Organic Chemistry 9 th Edition L G Wade


- Slides: 76

Organic Chemistry, 9 th Edition L. G. Wade, Jr. Chapter 22 Lecture Condensations and Alpha Substitutions of Carbonyl Compounds Chad Snyder, Ph. D Grace College © 2017 Pearson Education, Inc. © 2014 Pearson Education, Inc.

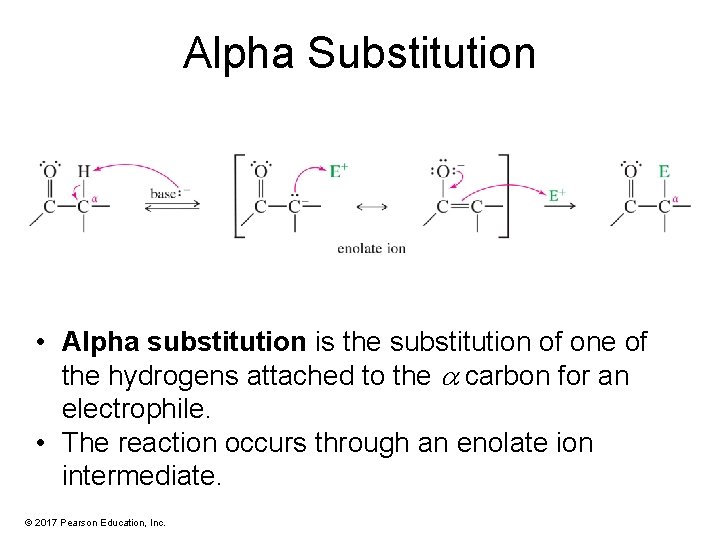
Alpha Substitution • Alpha substitution is the substitution of one of the hydrogens attached to the carbon for an electrophile. • The reaction occurs through an enolate ion intermediate. © 2017 Pearson Education, Inc.

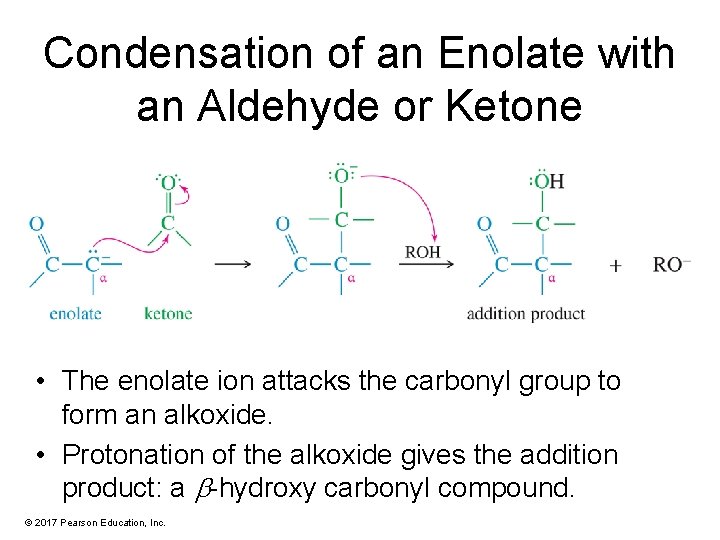
Condensation of an Enolate with an Aldehyde or Ketone • The enolate ion attacks the carbonyl group to form an alkoxide. • Protonation of the alkoxide gives the addition product: a -hydroxy carbonyl compound. © 2017 Pearson Education, Inc.

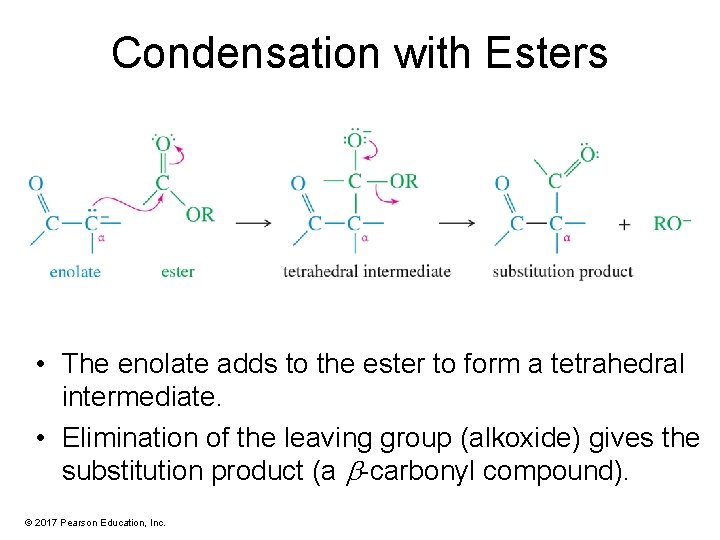
Condensation with Esters • The enolate adds to the ester to form a tetrahedral intermediate. • Elimination of the leaving group (alkoxide) gives the substitution product (a -carbonyl compound). © 2017 Pearson Education, Inc.

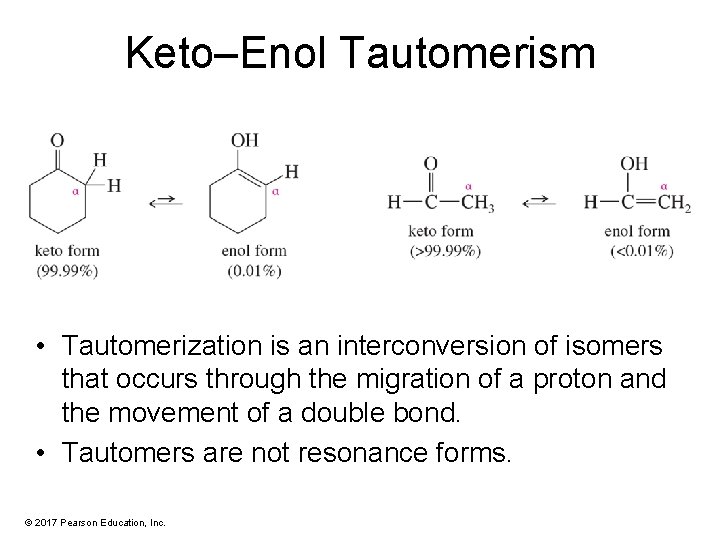
Keto–Enol Tautomerism • Tautomerization is an interconversion of isomers that occurs through the migration of a proton and the movement of a double bond. • Tautomers are not resonance forms. © 2017 Pearson Education, Inc.

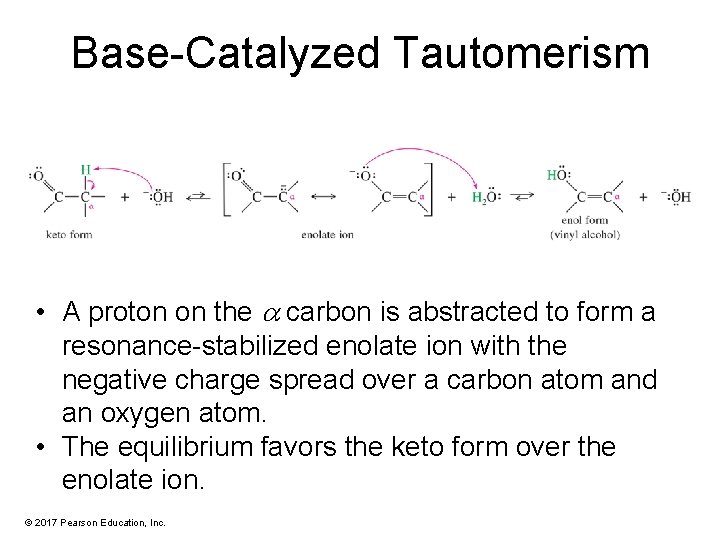
Base-Catalyzed Tautomerism • A proton on the carbon is abstracted to form a resonance-stabilized enolate ion with the negative charge spread over a carbon atom and an oxygen atom. • The equilibrium favors the keto form over the enolate ion. © 2017 Pearson Education, Inc.

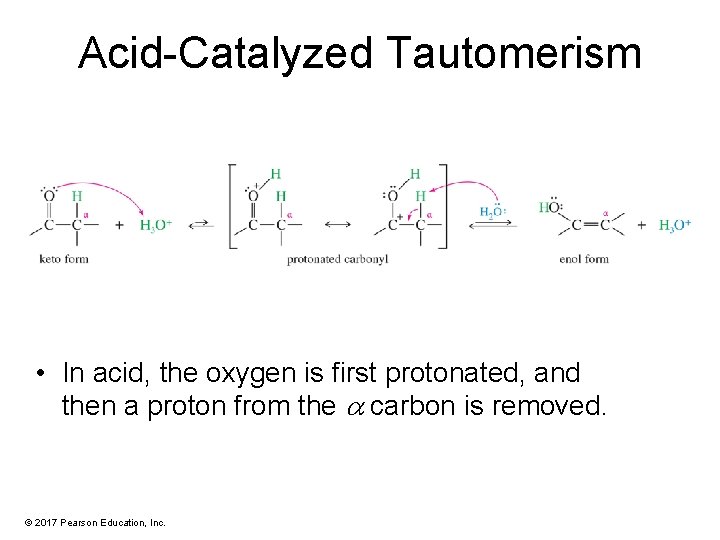
Acid-Catalyzed Tautomerism • In acid, the oxygen is first protonated, and then a proton from the carbon is removed. © 2017 Pearson Education, Inc.

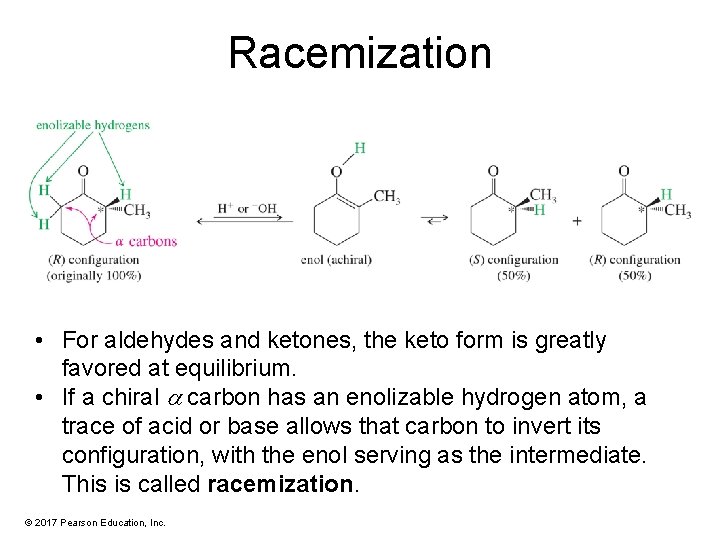
Racemization • For aldehydes and ketones, the keto form is greatly favored at equilibrium. • If a chiral carbon has an enolizable hydrogen atom, a trace of acid or base allows that carbon to invert its configuration, with the enol serving as the intermediate. This is called racemization. © 2017 Pearson Education, Inc.

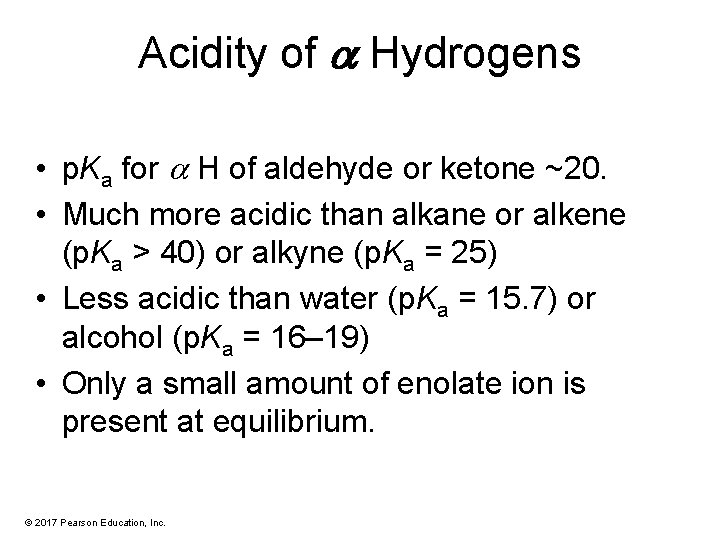
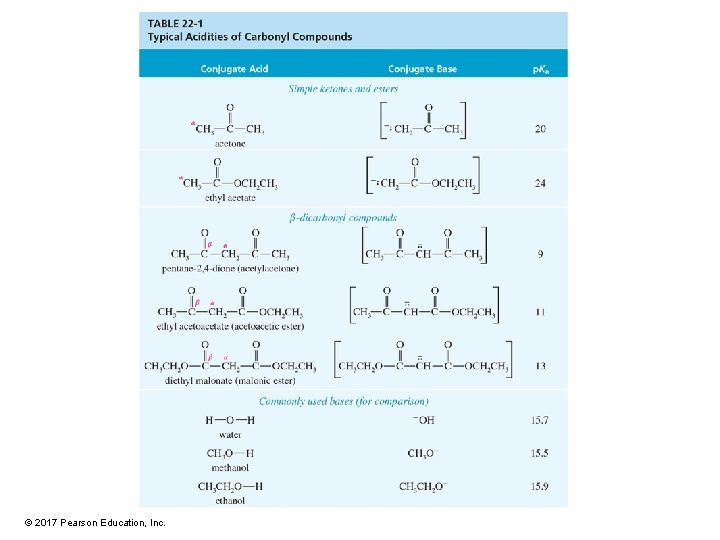
Acidity of a Hydrogens • p. Ka for H of aldehyde or ketone ~20. • Much more acidic than alkane or alkene (p. Ka > 40) or alkyne (p. Ka = 25) • Less acidic than water (p. Ka = 15. 7) or alcohol (p. Ka = 16– 19) • Only a small amount of enolate ion is present at equilibrium. © 2017 Pearson Education, Inc.

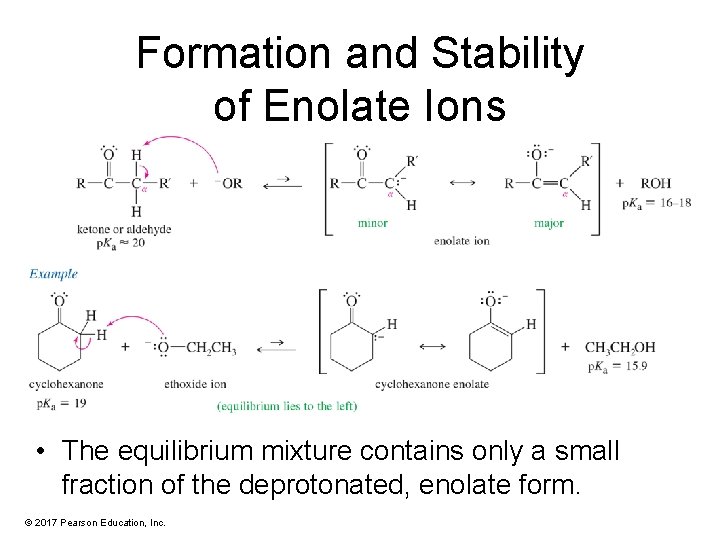
Formation and Stability of Enolate Ions • The equilibrium mixture contains only a small fraction of the deprotonated, enolate form. © 2017 Pearson Education, Inc.

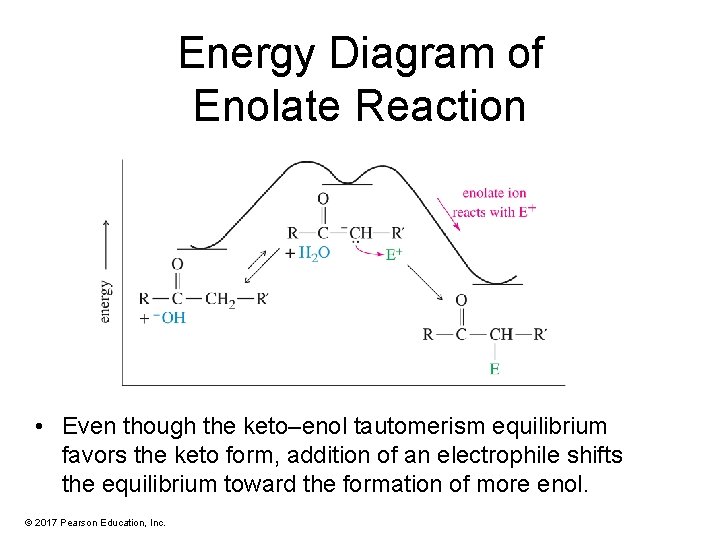
Energy Diagram of Enolate Reaction • Even though the keto–enol tautomerism equilibrium favors the keto form, addition of an electrophile shifts the equilibrium toward the formation of more enol. © 2017 Pearson Education, Inc.

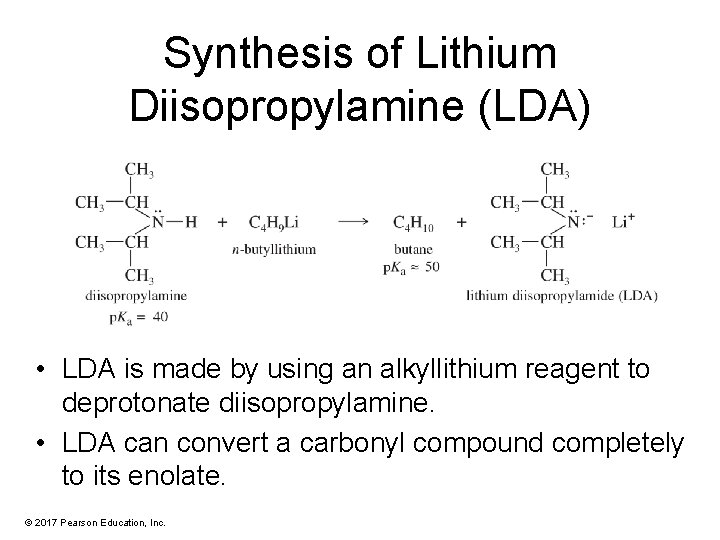
Synthesis of Lithium Diisopropylamine (LDA) • LDA is made by using an alkyllithium reagent to deprotonate diisopropylamine. • LDA can convert a carbonyl compound completely to its enolate. © 2017 Pearson Education, Inc.

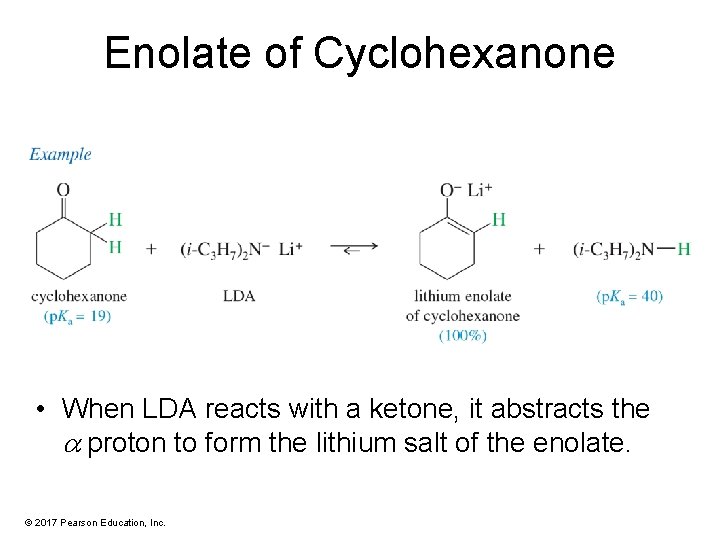
Enolate of Cyclohexanone • When LDA reacts with a ketone, it abstracts the proton to form the lithium salt of the enolate. © 2017 Pearson Education, Inc.

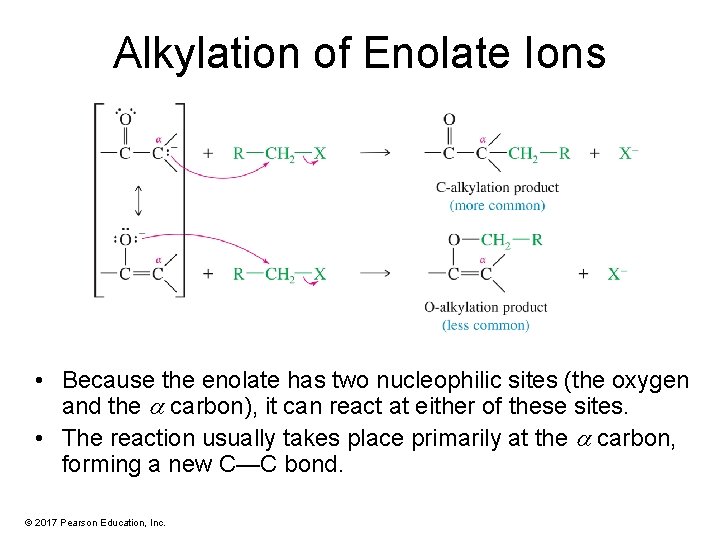
Alkylation of Enolate Ions • Because the enolate has two nucleophilic sites (the oxygen and the carbon), it can react at either of these sites. • The reaction usually takes place primarily at the carbon, forming a new C—C bond. © 2017 Pearson Education, Inc.

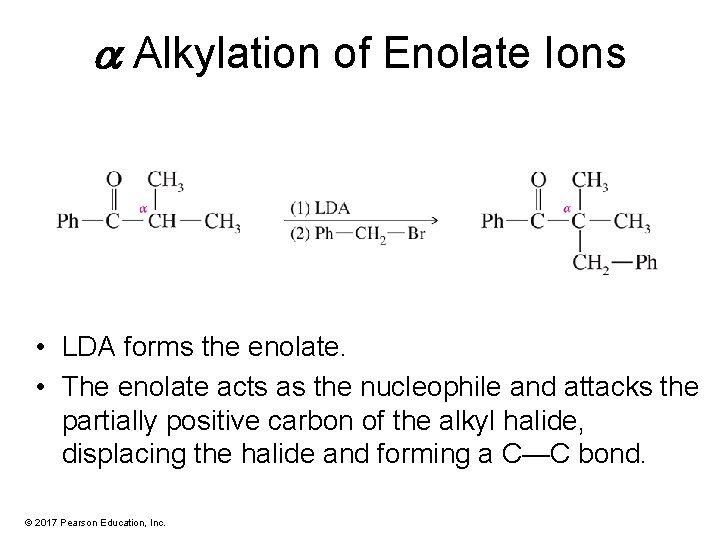
a Alkylation of Enolate Ions • LDA forms the enolate. • The enolate acts as the nucleophile and attacks the partially positive carbon of the alkyl halide, displacing the halide and forming a C—C bond. © 2017 Pearson Education, Inc.

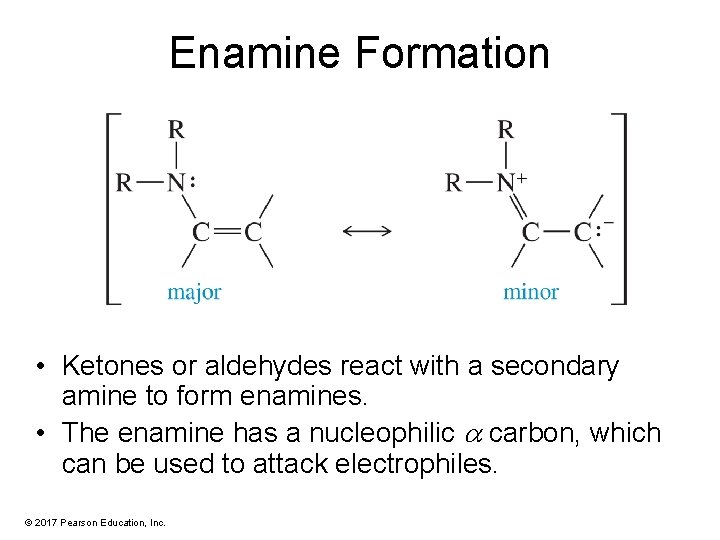
Enamine Formation • Ketones or aldehydes react with a secondary amine to form enamines. • The enamine has a nucleophilic carbon, which can be used to attack electrophiles. © 2017 Pearson Education, Inc.

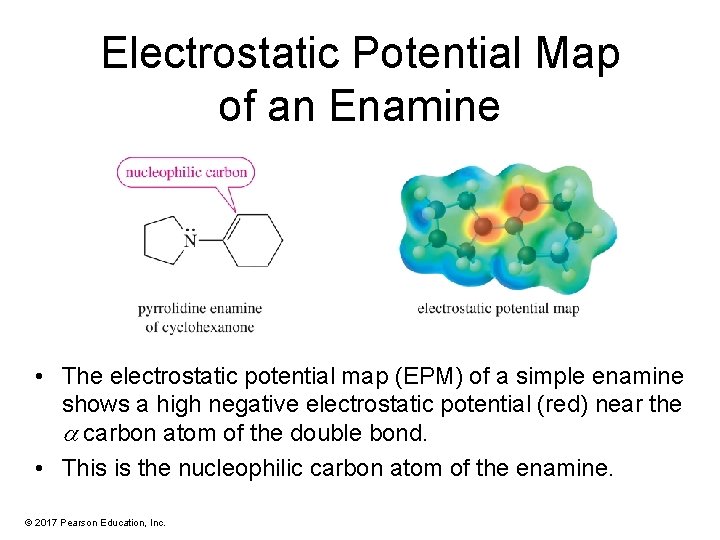
Electrostatic Potential Map of an Enamine • The electrostatic potential map (EPM) of a simple enamine shows a high negative electrostatic potential (red) near the carbon atom of the double bond. • This is the nucleophilic carbon atom of the enamine. © 2017 Pearson Education, Inc.

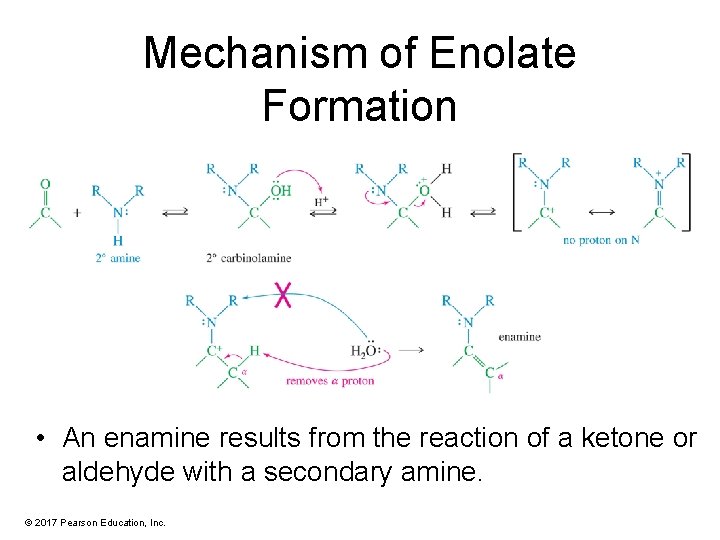
Mechanism of Enolate Formation • An enamine results from the reaction of a ketone or aldehyde with a secondary amine. © 2017 Pearson Education, Inc.

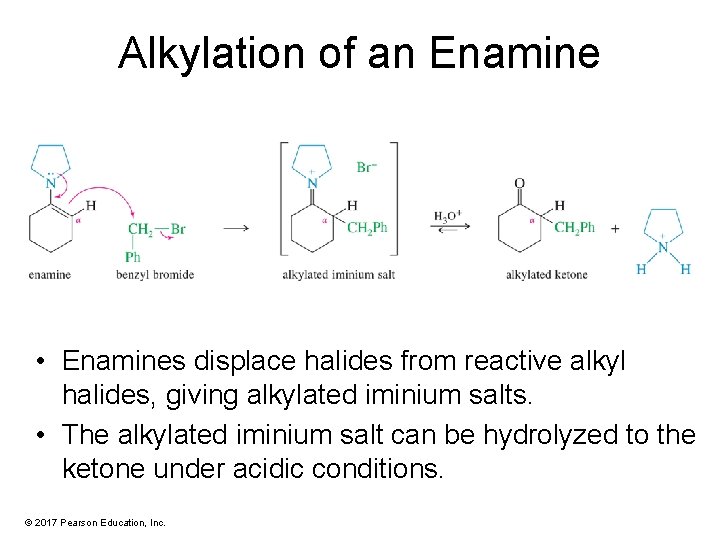
Alkylation of an Enamine • Enamines displace halides from reactive alkyl halides, giving alkylated iminium salts. • The alkylated iminium salt can be hydrolyzed to the ketone under acidic conditions. © 2017 Pearson Education, Inc.

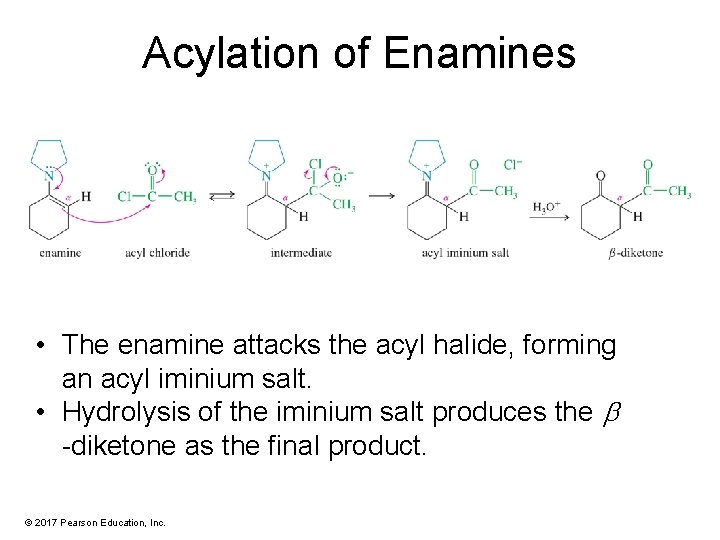
Acylation of Enamines • The enamine attacks the acyl halide, forming an acyl iminium salt. • Hydrolysis of the iminium salt produces the -diketone as the final product. © 2017 Pearson Education, Inc.

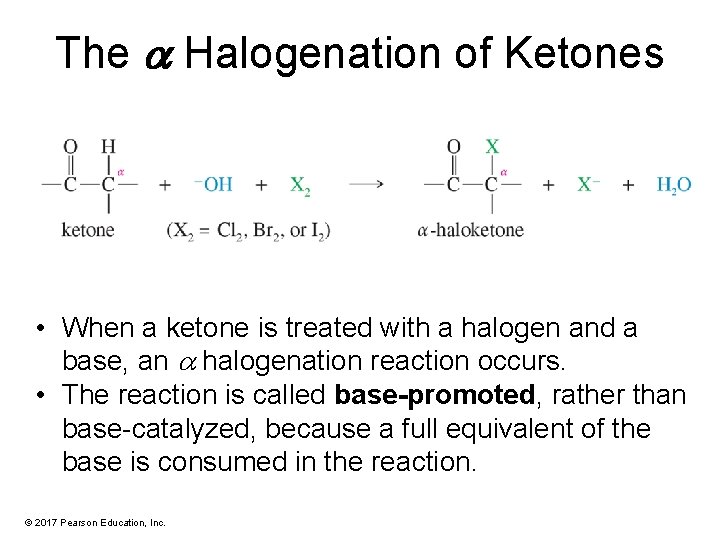
The a Halogenation of Ketones • When a ketone is treated with a halogen and a base, an halogenation reaction occurs. • The reaction is called base-promoted, rather than base-catalyzed, because a full equivalent of the base is consumed in the reaction. © 2017 Pearson Education, Inc.

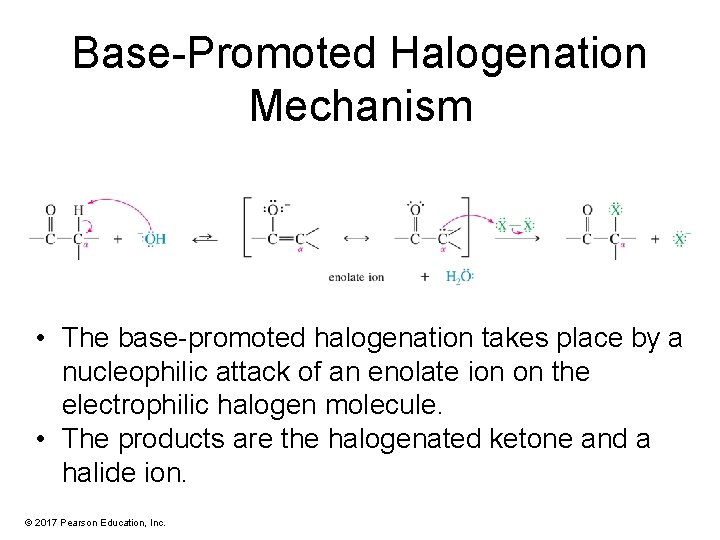
Base-Promoted Halogenation Mechanism • The base-promoted halogenation takes place by a nucleophilic attack of an enolate ion on the electrophilic halogen molecule. • The products are the halogenated ketone and a halide ion. © 2017 Pearson Education, Inc.

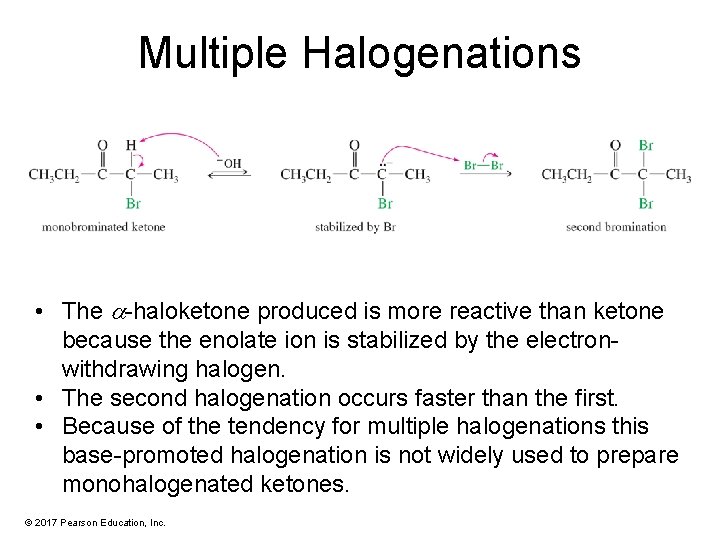
Multiple Halogenations • The -haloketone produced is more reactive than ketone because the enolate ion is stabilized by the electronwithdrawing halogen. • The second halogenation occurs faster than the first. • Because of the tendency for multiple halogenations this base-promoted halogenation is not widely used to prepare monohalogenated ketones. © 2017 Pearson Education, Inc.

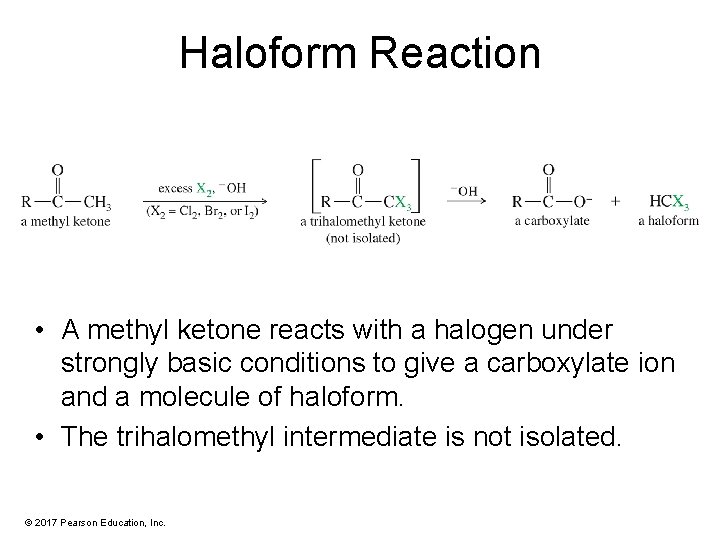
Haloform Reaction • A methyl ketone reacts with a halogen under strongly basic conditions to give a carboxylate ion and a molecule of haloform. • The trihalomethyl intermediate is not isolated. © 2017 Pearson Education, Inc.

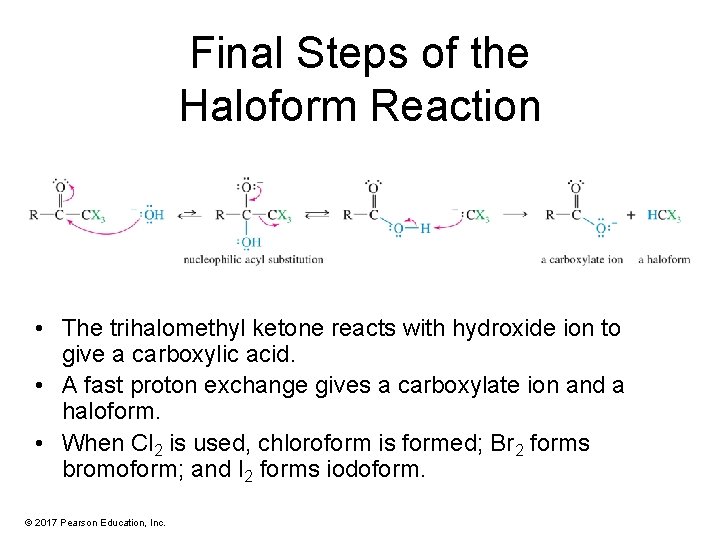
Final Steps of the Haloform Reaction • The trihalomethyl ketone reacts with hydroxide ion to give a carboxylic acid. • A fast proton exchange gives a carboxylate ion and a haloform. • When Cl 2 is used, chloroform is formed; Br 2 forms bromoform; and I 2 forms iodoform. © 2017 Pearson Education, Inc.

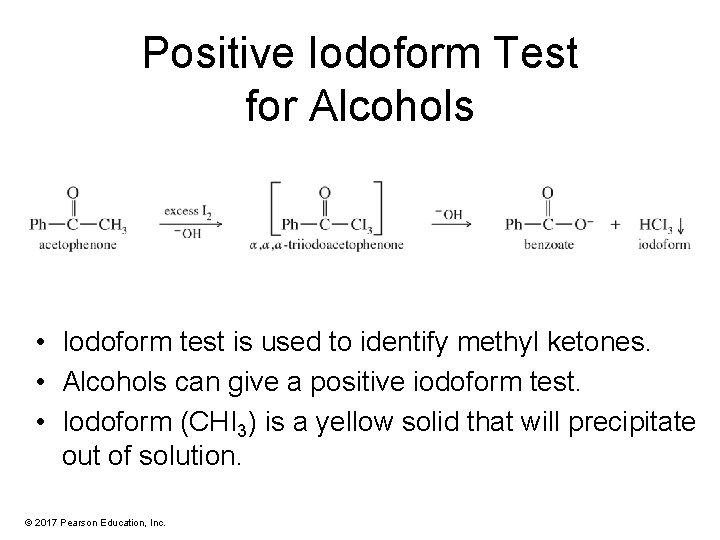
Positive Iodoform Test for Alcohols • Iodoform test is used to identify methyl ketones. • Alcohols can give a positive iodoform test. • Iodoform (CHI 3) is a yellow solid that will precipitate out of solution. © 2017 Pearson Education, Inc.

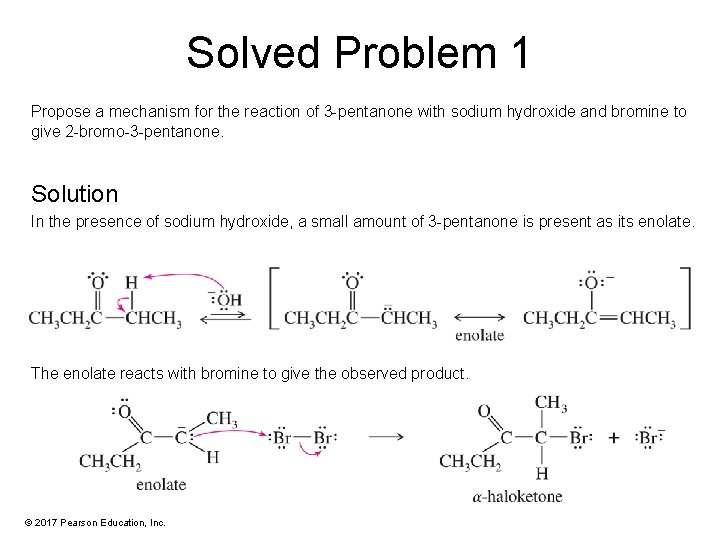
Solved Problem 1 Propose a mechanism for the reaction of 3 -pentanone with sodium hydroxide and bromine to give 2 -bromo-3 -pentanone. Solution In the presence of sodium hydroxide, a small amount of 3 -pentanone is present as its enolate. The enolate reacts with bromine to give the observed product. © 2017 Pearson Education, Inc.

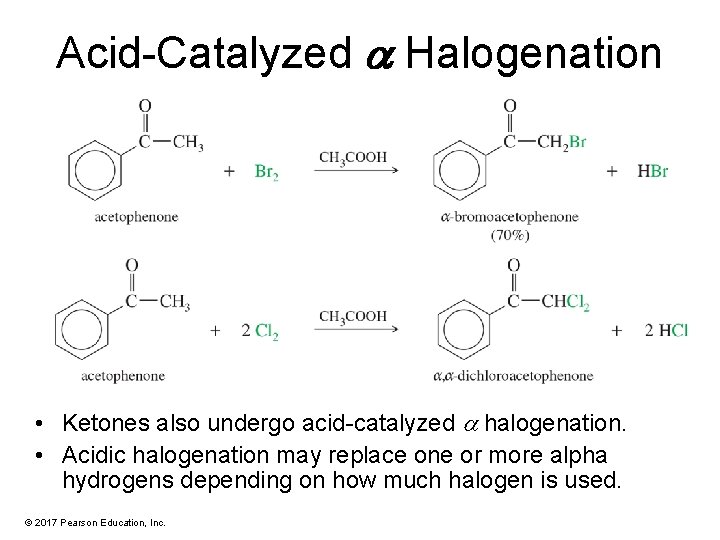
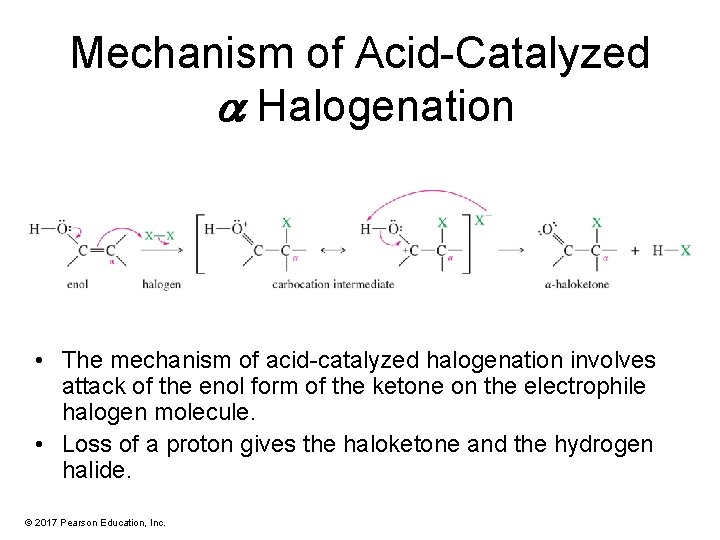
Acid-Catalyzed a Halogenation • Ketones also undergo acid-catalyzed halogenation. • Acidic halogenation may replace one or more alpha hydrogens depending on how much halogen is used. © 2017 Pearson Education, Inc.

Mechanism of Acid-Catalyzed a Halogenation • The mechanism of acid-catalyzed halogenation involves attack of the enol form of the ketone on the electrophile halogen molecule. • Loss of a proton gives the haloketone and the hydrogen halide. © 2017 Pearson Education, Inc.

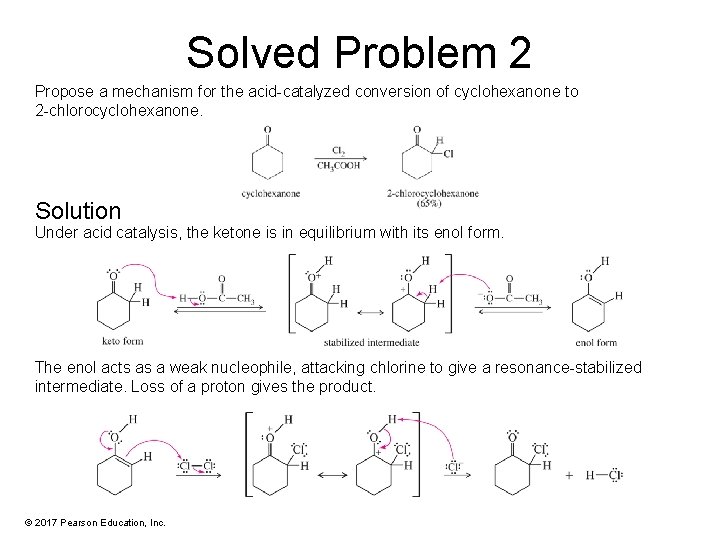
Solved Problem 2 Propose a mechanism for the acid-catalyzed conversion of cyclohexanone to 2 -chlorocyclohexanone. Solution Under acid catalysis, the ketone is in equilibrium with its enol form. The enol acts as a weak nucleophile, attacking chlorine to give a resonance-stabilized intermediate. Loss of a proton gives the product. © 2017 Pearson Education, Inc.

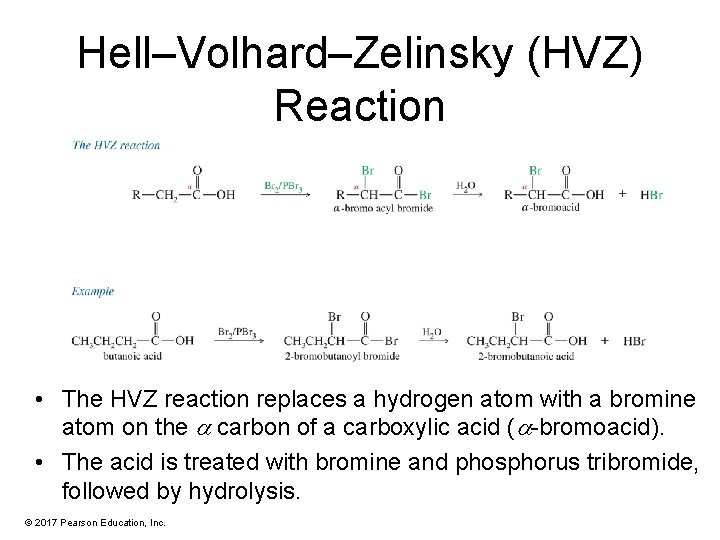
Hell–Volhard–Zelinsky (HVZ) Reaction • The HVZ reaction replaces a hydrogen atom with a bromine atom on the carbon of a carboxylic acid ( -bromoacid). • The acid is treated with bromine and phosphorus tribromide, followed by hydrolysis. © 2017 Pearson Education, Inc.

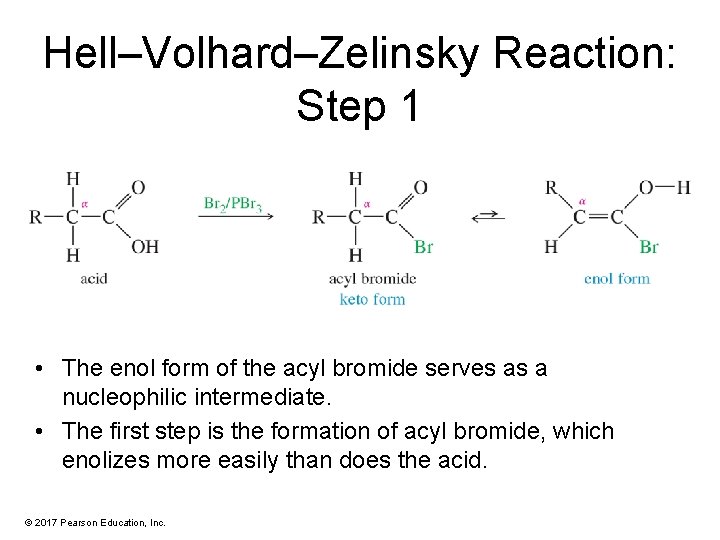
Hell–Volhard–Zelinsky Reaction: Step 1 • The enol form of the acyl bromide serves as a nucleophilic intermediate. • The first step is the formation of acyl bromide, which enolizes more easily than does the acid. © 2017 Pearson Education, Inc.

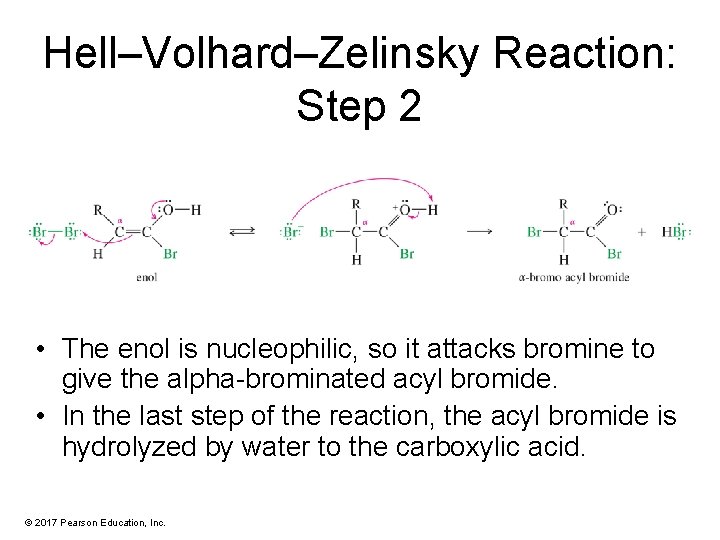
Hell–Volhard–Zelinsky Reaction: Step 2 • The enol is nucleophilic, so it attacks bromine to give the alpha-brominated acyl bromide. • In the last step of the reaction, the acyl bromide is hydrolyzed by water to the carboxylic acid. © 2017 Pearson Education, Inc.

Condensation of Ketones and Aldehydes • Condensations combine two or more molecules, often with the loss of a small molecule such as water or an alcohol. • The aldol condensation is the addition of an enolate ion to another carbonyl group. It occurs under basic conditions. © 2017 Pearson Education, Inc.

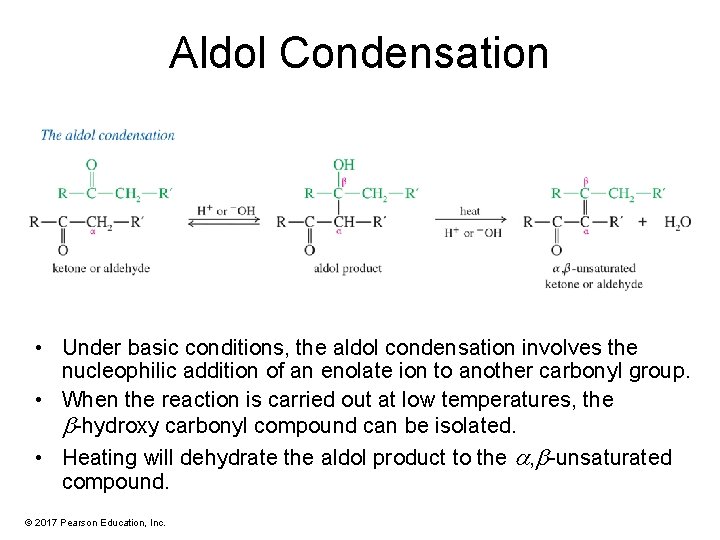
Aldol Condensation • Under basic conditions, the aldol condensation involves the nucleophilic addition of an enolate ion to another carbonyl group. • When the reaction is carried out at low temperatures, the -hydroxy carbonyl compound can be isolated. • Heating will dehydrate the aldol product to the , -unsaturated compound. © 2017 Pearson Education, Inc.

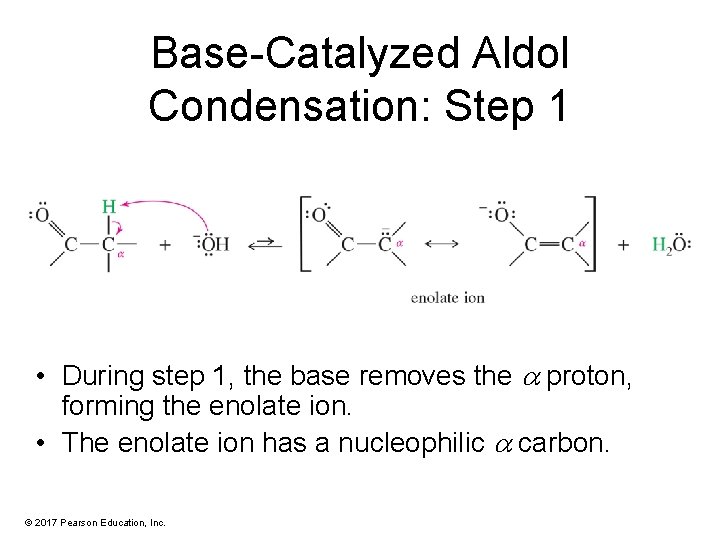
Base-Catalyzed Aldol Condensation: Step 1 • During step 1, the base removes the proton, forming the enolate ion. • The enolate ion has a nucleophilic carbon. © 2017 Pearson Education, Inc.

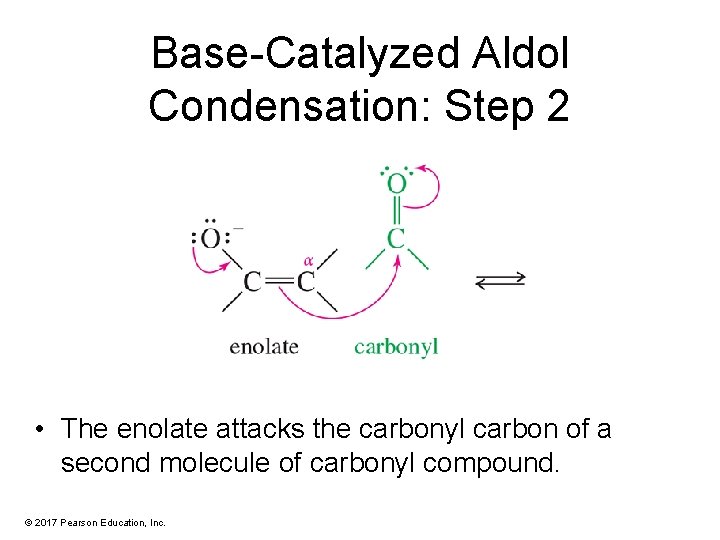
Base-Catalyzed Aldol Condensation: Step 2 • The enolate attacks the carbonyl carbon of a second molecule of carbonyl compound. © 2017 Pearson Education, Inc.

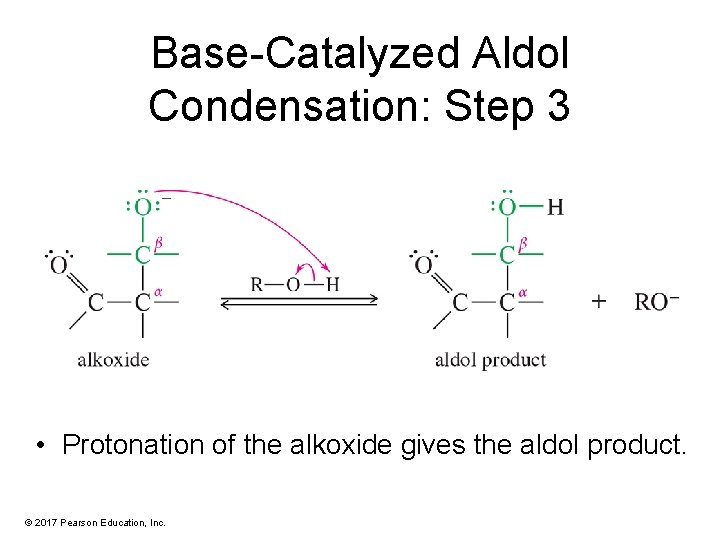
Base-Catalyzed Aldol Condensation: Step 3 • Protonation of the alkoxide gives the aldol product. © 2017 Pearson Education, Inc.

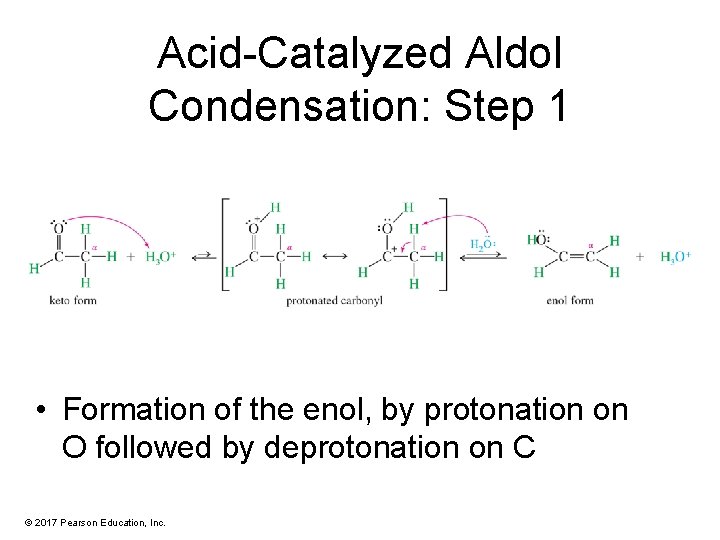
Acid-Catalyzed Aldol Condensation: Step 1 • Formation of the enol, by protonation on O followed by deprotonation on C © 2017 Pearson Education, Inc.

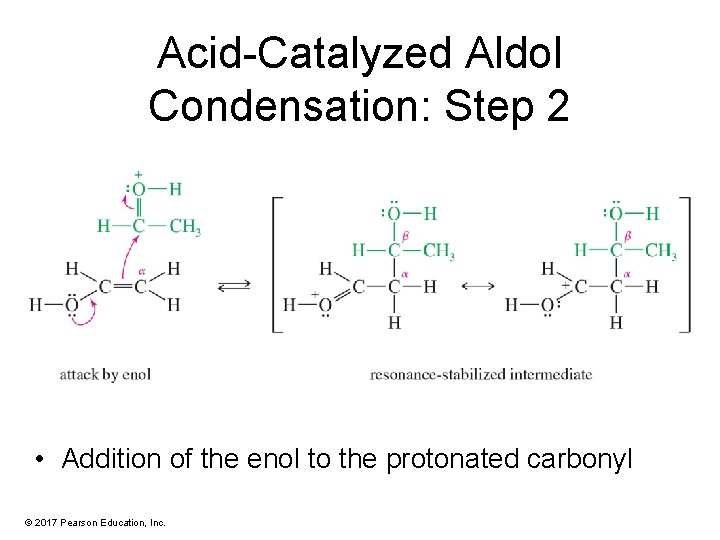
Acid-Catalyzed Aldol Condensation: Step 2 • Addition of the enol to the protonated carbonyl © 2017 Pearson Education, Inc.

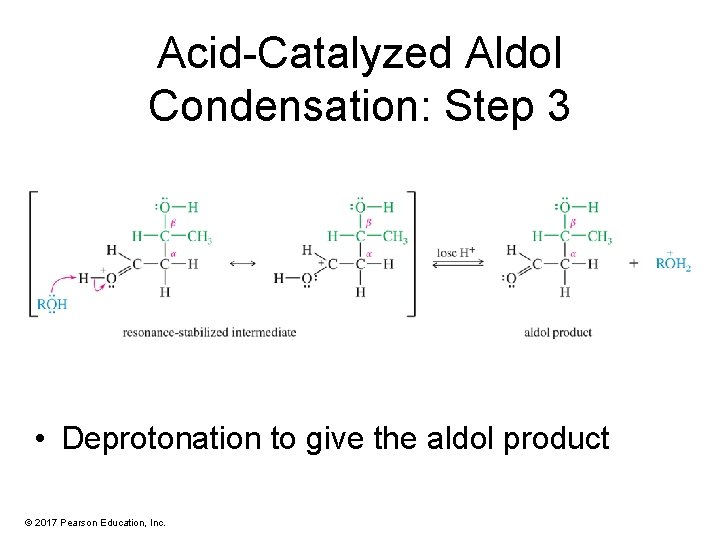
Acid-Catalyzed Aldol Condensation: Step 3 • Deprotonation to give the aldol product © 2017 Pearson Education, Inc.

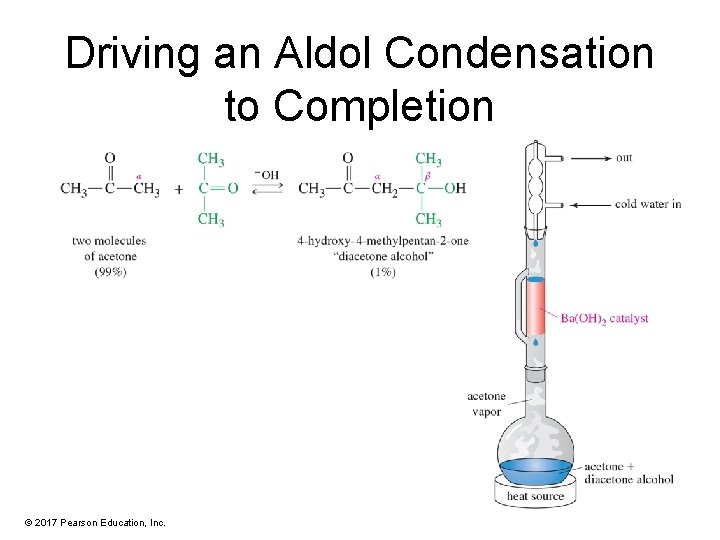
Driving an Aldol Condensation to Completion © 2017 Pearson Education, Inc.

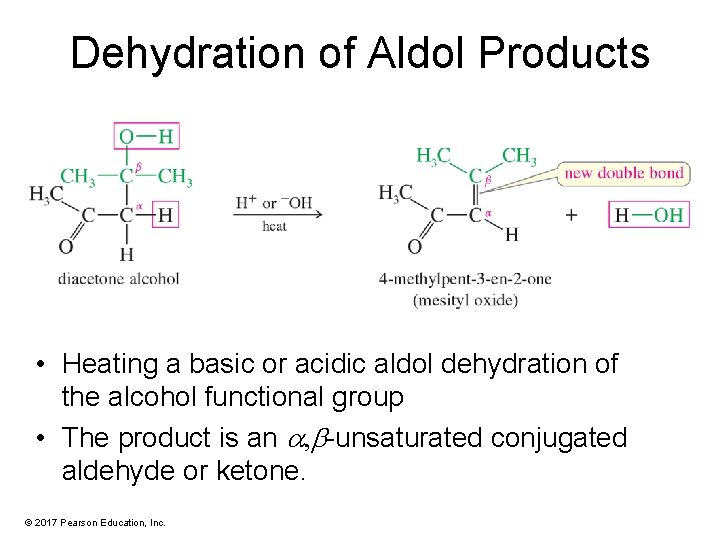
Dehydration of Aldol Products • Heating a basic or acidic aldol dehydration of the alcohol functional group • The product is an , -unsaturated conjugated aldehyde or ketone. © 2017 Pearson Education, Inc.

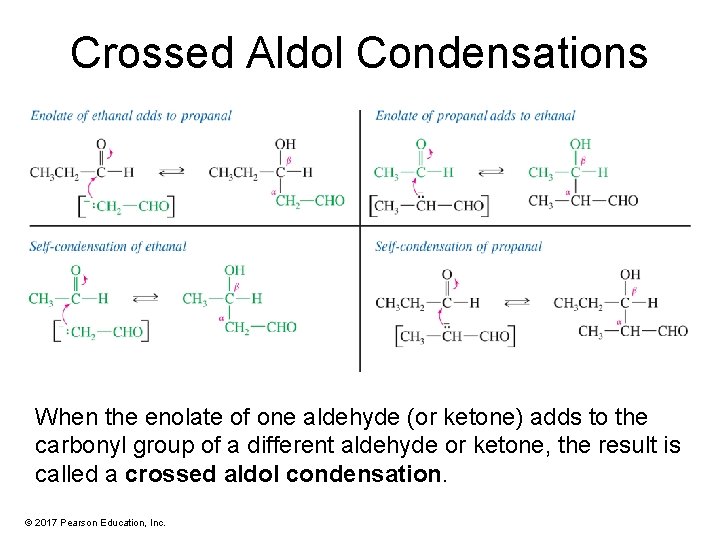
Crossed Aldol Condensations When the enolate of one aldehyde (or ketone) adds to the carbonyl group of a different aldehyde or ketone, the result is called a crossed aldol condensation. © 2017 Pearson Education, Inc.

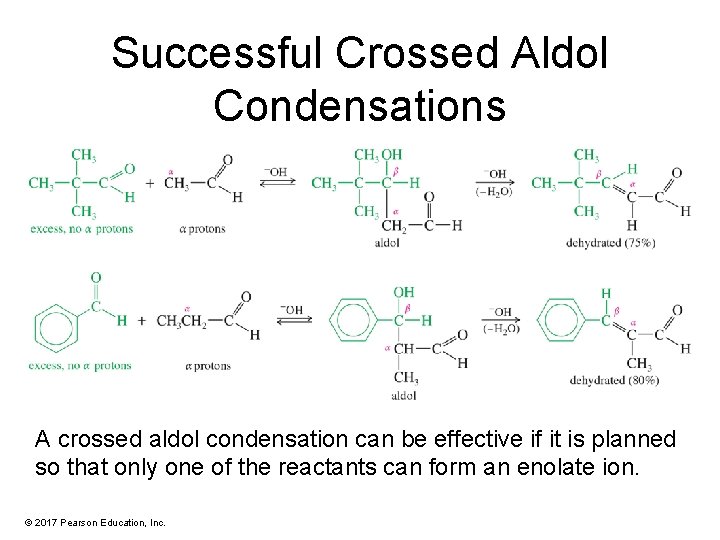
Successful Crossed Aldol Condensations A crossed aldol condensation can be effective if it is planned so that only one of the reactants can form an enolate ion. © 2017 Pearson Education, Inc.

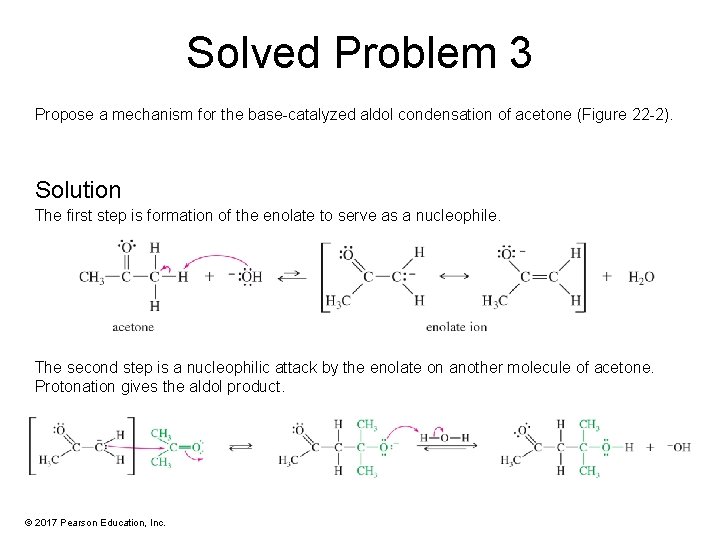
Solved Problem 3 Propose a mechanism for the base-catalyzed aldol condensation of acetone (Figure 22 -2). Solution The first step is formation of the enolate to serve as a nucleophile. The second step is a nucleophilic attack by the enolate on another molecule of acetone. Protonation gives the aldol product. © 2017 Pearson Education, Inc.

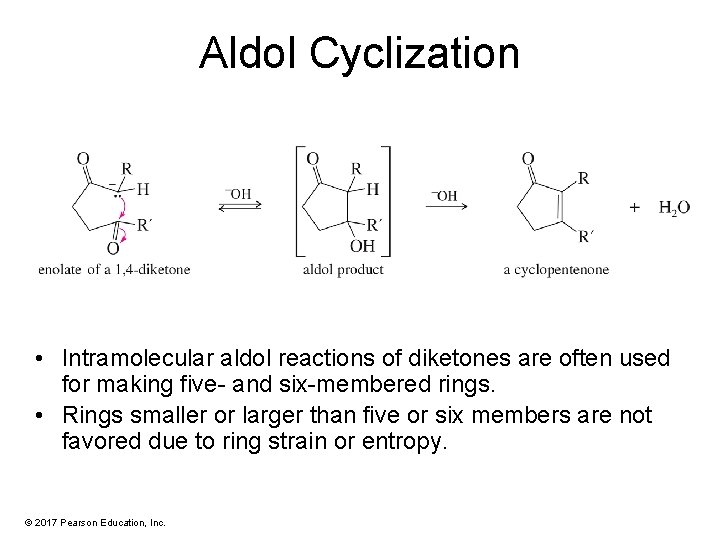
Aldol Cyclization • Intramolecular aldol reactions of diketones are often used for making five- and six-membered rings. • Rings smaller or larger than five or six members are not favored due to ring strain or entropy. © 2017 Pearson Education, Inc.

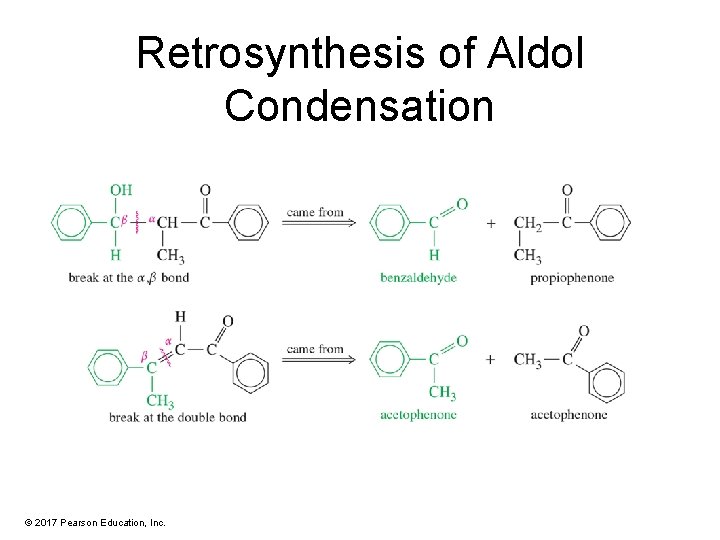
Retrosynthesis of Aldol Condensation © 2017 Pearson Education, Inc.

