Organic Chemistry Sh Javanshir Faculty of Chemistry Iran








- Slides: 41

Organic Chemistry Sh. Javanshir Faculty of Chemistry Iran University of Science & Technology 1

Chapter 4. Alkenes: Structure and Reactivity Based on Mc. Murry’s Organic Chemistry, 6 th edition 2

Alkene - Hydrocarbon With Carbon-Carbon Double Bond n n Includes many naturally occurring materials n Flavors, fragrances, vitamins Important industrial products n These are feedstocks for industrial processes 3

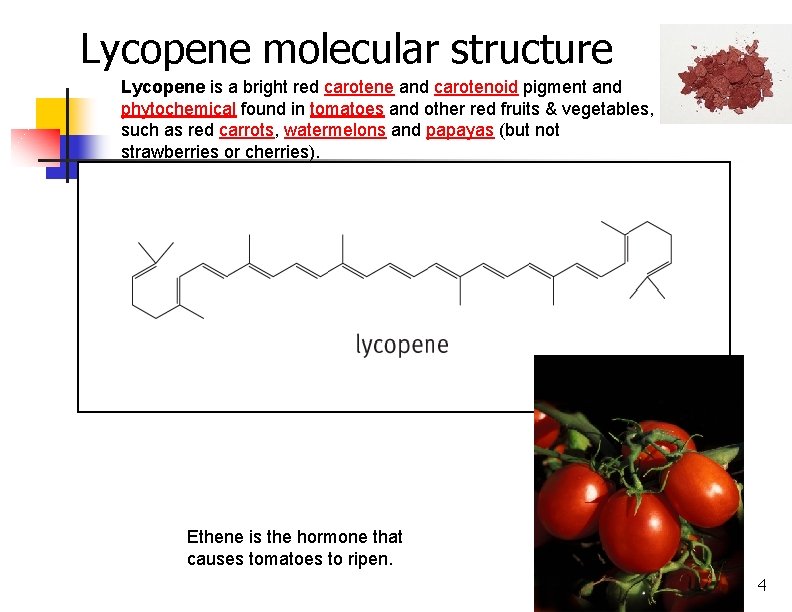
Lycopene molecular structure Lycopene is a bright red carotene and carotenoid pigment and phytochemical found in tomatoes and other red fruits & vegetables, such as red carrots, watermelons and papayas (but not strawberries or cherries). Ethene is the hormone that causes tomatoes to ripen. 4

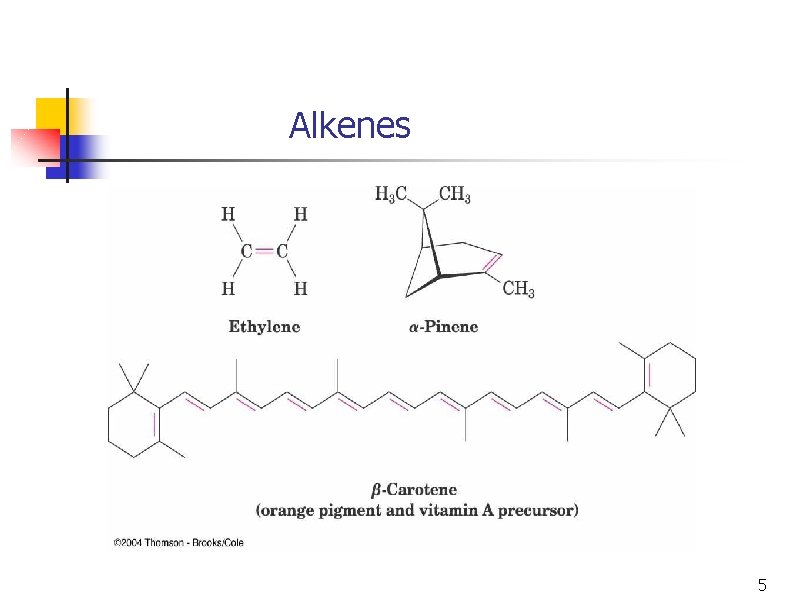
Alkenes 5

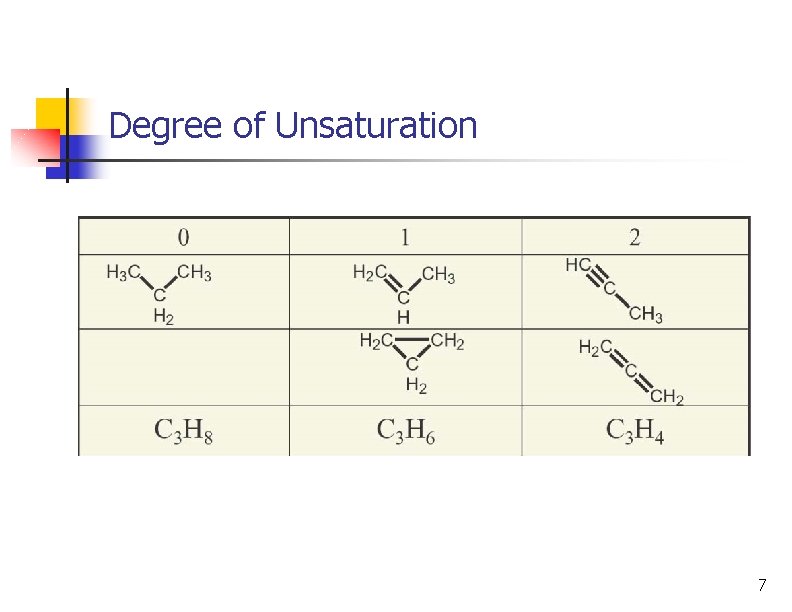
n Relates molecular formula to possible structures Degree of Unsaturation n Degree of unsaturation: number of multiple bonds or rings Formula for saturated a acyclic compound is Cn. H 2 n+2 Each ring or multiple bond replaces 2 H's 6

Degree of Unsaturation 7

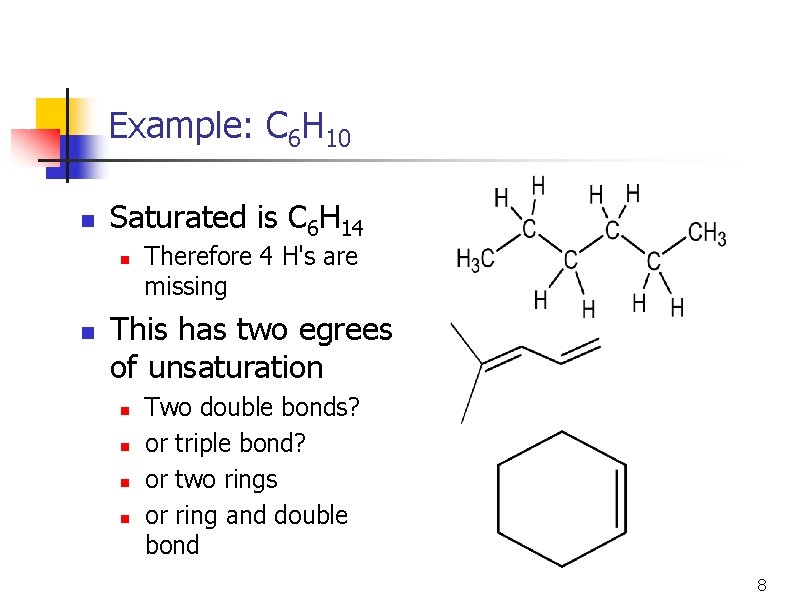
Example: C 6 H 10 n Saturated is C 6 H 14 n n Therefore 4 H's are missing This has two egrees of unsaturation n n Two double bonds? or triple bond? or two rings or ring and double bond 8

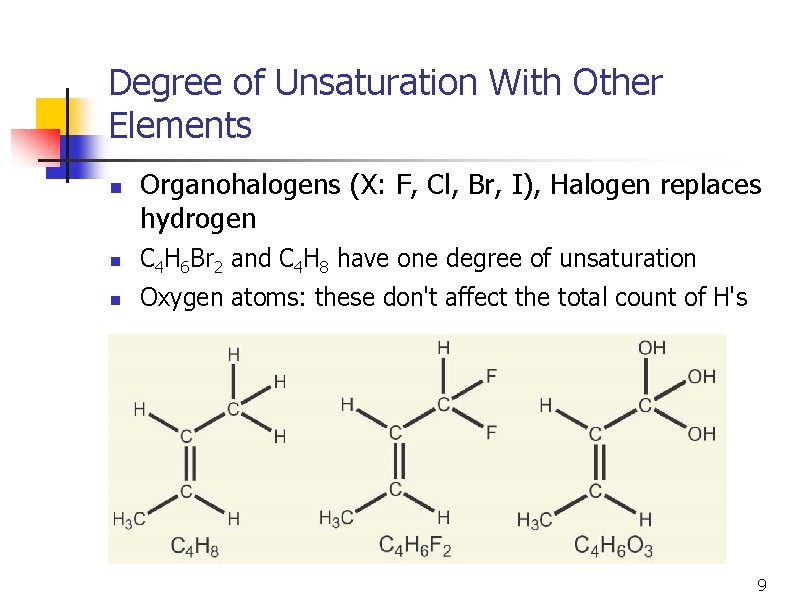
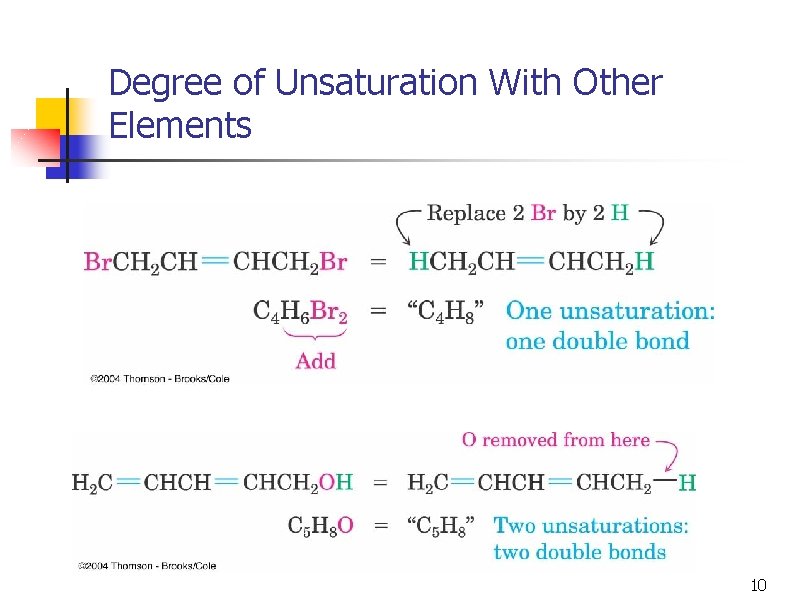
Degree of Unsaturation With Other Elements n Organohalogens (X: F, Cl, Br, I), Halogen replaces hydrogen n C 4 H 6 Br 2 and C 4 H 8 have one degree of unsaturation n Oxygen atoms: these don't affect the total count of H's 9

Degree of Unsaturation With Other Elements 10

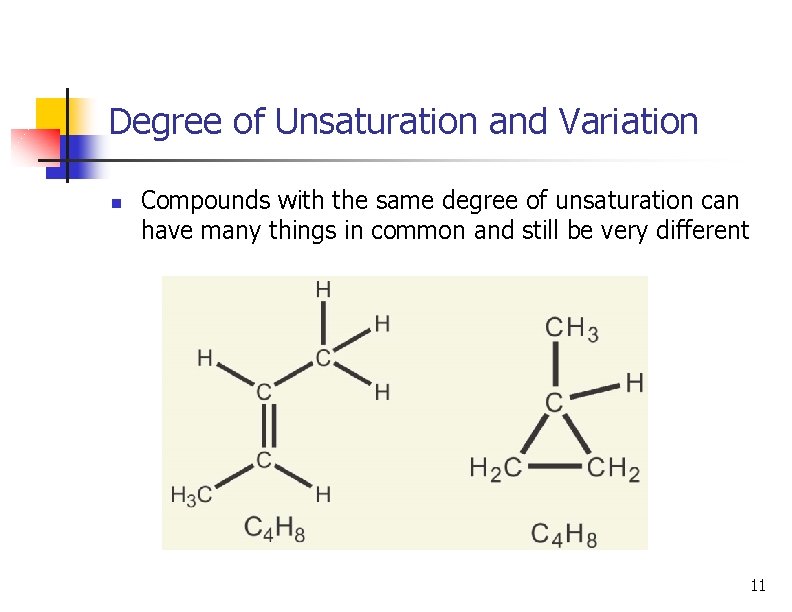
Degree of Unsaturation and Variation n Compounds with the same degree of unsaturation can have many things in common and still be very different 11

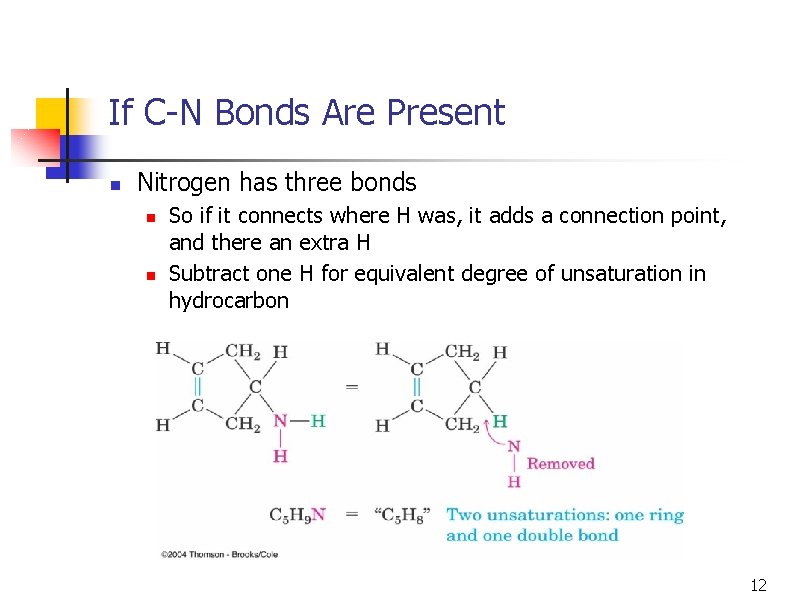
If C-N Bonds Are Present n Nitrogen has three bonds n n So if it connects where H was, it adds a connection point, and there an extra H Subtract one H for equivalent degree of unsaturation in hydrocarbon 12

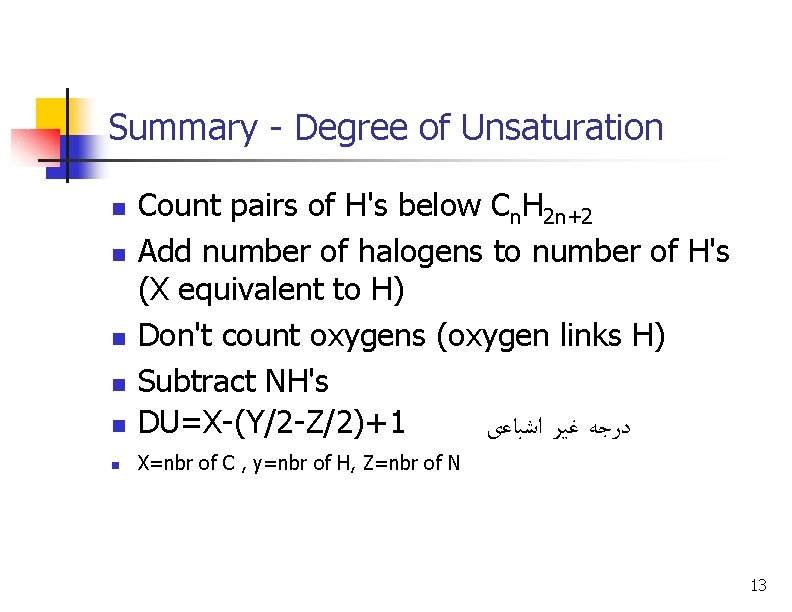
Summary - Degree of Unsaturation n Count pairs of H's below Cn. H 2 n+2 Add number of halogens to number of H's (X equivalent to H) Don't count oxygens (oxygen links H) Subtract NH's DU=X-(Y/2 -Z/2)+1 ﺩﺭﺟﻪ ﻏﻴﺮ ﺍﺷﺒﺎﻋی n X=nbr of C , y=nbr of H, Z=nbr of N n n 13

Naming of Alkenes n n n Find longest continuous carbon chain for root Number carbons in chain so that double bond carbons have lowest possible numbers Rings have “cyclo” prefix 14

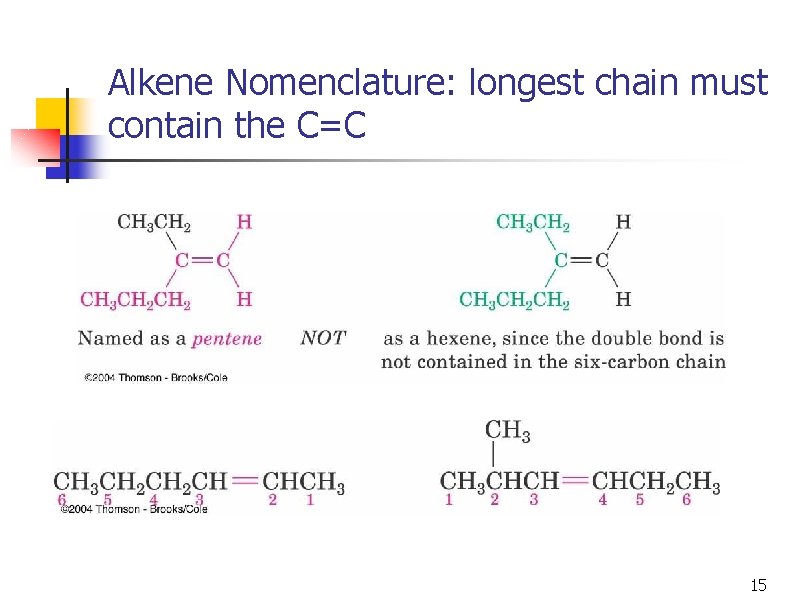
Alkene Nomenclature: longest chain must contain the C=C 15

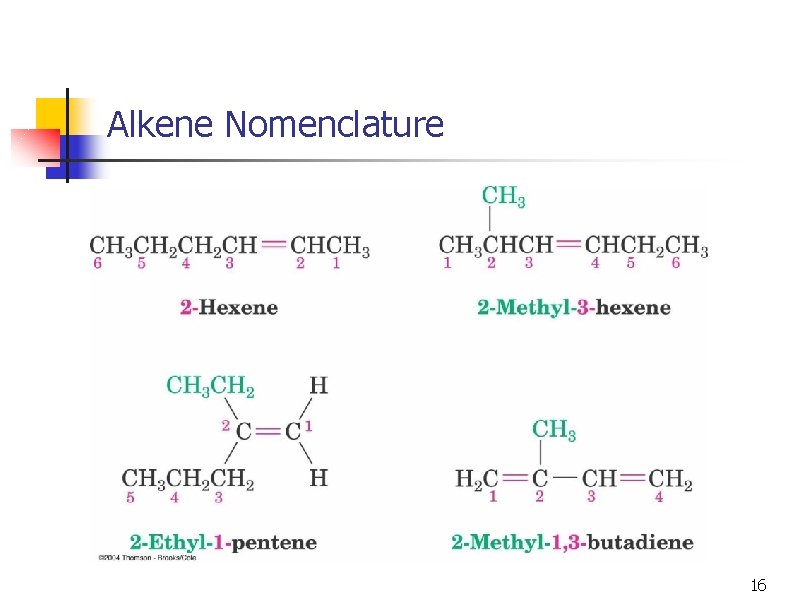
Alkene Nomenclature 16

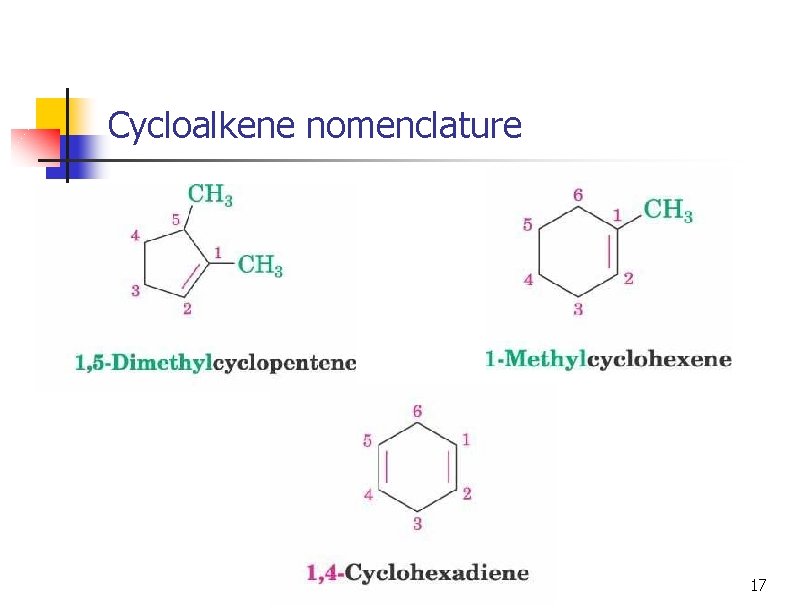
Cycloalkene nomenclature 17

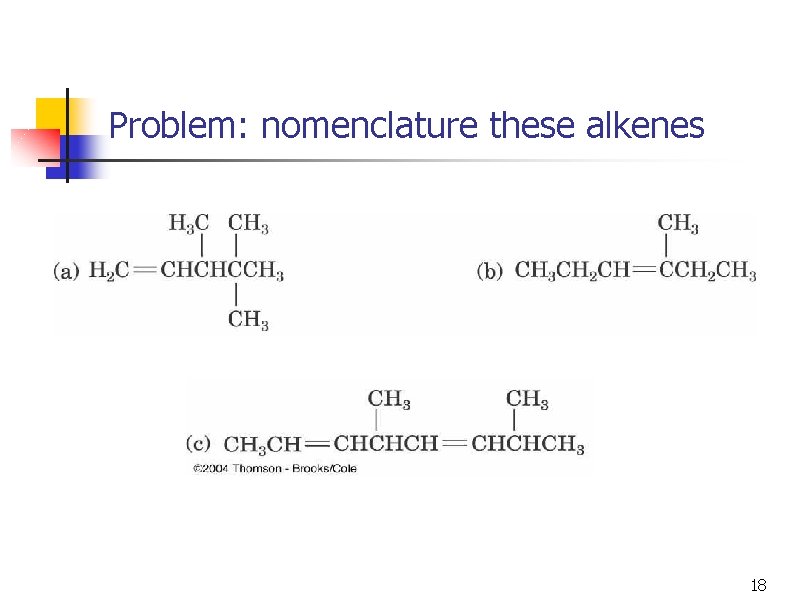
Problem: nomenclature these alkenes 18

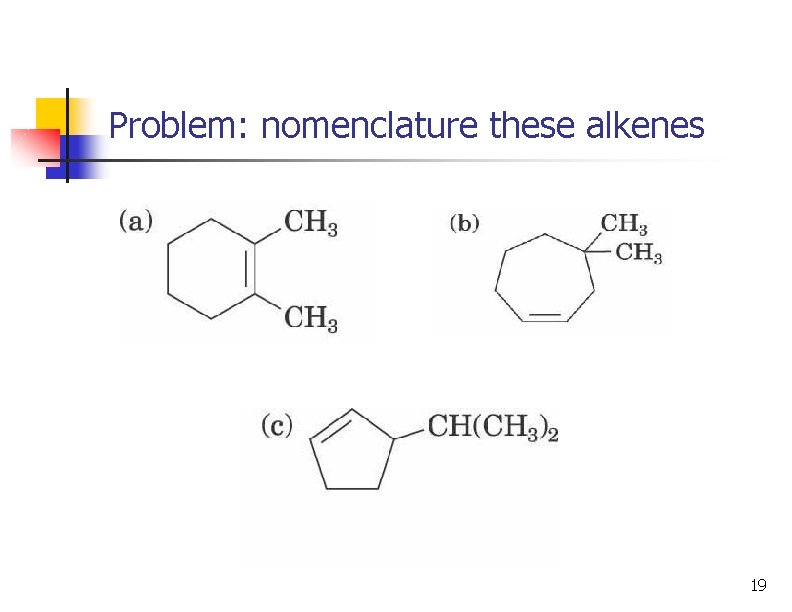
Problem: nomenclature these alkenes 19

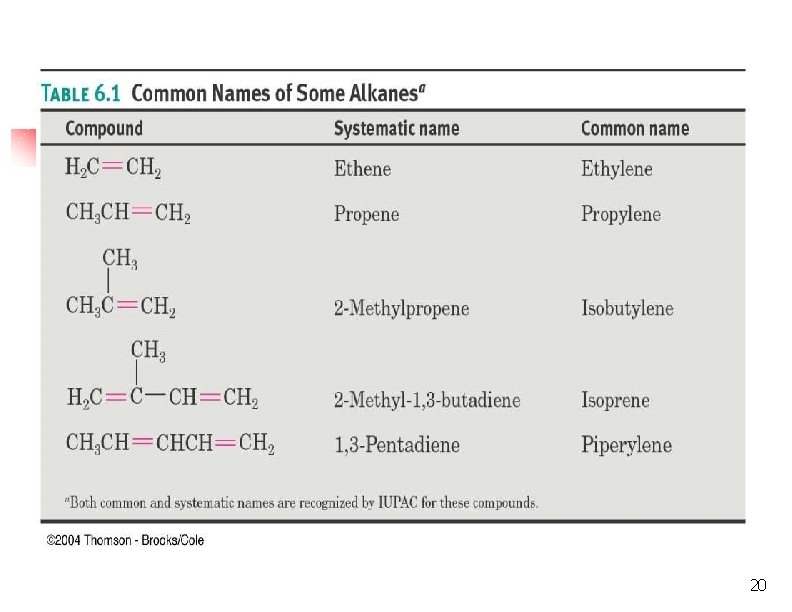
20

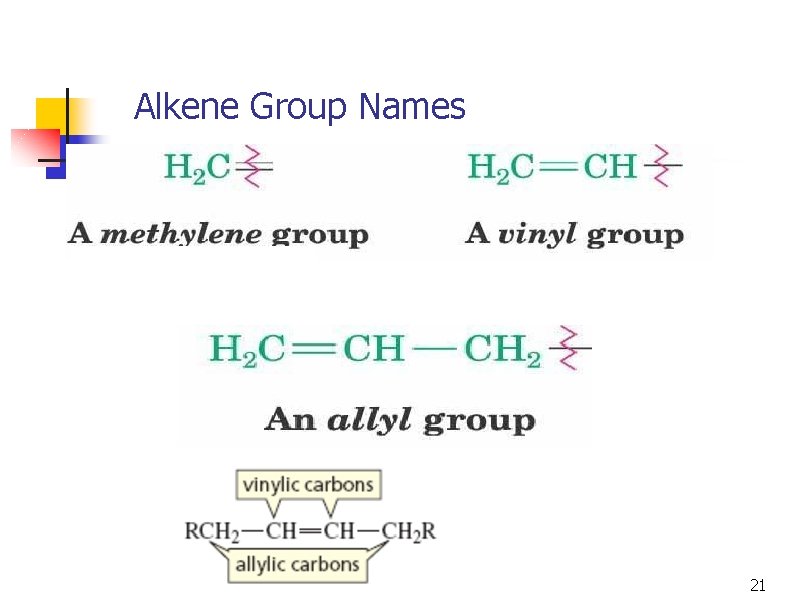
Alkene Group Names 21

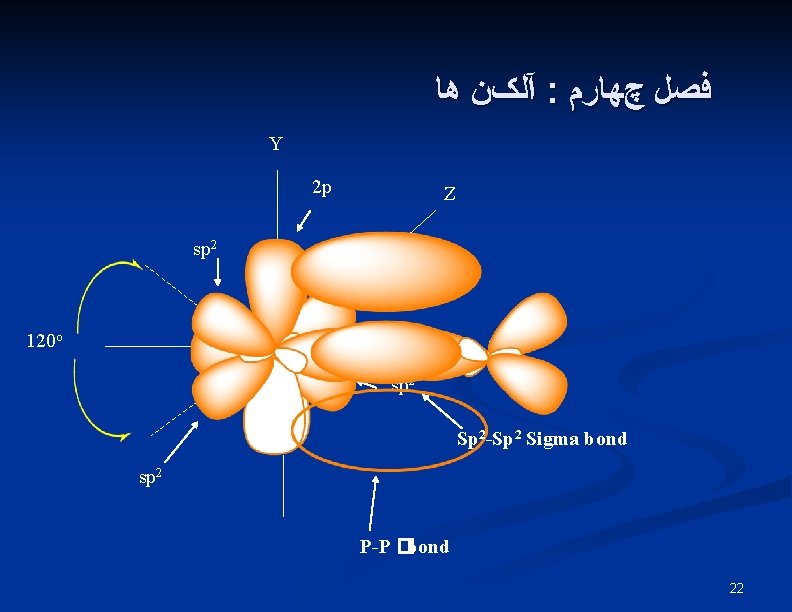
آﻠکﻦ ﻫﺎ : ﻓﺼﻞ چﻬﺎﺭﻡ Y 2 p Z sp 2 120 o X sp 2 Sp 2 -Sp 2 Sigma bond sp 2 P-P �bond 22



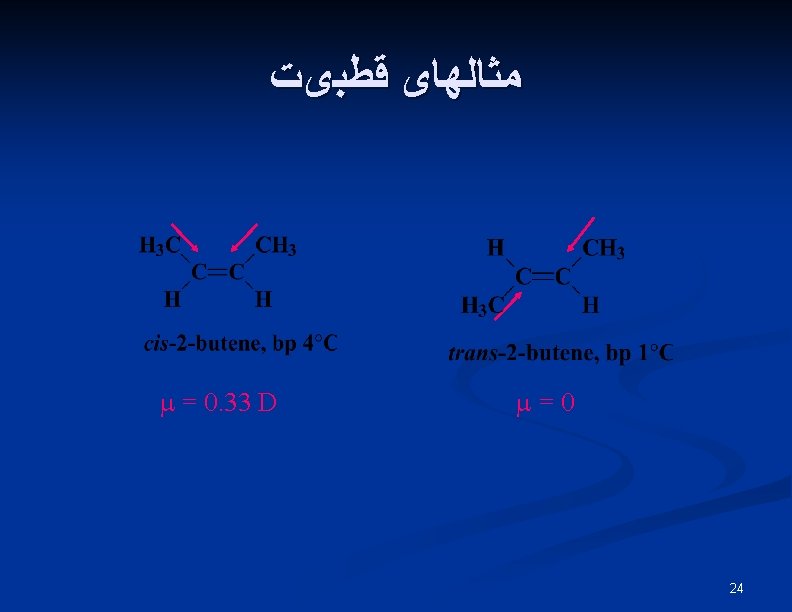
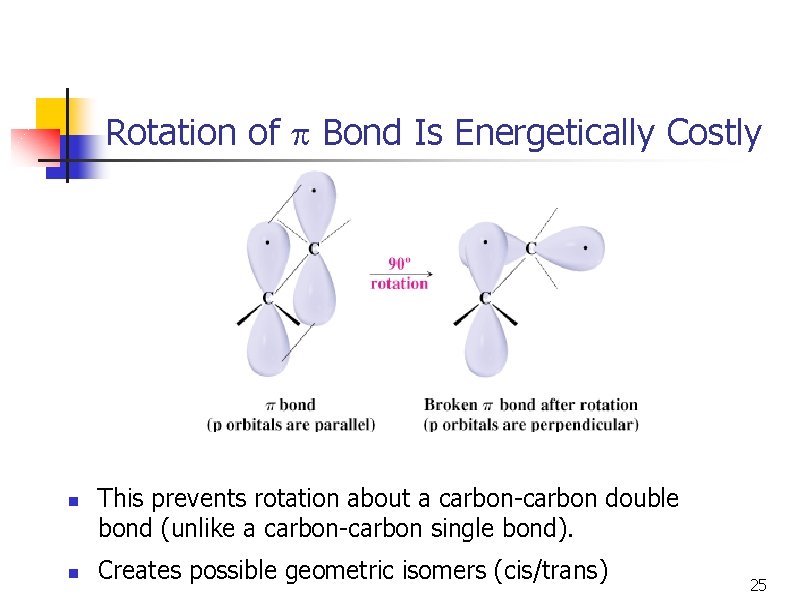
Rotation of Bond Is Energetically Costly n n This prevents rotation about a carbon-carbon double bond (unlike a carbon-carbon single bond). Creates possible geometric isomers (cis/trans) 25

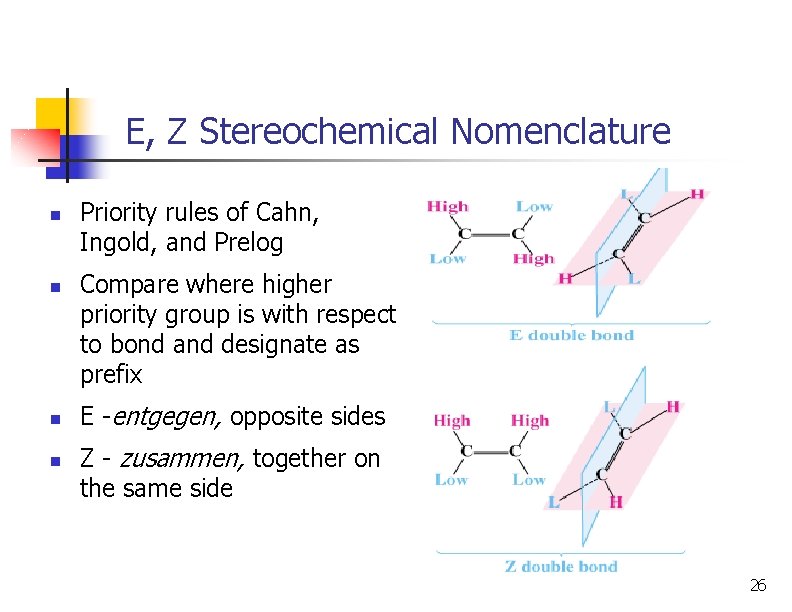
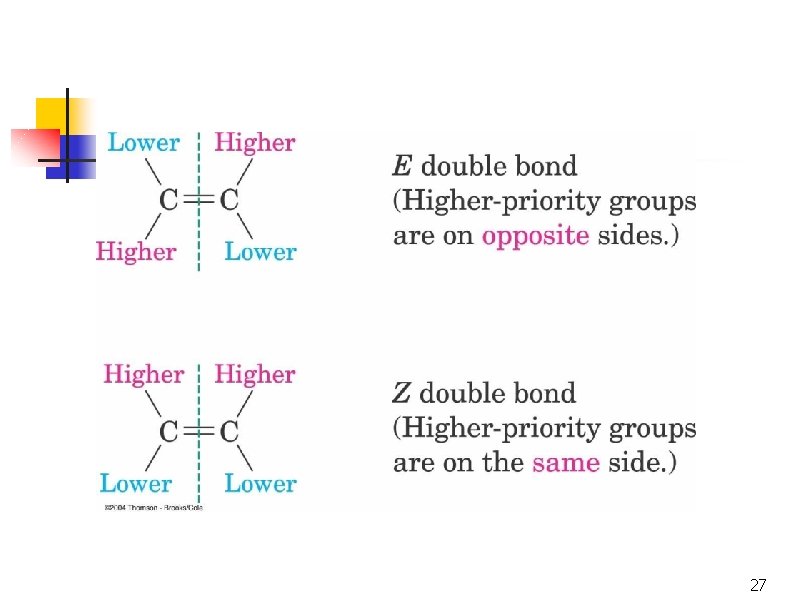
E, Z Stereochemical Nomenclature n n Priority rules of Cahn, Ingold, and Prelog Compare where higher priority group is with respect to bond and designate as prefix E -entgegen, opposite sides Z - zusammen, together on the same side 26

27

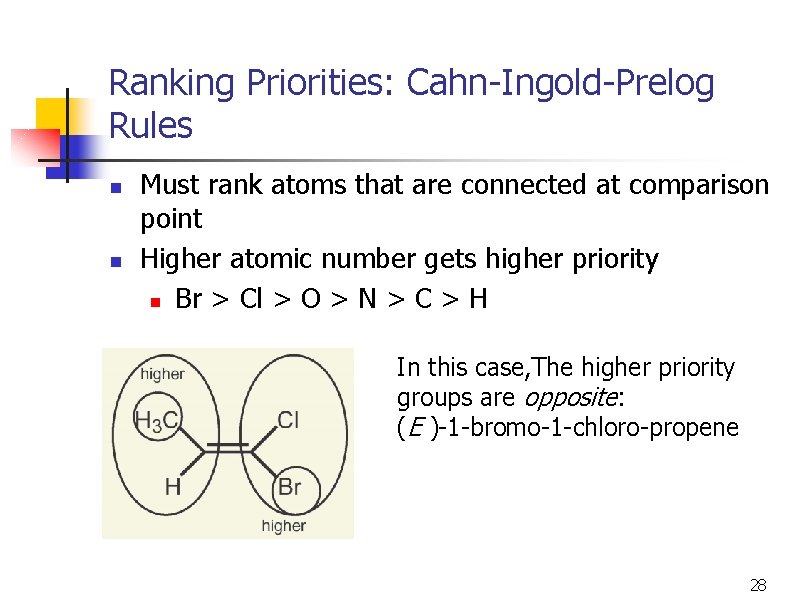
Ranking Priorities: Cahn-Ingold-Prelog Rules n n Must rank atoms that are connected at comparison point Higher atomic number gets higher priority n Br > Cl > O > N > C > H In this case, The higher priority groups are opposite: (E )-1 -bromo-1 -chloro-propene 28

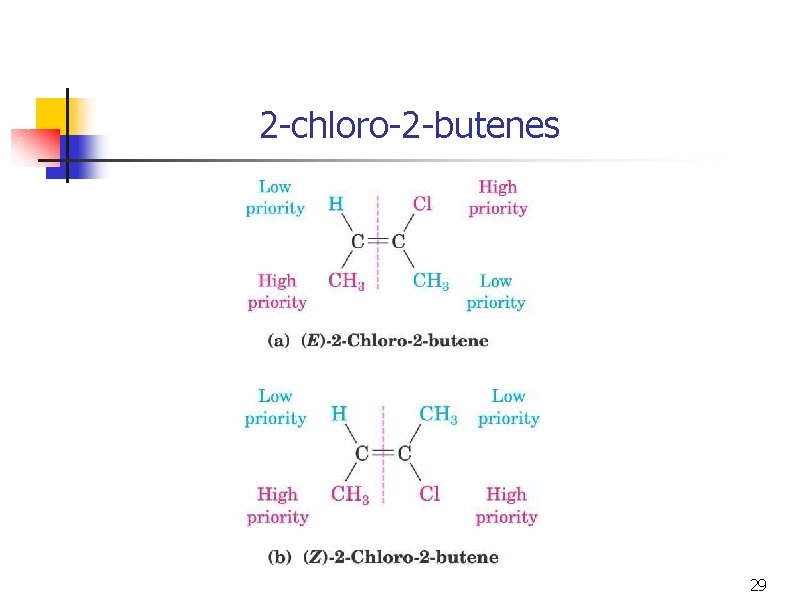
2 -chloro-2 -butenes 29

Extended Comparison n If atomic numbers are the same, compare at next connection point at same distance Compare until something has higher atomic number Do not combine – always compare 30

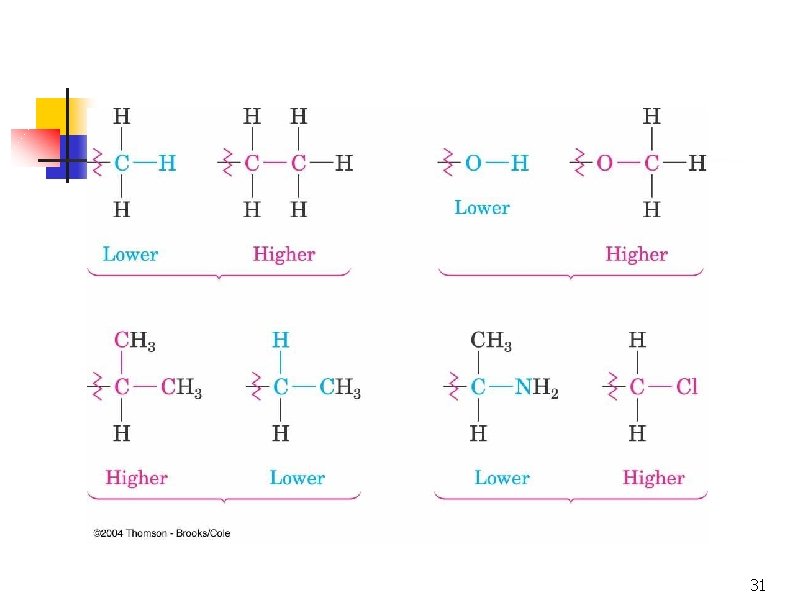
31

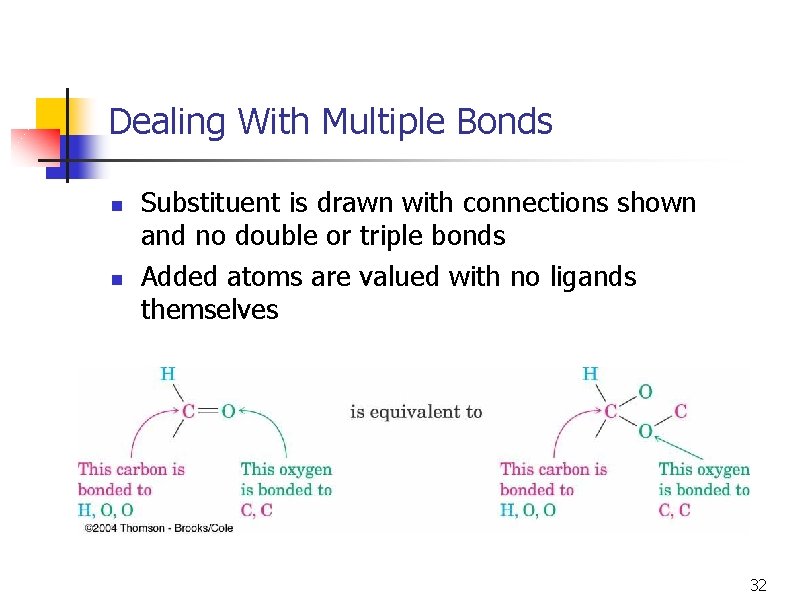
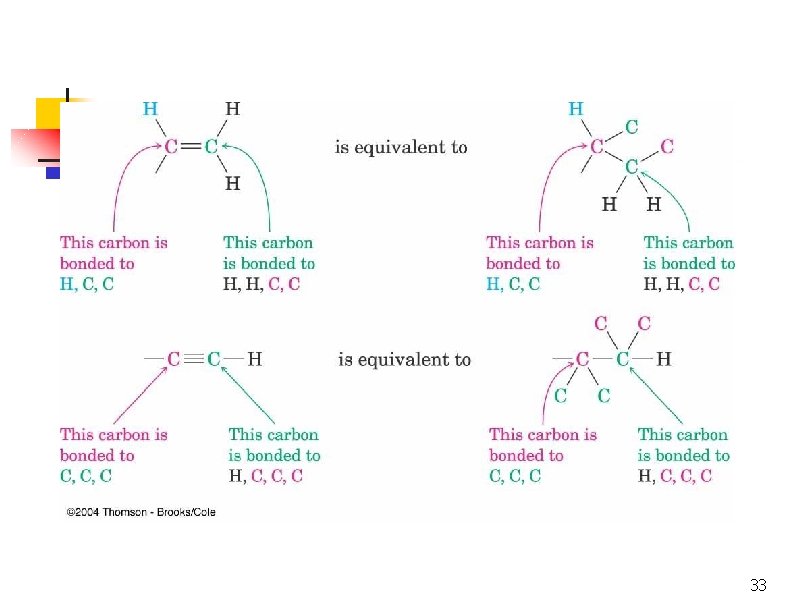
Dealing With Multiple Bonds n n Substituent is drawn with connections shown and no double or triple bonds Added atoms are valued with no ligands themselves 32

33

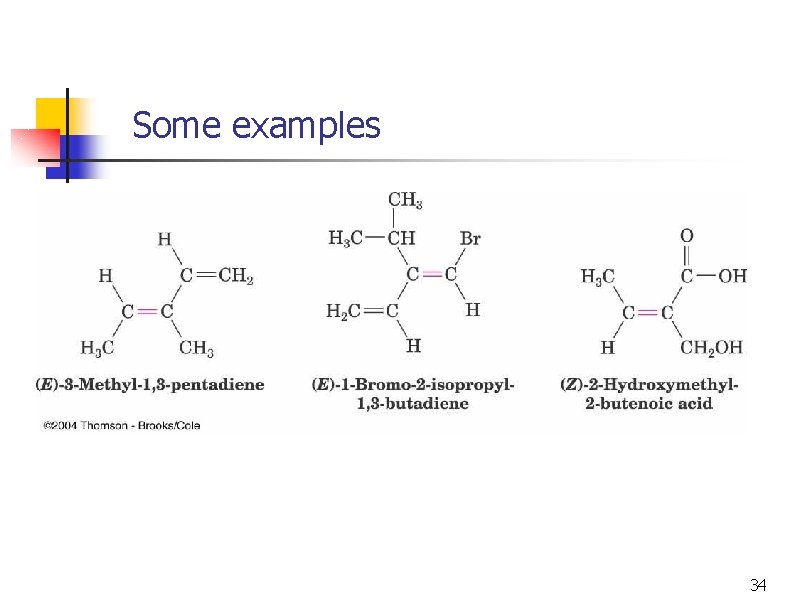
Some examples 34

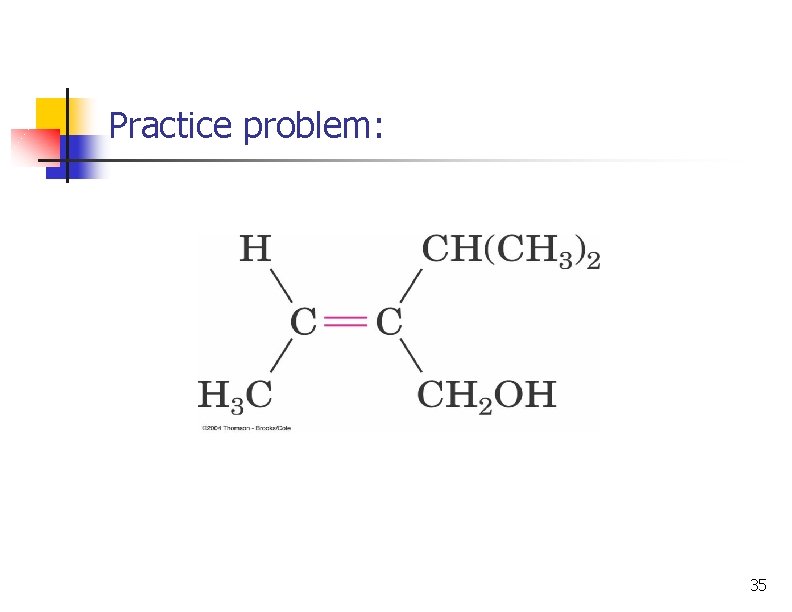
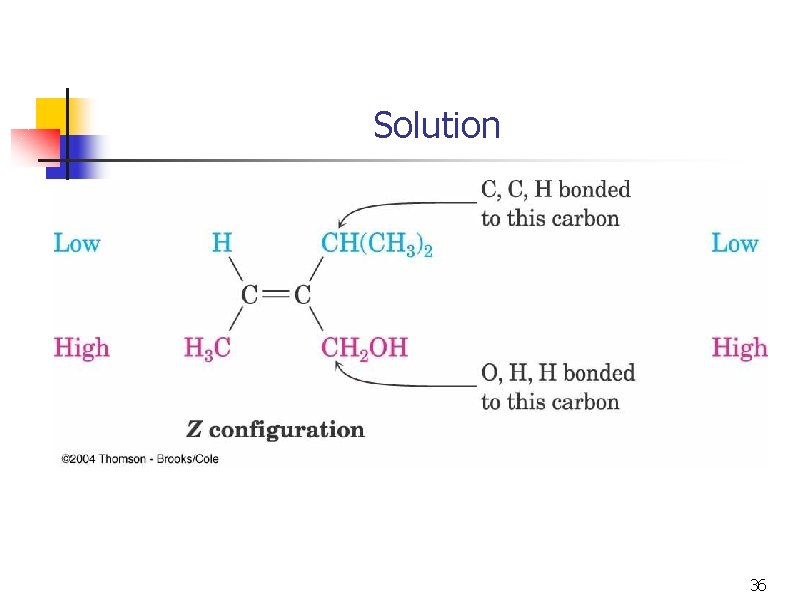
Practice problem: 35

Solution 36

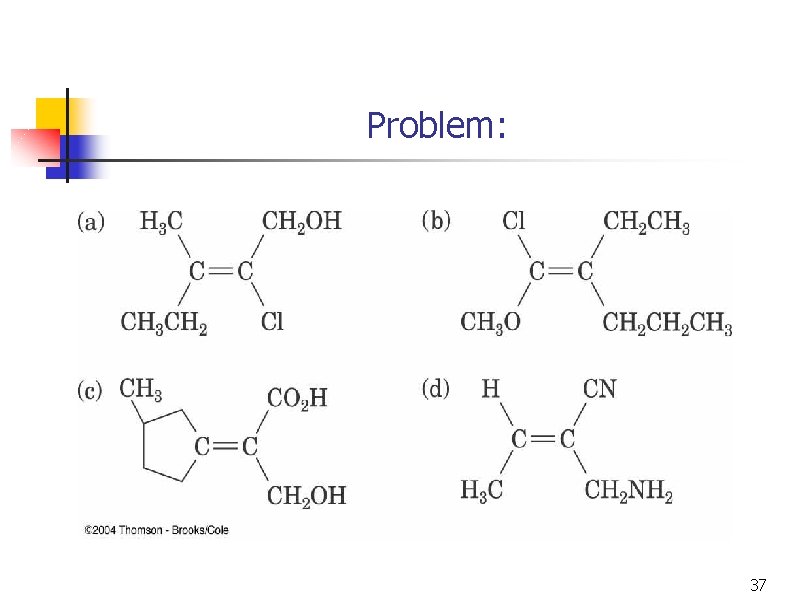
Problem: 37

38

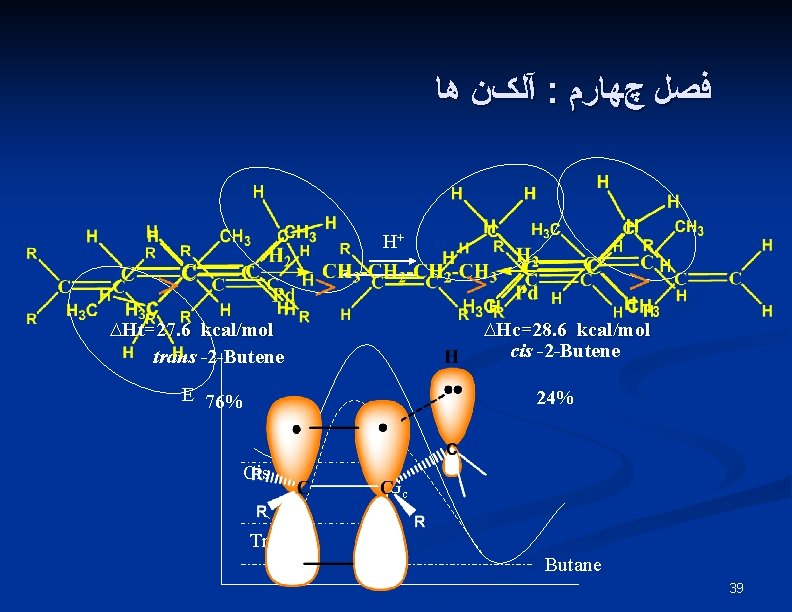
آﻠکﻦ ﻫﺎ : ﻓﺼﻞ چﻬﺎﺭﻡ H+ ∆Ht=27. 6 kcal/mol trans -2 -Butene ∆Hc=28. 6 kcal/mol cis -2 -Butene E 76% 24% Cis Trans ∆Gc ∆Gt Butane 39

Reaction of alkenes: Electrophilic Addition of HX to Alkenes n n n General reaction mechanism: electrophilic addition Attack of electrophile (such as HBr) on bond of alkene produces carbocation and bromide ion Carbocation is itself an electrophile, reacting with nucleophilic bromide ion 40

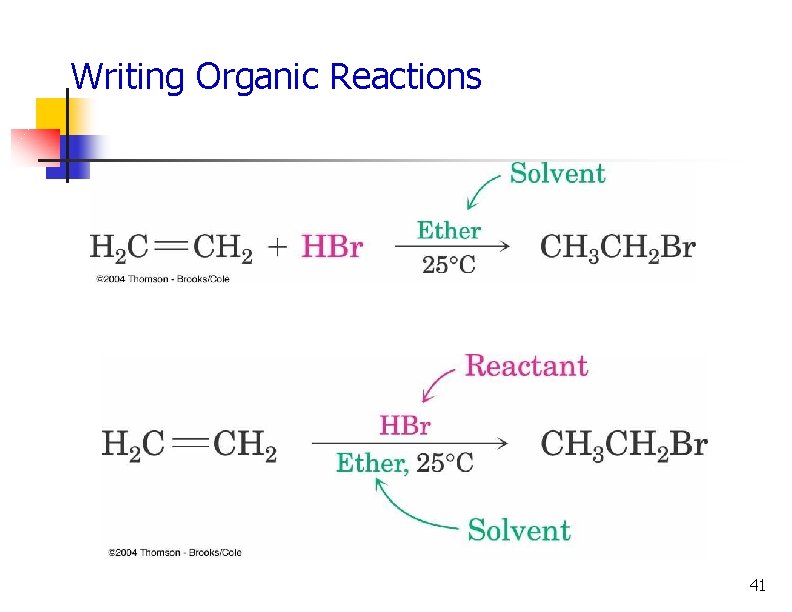
Writing Organic Reactions 41
 Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Ib organic chemistry
Ib organic chemistry Nit calicut chemistry department faculty
Nit calicut chemistry department faculty Organic chemistry nomenclature
Organic chemistry nomenclature Organic chemistry
Organic chemistry Organic chemistry chapter 9
Organic chemistry chapter 9 Butan 2 on
Butan 2 on Organic chemistry laboratory ch 2540 manual
Organic chemistry laboratory ch 2540 manual Hono organic chemistry
Hono organic chemistry Ee organic chemistry
Ee organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Which allotrope of carbon feels greasy and crumbles easily?
Which allotrope of carbon feels greasy and crumbles easily? Organic chemistry wade
Organic chemistry wade Organic chemistry
Organic chemistry Mind map organic chemistry
Mind map organic chemistry Organic chemistry
Organic chemistry Analytical chemistry chapter 1
Analytical chemistry chapter 1 Lassaigne test
Lassaigne test Hydrocarbon prefixes
Hydrocarbon prefixes Calculating percentage yield
Calculating percentage yield Wiley
Wiley Organic chemistry
Organic chemistry Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Organic vs inorganic chemistry
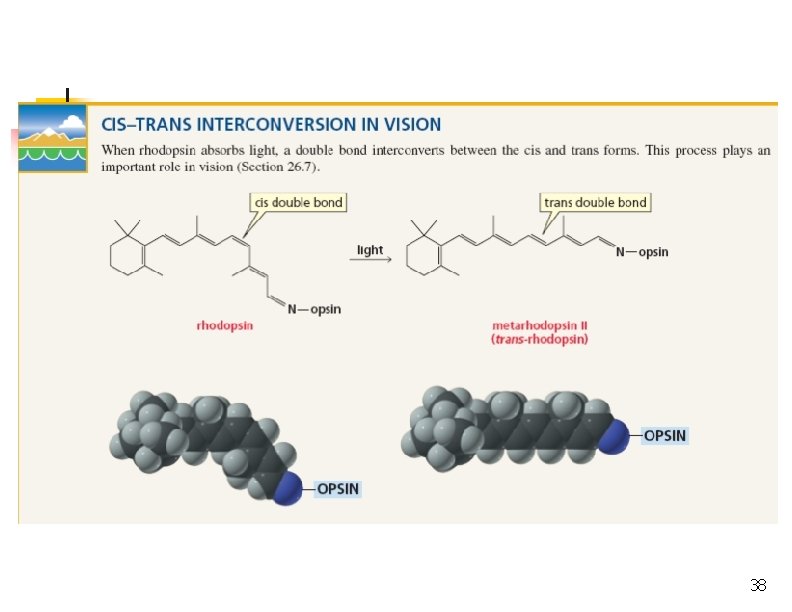
Organic vs inorganic chemistry Rhodopsin cgmp
Rhodopsin cgmp Organic chemistry myanmar
Organic chemistry myanmar Ester organic chemistry
Ester organic chemistry Grade 10 organic chemistry
Grade 10 organic chemistry Resonance in benzyl carbocation
Resonance in benzyl carbocation Functional groups in organic chemistry
Functional groups in organic chemistry Chemistry-ethics case studies
Chemistry-ethics case studies Chemistry organic
Chemistry organic A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Leaving group ability
Leaving group ability Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry reaction pathways
Organic chemistry reaction pathways Halohydrin formation
Halohydrin formation Organic chem crash course
Organic chem crash course John wiley & sons, inc.
John wiley & sons, inc. Organic chemistry stuart warren
Organic chemistry stuart warren Brooklyn college organic chemistry
Brooklyn college organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry