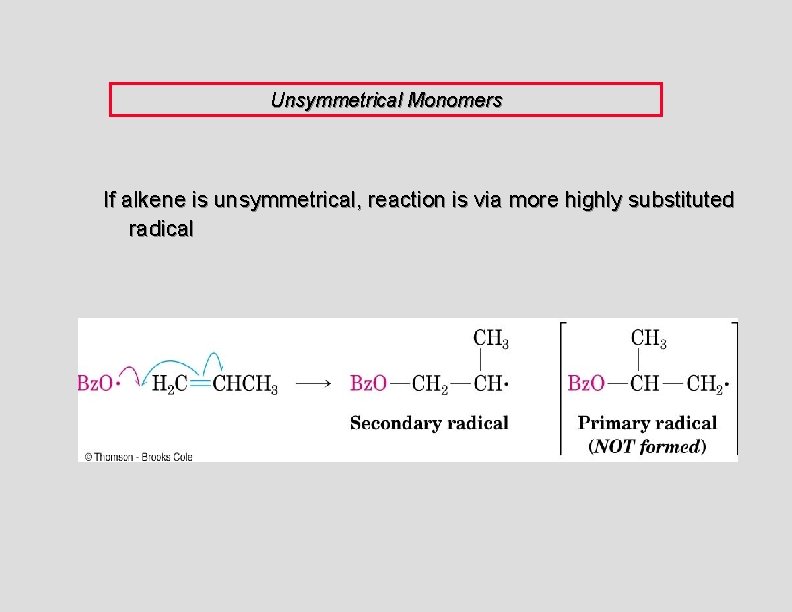
Unsymmetrical Monomers If alkene is unsymmetrical, reaction is via more highly substituted radical

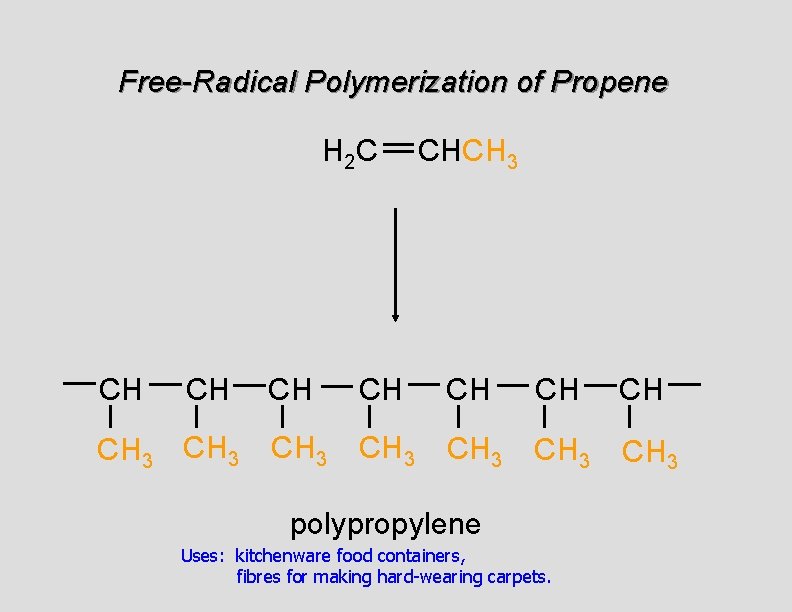
Free-Radical Polymerization of Propene H 2 C CH CH CH 3 CHCH 3 CH CH CH 3 CH 3 polypropylene Uses: kitchenware food containers, fibres for making hard-wearing carpets.

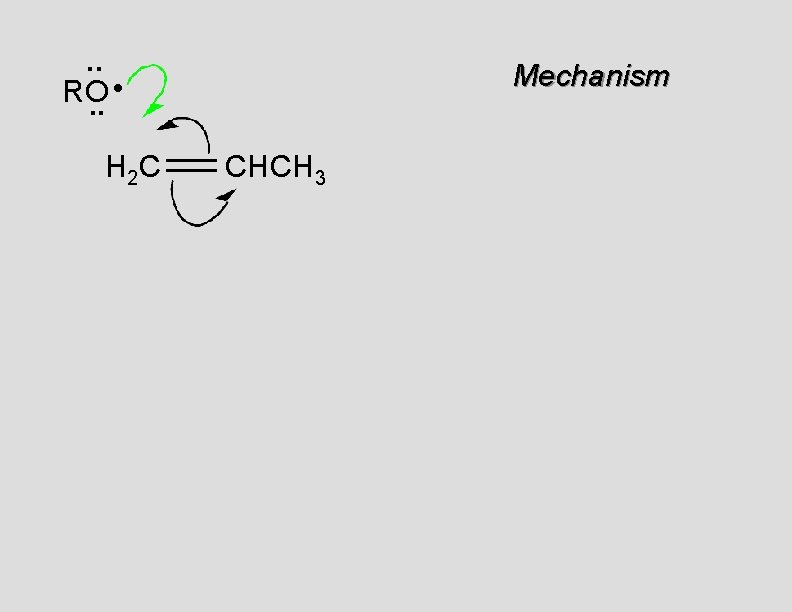
. . • RO. . H 2 C Mechanism CHCH 3

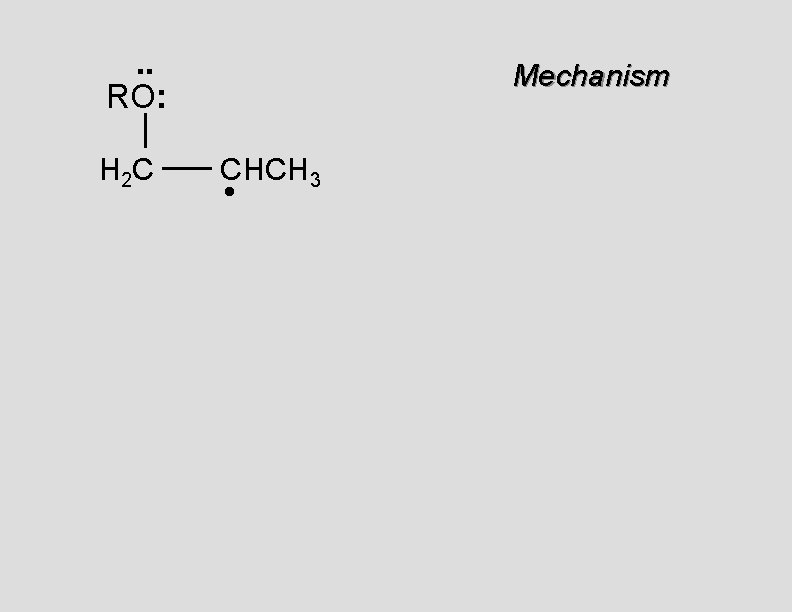
. . RO: H 2 C Mechanism CHCH 3 •

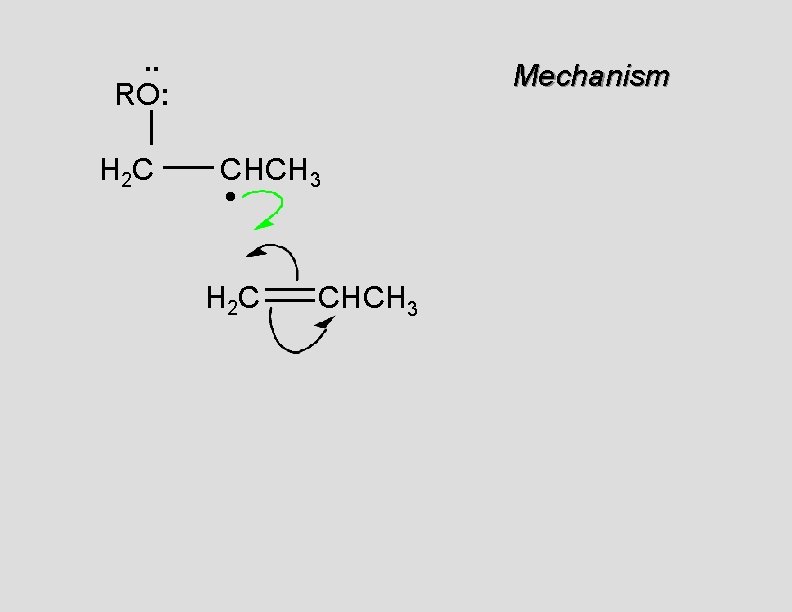
. . RO: H 2 C Mechanism CHCH 3 • H 2 C CHCH 3

. . RO: H 2 C Mechanism CHCH 3 H 2 C CHCH 3 •

. . RO: H 2 C Mechanism CHCH 3 H 2 C CHCH 3 • H 2 C CHCH 3

. . RO: H 2 C Mechanism CHCH 3 H 2 C CHCH 3 •

. . RO: H 2 C Mechanism CHCH 3 H 2 C CHCH 3 • H 2 C CHCH 3

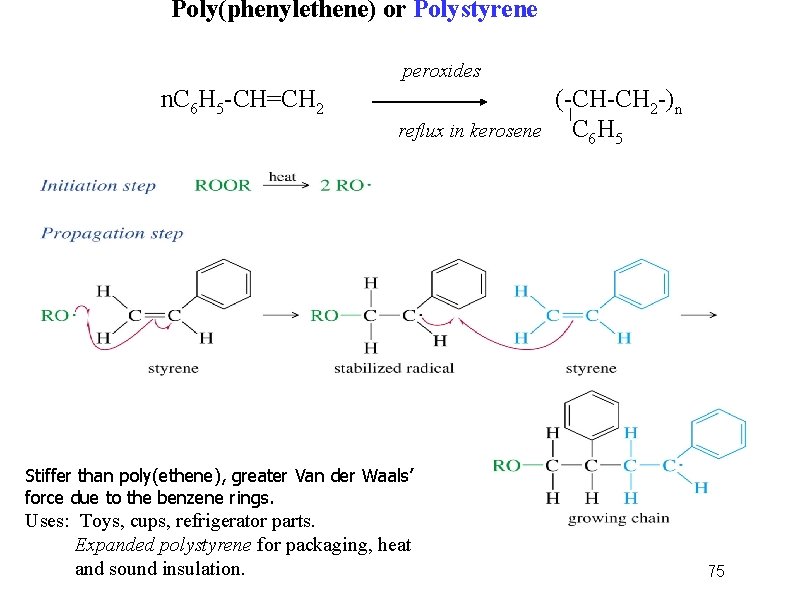
Poly(phenylethene) or Polystyrene peroxides n. C 6 H 5 -CH=CH 2 reflux in kerosene (-CH-CH 2 -)n C 6 H 5 Stiffer than poly(ethene), greater Van der Waals’ force due to the benzene rings. Uses: Toys, cups, refrigerator parts. Expanded polystyrene for packaging, heat and sound insulation. 75

76

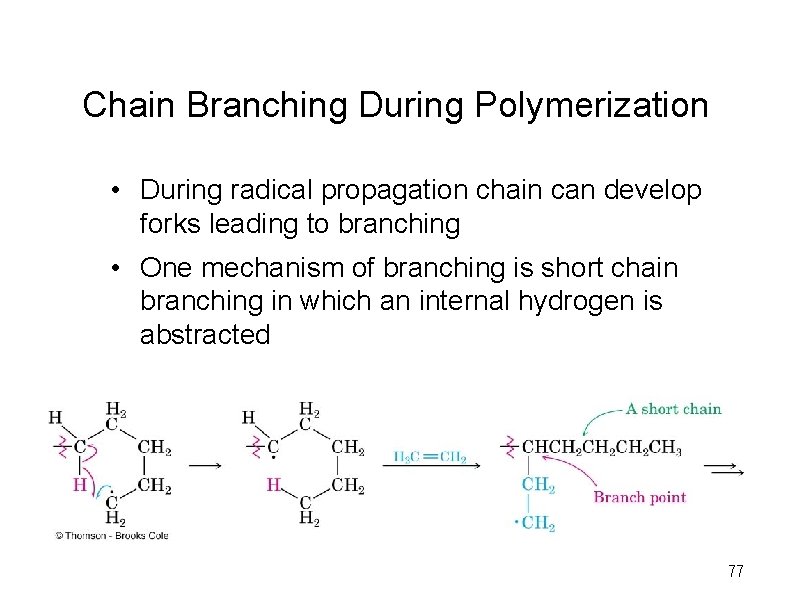
Chain Branching During Polymerization • During radical propagation chain can develop forks leading to branching • One mechanism of branching is short chain branching in which an internal hydrogen is abstracted 77

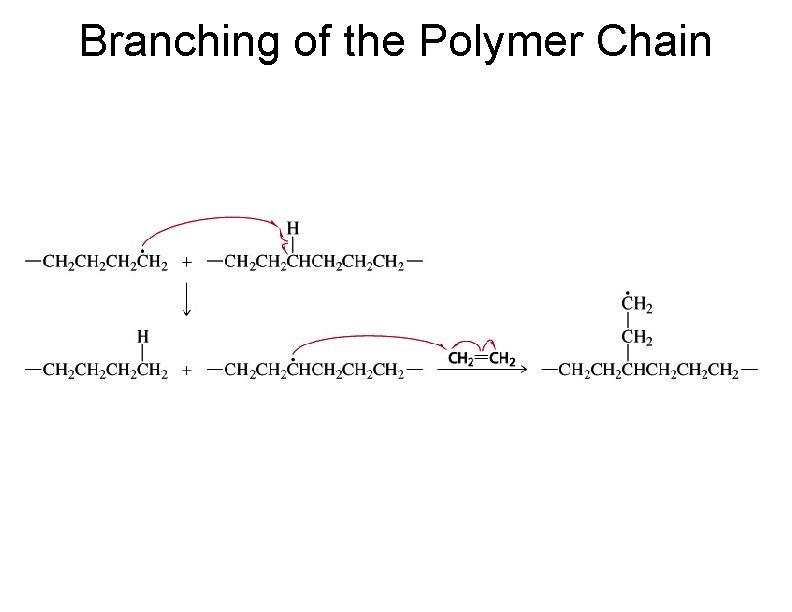
Branching of the Polymer Chain 78

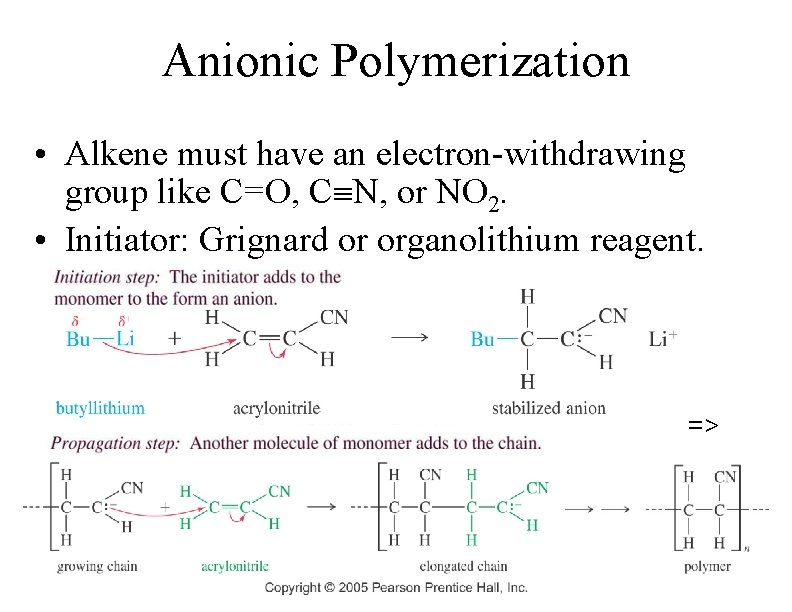
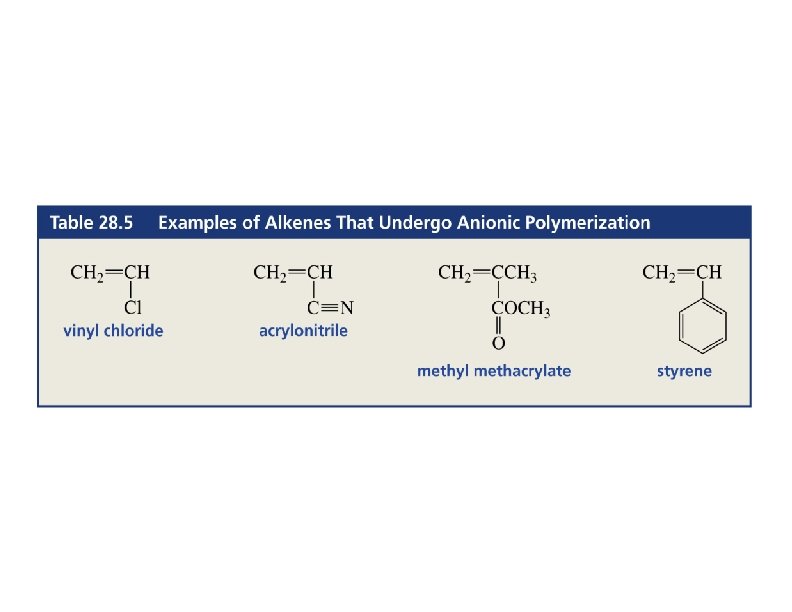
Anionic Polymerization • Alkene must have an electron-withdrawing group like C=O, C N, or NO 2. • Initiator: Grignard or organolithium reagent. => 79

80

81

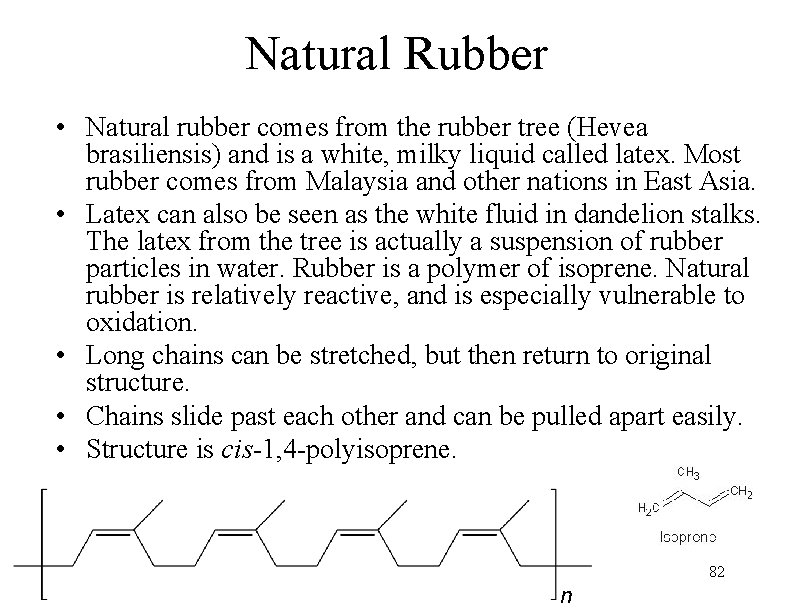
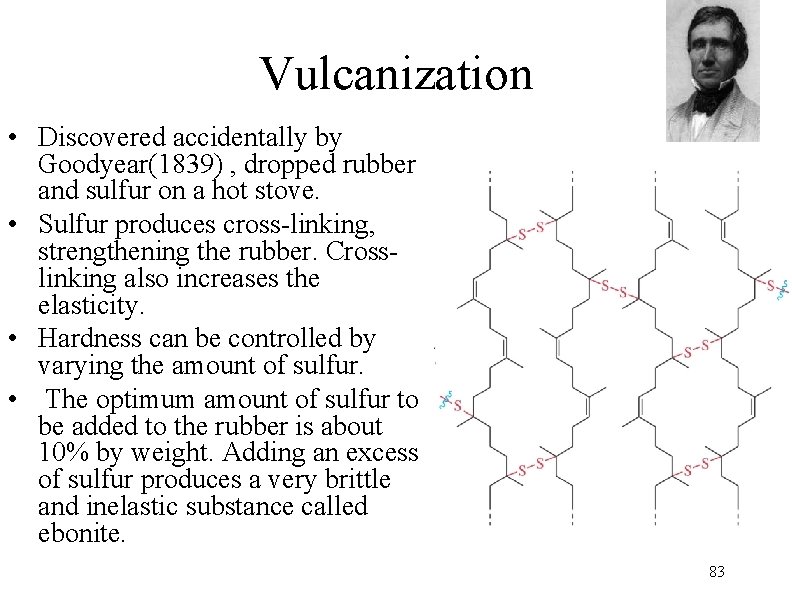
Natural Rubber • Natural rubber comes from the rubber tree (Hevea brasiliensis) and is a white, milky liquid called latex. Most rubber comes from Malaysia and other nations in East Asia. • Latex can also be seen as the white fluid in dandelion stalks. The latex from the tree is actually a suspension of rubber particles in water. Rubber is a polymer of isoprene. Natural rubber is relatively reactive, and is especially vulnerable to oxidation. • Long chains can be stretched, but then return to original structure. • Chains slide past each other and can be pulled apart easily. • Structure is cis-1, 4 -polyisoprene. 82 n

Vulcanization • Discovered accidentally by Goodyear(1839) , dropped rubber and sulfur on a hot stove. • Sulfur produces cross-linking, strengthening the rubber. Crosslinking also increases the elasticity. • Hardness can be controlled by varying the amount of sulfur. • The optimum amount of sulfur to be added to the rubber is about 10% by weight. Adding an excess of sulfur produces a very brittle and inelastic substance called ebonite. 83

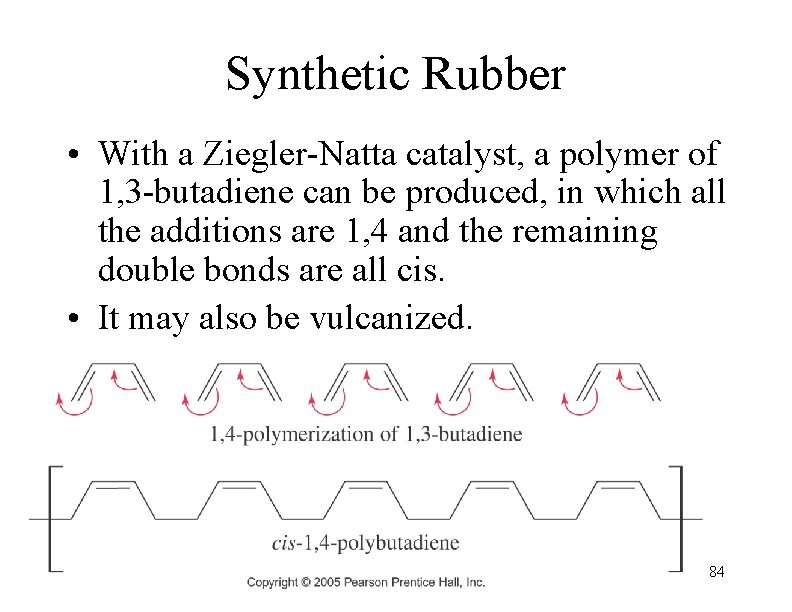
Synthetic Rubber • With a Ziegler-Natta catalyst, a polymer of 1, 3 -butadiene can be produced, in which all the additions are 1, 4 and the remaining double bonds are all cis. • It may also be vulcanized. 84

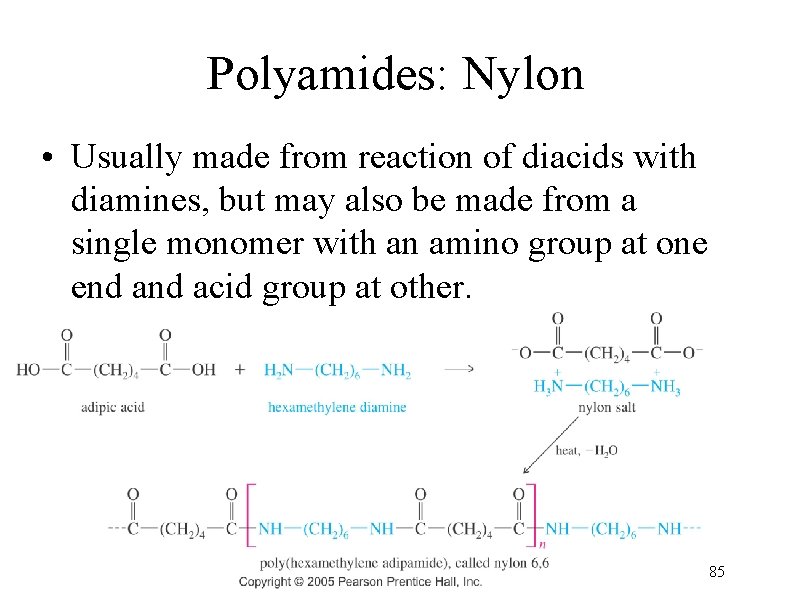
Polyamides: Nylon • Usually made from reaction of diacids with diamines, but may also be made from a single monomer with an amino group at one end acid group at other. 85

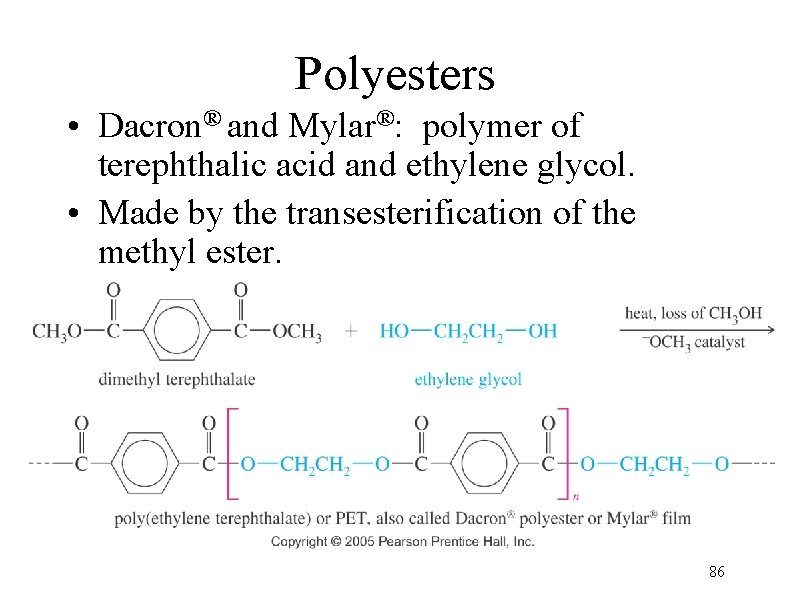
Polyesters • Dacron® and Mylar®: polymer of terephthalic acid and ethylene glycol. • Made by the transesterification of the methyl ester. 86

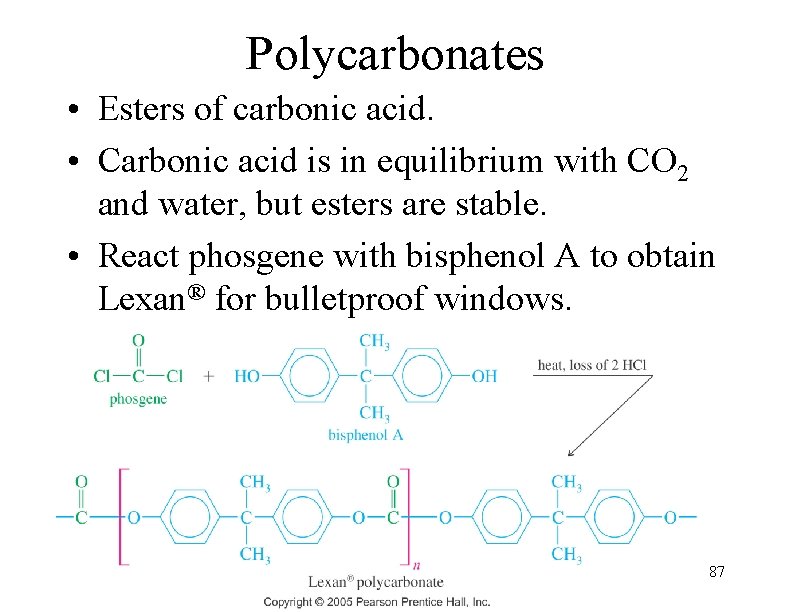
Polycarbonates • Esters of carbonic acid. • Carbonic acid is in equilibrium with CO 2 and water, but esters are stable. • React phosgene with bisphenol A to obtain Lexan® for bulletproof windows. 87

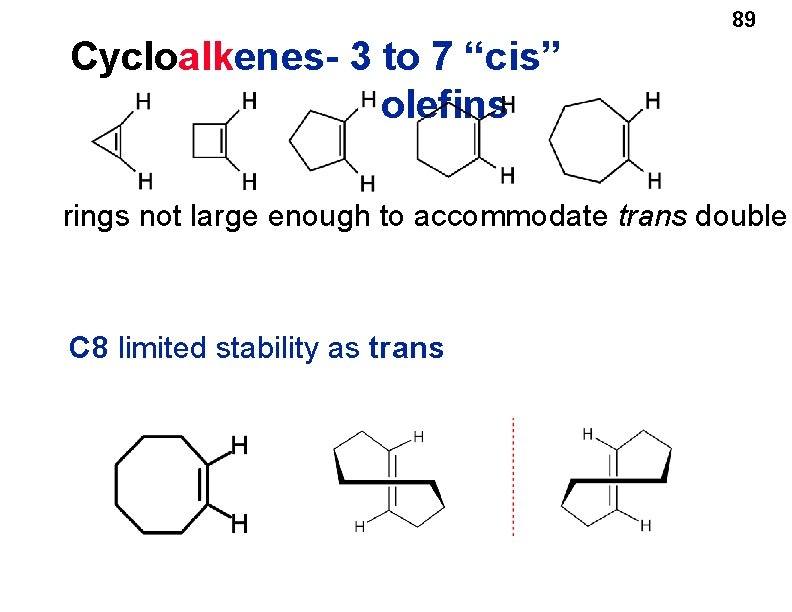
Cycloalkenes • Cyclopropene and cyclobutene have angle strain. • Larger cycloalkenes, such as cyclopentene and cyclohexene, can incorporate a double bond into the ring with little or no angle strain. 88

89 Cycloalkenes- 3 to 7 “cis” olefins rings not large enough to accommodate trans double C 8 limited stability as trans

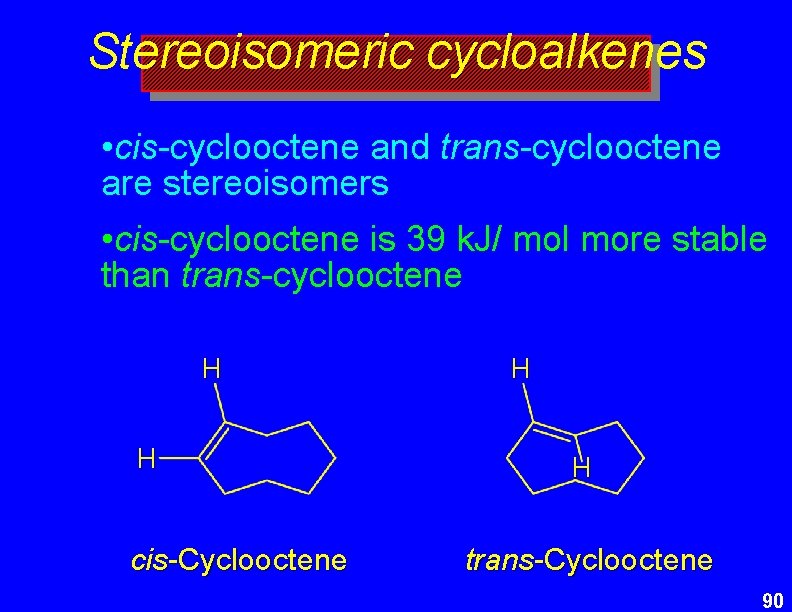
Stereoisomeric cycloalkenes • cis-cyclooctene and trans-cyclooctene are stereoisomers • cis-cyclooctene is 39 k. J/ mol more stable than trans-cyclooctene H H cis-Cyclooctene H H trans-Cyclooctene 90

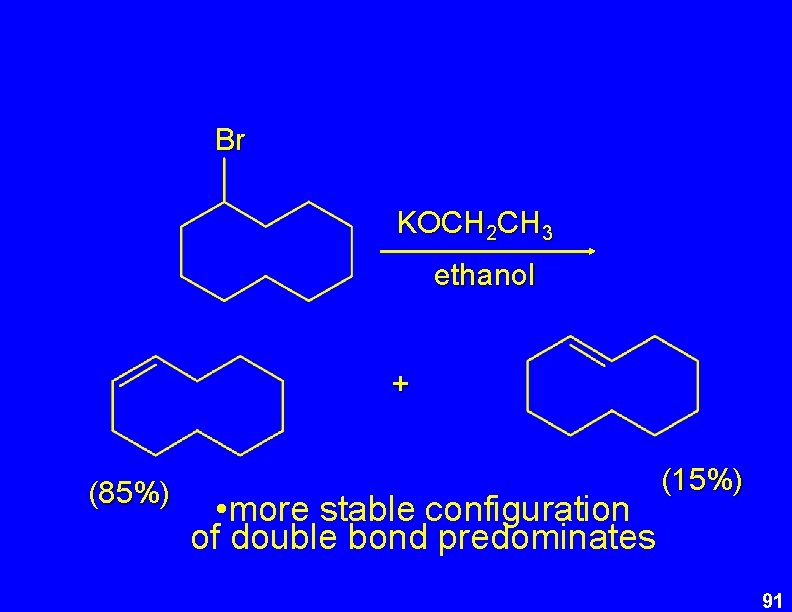
Br KOCH 2 CH 3 ethanol + (85%) • more stable configuration of double bond predominates (15%) 91

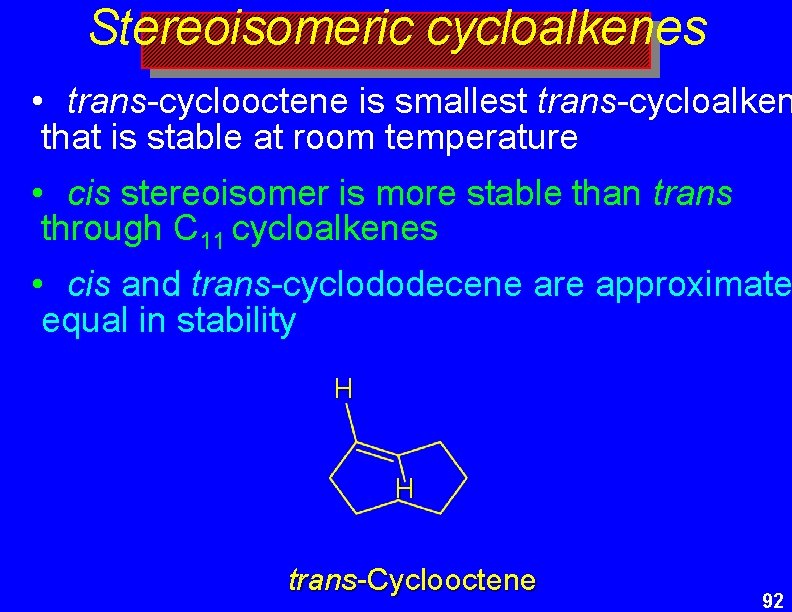
Stereoisomeric cycloalkenes • trans-cyclooctene is smallest trans-cycloalken that is stable at room temperature • cis stereoisomer is more stable than trans through C 11 cycloalkenes • cis and trans-cyclododecene are approximate equal in stability H H trans-Cyclooctene 92

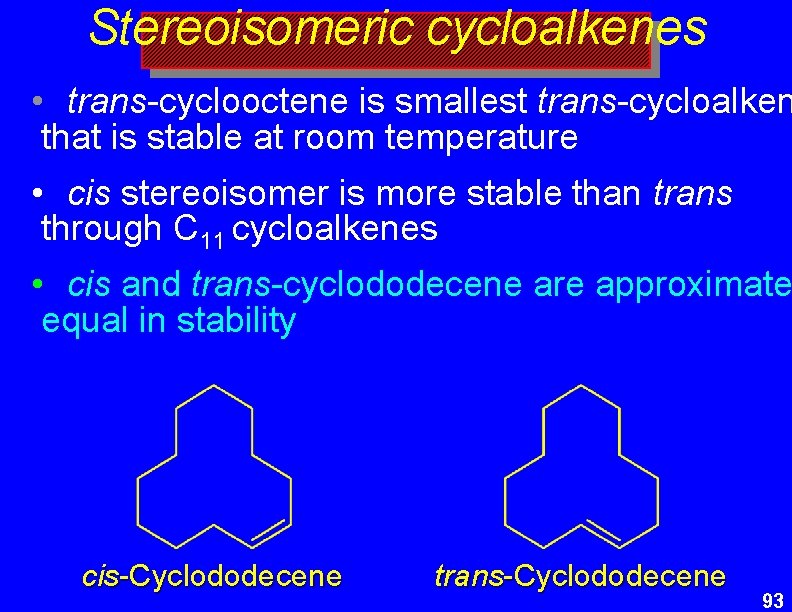
Stereoisomeric cycloalkenes • trans-cyclooctene is smallest trans-cycloalken that is stable at room temperature • cis stereoisomer is more stable than trans through C 11 cycloalkenes • cis and trans-cyclododecene are approximate equal in stability cis-Cyclododecene trans-Cyclododecene 93
 Inorganic vs organic chemistry
Inorganic vs organic chemistry Ib organic chemistry
Ib organic chemistry Nit calicut chemistry
Nit calicut chemistry Hono organic chemistry
Hono organic chemistry Ee organic chemistry
Ee organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Which allotrope of carbon feels greasy and crumbles easily?
Which allotrope of carbon feels greasy and crumbles easily? Kiliani fischer synthesis
Kiliani fischer synthesis Organic chemistry
Organic chemistry Mindup mind map
Mindup mind map Organic chemistry
Organic chemistry Analytical chemistry chapters
Analytical chemistry chapters Ag schnelltest beilstein
Ag schnelltest beilstein What is a branched hydrocarbon
What is a branched hydrocarbon How to calculate percent yield
How to calculate percent yield Is ch4o organic or inorganic
Is ch4o organic or inorganic Wiley
Wiley Organic chemistry
Organic chemistry Is alkane an organic compound
Is alkane an organic compound Conjugation organic chemistry
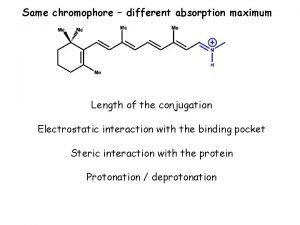
Conjugation organic chemistry Organic chemistry myanmar
Organic chemistry myanmar Ester organic chemistry
Ester organic chemistry Grade 10 organic chemistry
Grade 10 organic chemistry Resonance in benzyl carbocation
Resonance in benzyl carbocation Functional group
Functional group Chemistry ethics case studies
Chemistry ethics case studies Where is lysine found
Where is lysine found A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Vicinal dihalide
Vicinal dihalide Ario organic chemistry
Ario organic chemistry Organic chemistry reaction pathways
Organic chemistry reaction pathways Halohydrin formation
Halohydrin formation Pent hex hept oct
Pent hex hept oct Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Organic chemistry stuart warren
Organic chemistry stuart warren Brooklyn college organic chemistry
Brooklyn college organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Polarimetry organic chemistry
Polarimetry organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Organic chemistry nova
Organic chemistry nova What is the leveling effect organic chemistry
What is the leveling effect organic chemistry Organic chemistry vocabulary
Organic chemistry vocabulary David klein
David klein Organic chemistry
Organic chemistry Alkene formula
Alkene formula Organic chemistry
Organic chemistry Ethene hbr
Ethene hbr Organic chemistry
Organic chemistry Cyclo organic chemistry
Cyclo organic chemistry What functional group is ch3
What functional group is ch3 Organic chemistry
Organic chemistry Organic chemistry william h brown
Organic chemistry william h brown Organic chemistry class 11 notes
Organic chemistry class 11 notes Rancidity meaning
Rancidity meaning Organic chemistry topic 11
Organic chemistry topic 11 Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Alkene alcohol naming
Alkene alcohol naming All structural isomers of hexane
All structural isomers of hexane Ir spectroscopy
Ir spectroscopy How is cracking done
How is cracking done Father of organic chemistry
Father of organic chemistry Organic chemistry lab report example
Organic chemistry lab report example Octane lewis structure
Octane lewis structure Organic chemistry third edition david klein
Organic chemistry third edition david klein Organic chemistry second edition david klein
Organic chemistry second edition david klein Conjugation organic chemistry
Conjugation organic chemistry Iupac suffix table
Iupac suffix table Carboxylic acid h3o+ reaction
Carboxylic acid h3o+ reaction Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Rearranged most stable carbocation is
Rearranged most stable carbocation is Alkyl group example
Alkyl group example Mass spec of chlorine
Mass spec of chlorine Organic chemistry
Organic chemistry Organic chemistry cheat sheet
Organic chemistry cheat sheet Organic vs inorganic compounds
Organic vs inorganic compounds This name
This name Meth eth prop but table
Meth eth prop but table Organic synthesis via enolates
Organic synthesis via enolates Organic chemistry
Organic chemistry Ario organic chemistry
Ario organic chemistry Organic chemistry
Organic chemistry David klein organic chemistry
David klein organic chemistry What is organic chemistry like
What is organic chemistry like The art of writing reasonable organic reaction mechanisms
The art of writing reasonable organic reaction mechanisms Eth meth prop but pent
Eth meth prop but pent Canola oil
Canola oil Alkane organic chemistry
Alkane organic chemistry Oxidation of carbohydrates
Oxidation of carbohydrates Iupac
Iupac Organic chemistry
Organic chemistry Organic chemistry chapter 9
Organic chemistry chapter 9 Organic chemistry
Organic chemistry Extraction of caffeine from vivarin tablets lab report
Extraction of caffeine from vivarin tablets lab report