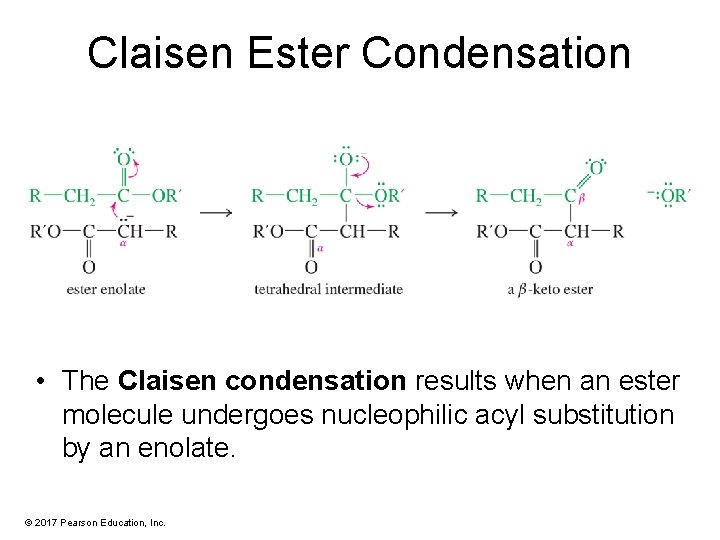
Claisen Ester Condensation • The Claisen condensation results when an ester molecule undergoes nucleophilic acyl substitution by an enolate. © 2017 Pearson Education, Inc.

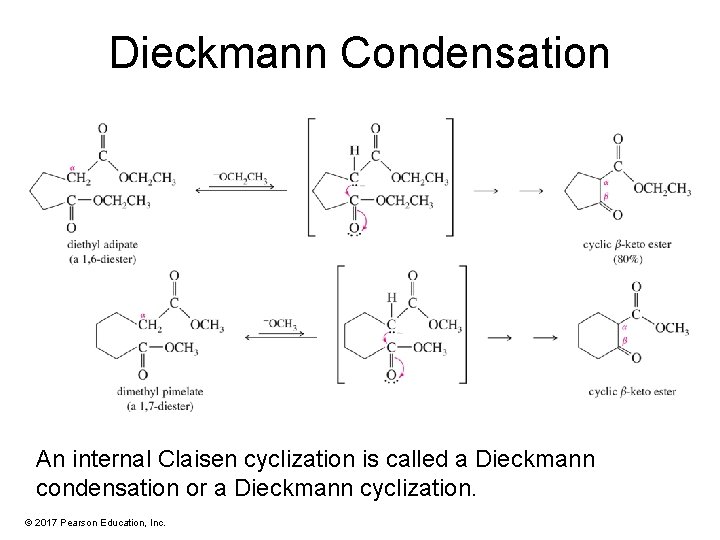
Dieckmann Condensation An internal Claisen cyclization is called a Dieckmann condensation or a Dieckmann cyclization. © 2017 Pearson Education, Inc.

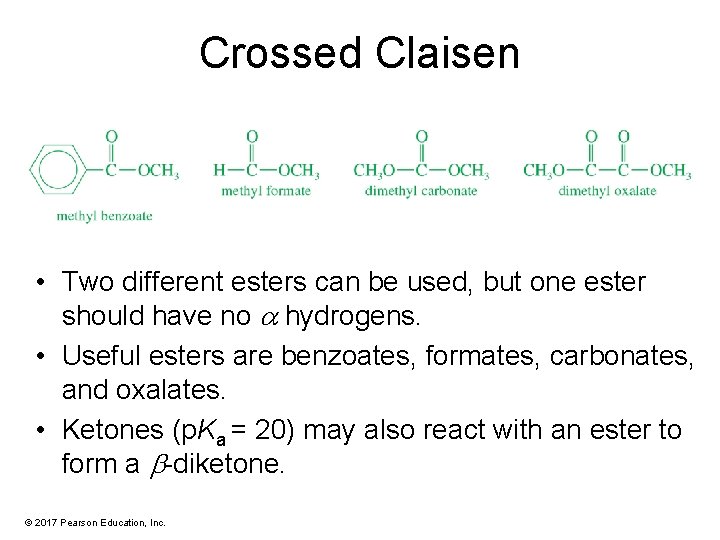
Crossed Claisen • Two different esters can be used, but one ester should have no hydrogens. • Useful esters are benzoates, formates, carbonates, and oxalates. • Ketones (p. Ka = 20) may also react with an ester to form a -diketone. © 2017 Pearson Education, Inc.

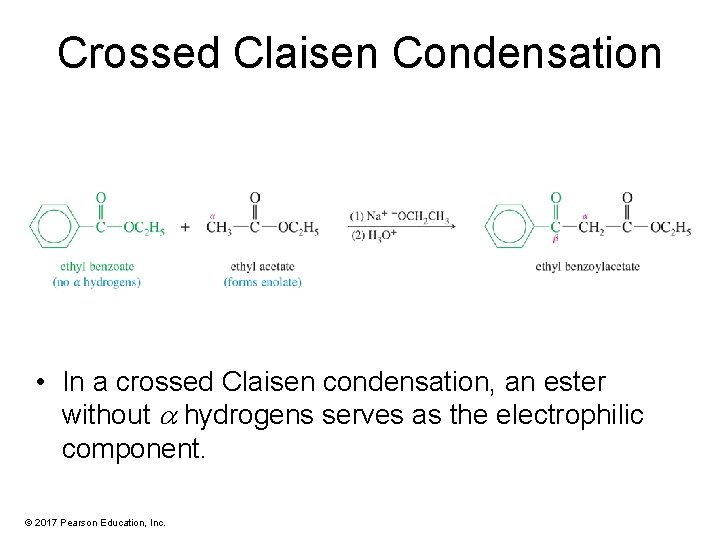
Crossed Claisen Condensation • In a crossed Claisen condensation, an ester without hydrogens serves as the electrophilic component. © 2017 Pearson Education, Inc.

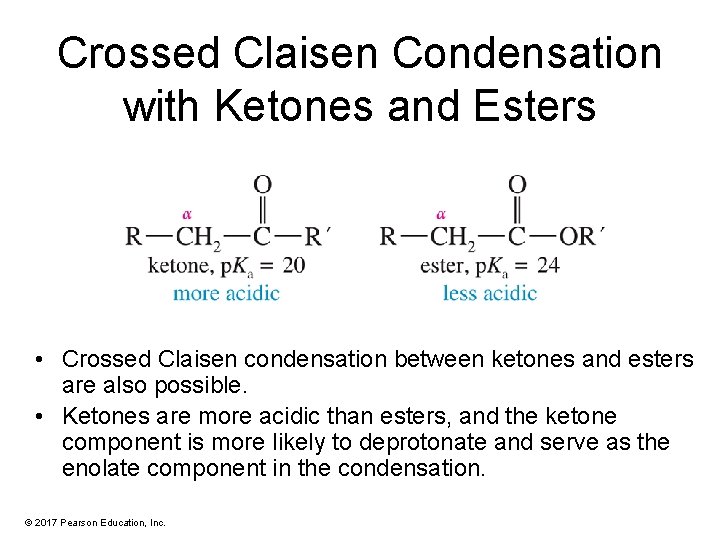
Crossed Claisen Condensation with Ketones and Esters • Crossed Claisen condensation between ketones and esters are also possible. • Ketones are more acidic than esters, and the ketone component is more likely to deprotonate and serve as the enolate component in the condensation. © 2017 Pearson Education, Inc.

Crossed Claisen Mechanism • The ketone enolate attacks the ester, which undergoes nucleophilic acyl substitution and, thereby, acylates the ketone. © 2017 Pearson Education, Inc.

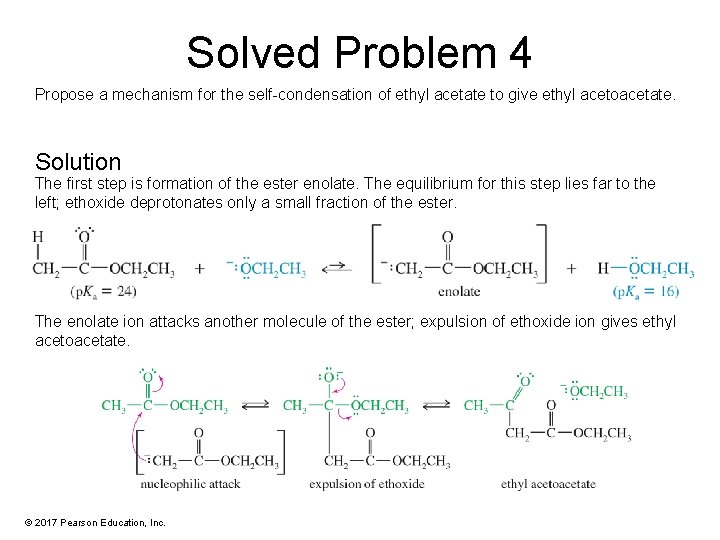
Solved Problem 4 Propose a mechanism for the self-condensation of ethyl acetate to give ethyl acetoacetate. Solution The first step is formation of the ester enolate. The equilibrium for this step lies far to the left; ethoxide deprotonates only a small fraction of the ester. The enolate ion attacks another molecule of the ester; expulsion of ethoxide ion gives ethyl acetoacetate. © 2017 Pearson Education, Inc.

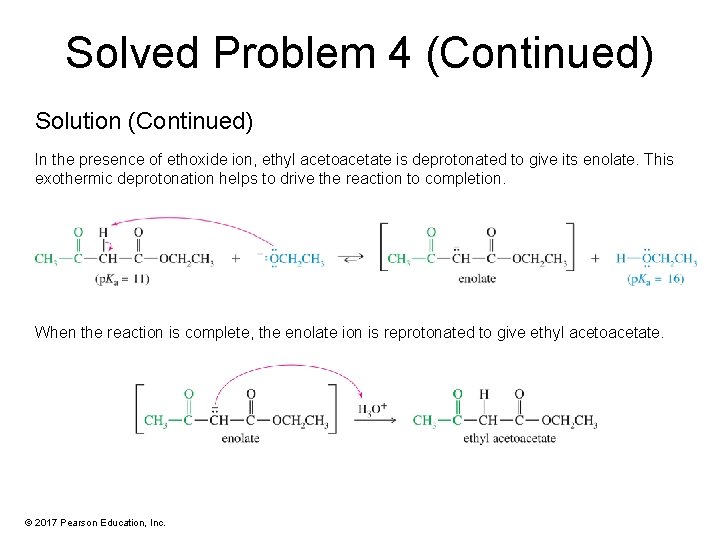
Solved Problem 4 (Continued) Solution (Continued) In the presence of ethoxide ion, ethyl acetoacetate is deprotonated to give its enolate. This exothermic deprotonation helps to drive the reaction to completion. When the reaction is complete, the enolate ion is reprotonated to give ethyl acetoacetate. © 2017 Pearson Education, Inc.

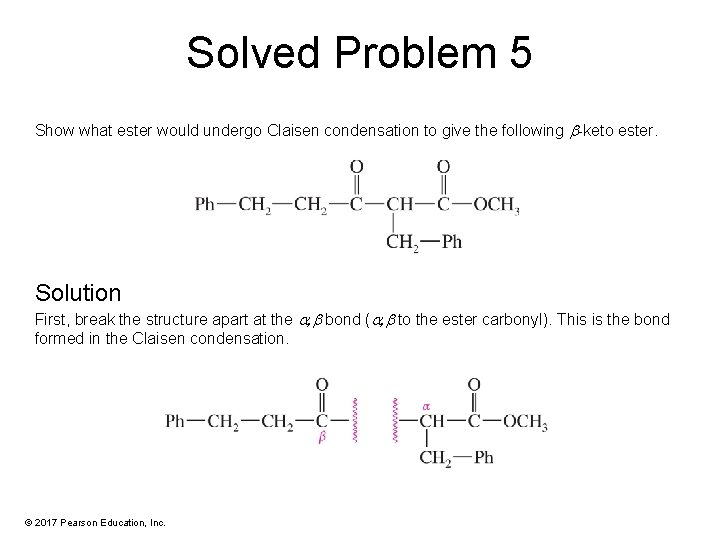
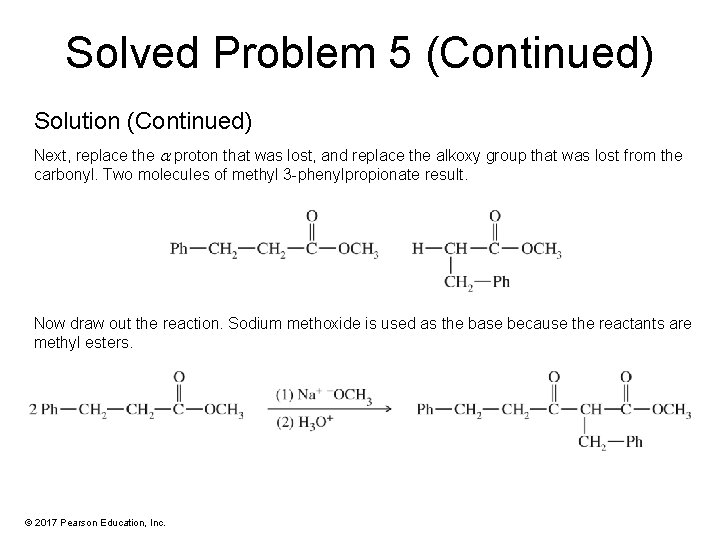
Solved Problem 5 Show what ester would undergo Claisen condensation to give the following -keto ester. Solution First, break the structure apart at the , bond ( , to the ester carbonyl). This is the bond formed in the Claisen condensation. © 2017 Pearson Education, Inc.

Solved Problem 5 (Continued) Solution (Continued) Next, replace the proton that was lost, and replace the alkoxy group that was lost from the carbonyl. Two molecules of methyl 3 -phenylpropionate result. Now draw out the reaction. Sodium methoxide is used as the base because the reactants are methyl esters. © 2017 Pearson Education, Inc.

© 2017 Pearson Education, Inc.

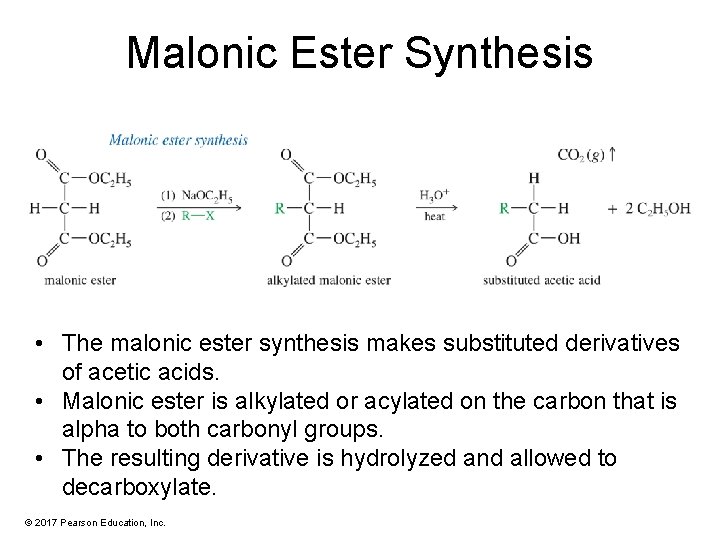
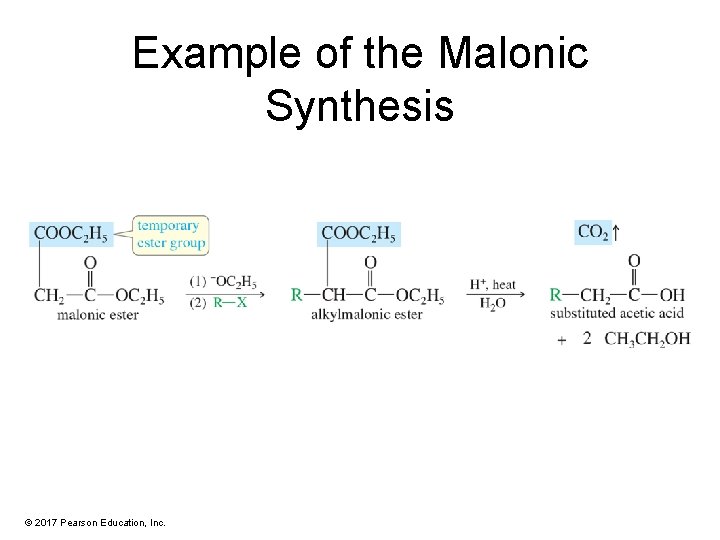
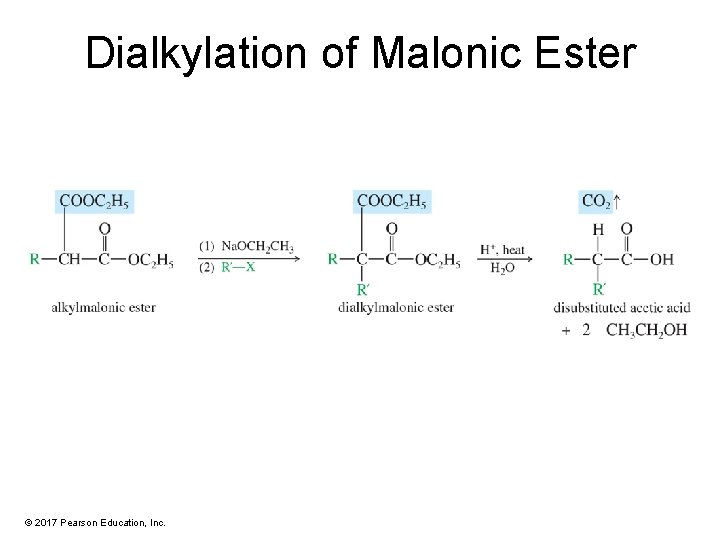
Malonic Ester Synthesis • The malonic ester synthesis makes substituted derivatives of acetic acids. • Malonic ester is alkylated or acylated on the carbon that is alpha to both carbonyl groups. • The resulting derivative is hydrolyzed and allowed to decarboxylate. © 2017 Pearson Education, Inc.

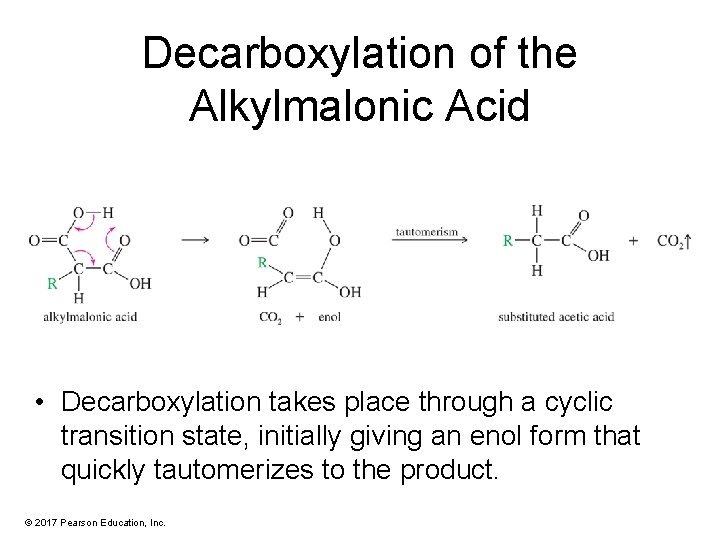
Decarboxylation of the Alkylmalonic Acid • Decarboxylation takes place through a cyclic transition state, initially giving an enol form that quickly tautomerizes to the product. © 2017 Pearson Education, Inc.

Example of the Malonic Synthesis © 2017 Pearson Education, Inc.

Dialkylation of Malonic Ester © 2017 Pearson Education, Inc.

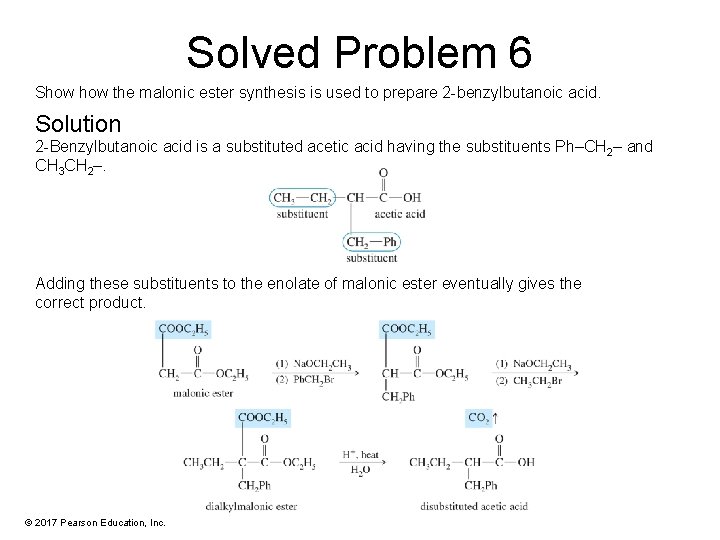
Solved Problem 6 Show the malonic ester synthesis is used to prepare 2 -benzylbutanoic acid. Solution 2 -Benzylbutanoic acid is a substituted acetic acid having the substituents Ph–CH 2– and CH 3 CH 2–. Adding these substituents to the enolate of malonic ester eventually gives the correct product. © 2017 Pearson Education, Inc.

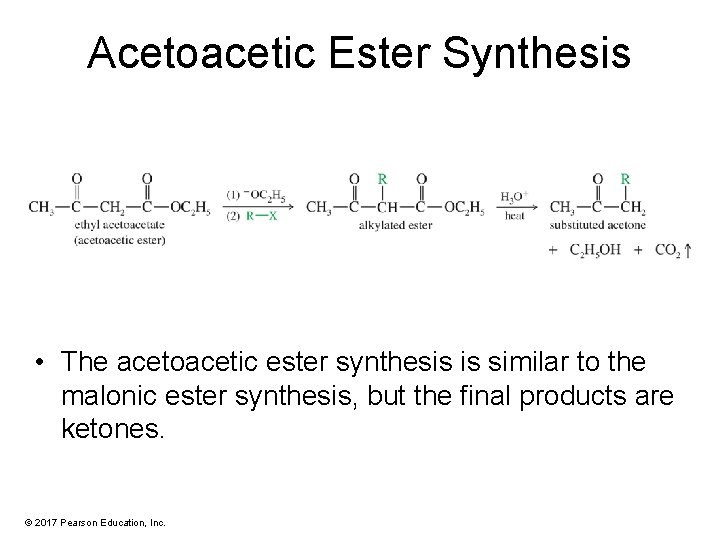
Acetoacetic Ester Synthesis • The acetoacetic ester synthesis is similar to the malonic ester synthesis, but the final products are ketones. © 2017 Pearson Education, Inc.

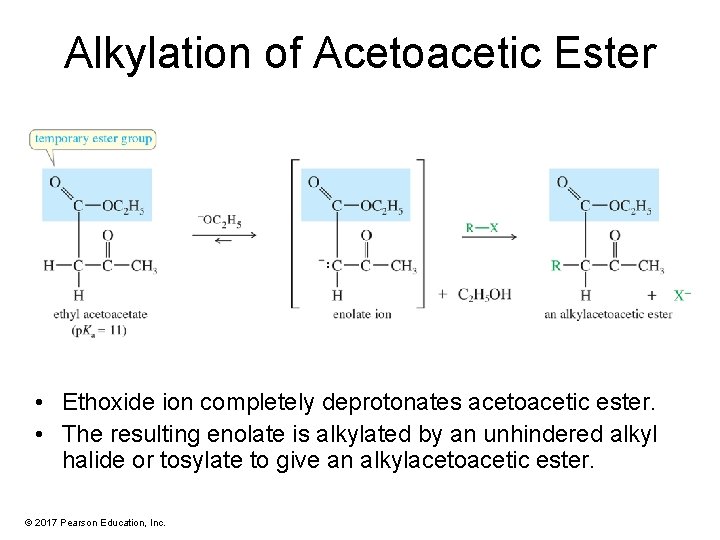
Alkylation of Acetoacetic Ester • Ethoxide ion completely deprotonates acetoacetic ester. • The resulting enolate is alkylated by an unhindered alkyl halide or tosylate to give an alkylacetoacetic ester. © 2017 Pearson Education, Inc.

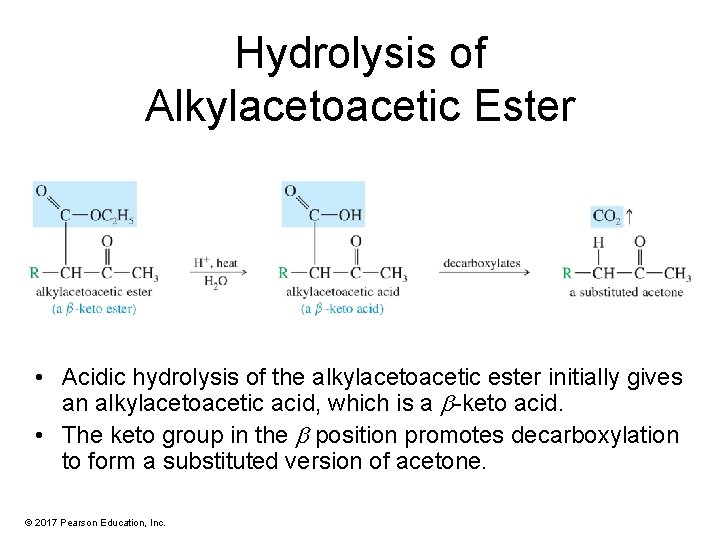
Hydrolysis of Alkylacetoacetic Ester • Acidic hydrolysis of the alkylacetoacetic ester initially gives an alkylacetoacetic acid, which is a -keto acid. • The keto group in the position promotes decarboxylation to form a substituted version of acetone. © 2017 Pearson Education, Inc.

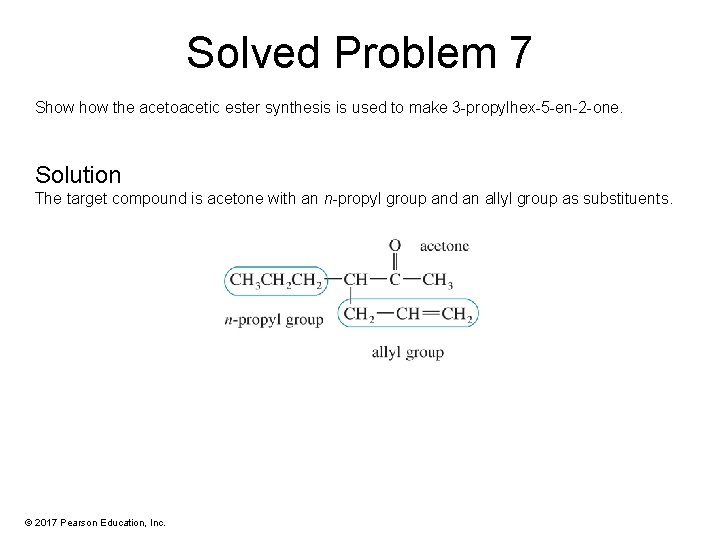
Solved Problem 7 Show the acetoacetic ester synthesis is used to make 3 -propylhex-5 -en-2 -one. Solution The target compound is acetone with an n-propyl group and an allyl group as substituents. © 2017 Pearson Education, Inc.

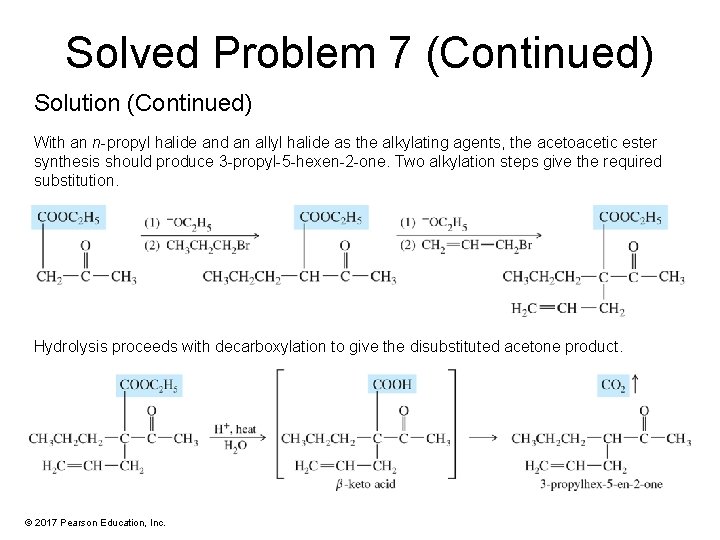
Solved Problem 7 (Continued) Solution (Continued) With an n-propyl halide and an allyl halide as the alkylating agents, the acetoacetic ester synthesis should produce 3 -propyl-5 -hexen-2 -one. Two alkylation steps give the required substitution. Hydrolysis proceeds with decarboxylation to give the disubstituted acetone product. © 2017 Pearson Education, Inc.

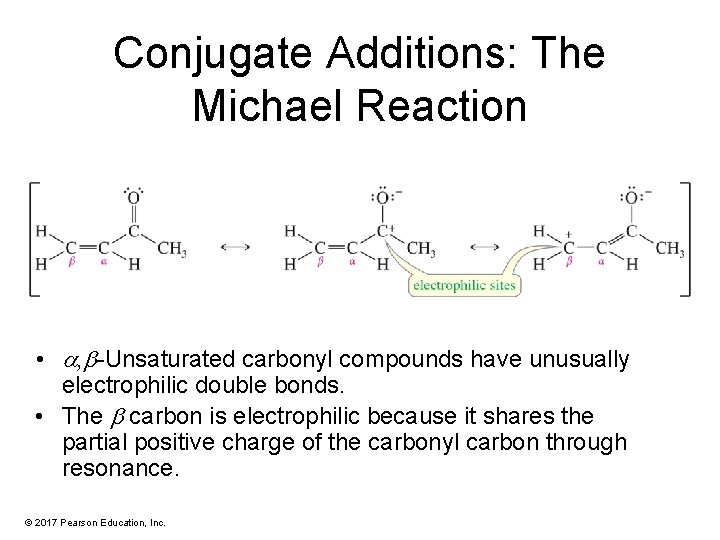
Conjugate Additions: The Michael Reaction • , -Unsaturated carbonyl compounds have unusually electrophilic double bonds. • The carbon is electrophilic because it shares the partial positive charge of the carbonyl carbon through resonance. © 2017 Pearson Education, Inc.

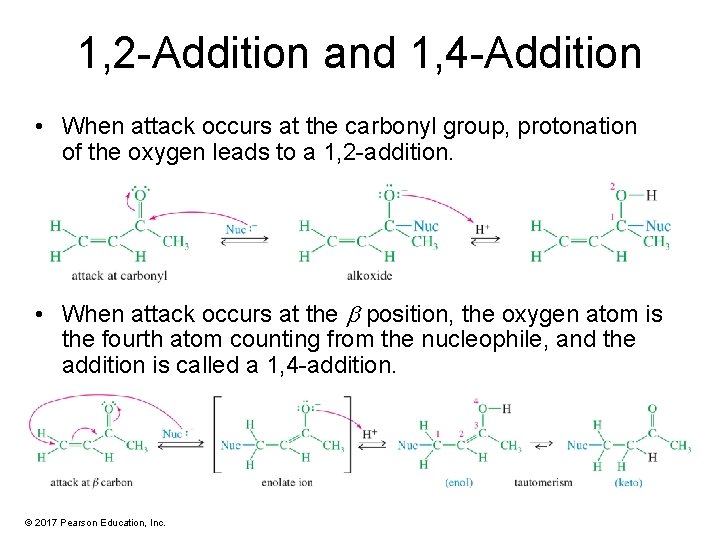
1, 2 -Addition and 1, 4 -Addition • When attack occurs at the carbonyl group, protonation of the oxygen leads to a 1, 2 -addition. • When attack occurs at the position, the oxygen atom is the fourth atom counting from the nucleophile, and the addition is called a 1, 4 -addition. © 2017 Pearson Education, Inc.

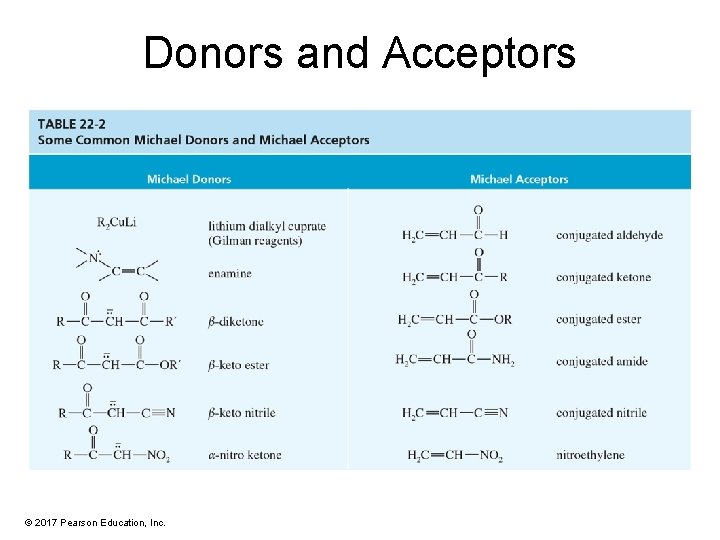
Donors and Acceptors © 2017 Pearson Education, Inc.

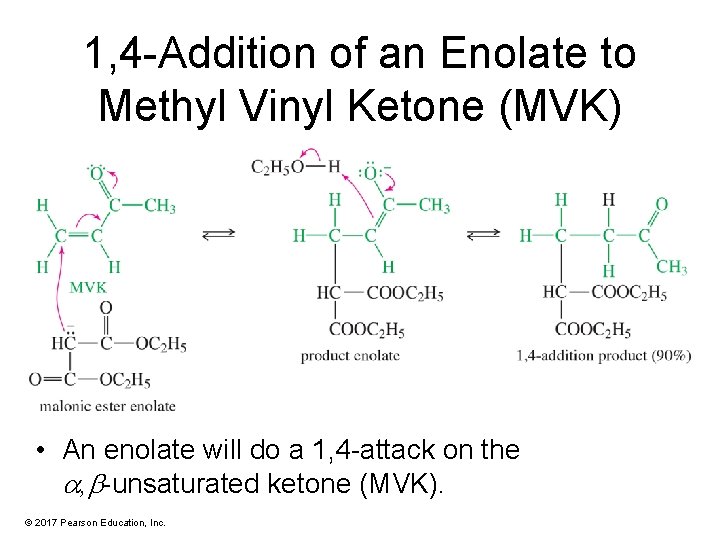
1, 4 -Addition of an Enolate to Methyl Vinyl Ketone (MVK) • An enolate will do a 1, 4 -attack on the , -unsaturated ketone (MVK). © 2017 Pearson Education, Inc.

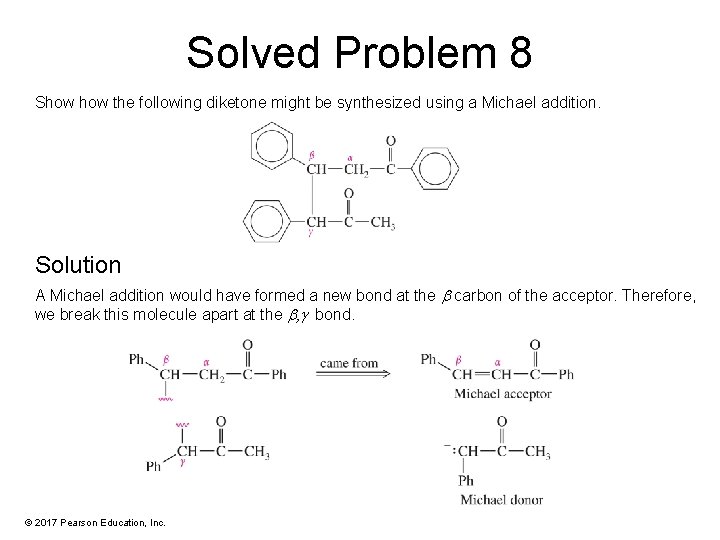
Solved Problem 8 Show the following diketone might be synthesized using a Michael addition. Solution A Michael addition would have formed a new bond at the carbon of the acceptor. Therefore, we break this molecule apart at the , g bond. © 2017 Pearson Education, Inc.

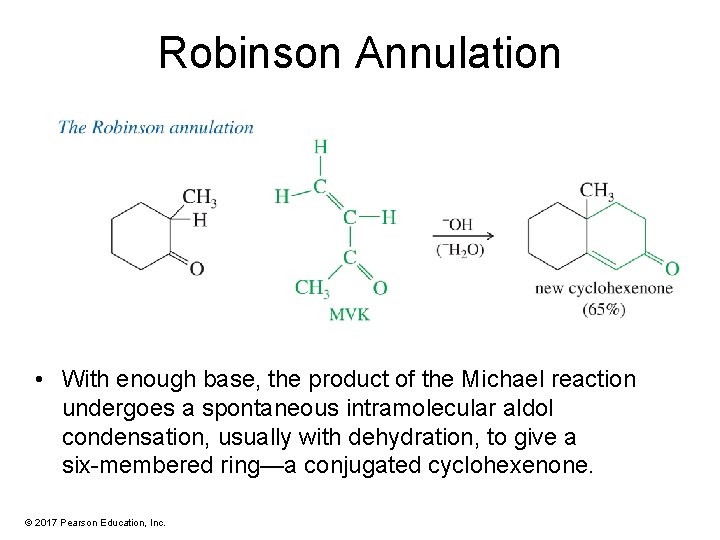
Robinson Annulation • With enough base, the product of the Michael reaction undergoes a spontaneous intramolecular aldol condensation, usually with dehydration, to give a six-membered ring—a conjugated cyclohexenone. © 2017 Pearson Education, Inc.

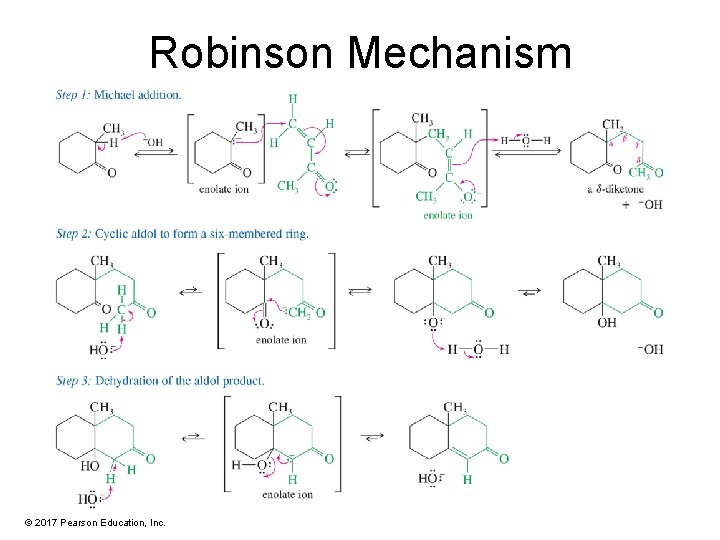
Robinson Mechanism © 2017 Pearson Education, Inc.
 Kiliani fischer synthesis
Kiliani fischer synthesis Organic chemistry third edition david klein
Organic chemistry third edition david klein Is alkane an organic compound
Is alkane an organic compound Halohydrin formation
Halohydrin formation Reaction of grignard reagent with acid chloride
Reaction of grignard reagent with acid chloride Organic chemistry
Organic chemistry Transition state energy diagram
Transition state energy diagram David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Organic chemistry
Organic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Polarimetry organic chemistry
Polarimetry organic chemistry Resonance hybrid
Resonance hybrid Rancidity chemical reaction
Rancidity chemical reaction Organic chemistry
Organic chemistry Organic composition definition
Organic composition definition Canola oil
Canola oil Organic chemistry reaction pathways
Organic chemistry reaction pathways Organic chemistry
Organic chemistry Meth eth but prop
Meth eth but prop What is the leveling effect organic chemistry
What is the leveling effect organic chemistry Organic chemistry
Organic chemistry Prop but
Prop but Organic synthesis via enolates
Organic synthesis via enolates Chemistry-ethics case studies
Chemistry-ethics case studies Hybridisation
Hybridisation Organic chemistry vocabulary
Organic chemistry vocabulary Propyl bromide
Propyl bromide Organic chemistry
Organic chemistry Hhcchh
Hhcchh Grade 10 organic chemistry
Grade 10 organic chemistry Ir spectroscopy
Ir spectroscopy What is the displayed formula
What is the displayed formula Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Radicals
Radicals Organic chemistry nomenclature
Organic chemistry nomenclature Ario organic chemistry
Ario organic chemistry Organic chemistry
Organic chemistry C5h12
C5h12 Ester organic chemistry
Ester organic chemistry Organic chemistry nomenclature
Organic chemistry nomenclature Organic chemistry formulas
Organic chemistry formulas How is cracking done
How is cracking done Macromolecule cheat sheet
Macromolecule cheat sheet Priority of functional groups
Priority of functional groups Organic chemistry
Organic chemistry Ferrox test for oxygen
Ferrox test for oxygen Organic chemistry
Organic chemistry Chapter 7 organic chemistry
Chapter 7 organic chemistry Organic chemistry
Organic chemistry Organic chemistry laboratory ch 2540 manual
Organic chemistry laboratory ch 2540 manual Organic chemistry topic 11
Organic chemistry topic 11 Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry The art of writing reasonable organic reaction mechanisms
The art of writing reasonable organic reaction mechanisms Ee organic chemistry
Ee organic chemistry Octane lewis structure
Octane lewis structure How to calculate percent yield in organic chemistry
How to calculate percent yield in organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Functional groups in organic chemistry
Functional groups in organic chemistry What is organic chemistry like
What is organic chemistry like Chemistry organic
Chemistry organic Mass spec of chlorine
Mass spec of chlorine Organic chemistry
Organic chemistry Organic and biochemistry
Organic and biochemistry Mind map organic chemistry
Mind map organic chemistry Organic chemistry lab report format
Organic chemistry lab report format Organic chemistry
Organic chemistry Entane
Entane Cis-2 3-dimethyloxirane
Cis-2 3-dimethyloxirane Brooklyn college organic chemistry
Brooklyn college organic chemistry Danswer
Danswer A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Nbs chemistry
Nbs chemistry Cycloalkanes
Cycloalkanes Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers