Internal Quality Auditing Terminologies TERMINOLOGIES Fitness for use





























































































- Slides: 100

• Internal Quality Auditing • Terminologies

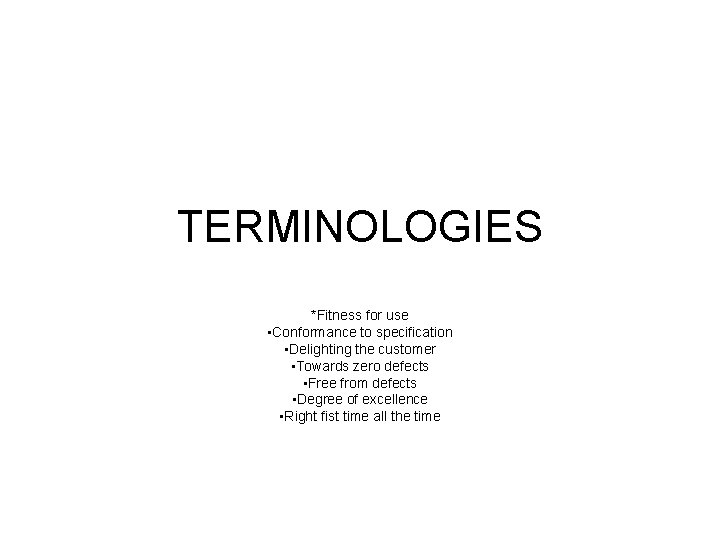
TERMINOLOGIES *Fitness for use • Conformance to specification • Delighting the customer • Towards zero defects • Free from defects • Degree of excellence • Right fist time all the time

Quality Inspection State (QI) Inspection is: • Conformity evaluation by observation and judgment accompanied as appropriate by measurement, testing or gauging (3. . 8. 2) • After-the-event screening process • Application point within the process • Thirty party inspection or operator sell inspection • Decision scrap, rework, medication, concession • No prevention content-does not provide any basis for process improvement

Outline • Definition of quality and other related terms • Historical development of quality management • Functions affecting quality • Achieving quality assurance

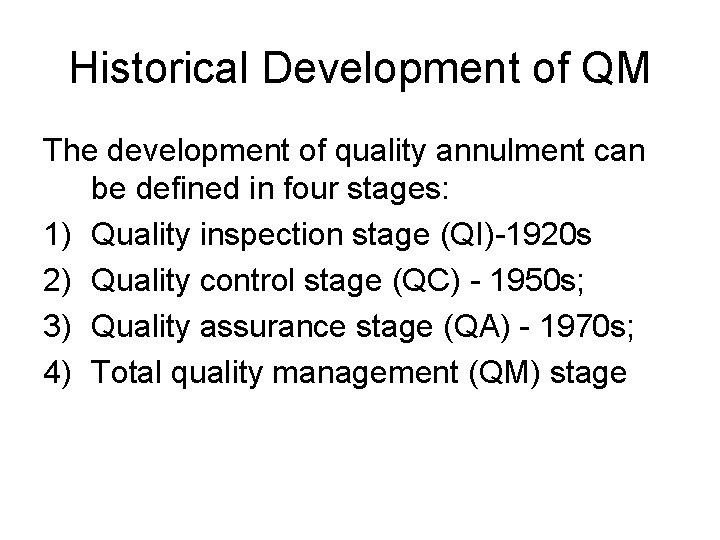
Historical Development of QM The development of quality annulment can be defined in four stages: 1) Quality inspection stage (QI)-1920 s 2) Quality control stage (QC) - 1950 s; 3) Quality assurance stage (QA) - 1970 s; 4) Total quality management (QM) stage

Quality Assurance Stage (QA) Quality Assurance is: • Part of quality management focused on providing confidence that quality requirements will be fulfilled (3. 1. 11) • Issues of progression from QC to QA -Change n emphasis from production to process • Third level of quality development -Represents a shift from detection to prevention -Planed and systematic actions defined • Existence of motive quality system –ISO 9000 • Gender use of QM tools – SPC, QFD, FMEA • Quality performance and costs • Major customer approval

The ISO 9000 Concept…. • Represents an international consensus on good management practices for a systematic and generic application of principles and practices based on quality • ISO 9000 is a written standard that defines the system that organizations should use to ensure that their products and / or services meet or exceed customer expectations • A set of standardized requirements for quality management systems and is applicable to any organization regardless of it’s size or whether public or private sector

Quality Defined: ISO 9000: 2000 • Degree to which a set of inherent characteristic fulfils requirements (3. 11) -Characteristic – distinguishing feature, i. e. physical, sensory, temporal or functional etc (3, 5, 1) -Requirement –need or expectation that is stated or implied or obligatory, i. e. custom or common practices for you (3. 1. 2)

Total Quality Management Stage • Represents the most advanced stage of quality development today (TQM was first used in 1985 to describe the Japanese style at quality improvement – Naval Air Command System – UK) • A Management philosophy • Company wide approached centred on application of QM to all aspects of business -Focused on the requirements of the customer satisfaction -Participation of all employees -Recognized the important of suppliers -Aimed at long term success for • Development of organization culture • Continue improvement • Benefit of all employees, stakeholders and society

The ISO 9000 Concept … • ISO 9000 is seen as a vehicle towards TQM • The principal goal of the ISO 9000 Standards is to demonstrate quality assurance • “Quality culture” refers to the degree of awareness, commitment, collective attitude and behaviour of the organization with regard to quality

What is Quality Management? • Quality Management (QM) • Coordinated activities to direct and control an organization with regard to quality (3, 2, 8) • Management System (SM) • System to establish policy and objectives and to achieve those objectives (3, 2, 2) • Quality Management System (QMS) • Management system to direct and control and organization with regard to quality (3, 2, 2)

Quality: Meeting Requirements • Specifications are imprecise means of conveying subjective aspects i. e. -Not everything is measurable, e. g. courtesy or friendliness • Thus, conformance to requirements is not necessarily all there is to achieving quality

QUALITY CLASSIFICATION Product quality may be classified into two categories; Quality of design • Quality of conformance

FUNCTIONS AFFECTING QULTITY QUALITY LOOP

Quality: Customer Satisfaction • Customer Satisfaction or is it meeting requirements? • Only true measure of acceptable quality. . . -Takes account of both subjective and objective interpretations of needs and expectations -Correct interpretation of needs and expectation. . Acceptable quality

TERMINOLOGIES • QUALITY POLICY Overall intentions and direction of an organization related to quality as formally expressed by top management QUALITY OBJECTIVES Something sought, or aimed for, related to quality

ACHIEVING QUALITY ASSURANCE The organization’s concern is to provide quality products / services To succeed the company must offer products that fulfill the following objectives. • Meet well define need, use or purpose • Satisfy customer’s expectations • Comply with applicable standards and specifications • Comply with statutory requirements • Are made available to competitive prices • Are provided at a cost that will yield profits

TO MEET THIS OBJECTIVES The company must organize itself in a way that: The technical ) Administrative ) Control Human factors ) Hence the need for a quality system

• Internal Quality auditing to ISO 19011: 2000

Definitions (1) Audit A systematic and independent examination to determine whether quality activities and related results comply with the planned arrangements and whether these arrangements are implemented effectively and are suitable to achieve the objectives

Auditing Standard • IS 0 19011 : 2002 Guidelines on Quality and / or Environmental Systems Auditing

Outline • • Definition of a quality audit Types of audit Purpose and objectives of internal audits Creating an environment for successful audits • Benefits of an effective audit process

Definitions (2) • Quality Audit Systematic, independent and documented process for obtaining audit evidence and evaluating it objectively to determine the extent to which audit criteria are fulfilled.

Types of audit 3 types • Process audits • Product audits • System audits

Process audits • Evaluation of the content and effectiveness of specific processes and work activities • To confirm the process parameters and improve capability of the process • To ensure the realization of process quality characteristics • To ensure improvement of process control during service provision

Product audits -To identify opportunities for improvement to establish the quality level of units before final inspection and testing -To establish the capability of the inspection function -To determine the usefulness of inspection/tests

Audits are distinguished by the party requesting: • 1 st party audit (internal audits) – the auditee audits own quality system according to a quality standard. • 2 nd part audits (supplier audits) – the customer audits the supplier’s quality system • 3 rd party audits – these are external certification audits by an independent institution in order to certify the quality system

Products audits Investigation of products conformance to specified characteristics -To obtain additional neutral assessment of product’s level of quality -To obtain additional assurance that specified quality requirements are met

System audits • Evaluation all the elements of the quaity system in order to: • Verify usefulness, suitability and effectiveness • Verify adequate documentation • Verify compliance with requirements • Determine weak points

Purpose of audits • Registration / certification audit • Verify that the organization's QMS meet the requirements of ISO 9001 : 2000 • Internal audit • Identify opportunities for improvement • Maintain ISO 9001 certification

Objectives of the Internal audit • To verify conformance to applicable standards • To verify conformance to documented procedures • To verify effectiveness of the processes in the system • To identify opportunities to improve the system

Creating and environment for successful audits (1) • Gaining management support -Documenting audit findings in away that ensures management understands the effect on system -Linking non-conformities to losses especially in income -Making clear the consequence of deviating form system requirements e. g. y quantifying the losses

Benefits of an effective audit process • Reduced operating costs through better efficiency • Improved customer satisfaction • Improved morale • Reduced barriers between departments • Survival through continual improvement

Creating an environment for successful audits (2) • Gaining employees support -Provide basic training so people understand the purpose of the audit -Ensuring people understand their roles -Making clear that the auditees is the customer of the audit not the ‘victim’ -Ensuring that punitive action is not taken based on audit findings

• Audit Planning and Scheduling

Audit Planning and Scheduling The MR plans the audit • The schedule is drawn by the MR well in advance in consultation with the Management Review Board • The schedule must be comprehensive to ensure that each activity is examined at least once in a year MR has overall responsibility

Drawing an Audit Schedule (2) • Frequency of audits is determined by: - Status of activity - Importance of activity • Provide for unscheduled audits - Due to failures in the system - Due to new features added to the systme

Outline • Responsibilities for planning and scheduling • Drawing of a schedule • Selecting auditors • Audit cycle

Drawing an Audit Schedule (1) Can be: • Departmental • Section • Project • Corporate Period covered by schedule usually 12 months

Selecting Auditors Identify independent auditors • Train them to form a pool of auditors • Assign them the audit task

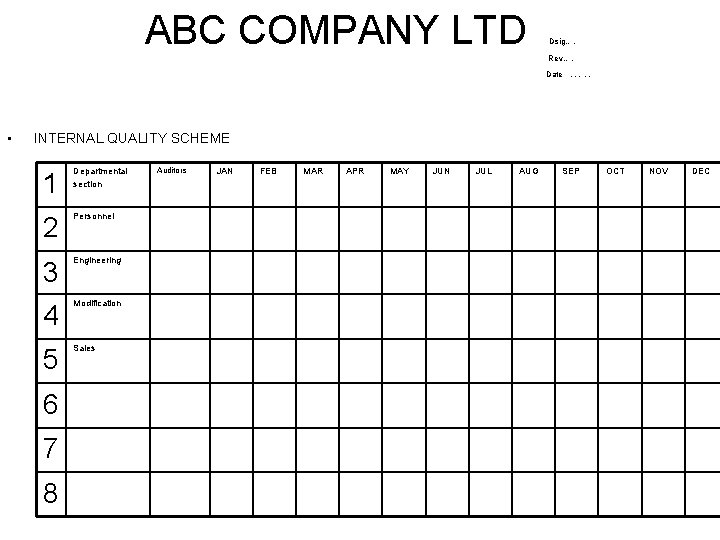
ABC COMPANY LTD Dsig…. Rev…. Date • …. . INTERNAL QUALITY SCHEME 1 Departmental section 2 Personnel 3 Engineering 4 Modification 5 Sales 6 7 8 Auditors JAN FEB MAR APR MAY JUN JUL AUG SEP OCT NOV DEC

The Audit Cycle (2) • Identification of deficiencies or non conformance • Proposal and agreement on corrective action • Corrective action taken • Follow up audit to evaluate effectiveness of corrective action • Report to management

Agenda for Management Review Meeting (5, 6, 2) • • • Review of audits Customer feedback Process performance & product conformity Status of conformity & preventive action Follow up action on previous management reviews • Changes that affect the quality management system and • Recommendations for improvement

The Audit Cycle (1) • Identification of the need for the audit (From the management) • Planning and preparation • Internal audits performed as per the schedule

Management Review • Management review meeting (at least annually) • Management issues the internal audit schedule • After audits Management analyses results of the audit

Preparation for an Individual Audit

2. Establish audit scope 3. Possible ways: • Audit of entire organization at one time • Audit y department, area or product • Audit by process in the quality system

4. Establish audit basis • Usually a combination of the quality system standard and the system documentation (manual, plans, procedures, work instructions, standard specification, etc)

1. Establish audit type and purpose Especially if the audits are: • Unscheduled • Occasioned by a problem in the system

3. Establish audit personnel • Usually appointed by MR • Must be independent of area being audited • Must have sound understanding of area to be audited • Usually from pool of trained auditors • Team leaders should be identified

5. Review basis documentation • A good understanding of the basis documentation very important • For the standard may choose to underline all sentences with the work shall • For system documentation useful to start with the manual, followed by procedures, etc

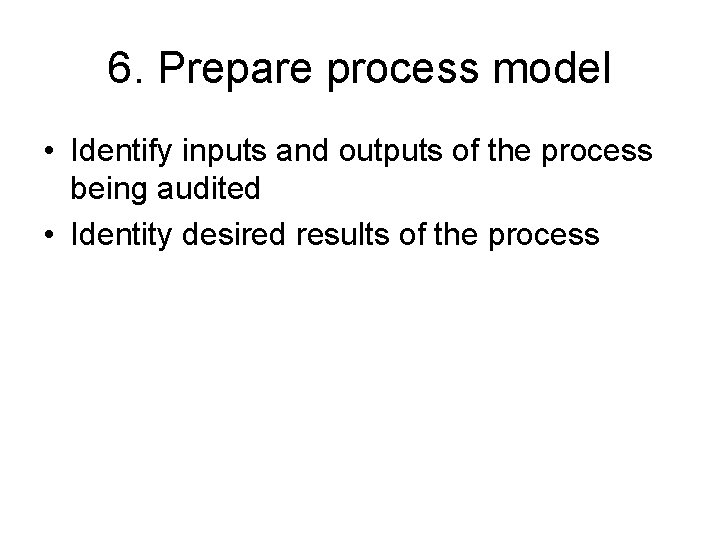
6. Prepare process model • Identify inputs and outputs of the process being audited • Identity desired results of the process

8. Notify the auditee • • • Notify auditee in advance of: Time Date The scope The basis Timetable of the audit issue notification in writing

Notification Timetable allows auditee to: • Organize who will attend • Make himself available or assign a guide • Organize resources (guide, meeting room etc) Timetable should be sent to auditee well in advance

7. Review previous audit results • Should be done both for internal and external audits • Effectiveness of corrective action from previous audits can be evaluated • Adds value by not focusing on issues that have been fully resolved • Ensures that unresolved problems are revisited until resolved.

Notification

9. Prepare audit checklist • Acts as a reminder / guide during audits • Be based on audit basis document - ISO 9001 : 2000 - Manuals - Procedures - Work instruction • Use key words

Checklist preparation • Ensure the following comes out • Quality system is understood and implemented • Procedures are conformed to • Procedures are effective

Remember • Audit preparation and planning • Carrying out the audit • Reporting audit and follow up 50% 30% 20%

Checklist preparation Audit questions • Use open ended questions which prevent the auditee from answering YES or NO • Do not use leading questions • Open questions start with How, Who, When, what, Which, Why • On your checklist indicate against each question the section of the standard manual or procedure

Performing the Audit

Performing the audit Before start of audit • Auditees management informs personnel in the involved departments about expected audit • Lead auditors and rest of the team must be acceptable to auditee management

Personal attributes • • • God outward impression Ethical Open minded Alert, observant Perceptive Well spoken and reasoning, diplomatic Emotionally stable Calm Self confident Persistent, insistent, through , curious

Outline • • Auditor qualifications Phases of an audit The opening meeting Rules for auditors Objectives evidence Ending difficult situations Raising non-conformities Closing meetings

Auditor qualifications The qualification on an auditor is founded on a solid • Education • Training • Experience

Four phases of an Audit • • Opening meeting Executive of audit Auditor’s meeting Closing meeting

Opening Meeting • Always arrive on time • Keep it brief • Auditee and others to be present • Cover the following - Introductions - Confirm purpose - Confirm Scope and Basic of audit - Confirm timetable (plan)

Performing the audit • • • You my need to start with …. . Carry out audit as per the plan He checklist but don’t be a salve of it You may deviate but don’t get disorganized Met areas of audit with the guide Observe operations at work Interview personnel Ask open ended questions Book all notices, walls, work instruction and procedures Note equipments calibration and maintenance status Don’t ask more than one question at time Don mope listening

Some rules for auditors • • • Keep calm and relaxed Book at the other person eye contact Keep one question at a time Let the other person finish talking Keep the right distance from the person If your question cause uneasiness or uncertainly, formulate differently • Involve all participants • Let the right g

Opening Meeting contd. • • • Confirm guide and his role Not an arbitrator May sign for observations Not to interfere Confirm resources availability (tools, office) Confidentality if need be Confirm time of closing NB: Keep meeting professional Tem leader chairs the meeting Don’t allow auditee to take over the control of the meeting

Audit lozenge • Drawing…. . • Ask one question a time • Wait for answers from the auditee • Book and observe the answer, person

Basic communication skills • • Tone of voice Facial expressions Body language, gestures and posture Listening and not ignoring auditee

Performing the audit CHECK – Compare what you see and hear with what is written in the Standards, Procedures and Manual SHOW ME – Collaborate with evidence e. g. - Are signatures by authorized personnel? - Are documents dated, traceable? RECORD – Objective evidence to confirm that activities conform ( or not) with requirements of QMS -Reference numbers of documents -Persons, department and try to answering - What, Where, When, Who, How etc

Objective Evidence Collect all the details • Exact observation • What was seen / heard • Where • When • Who • Why / Creteria

Handling difficult situations • The nervous, anxious auditee - Clarify purpose of audit light conversation on topic of interest before interview • Angry resentful auditee -Treat with respect and find out causes • Excessively proud auditee - Admire work, then move quickly to business at hand • The easily distracted auditee -Auditee away from source of distraction

Performing the Audit OBJECTIVE / EVIDENCE Data supporting the existence or verify of something

Objective Evidence • • ESTABLISH RECORD EXTRACT CLARIFY the facts the evidence for compliance for significant

Auditors’ Meeting • • Compilation of findings / observation Recording of non – conformities Preparation o summary report Formulation of opinions to be presented at Closing meeting

Raising non-conformities • Any considered deviation to be discussed with auditee • Say what clause it is against • Ensure it is understood • Record the objectives evidence • Commit observation to paper • Have the auditee / guide sign for it

Evaluating Corrective Action ……. . insert table

Response to a Non Conformity? There are two routes with which the response of an organization to a non conformity Route 1: • Correction action to eliminate a detected non-conformity (ISO 9000: 2000 cause (3, 6, 5) • Analysis of cause • Corrective action: action to eliminate the cause of a detected non conformity or other undesirable situation (ISO 9000: 2000 cause 3, 6, 5)

Response at a Non – Conformity? My 2 different sequences or routes; • Depends on the product type or • The situation of the nonconformity; as to which is the correct one to be followed Example: • For software, it is inadvisable to implement a correction until the cause is known • For a hardware, if a low brake paid warning light were to illuminate in a vehicle and you immediately implemented the correction of replacing the brake pads before examining II the sensor was faulty, you might fall to resolve the problem and would have wasted time and resources.

Outline • • Response to non conformity Reviewing responses Objective of the QMS Informed judgment

Response to a Non – Conformity? There are two routes with which the response of an organization to a non-conformity: Route 2. Analysis of cause. Correction: action to eliminate a detected nonconformity (ISO 9000: 2000 cause( 3, 6, 5). Corrective action: action to eliminate the cause of a detected non conformity or other undesirable situation (ISO 9000: 2000 cause (3, 6, 5)

Response to a Non Conformity? • Both Correction and Corrective action should be expected when there is a deducted nonconformity Correction for example, • Correction may involve replacing nonconforming production or replacing an obsolete procedure with the current issue Correction action: • Cannot be taken without list making a determination of the cause of nonconformity • May methods and tools available from simple brainstorming to more complex, systematic problems soling techniques (e. g. root cause analysis, fish bone diagrams, five why's etc

Reviewing responses! • Documentation and objective evidence for corrections causes and corrective action: • Effective corrective action should eliminate the cause; • Corrective action should not be confused with preventive action • Preventive action –to eliminate the cause of a potential nonconformity or other undesirable situation (ISO 9000: 2000 cause 3, 6, 4, )

QMS Objective? • Improvement - Quality improvement (3. 2. 12)= part of quality management focused on increasing the equality to (NOTE the requirements can be related to any aspect such as effectiveness) • Continual improvement (3, 2, 13) = recruiting quality to increase the quality to ……. Requirements • Improvement - Further requirements specify the need for continual improvements to the quality management system – not just sporadic quality campaigns - The term “continual improvement” is stated 10 times in the standard (5, 8 important) - The term “improvement” is stated 27 times in the standard

Informed Judgment Conclusion drawn (positive and negative) findings • Number of major nonconformities raised • Number of nonconformities raised during audit of documentation • Number of nonconformities raised during audit of implementation • Number of nonconformities related to the effectiveness of the QMS • Number of non conformities raised against the clause of the standard • Number of nonconformist raised to each • Number of nonconformities raised in each department or area of responsibility

…QMS Objectives? • Effectiveness: - Extent to which planned activities are realized and planned results achieved (ISO 9000: 2000 defn). Effectiveness - ISO 9001 specifies requirements for a quality management system that can be used for internal application by organization, or for certification or for contractual purposes. - It locuses on the effectiveness of the quality management system in meeting customer requirements - The term effectiveness of is state 10 times in the standard (5, 3 important) - The term effectiveness is stated 16 times in the standard (8, 5 important

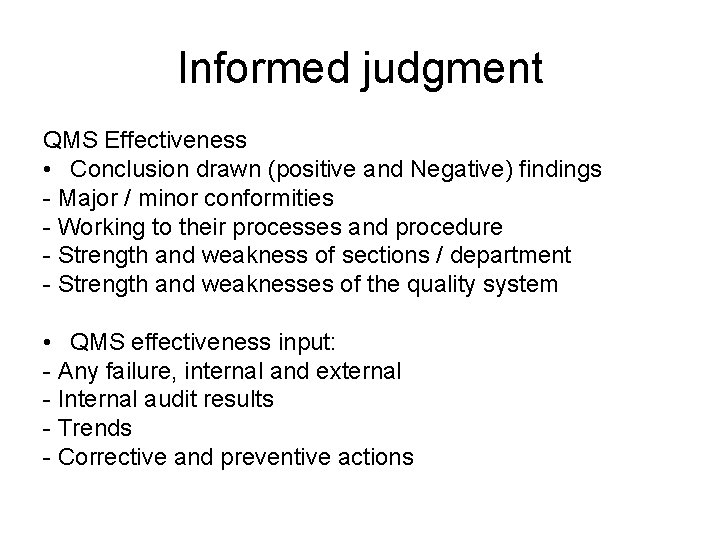
Informed judgment QMS Effectiveness • Conclusion drawn (positive and Negative) findings - Major / minor conformities - Working to their processes and procedure - Strength and weakness of sections / department - Strength and weaknesses of the quality system • QMS effectiveness input: - Any failure, internal and external - Internal audit results - Trends - Corrective and preventive actions

• Reporting and Analysis of audits

Reporting of Audits • Auditors not responsible for carrying out corrective action but should facilitate timely action by: • Issuing audit report immediately (within 24 hrs) • Ensuring that CARs are clear – with reference to finding and specific objective evidence

Reporting of Audits Report should cover the following headings • Date of audit • Scope and purpose of audit • Basis of the audit • Audit tem • Findings - Summary of the non conformities - Observations noted - Opportunities for improvement - Examples of outstanding performance / improvement since last audit • Summary • Attachment (including copies of CARs) The audit report including any nonconformity report/corrective action notices are issued to the auditee or MR

Outline • • Responsibilities for reporting Format for reports Analysis of audits Records and administration follow-up of audits

Reporting of Audits • A standard Proforma for the reporting of audit is usually used • Ensure that positive as well as negative findings are summarized

Reporting of Audits • ISO 19011: 2002 includes recommendations for report content

Analysis of Audits • MR will analyze audit reports to identify areas of common deficiency within QMS • This will initiate changes to the audit schedule • A full analysis of audit reports is presented to management review meeting

Following up Can be done in two ways • By the auditors at an agreed time • By the MR after collection of all reports

Records and administration of audits • The MR should enter the audits in the audit log • Original checklist, audit reports and related documents are retained QMS departmental files as records

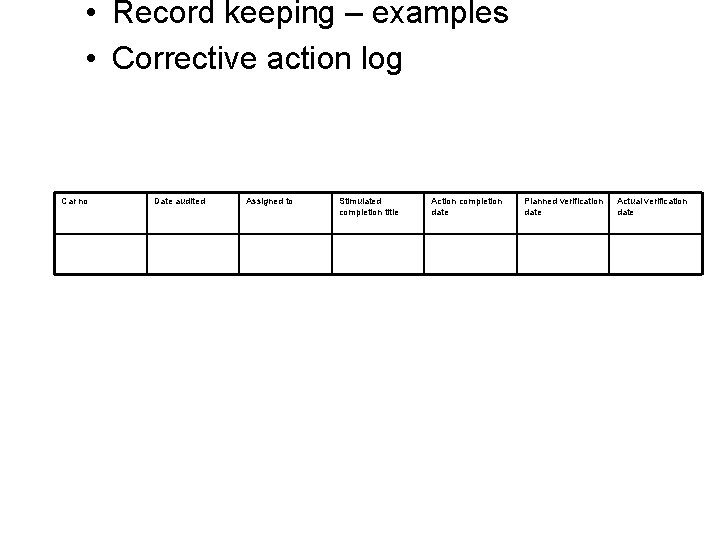
• Record keeping – examples • Corrective action log Car no Date audited Assigned to Stimulated completion title Action completion date Planned verification date Actual verification date
 How do auditing standards differ from auditing procedures
How do auditing standards differ from auditing procedures Parallel simulation audit
Parallel simulation audit Internal auditing assurance & advisory services
Internal auditing assurance & advisory services Credit union internal audit program
Credit union internal audit program Skill related fitness vs health related fitness
Skill related fitness vs health related fitness Itsm terms and terminologies
Itsm terms and terminologies Ethical hacking terminologies
Ethical hacking terminologies Security terminologies and principle
Security terminologies and principle Basic research terminology
Basic research terminology Define dominant trait
Define dominant trait Three authors citation
Three authors citation Fitness club management system project
Fitness club management system project Use case diagram for fitness app
Use case diagram for fitness app Internal control introduction
Internal control introduction Importance of vouching
Importance of vouching Quality control in blood bank
Quality control in blood bank Internal quality control
Internal quality control Internal qc
Internal qc Westgard kuralları
Westgard kuralları 41s westgard rule
41s westgard rule Perform quality assurance
Perform quality assurance Pmp quality management
Pmp quality management What are quality standards in project management
What are quality standards in project management Quality assurance model in nursing management
Quality assurance model in nursing management Compliance vs quality
Compliance vs quality Basic quality concepts
Basic quality concepts Gurus of tqm
Gurus of tqm Crosby quality is free
Crosby quality is free What is tqm
What is tqm Iso 22301 utbildning
Iso 22301 utbildning Typiska drag för en novell
Typiska drag för en novell Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Returpilarna
Returpilarna Shingelfrisyren
Shingelfrisyren En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Kassaregister ideell förening
Kassaregister ideell förening Personlig tidbok fylla i
Personlig tidbok fylla i Anatomi organ reproduksi
Anatomi organ reproduksi Densitet vatten
Densitet vatten Datorkunskap för nybörjare
Datorkunskap för nybörjare Boverket ka
Boverket ka Debattinlägg mall
Debattinlägg mall För och nackdelar med firo
För och nackdelar med firo Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Påbyggnader för flakfordon
Påbyggnader för flakfordon Lufttryck formel
Lufttryck formel Svenskt ramverk för digital samverkan
Svenskt ramverk för digital samverkan Lyckans minut erik lindorm analys
Lyckans minut erik lindorm analys Presentera för publik crossboss
Presentera för publik crossboss Teckenspråk minoritetsspråk argument
Teckenspråk minoritetsspråk argument Plats för toran ark
Plats för toran ark Treserva lathund
Treserva lathund Epiteltyper
Epiteltyper Claes martinsson
Claes martinsson Centrum för kunskap och säkerhet
Centrum för kunskap och säkerhet Verifikationsplan
Verifikationsplan Mat för idrottare
Mat för idrottare Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Rutin för avvikelsehantering
Rutin för avvikelsehantering Smärtskolan kunskap för livet
Smärtskolan kunskap för livet Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tack för att ni har lyssnat
Tack för att ni har lyssnat Referat mall
Referat mall Redogör för vad psykologi är
Redogör för vad psykologi är Stål för stötfångarsystem
Stål för stötfångarsystem Atmosfr
Atmosfr Borra hål för knoppar
Borra hål för knoppar Orubbliga rättigheter
Orubbliga rättigheter R formel
R formel Tack för att ni har lyssnat
Tack för att ni har lyssnat Rita perspektiv
Rita perspektiv Ledningssystem för verksamhetsinformation
Ledningssystem för verksamhetsinformation Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Toppslätskivling dos
Toppslätskivling dos Mästare lärling modell
Mästare lärling modell Egg för emanuel
Egg för emanuel Elektronik för barn
Elektronik för barn Mantel som bars av kvinnor i antikens rom
Mantel som bars av kvinnor i antikens rom Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning Kung dog 1611
Kung dog 1611 Ellika andolf
Ellika andolf Ro i rom pax
Ro i rom pax Tack för att ni lyssnade
Tack för att ni lyssnade Mindre än tecken
Mindre än tecken Bra rim texter
Bra rim texter Inköpsprocessen steg för steg
Inköpsprocessen steg för steg Rådet för byggkompetens
Rådet för byggkompetens Etik och ledarskap etisk kod för chefer
Etik och ledarskap etisk kod för chefer Expektans eller exspektans
Expektans eller exspektans Myndigheten för delaktighet
Myndigheten för delaktighet Frgar
Frgar Tillitsbaserad ledning
Tillitsbaserad ledning Läkarutlåtande för livränta
Läkarutlåtande för livränta Karttecken brant
Karttecken brant Ramsa geometriska former
Ramsa geometriska former Vishnuiter
Vishnuiter Vad är vanlig celldelning
Vad är vanlig celldelning Bris för vuxna
Bris för vuxna Jätte råtta
Jätte råtta Company confidential internal use only
Company confidential internal use only Confidential internal use only
Confidential internal use only