General Chemistry CHEM 110 Dr Nuha Wazzan Chapter



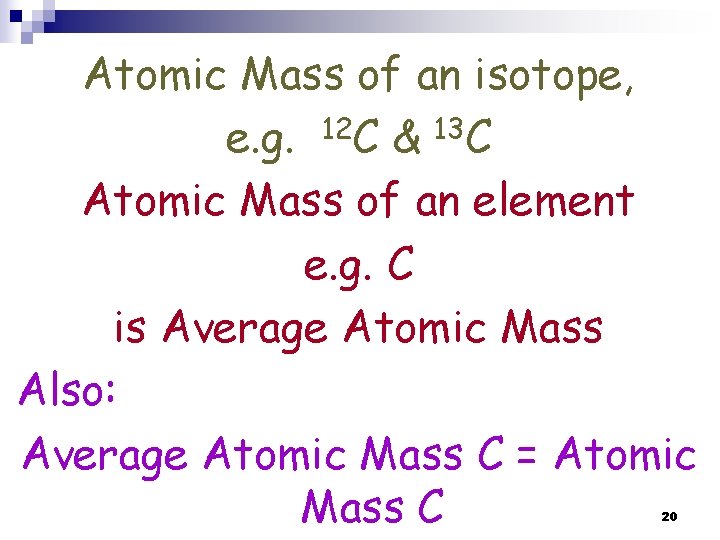

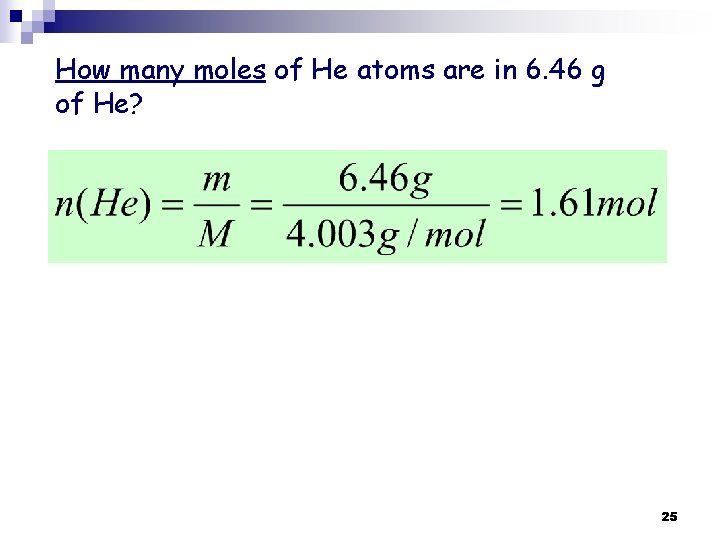





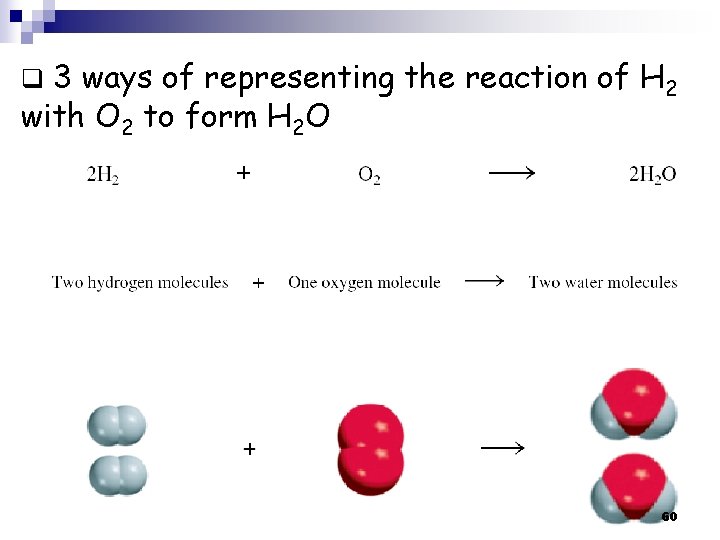




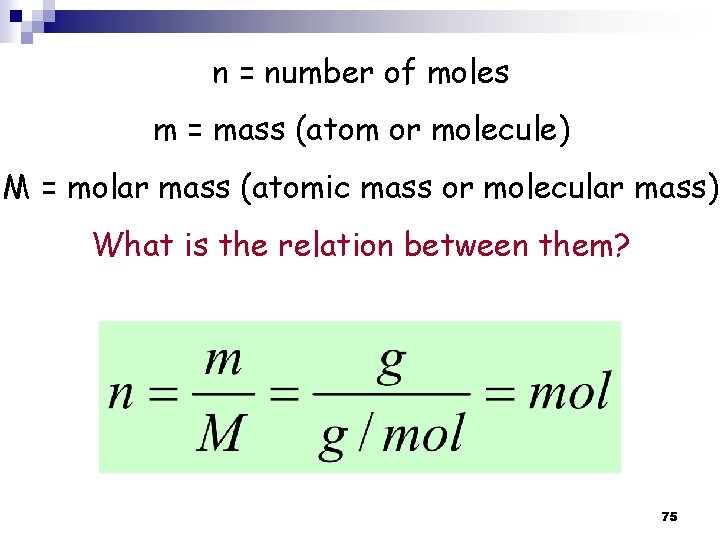





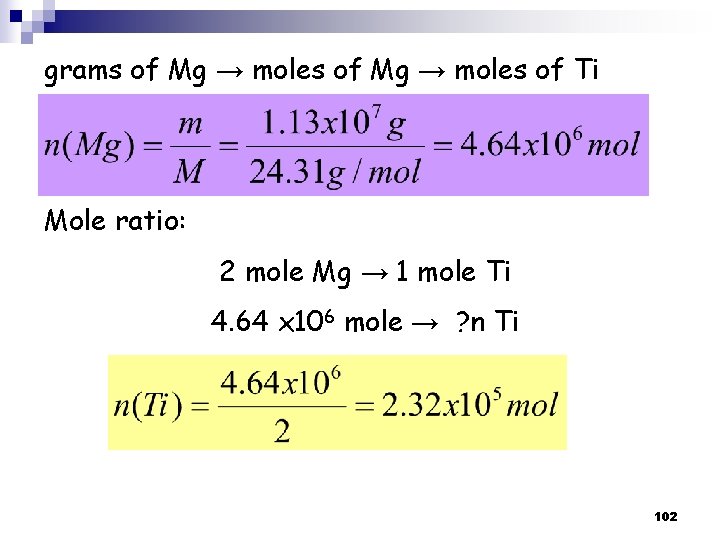


- Slides: 105

General Chemistry CHEM 110 Dr. Nuha Wazzan Chapter 3 Mass Relationships in Chemical Reactions 1

Chapter 3 Mass Relationships in Chemical Reactions Chang Pages 80 -107 H. W. p 110 -114 3. 5, 3. 6, 3. 7, 3. 8, 3. 14, 3. 16, 3. 18, 3. 20, 3. 22, 3. 24, 3. 26, 3. 28, 3. 40, 3. 42, 3. 44, 3. 46, 3. 48, 3. 50, 3. 52, 3. 60, 3. 84, 3. 86 2

Chapter 3: Mass Relationships in Chemical Reactions: Outline 3. 1 Atomic Mass 3. 2 Avogadro’s Number and Molar Mass of an Element 3. 3 Molecular Mass 3. 5 Percent Composition of Compounds 3. 6 Experimental Determination of Empirical Formula 3. 9 Limiting Reagents 3. 10 Reaction Yield 3

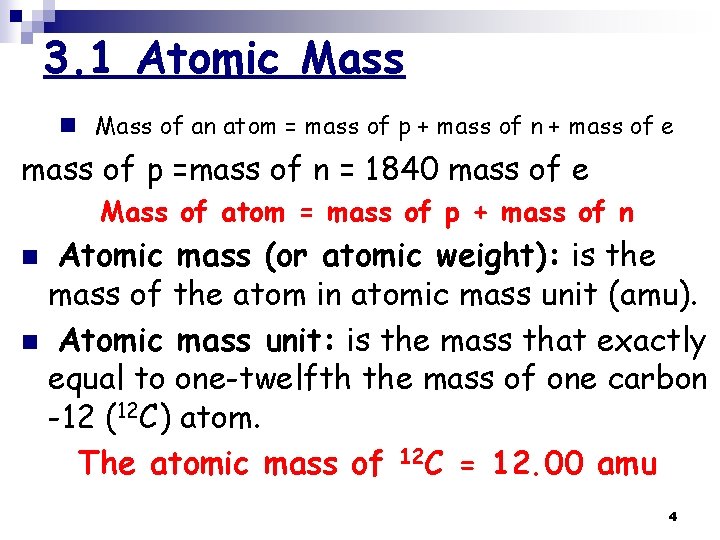
3. 1 Atomic Mass n Mass of an atom = mass of p + mass of n + mass of e mass of p =mass of n = 1840 mass of e Mass of atom = mass of p + mass of n Atomic mass (or atomic weight): is the mass of the atom in atomic mass unit (amu). n Atomic mass unit: is the mass that exactly equal to one-twelfth the mass of one carbon -12 (12 C) atom. The atomic mass of 12 C = 12. 00 amu n 4

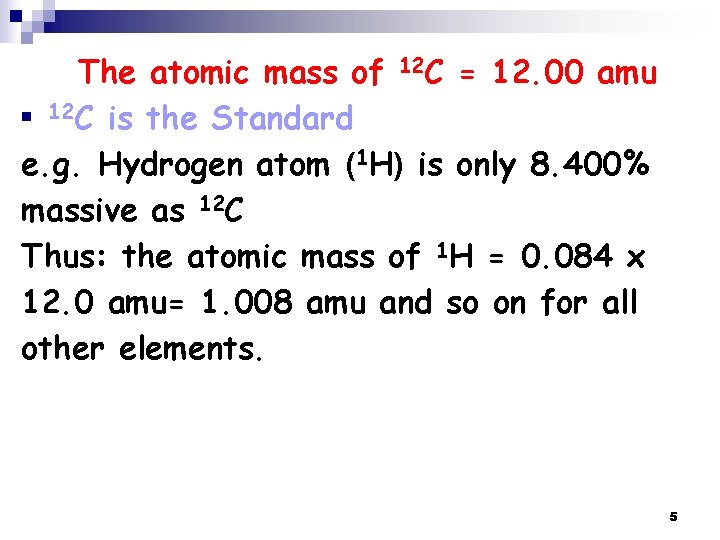
The atomic mass of 12 C = 12. 00 amu n 12 C is the Standard e. g. Hydrogen atom (1 H) is only 8. 400% massive as 12 C Thus: the atomic mass of 1 H = 0. 084 x 12. 0 amu= 1. 008 amu and so on for all other elements. 5

6

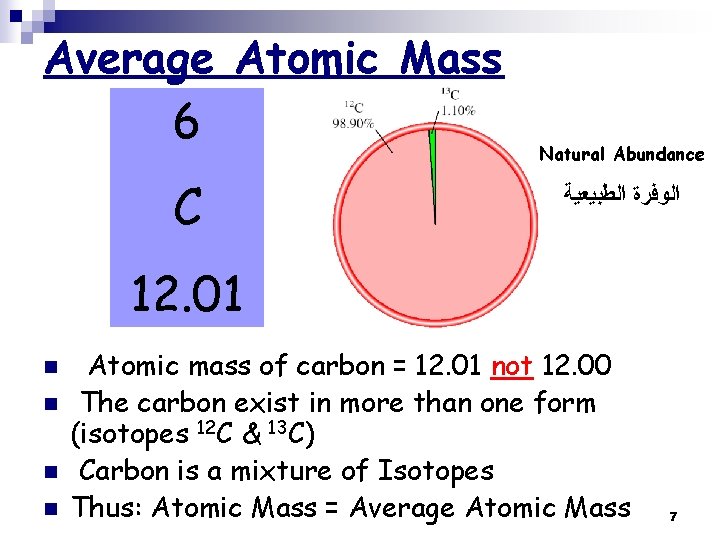
Average Atomic Mass 6 C Natural Abundance ﺍﻟﻮﻓﺮﺓ ﺍﻟﻄﺒﻴﻌﻴﺔ 12. 01 n n Atomic mass of carbon = 12. 01 not 12. 00 The carbon exist in more than one form (isotopes 12 C & 13 C) Carbon is a mixture of Isotopes Thus: Atomic Mass = Average Atomic Mass 7

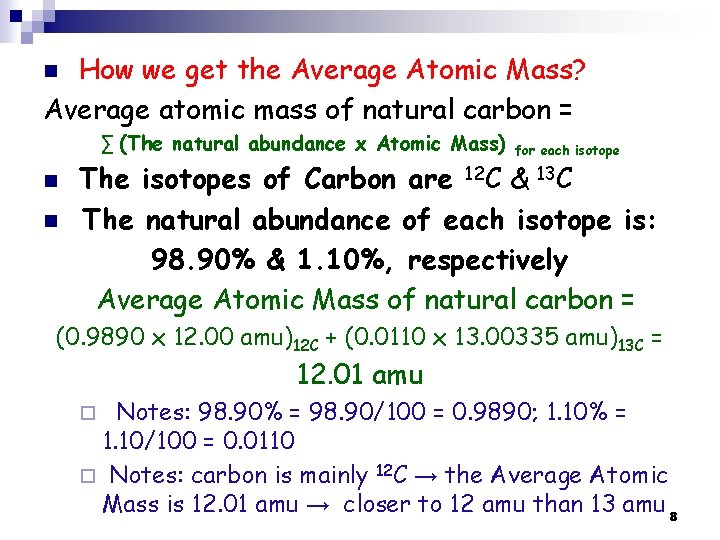
How we get the Average Atomic Mass? Average atomic mass of natural carbon = n ∑ (The natural abundance x Atomic Mass) n n for each isotope The isotopes of Carbon are 12 C & 13 C The natural abundance of each isotope is: 98. 90% & 1. 10%, respectively Average Atomic Mass of natural carbon = (0. 9890 x 12. 00 amu)12 C + (0. 0110 x 13. 00335 amu)13 C = 12. 01 amu Notes: 98. 90% = 98. 90/100 = 0. 9890; 1. 10% = 1. 10/100 = 0. 0110 ¨ Notes: carbon is mainly 12 C → the Average Atomic Mass is 12. 01 amu → closer to 12 amu than 13 amu 8 ¨

Average atomic mass (12. 01) 9

What information would you need to calculate the average atomic mass of an element? (a) The number of neutrons in the element. (b) The atomic number of the element. (c) The mass and abundance of each isotope of the element. (d) The position in the periodic table of the element. 10

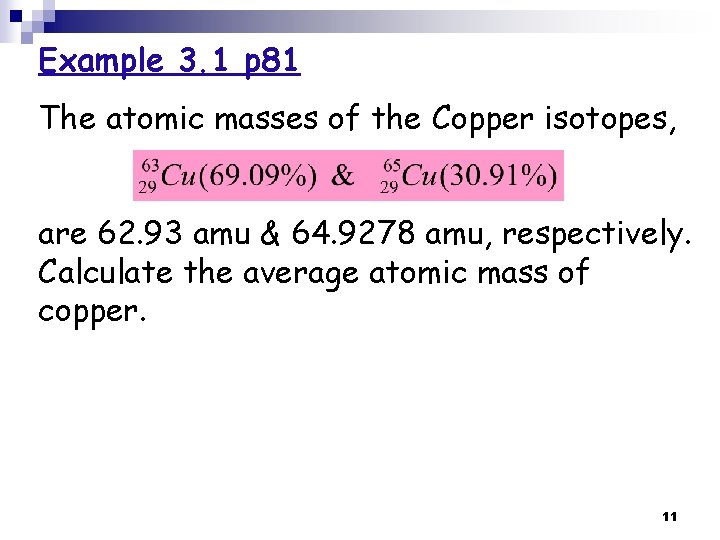
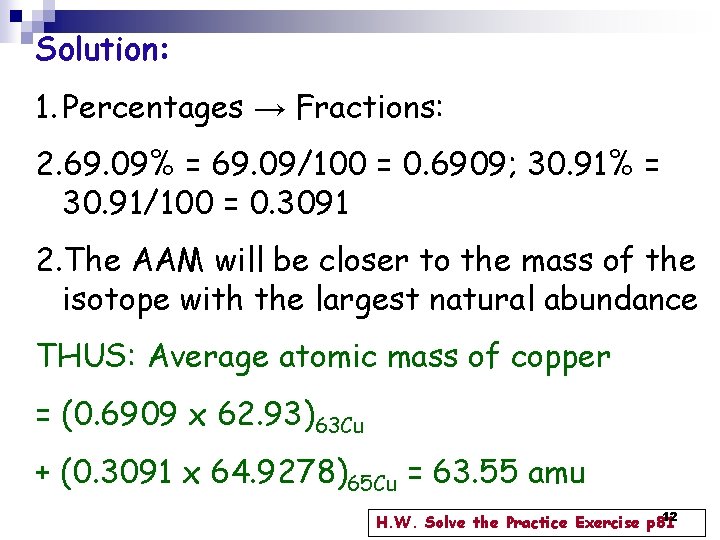
Example 3. 1 p 81 The atomic masses of the Copper isotopes, are 62. 93 amu & 64. 9278 amu, respectively. Calculate the average atomic mass of copper. 11

Solution: 1. Percentages → Fractions: 2. 69. 09% = 69. 09/100 = 0. 6909; 30. 91% = 30. 91/100 = 0. 3091 2. The AAM will be closer to the mass of the isotope with the largest natural abundance THUS: Average atomic mass of copper = (0. 6909 x 62. 93)63 Cu + (0. 3091 x 64. 9278)65 Cu = 63. 55 amu 12 H. W. Solve the Practice Exercise p 81

Iodine has two isotopes 126 I and 127 I, with the equal abundance. Calculate the average atomic mass of Iodine (53 I). (a) 126. 5 amu (b) 35. 45 amu (c) 1. 265 amu (d) 71. 92 amu 13

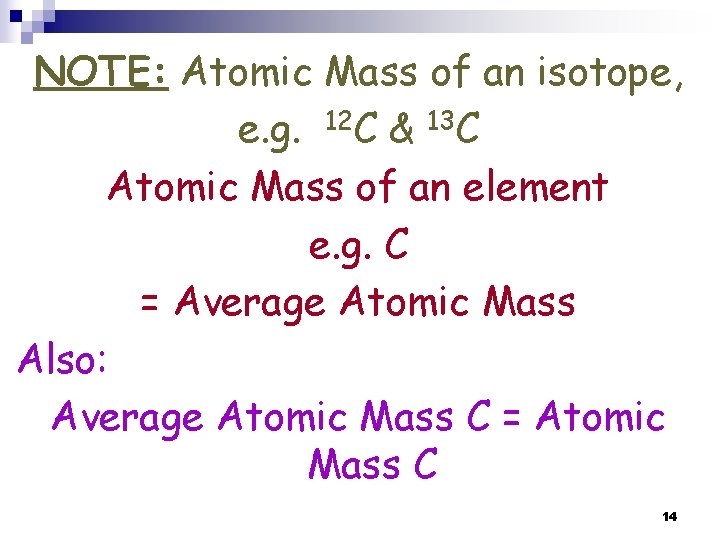
NOTE: Atomic Mass of an isotope, e. g. 12 C & 13 C Atomic Mass of an element e. g. C = Average Atomic Mass Also: Average Atomic Mass C = Atomic Mass C 14

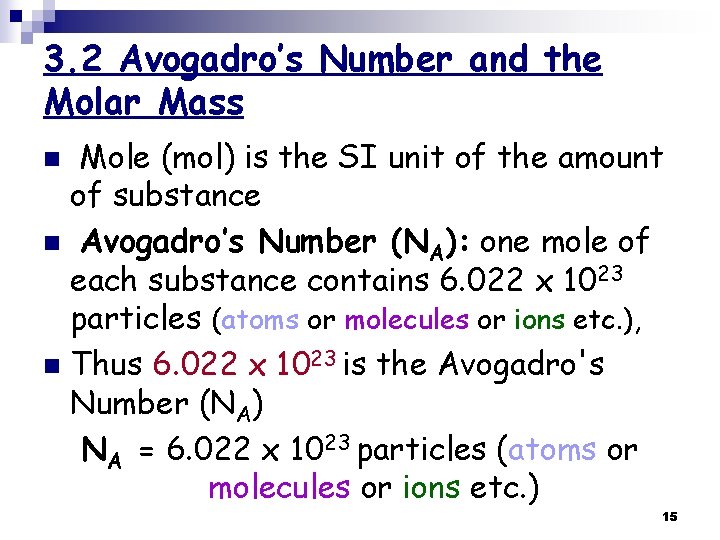
3. 2 Avogadro’s Number and the Molar Mass Mole (mol) is the SI unit of the amount of substance n Avogadro’s Number (NA): one mole of each substance contains 6. 022 x 1023 particles (atoms or molecules or ions etc. ), n Thus 6. 022 x 1023 is the Avogadro's Number (NA) NA = 6. 022 x 1023 particles (atoms or molecules or ions etc. ) n 15

Dozen = 12 1 dozen = 12 Anything 1 mol = 6. 022 x 1023 particles 16

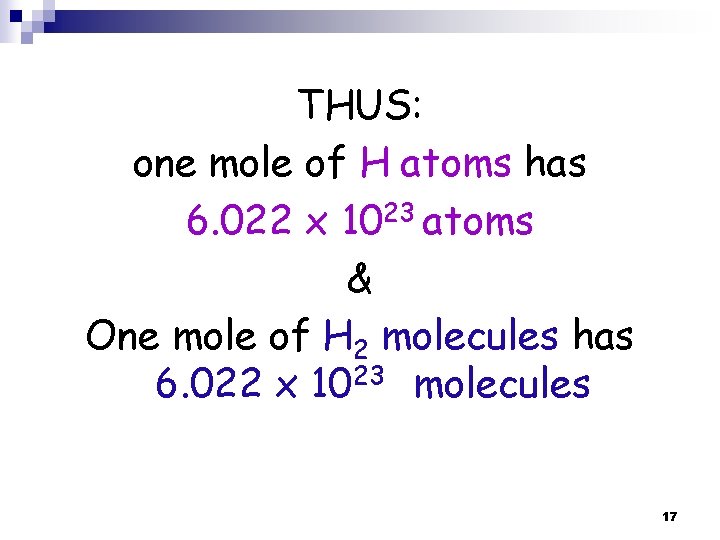
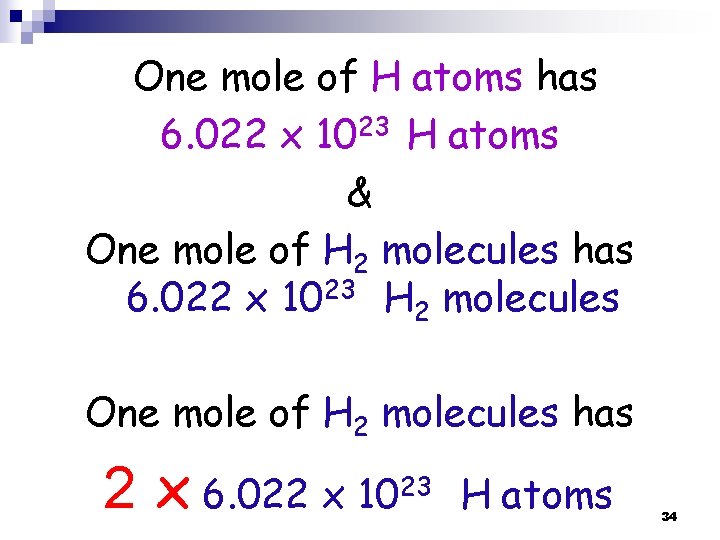
THUS: one mole of H atoms has 6. 022 x 1023 atoms & One mole of H 2 molecules has 6. 022 x 1023 molecules 17

One mole of Carbon One mole of Copper One mole of Sulphur One mole of Mercury One mole of Iron 18

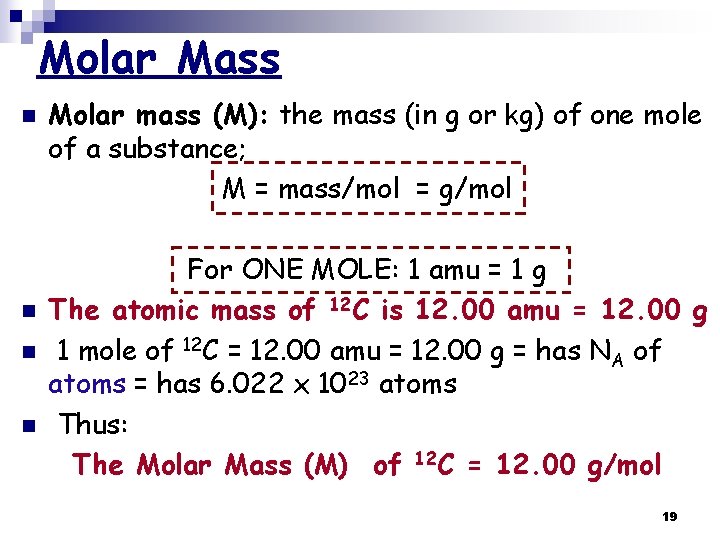
Molar Mass n n Molar mass (M): the mass (in g or kg) of one mole of a substance; M = mass/mol = g/mol For ONE MOLE: 1 amu = 1 g The atomic mass of 12 C is 12. 00 amu = 12. 00 g 1 mole of 12 C = 12. 00 amu = 12. 00 g = has NA of atoms = has 6. 022 x 1023 atoms Thus: The Molar Mass (M) of 12 C = 12. 00 g/mol 19
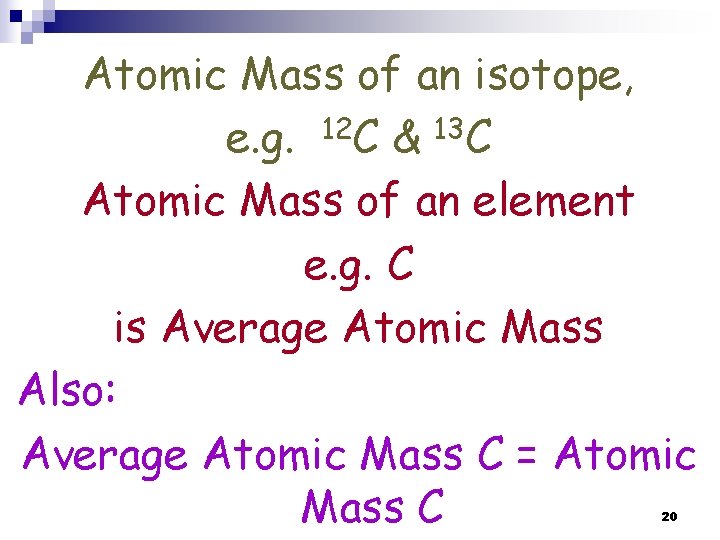
Atomic Mass of an isotope, e. g. 12 C & 13 C Atomic Mass of an element e. g. C is Average Atomic Mass Also: Average Atomic Mass C = Atomic Mass C 20

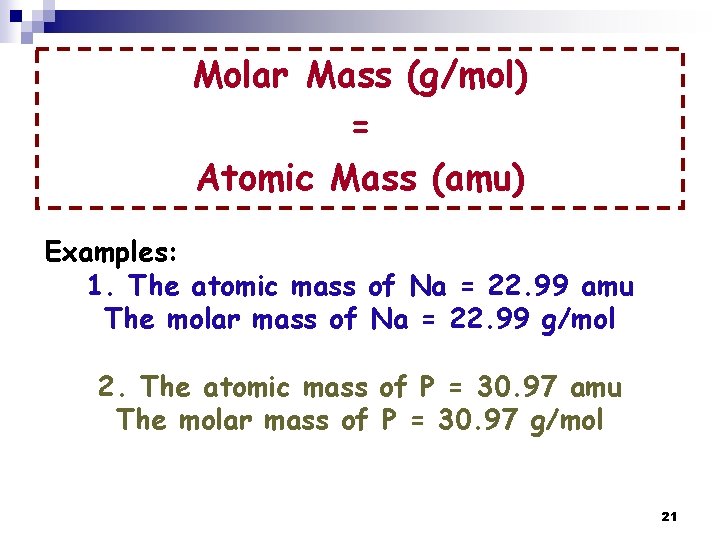
Molar Mass (g/mol) = Atomic Mass (amu) Examples: 1. The atomic mass of Na = 22. 99 amu The molar mass of Na = 22. 99 g/mol 2. The atomic mass of P = 30. 97 amu The molar mass of P = 30. 97 g/mol 21

Example 3. 2 p 83: How many moles of He atoms are in 6. 46 g of He? 22

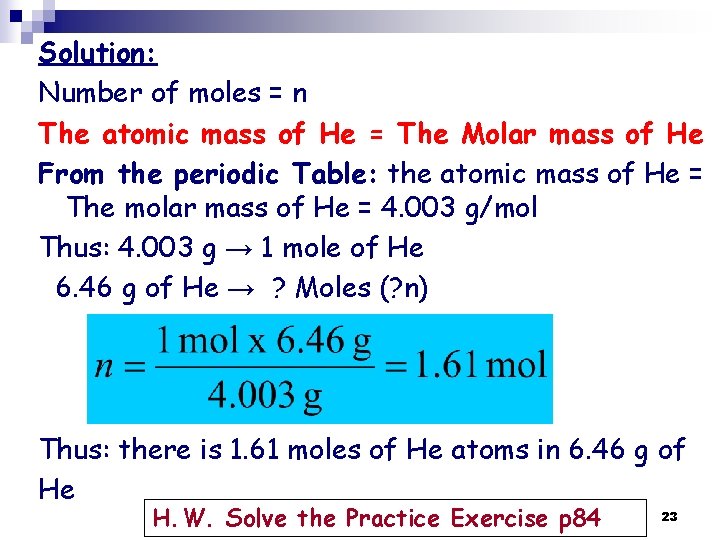
Solution: Number of moles = n The atomic mass of He = The Molar mass of He From the periodic Table: the atomic mass of He = The molar mass of He = 4. 003 g/mol Thus: 4. 003 g → 1 mole of He 6. 46 g of He → ? Moles (? n) Thus: there is 1. 61 moles of He atoms in 6. 46 g of He H. W. Solve the Practice Exercise p 84 23

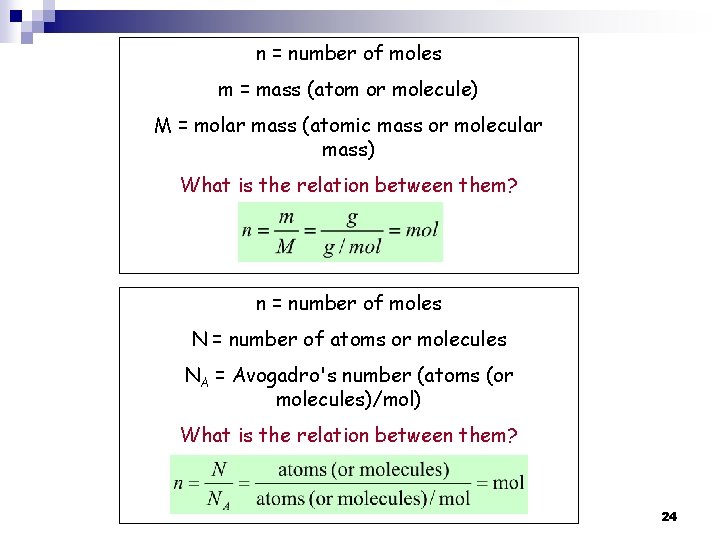
n = number of moles m = mass (atom or molecule) M = molar mass (atomic mass or molecular mass) What is the relation between them? n = number of moles N = number of atoms or molecules NA = Avogadro's number (atoms (or molecules)/mol) What is the relation between them? 24
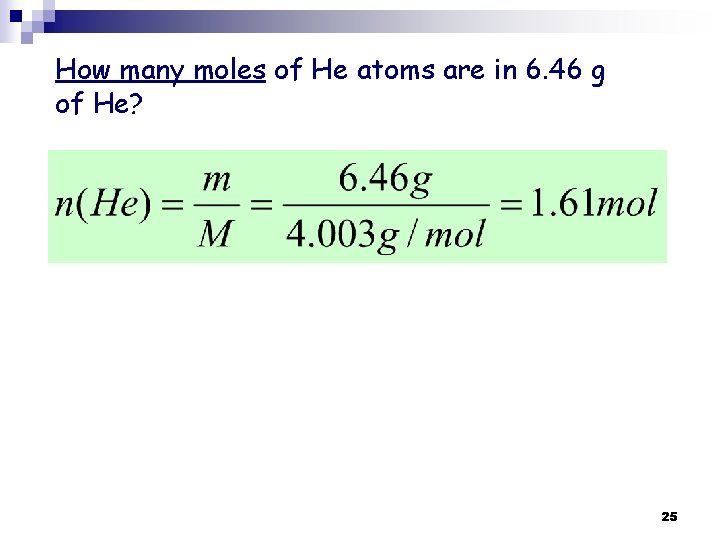
How many moles of He atoms are in 6. 46 g of He? 25

Example 3. 3 p 84: How many grams of Zn are in 0. 356 mole of Zn? 26

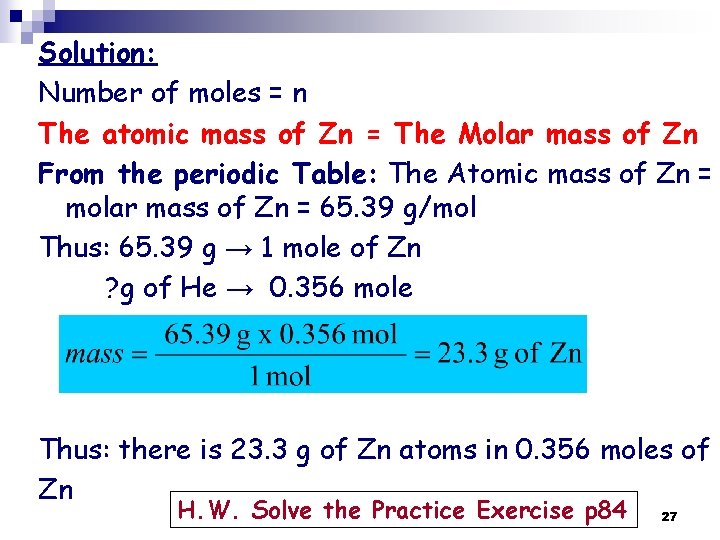
Solution: Number of moles = n The atomic mass of Zn = The Molar mass of Zn From the periodic Table: The Atomic mass of Zn = molar mass of Zn = 65. 39 g/mol Thus: 65. 39 g → 1 mole of Zn ? g of He → 0. 356 mole Thus: there is 23. 3 g of Zn atoms in 0. 356 moles of Zn H. W. Solve the Practice Exercise p 84 27

ﺣﻞ ﻣﺨﺘﺼﺮ How many grams of Zn are in 0. 356 mole of Zn? 28

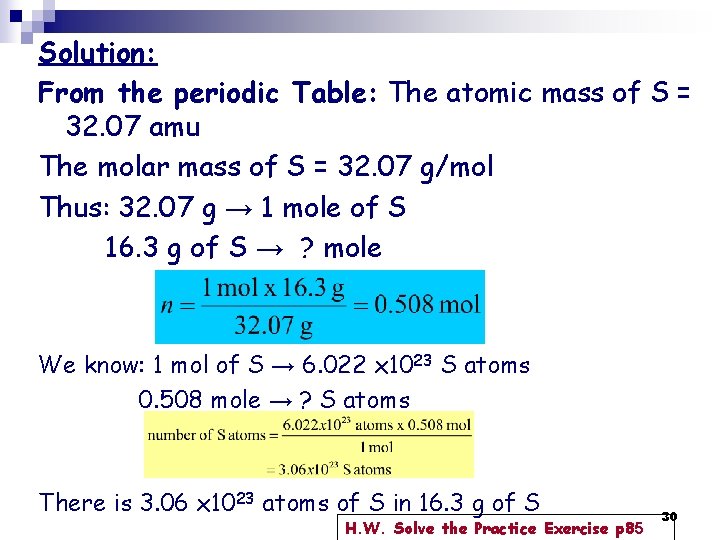
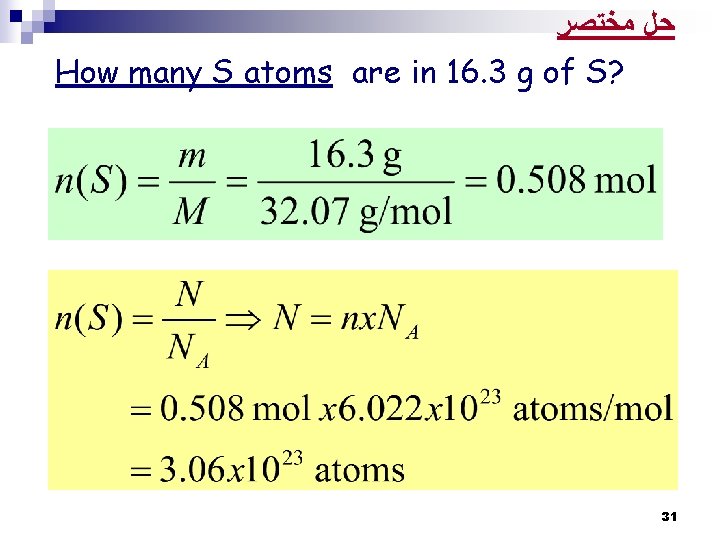
Example 3. 4 p 84: How many S atoms are in 16. 3 g of S? Strategy: 1. How many moles in 16. 3 g of S = X mol 2. 1 mole → 6. 022 x 1023 S atoms X moles → ? atoms 29

Solution: From the periodic Table: The atomic mass of S = 32. 07 amu The molar mass of S = 32. 07 g/mol Thus: 32. 07 g → 1 mole of S 16. 3 g of S → ? mole We know: 1 mol of S → 6. 022 x 1023 S atoms 0. 508 mole → ? S atoms There is 3. 06 x 1023 atoms of S in 16. 3 g of S H. W. Solve the Practice Exercise p 85 30

ﺣﻞ ﻣﺨﺘﺼﺮ How many S atoms are in 16. 3 g of S? 31

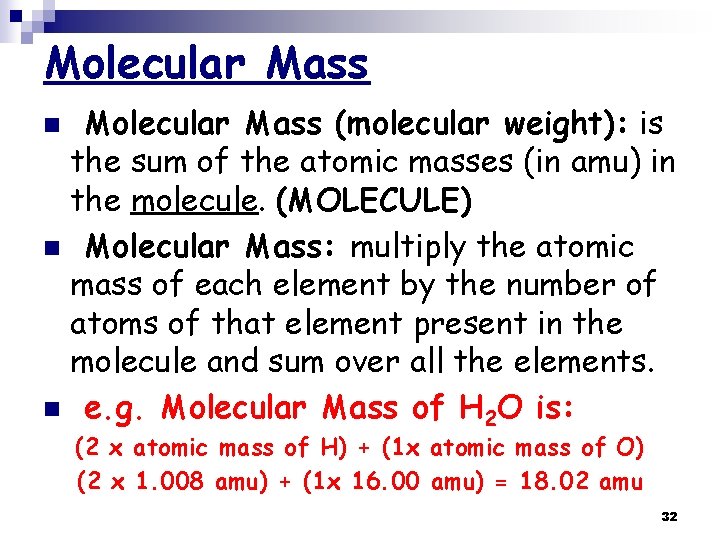
Molecular Mass (molecular weight): is the sum of the atomic masses (in amu) in the molecule. (MOLECULE) n Molecular Mass: multiply the atomic mass of each element by the number of atoms of that element present in the molecule and sum over all the elements. n e. g. Molecular Mass of H 2 O is: n (2 x atomic mass of H) + (1 x atomic mass of O) (2 x 1. 008 amu) + (1 x 16. 00 amu) = 18. 02 amu 32

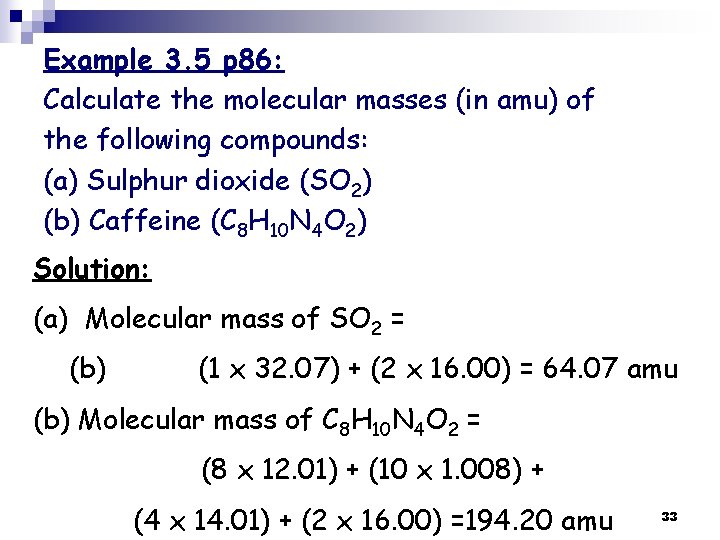
Example 3. 5 p 86: Calculate the molecular masses (in amu) of the following compounds: (a) Sulphur dioxide (SO 2) (b) Caffeine (C 8 H 10 N 4 O 2) Solution: (a) Molecular mass of SO 2 = (b) (1 x 32. 07) + (2 x 16. 00) = 64. 07 amu (b) Molecular mass of C 8 H 10 N 4 O 2 = (8 x 12. 01) + (10 x 1. 008) + (4 x 14. 01) + (2 x 16. 00) =194. 20 amu 33

One mole of H atoms has 6. 022 x 1023 H atoms & One mole of H 2 molecules has 6. 022 x 1023 H 2 molecules One mole of H 2 molecules has 2 x 6. 022 x 1023 H atoms 34

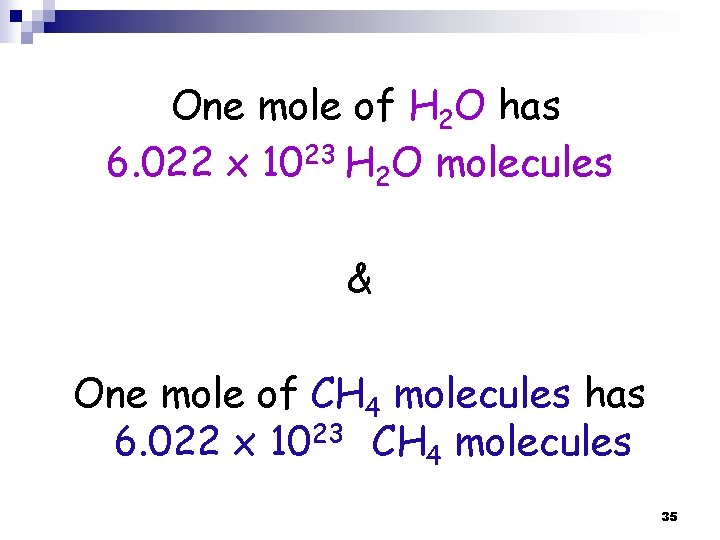
One mole of H 2 O has 6. 022 x 1023 H 2 O molecules & One mole of CH 4 molecules has 6. 022 x 1023 CH 4 molecules 35

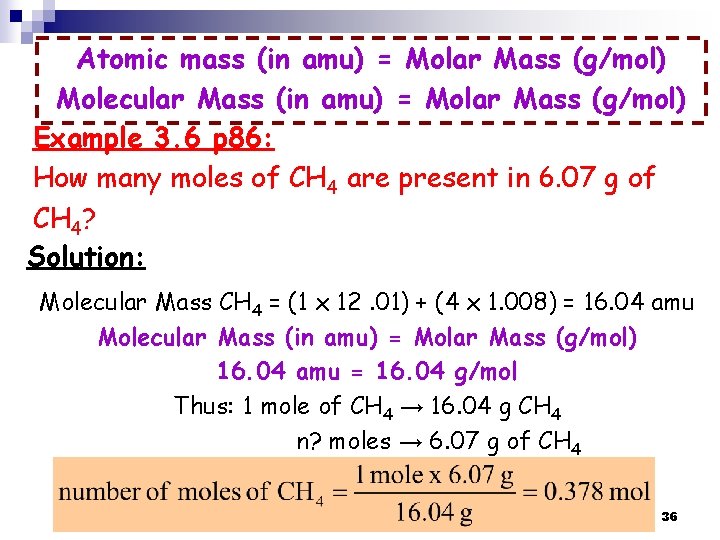
Atomic mass (in amu) = Molar Mass (g/mol) Molecular Mass (in amu) = Molar Mass (g/mol) Example 3. 6 p 86: How many moles of CH 4 are present in 6. 07 g of CH 4? Solution: Molecular Mass CH 4 = (1 x 12. 01) + (4 x 1. 008) = 16. 04 amu Molecular Mass (in amu) = Molar Mass (g/mol) 16. 04 amu = 16. 04 g/mol Thus: 1 mole of CH 4 → 16. 04 g CH 4 n? moles → 6. 07 g of CH 4 36

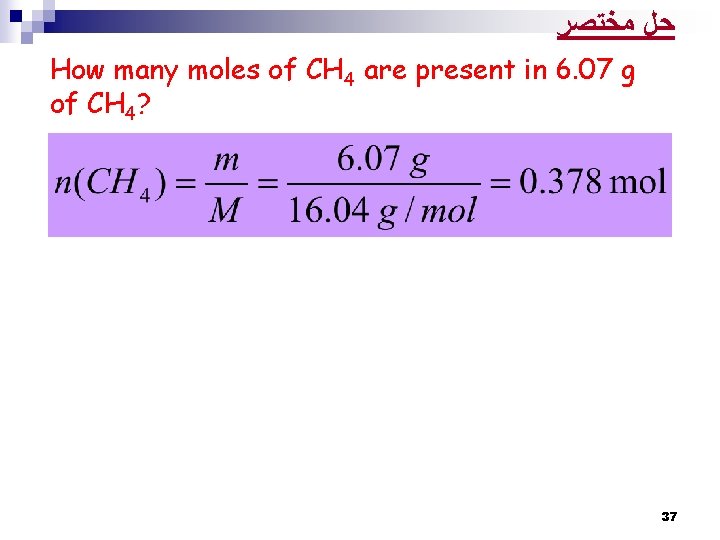
ﺣﻞ ﻣﺨﺘﺼﺮ How many moles of CH 4 are present in 6. 07 g of CH 4? 37

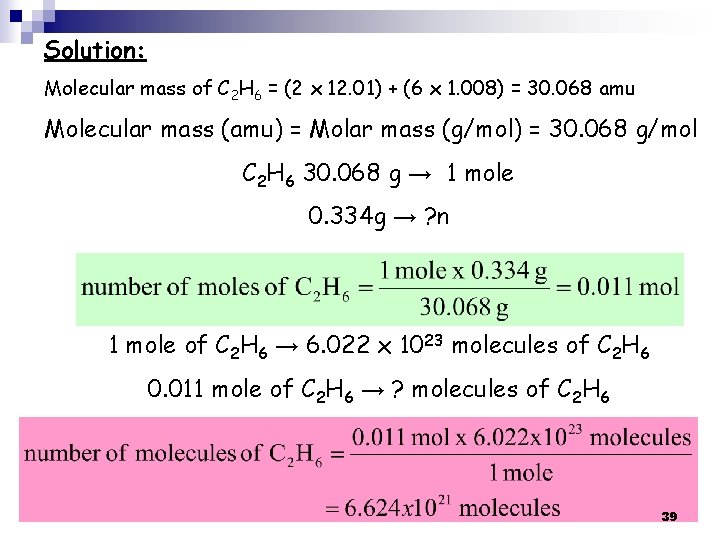
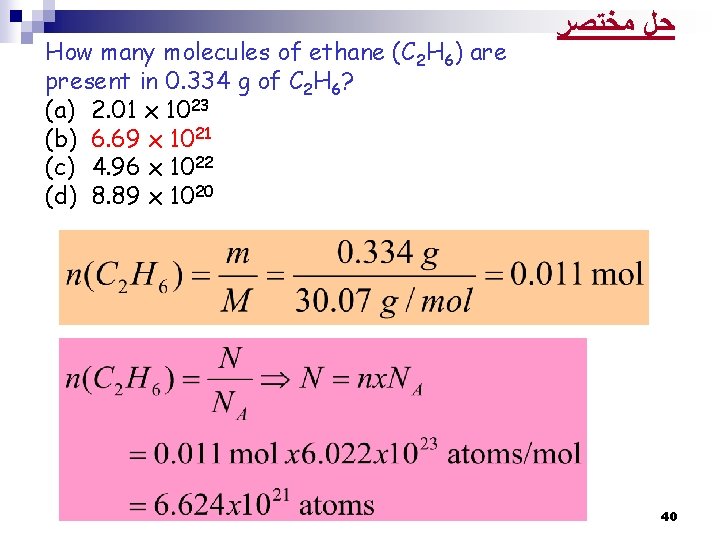
How many molecules of ethane (C 2 H 6) are present in 0. 334 g of C 2 H 6? (a) 2. 01 x 1023 (b) 6. 69 x 1021 (c) 4. 96 x 1022 (d) 8. 89 x 1020 38

Solution: Molecular mass of C 2 H 6 = (2 x 12. 01) + (6 x 1. 008) = 30. 068 amu Molecular mass (amu) = Molar mass (g/mol) = 30. 068 g/mol C 2 H 6 30. 068 g → 1 mole 0. 334 g → ? n 1 mole of C 2 H 6 → 6. 022 x 1023 molecules of C 2 H 6 0. 011 mole of C 2 H 6 → ? molecules of C 2 H 6 39

How many molecules of ethane (C 2 H 6) are present in 0. 334 g of C 2 H 6? (a) 2. 01 x 1023 (b) 6. 69 x 1021 (c) 4. 96 x 1022 (d) 8. 89 x 1020 ﺣﻞ ﻣﺨﺘﺼﺮ 40

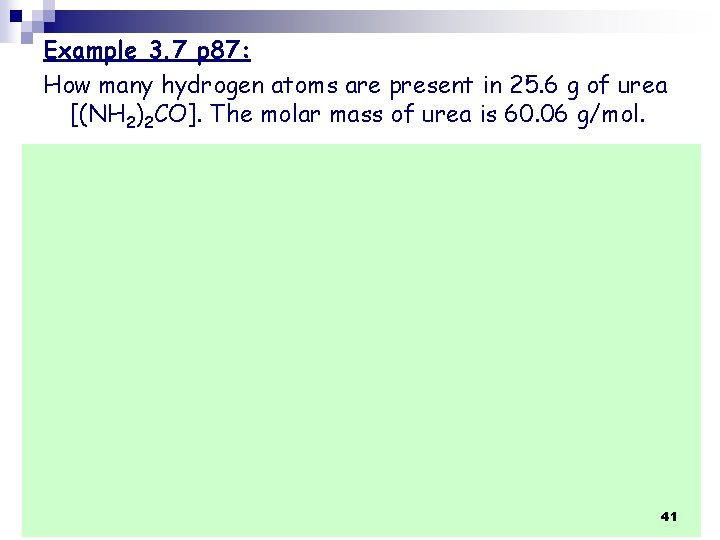
Example 3. 7 p 87: How many hydrogen atoms are present in 25. 6 g of urea [(NH 2)2 CO]. The molar mass of urea is 60. 06 g/mol. 41

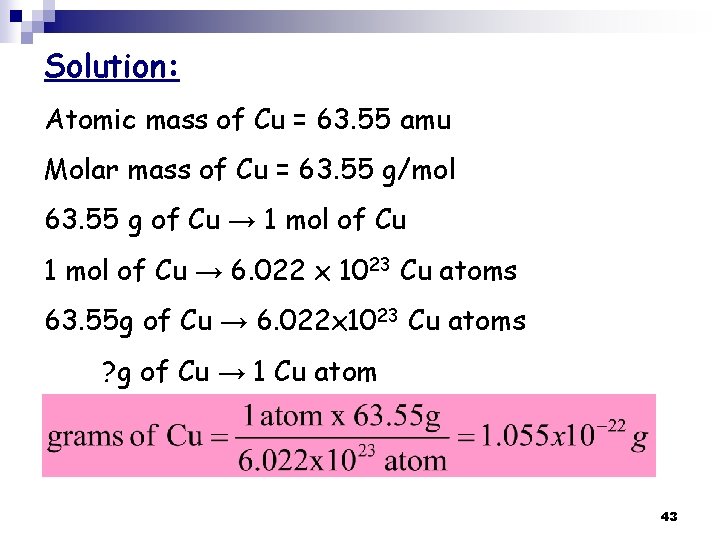
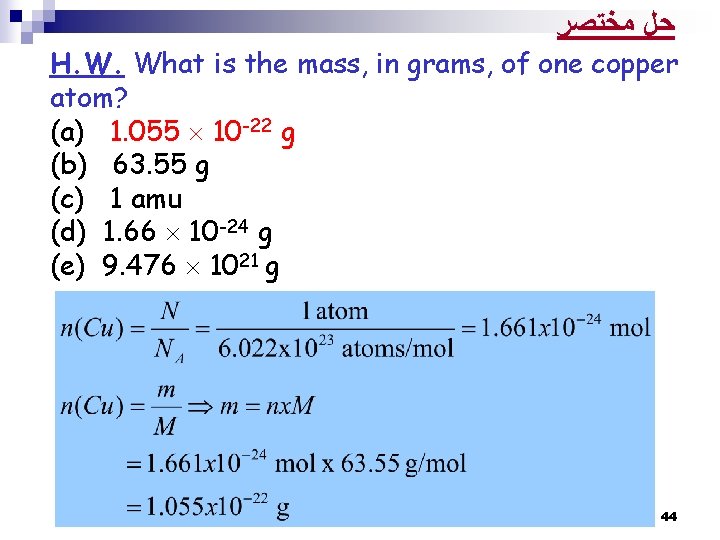
H. W. What is the mass, in grams, of one copper atom? (a) 1. 055 10 -22 g (b) 63. 55 g (c) 1 amu (d) 1. 66 10 -24 g (e) 9. 476 1021 g 42

Solution: Atomic mass of Cu = 63. 55 amu Molar mass of Cu = 63. 55 g/mol 63. 55 g of Cu → 1 mol of Cu → 6. 022 x 1023 Cu atoms 63. 55 g of Cu → 6. 022 x 1023 Cu atoms ? g of Cu → 1 Cu atom 43

ﺣﻞ ﻣﺨﺘﺼﺮ H. W. What is the mass, in grams, of one copper atom? (a) 1. 055 10 -22 g (b) 63. 55 g (c) 1 amu (d) 1. 66 10 -24 g (e) 9. 476 1021 g 44

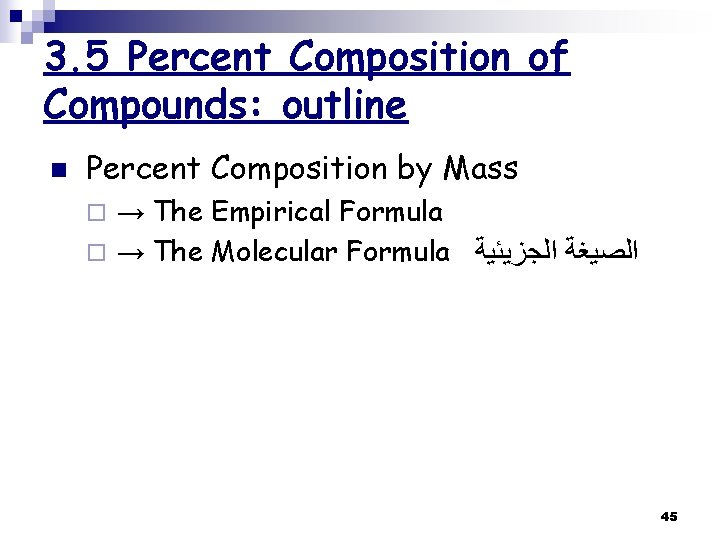
3. 5 Percent Composition of Compounds: outline n Percent Composition by Mass → The Empirical Formula ¨ → The Molecular Formula ﺍﻟﺼﻴﻐﺔ ﺍﻟﺠﺰﻳﺌﻴﺔ ¨ 45

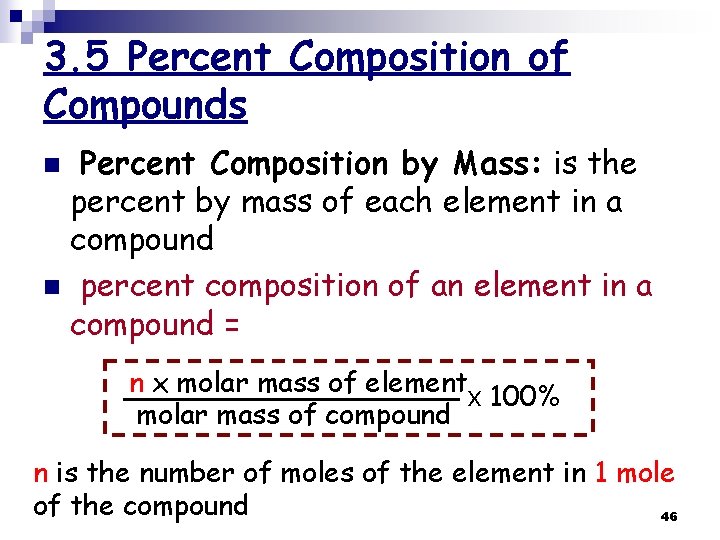
3. 5 Percent Composition of Compounds Percent Composition by Mass: is the percent by mass of each element in a compound n percent composition of an element in a compound = n n x molar mass of element x 100% molar mass of compound n is the number of moles of the element in 1 mole of the compound 46

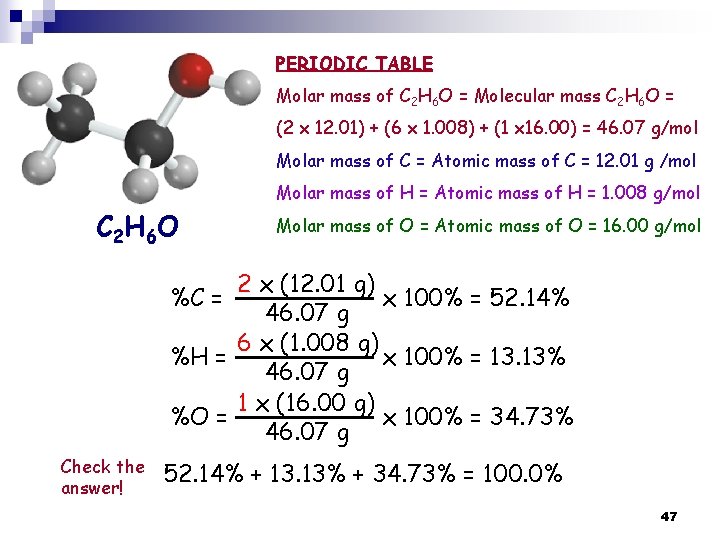
PERIODIC TABLE Molar mass of C 2 H 6 O = Molecular mass C 2 H 6 O = (2 x 12. 01) + (6 x 1. 008) + (1 x 16. 00) = 46. 07 g/mol Molar mass of C = Atomic mass of C = 12. 01 g /mol Molar mass of H = Atomic mass of H = 1. 008 g/mol C 2 H 6 O Molar mass of O = Atomic mass of O = 16. 00 g/mol 2 x (12. 01 g) %C = x 100% = 52. 14% 46. 07 g 6 x (1. 008 g) %H = x 100% = 13. 13% 46. 07 g 1 x (16. 00 g) %O = x 100% = 34. 73% 46. 07 g Check the answer! 52. 14% + 13. 13% + 34. 73% = 100. 0% 47

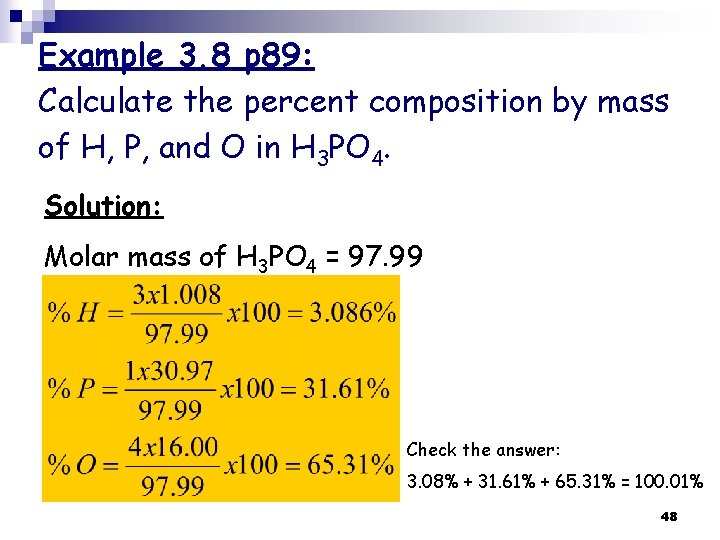
Example 3. 8 p 89: Calculate the percent composition by mass of H, P, and O in H 3 PO 4. Solution: Molar mass of H 3 PO 4 = 97. 99 Check the answer: 3. 08% + 31. 61% + 65. 31% = 100. 01% 48

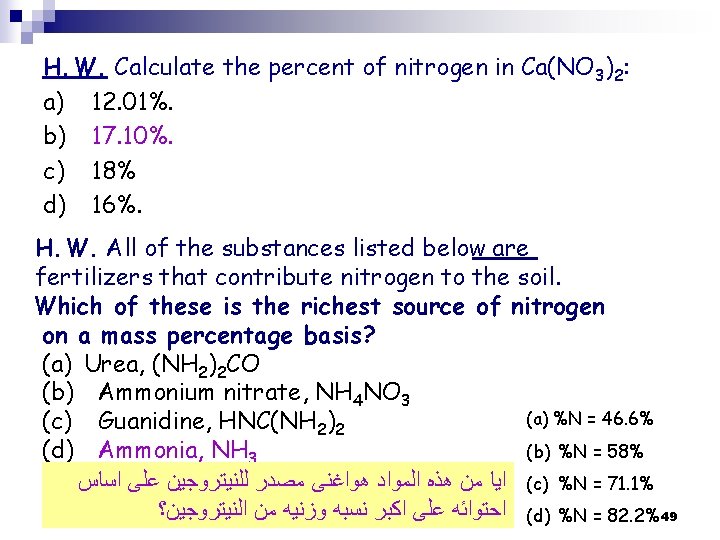
H. W. Calculate the percent of nitrogen in Ca(NO 3)2: a) 12. 01%. b) 17. 10%. c) 18% d) 16%. H. W. All of the substances listed below are fertilizers that contribute nitrogen to the soil. Which of these is the richest source of nitrogen on a mass percentage basis? (a) Urea, (NH 2)2 CO (b) Ammonium nitrate, NH 4 NO 3 (a) %N = 46. 6% (c) Guanidine, HNC(NH 2)2 (b) %N = 58% (d) Ammonia, NH 3 ( ﺍﻳﺎ ﻣﻦ ﻫﺬﻩ ﺍﻟﻤﻮﺍﺩ ﻫﻮﺍﻏﻨﻰ ﻣﺼﺪﺭ ﻟﻠﻨﻴﺘﺮﻭﺟﻴﻦ ﻋﻠﻰ ﺍﺳﺎﺱ c) %N = 71. 1% ( ﺍﺣﺘﻮﺍﺋﻪ ﻋﻠﻰ ﺍﻛﺒﺮ ﻧﺴﺒﻪ ﻭﺯﻧﻴﻪ ﻣﻦ ﺍﻟﻨﻴﺘﺮﻭﺟﻴﻦ؟ d) %N = 82. 2% 49

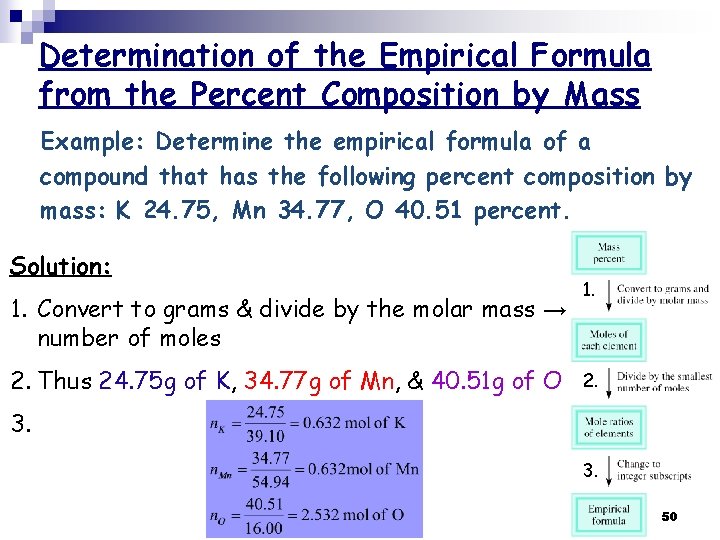
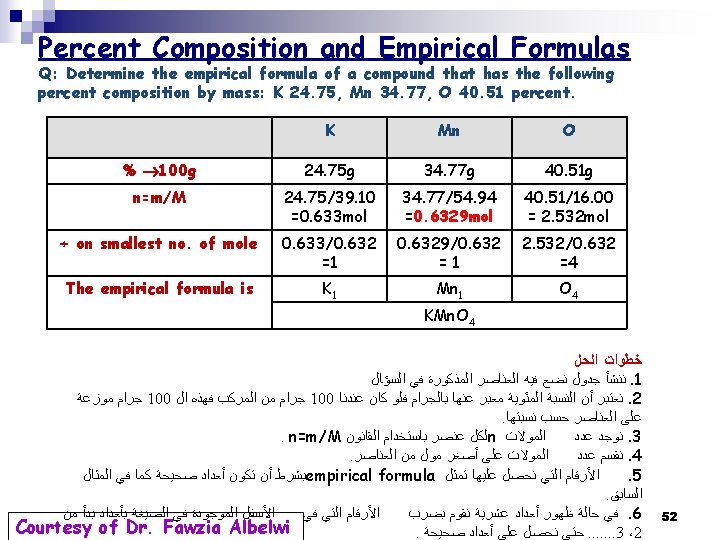
Determination of the Empirical Formula from the Percent Composition by Mass Example: Determine the empirical formula of a compound that has the following percent composition by mass: K 24. 75, Mn 34. 77, O 40. 51 percent. Solution: 1. Convert to grams & divide by the molar mass → number of moles 2. Thus 24. 75 g of K, 34. 77 g of Mn, & 40. 51 g of O 1. 2. 3. 3. 50

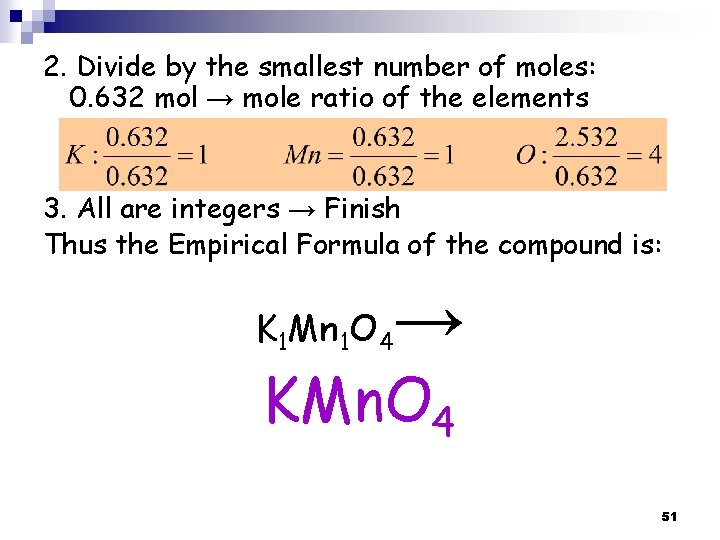
2. Divide by the smallest number of moles: 0. 632 mol → mole ratio of the elements 3. All are integers → Finish Thus the Empirical Formula of the compound is: K 1 Mn 1 O 4 → KMn. O 4 51


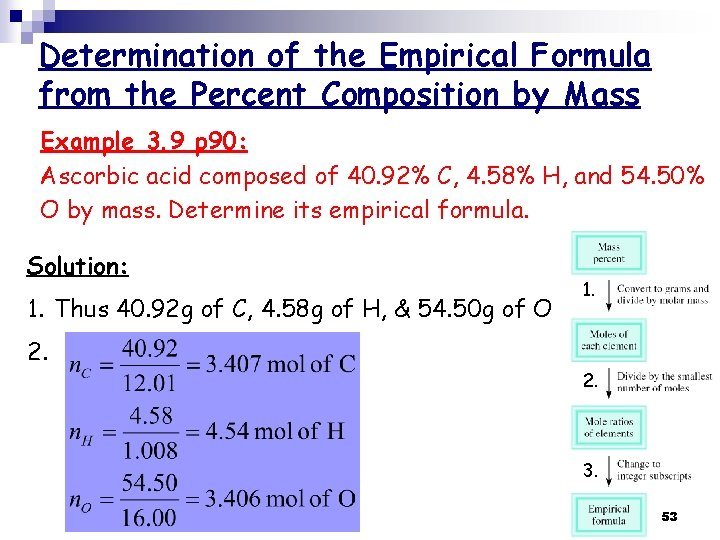
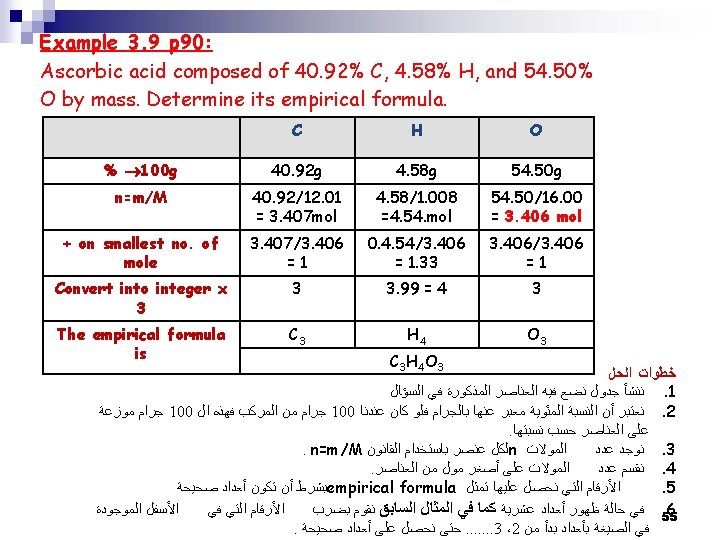
Determination of the Empirical Formula from the Percent Composition by Mass Example 3. 9 p 90: Ascorbic acid composed of 40. 92% C, 4. 58% H, and 54. 50% O by mass. Determine its empirical formula. Solution: 1. Thus 40. 92 g of C, 4. 58 g of H, & 54. 50 g of O 2. 1. 2. 3. 53

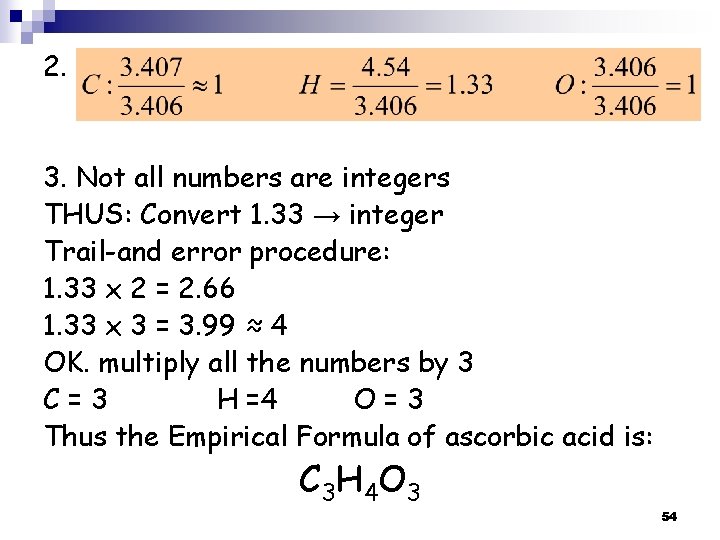
2. 3. Not all numbers are integers THUS: Convert 1. 33 → integer Trail-and error procedure: 1. 33 x 2 = 2. 66 1. 33 x 3 = 3. 99 ≈ 4 OK. multiply all the numbers by 3 C=3 H =4 O=3 Thus the Empirical Formula of ascorbic acid is: C 3 H 4 O 3 54


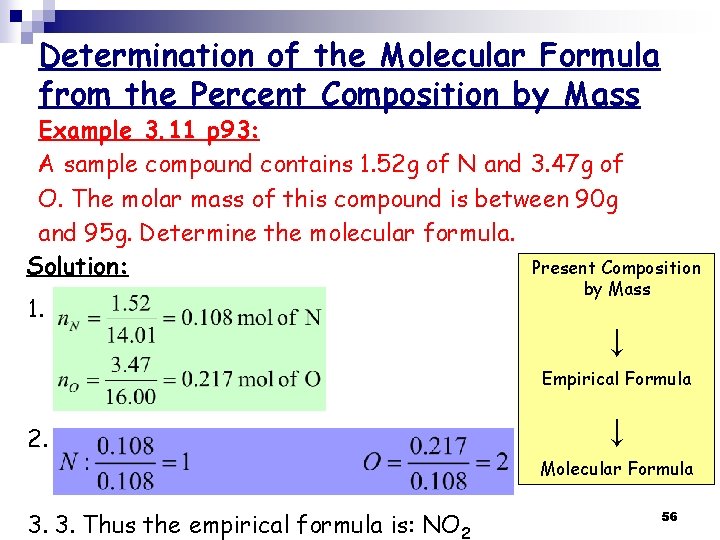
Determination of the Molecular Formula from the Percent Composition by Mass Example 3. 11 p 93: A sample compound contains 1. 52 g of N and 3. 47 g of O. The molar mass of this compound is between 90 g and 95 g. Determine the molecular formula. Present Composition Solution: 1. by Mass ↓ Empirical Formula 2. 2. ↓ Molecular Formula 3. 3. Thus the empirical formula is: NO 2 56

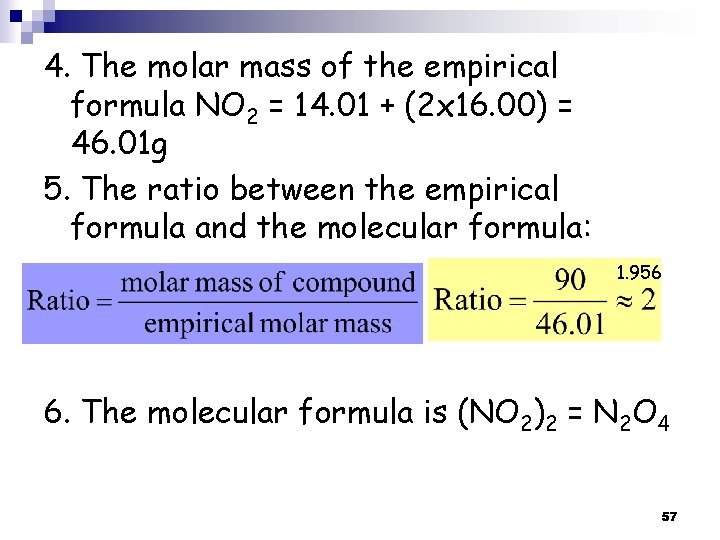
4. The molar mass of the empirical formula NO 2 = 14. 01 + (2 x 16. 00) = 46. 01 g 5. The ratio between the empirical formula and the molecular formula: 1. 956 6. The molecular formula is (NO 2)2 = N 2 O 4 57

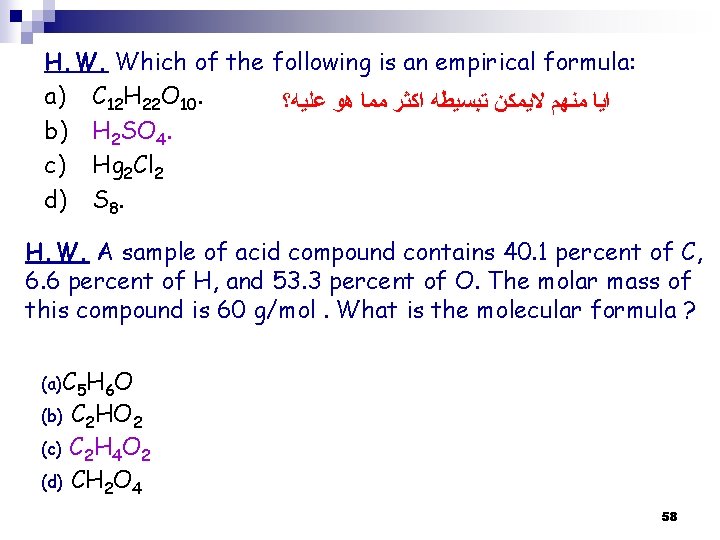
H. W. Which of the following is an empirical formula: a) C 12 H 22 O 10. ﺍﻳﺎ ﻣﻨﻬﻢ ﻻﻳﻤﻜﻦ ﺗﺒﺴﻴﻄﻪ ﺍﻛﺜﺮ ﻣﻤﺎ ﻫﻮ ﻋﻠﻴﻪ؟ b) H 2 SO 4. c) Hg 2 Cl 2 d) S 8. H. W. A sample of acid compound contains 40. 1 percent of C, 6. 6 percent of H, and 53. 3 percent of O. The molar mass of this compound is 60 g/mol. What is the molecular formula ? (a)C 5 H 6 O C 2 HO 2 (c) C 2 H 4 O 2 (d) CH 2 O 4 (b) 58

3. 7 Chemical Reactions and Chemical Equations q Chemical Reaction: is a process in which one or more substances is changed into one or more new substances q Chemical Equation: uses chemical symbols to show what happens during a chemical reaction reactants products 59
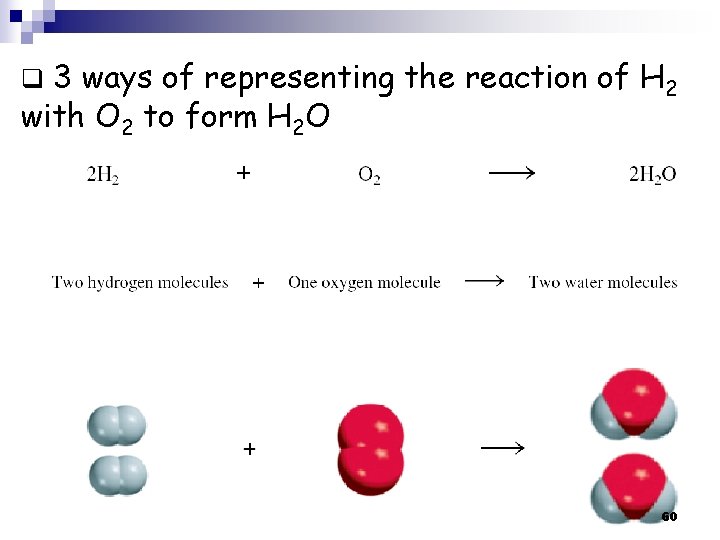
q 3 ways of representing the reaction of H 2 with O 2 to form H 2 O 60

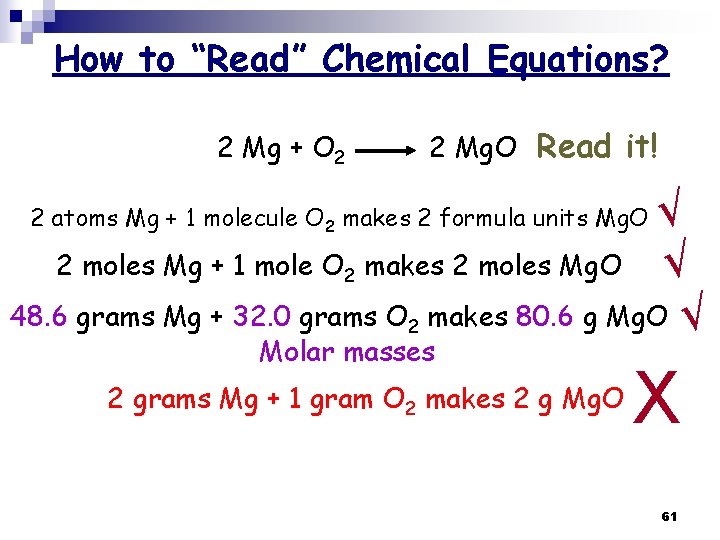
How to “Read” Chemical Equations? 2 Mg + O 2 2 Mg. O Read it! √ 2 moles Mg + 1 mole O 2 makes 2 moles Mg. O √ 48. 6 grams Mg + 32. 0 grams O 2 makes 80. 6 g Mg. O √ 2 atoms Mg + 1 molecule O 2 makes 2 formula units Mg. O Molar masses 2 grams Mg + 1 gram O 2 makes 2 g Mg. O X 61

Balancing Chemical Equations 1. Write the correct formula/s for the reactant/s on the left side and the correct formula/s for the product/s on the right side of the equation. ﻧﻜﺘﺐ ﺍﻟﺼﻴﻐﻪ ﺍﻟﺼﺤﻴﺤﻪ ﻟﻜﻞ ﻣﺘﻔﺎﻋﻞ )ﻋﻠﻰ ﺍﻟﻄﺮﻑ ﺍﻻﻳﺴﺮ( ﻭﻟﻜﻞ ( ﻧﺎﺗﺞ )ﻋﻠﻰ ﺍﻟﻄﺮﻑ ﺍﻻﻳﻤﻦ 62


3. Start by balancing those elements that appear in only one reactant and one product. ﺗﻮﺯﻥ ﺍﻭﻻ ﺍﻟﻌﻨﺎﺻﺮ ﺍﻻﻗﻞ ﻇﻬﻮﺭﺍ 4. Balance those elements that appear in two or more reactants or products. ﺛﻢ ﺗﻮﺯﻥ ﺍﻟﻌﻨﺎﺻﺮ ﺍﻻﻛﺜﺮ ﻇﻬﻮﺭﺍ 5. Check to make sure that you have the same number of each type of atom on both sides of the equation. ﺍﻟﺨﻄﻮﻩ ﺍﻻﺧﻴﺮﻩ ﻫﻲ ﺍﻟﺘﺄﻜﺪ ﻣﻦ ﺍﻥ ﻟﺪﻳﻚ ﻧﻔﺲ ﺍﻟﻌﺪﺩ ﻣﻦ ﺍﻟﺬﺭﺍﺕ ﻟﻜﻞ ﻋﻨﺼﺮ ﻋﻠﻰ ﻃﺮﻓﻲ ﺍﻟﻤﻌﺎﺩﻟﻪ 64

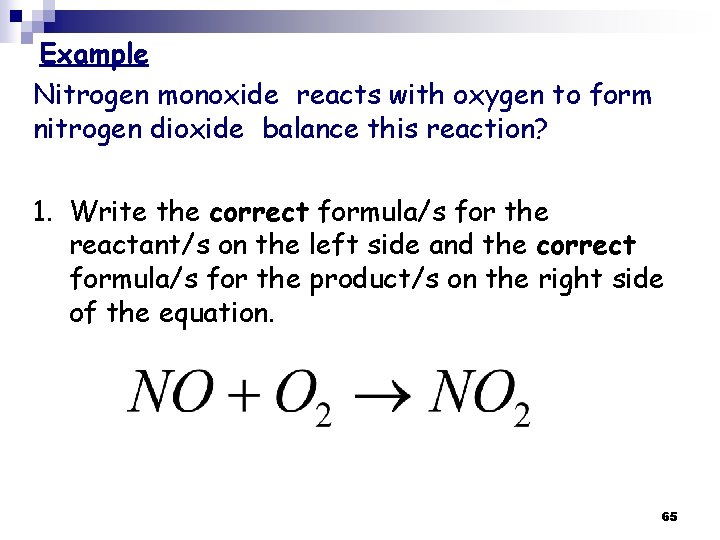
Example Nitrogen monoxide reacts with oxygen to form nitrogen dioxide balance this reaction? 1. Write the correct formula/s for the reactant/s on the left side and the correct formula/s for the product/s on the right side of the equation. 65

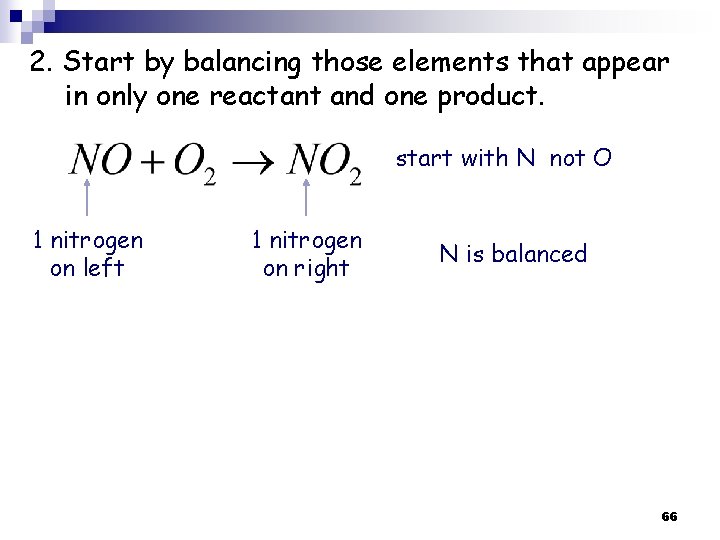
2. Start by balancing those elements that appear in only one reactant and one product. start with N not O 1 nitrogen on left 1 nitrogen on right N is balanced 66

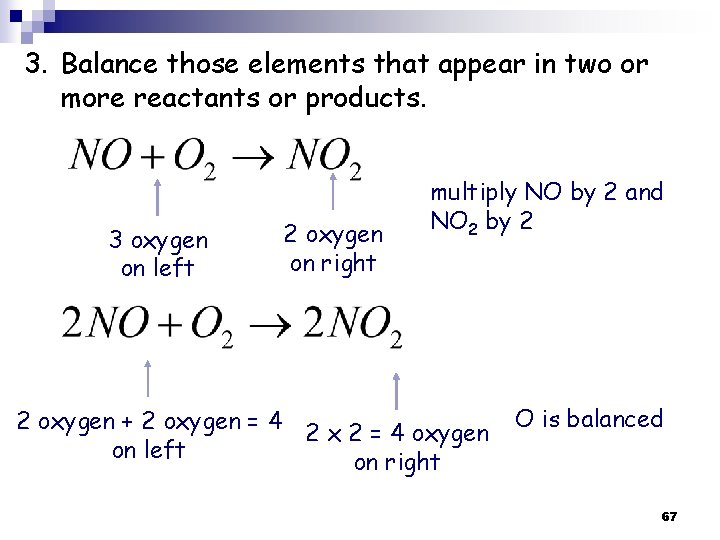
3. Balance those elements that appear in two or more reactants or products. 3 oxygen on left 2 oxygen on right multiply NO by 2 and NO 2 by 2 2 oxygen + 2 oxygen = 4 2 x 2 = 4 oxygen on left on right O is balanced 67

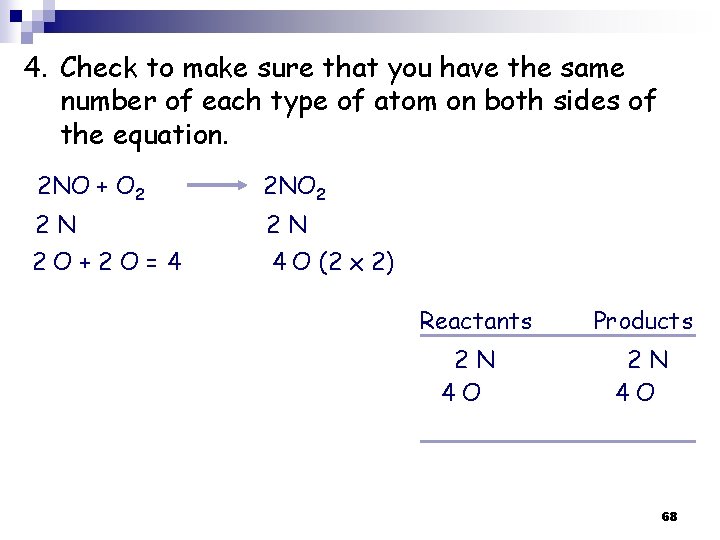
4. Check to make sure that you have the same number of each type of atom on both sides of the equation. 2 NO + O 2 2 N 2 N 2 O+2 O=4 4 O (2 x 2) Reactants 2 N 4 O Products 2 N 4 O 68

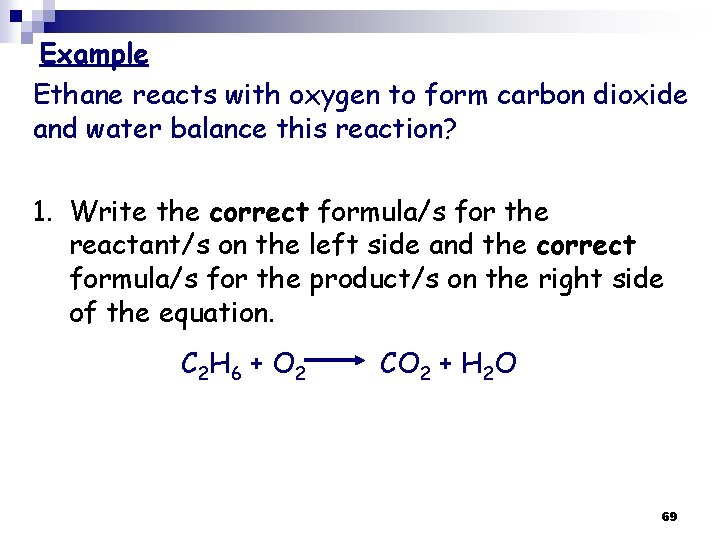
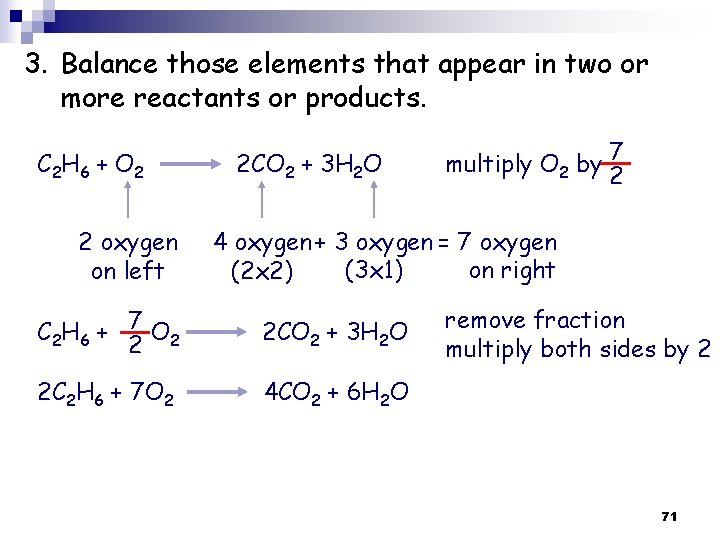
Example Ethane reacts with oxygen to form carbon dioxide and water balance this reaction? 1. Write the correct formula/s for the reactant/s on the left side and the correct formula/s for the product/s on the right side of the equation. C 2 H 6 + O 2 CO 2 + H 2 O 69

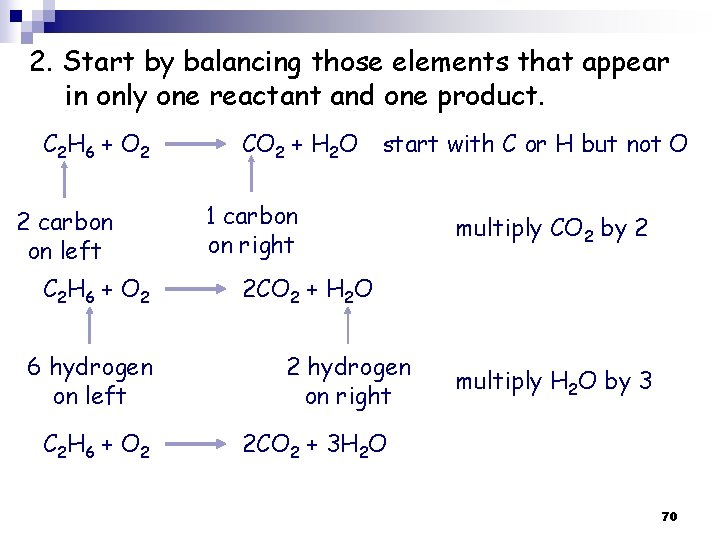
2. Start by balancing those elements that appear in only one reactant and one product. C 2 H 6 + O 2 2 carbon on left C 2 H 6 + O 2 6 hydrogen on left C 2 H 6 + O 2 CO 2 + H 2 O start with C or H but not O 1 carbon on right multiply CO 2 by 2 2 CO 2 + H 2 O 2 hydrogen on right multiply H 2 O by 3 2 CO 2 + 3 H 2 O 70

3. Balance those elements that appear in two or more reactants or products. C 2 H 6 + O 2 2 oxygen on left 2 CO 2 + 3 H 2 O multiply O 2 by 7 2 4 oxygen + 3 oxygen = 7 oxygen (3 x 1) on right (2 x 2) C 2 H 6 + 7 O 2 2 2 CO 2 + 3 H 2 O 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O remove fraction multiply both sides by 2 71

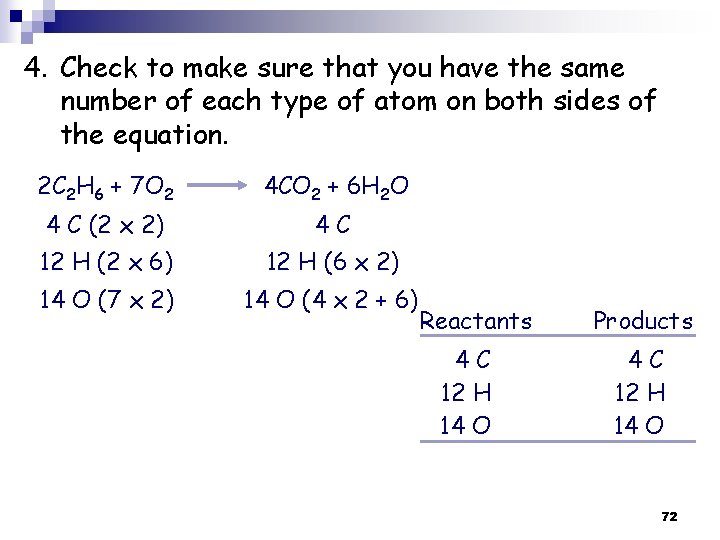
4. Check to make sure that you have the same number of each type of atom on both sides of the equation. 2 C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O 4 C (2 x 2) 4 C 12 H (2 x 6) 12 H (6 x 2) 14 O (7 x 2) 14 O (4 x 2 + 6) Reactants 4 C 12 H 14 O Products 4 C 12 H 14 O 72

Example 3. 12 p 98 : Balance the following reaction Nitrogen monoxide reacts with oxygen to form nitrogen dioxide and balance this reaction? 73 3

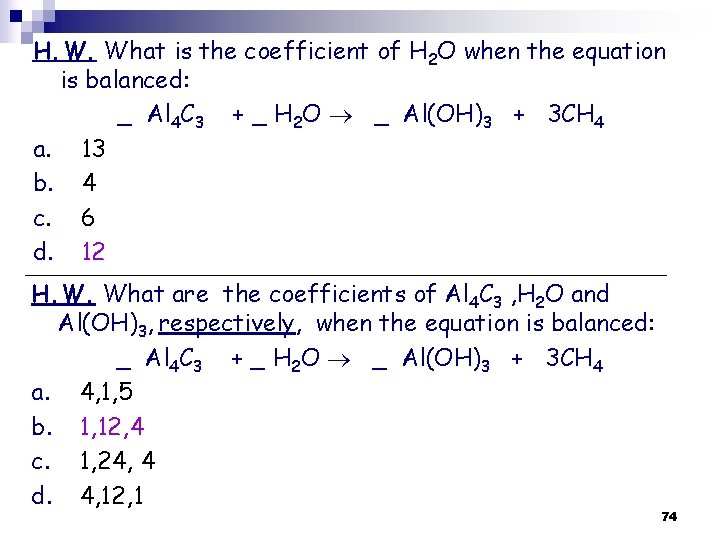
H. W. What is the coefficient of H 2 O when the equation is balanced: _ Al 4 C 3 + _ H 2 O _ Al(OH)3 + 3 CH 4 a. 13 b. 4 c. 6 d. 12 H. W. What are the coefficients of Al 4 C 3 , H 2 O and Al(OH)3, respectively, when the equation is balanced: _ Al 4 C 3 + _ H 2 O _ Al(OH)3 + 3 CH 4 a. 4, 1, 5 b. 1, 12, 4 c. 1, 24, 4 d. 4, 12, 1 74
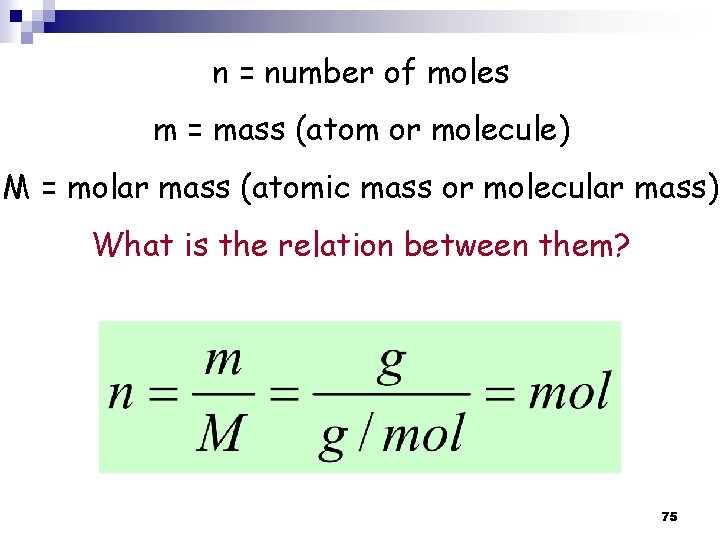
n = number of moles m = mass (atom or molecule) M = molar mass (atomic mass or molecular mass) What is the relation between them? 75

3. 8 Amounts of Reactants and Products n Two important questions: ¨ How much product will be formed from specific amount of reactants? n ¨ e. g. 6. 0 g reactant→ ? product How much starting reactants must be used to obtain a specific amount of product? n e. g. ? reactant → 6. 0 g product 76

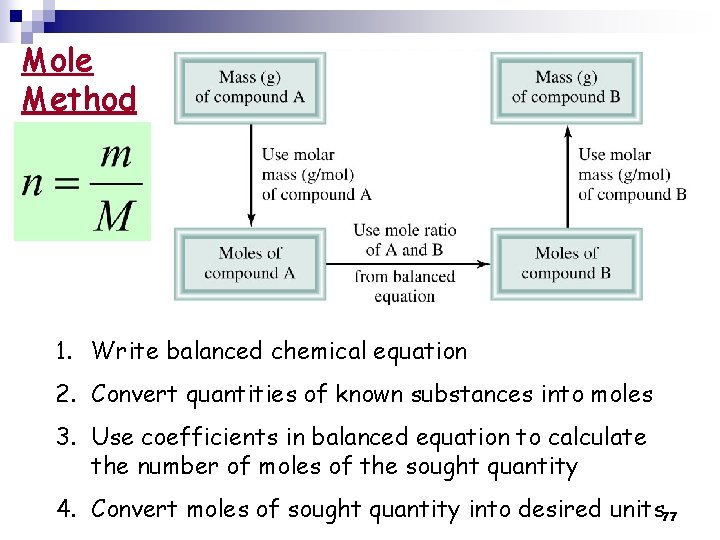
Mole Method 1. Write balanced chemical equation 2. Convert quantities of known substances into moles 3. Use coefficients in balanced equation to calculate the number of moles of the sought quantity 4. Convert moles of sought quantity into desired units 77

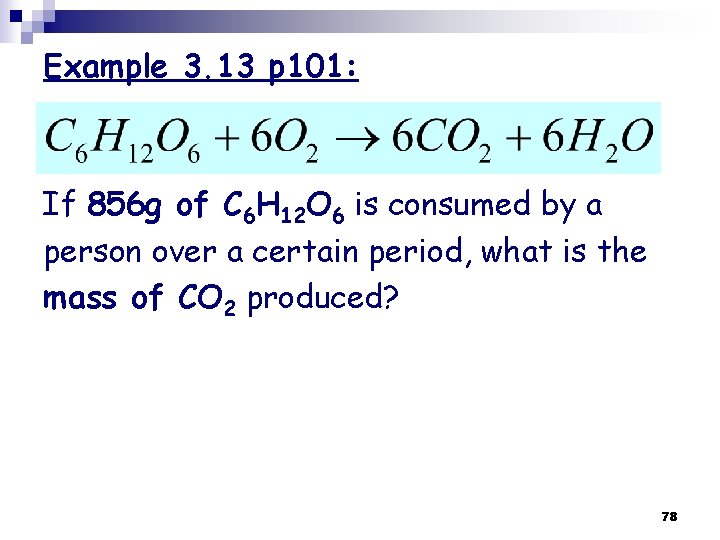
Example 3. 13 p 101: If 856 g of C 6 H 12 O 6 is consumed by a person over a certain period, what is the mass of CO 2 produced? 78

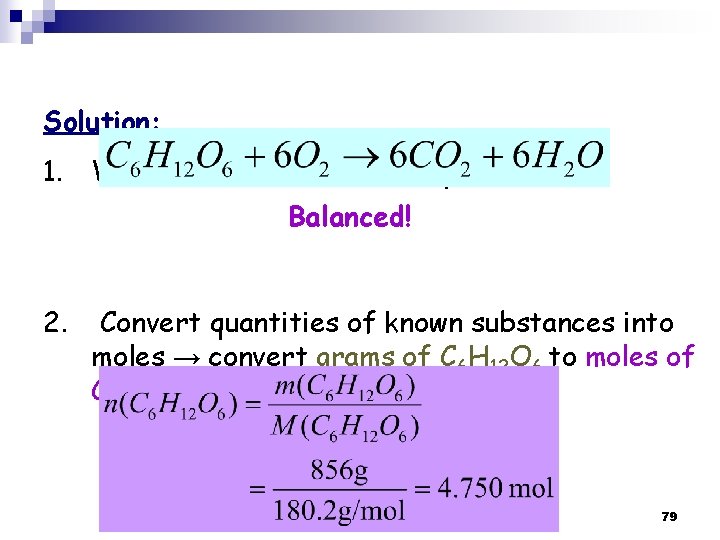
Solution: 1. Write balanced chemical equation Balanced! 2. Convert quantities of known substances into moles → convert grams of C 6 H 12 O 6 to moles of C 6 H 12 O 6 79

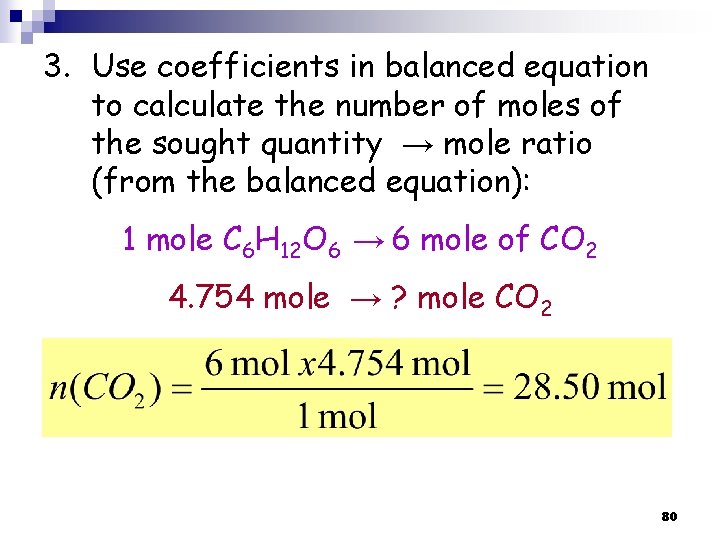
3. Use coefficients in balanced equation to calculate the number of moles of the sought quantity → mole ratio (from the balanced equation): 1 mole C 6 H 12 O 6 → 6 mole of CO 2 4. 754 mole → ? mole CO 2 80

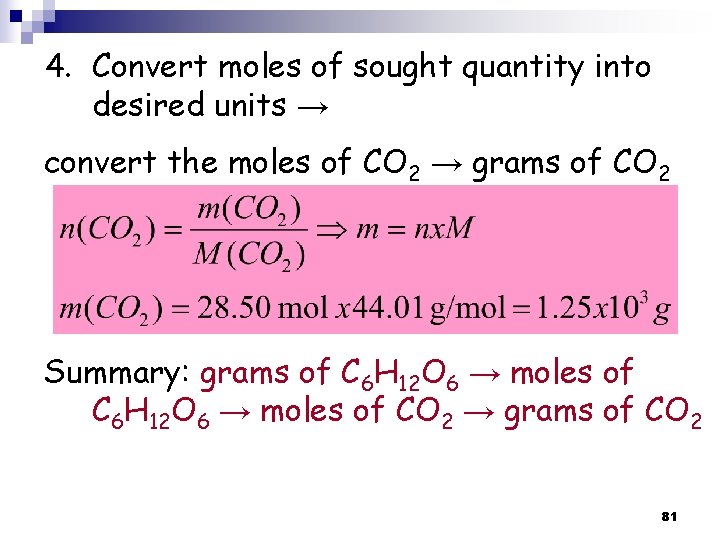
4. Convert moles of sought quantity into desired units → convert the moles of CO 2 → grams of CO 2 Summary: grams of C 6 H 12 O 6 → moles of CO 2 → grams of CO 2 81

Example 3. 14 p 101: How many grams of Li are needed to produce 9. 89 g of H 2? Strategy: grams of H 2 → moles of Li→ grams of Li 82

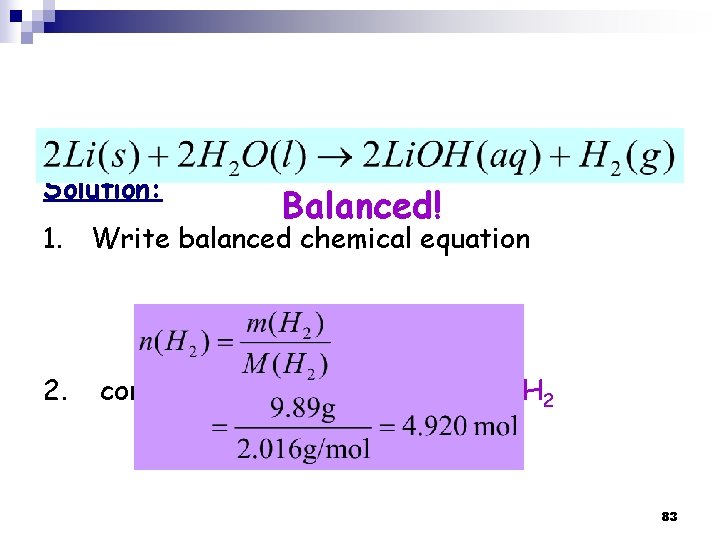
Solution: Balanced! 1. Write balanced chemical equation 2. convert grams of H 2 to moles of H 2 83

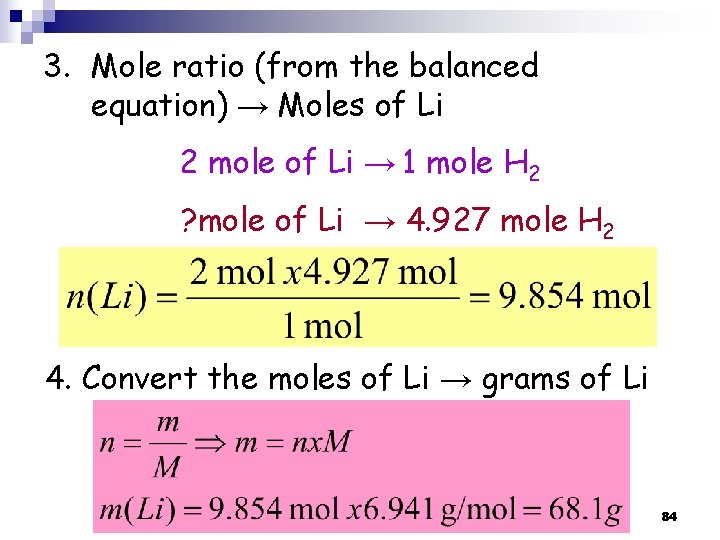
3. Mole ratio (from the balanced equation) → Moles of Li 2 mole of Li → 1 mole H 2 ? mole of Li → 4. 927 mole H 2 4. Convert the moles of Li → grams of Li 84

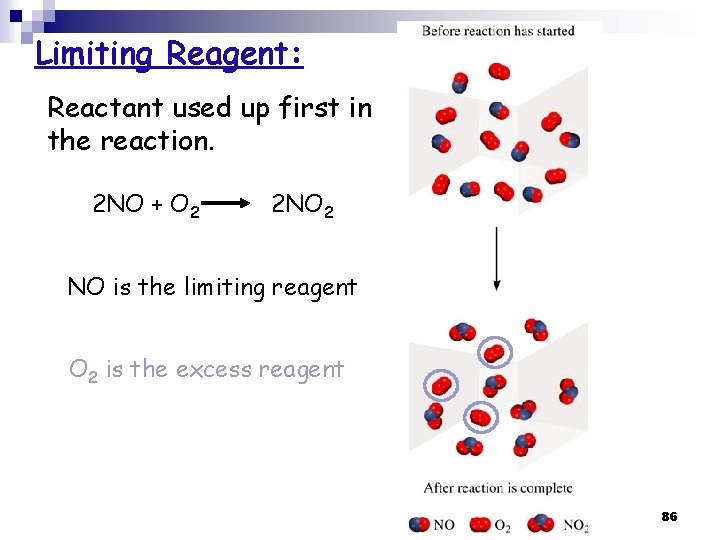
3. 9 Limiting Reagent ﺍﻟﻜﺎﺷﻒ ﺍﻟﻤﺤﺪﺩ Limiting Reagent: is the reactant used up first in a reaction and thus determine the amount of product n Excess Reagent ﺍﻟﻜﺎﺷﻒ ﺍﻟﻔﺎﺋﺾ : is the reactant present in quantities greater than necessary to react with the quantity of the limiting reagent (the one that is left at the end of the reaction). n → Limiting reagent is in a reaction of more than one reactant! n 85

Limiting Reagent: Reactant used up first in the reaction. 2 NO + O 2 2 NO 2 NO is the limiting reagent O 2 is the excess reagent 86

Questions in Limiting Reagent: n First: we have to determine which reactant is the limiting reagent and which is the excess reagent! n Second: after we know which one is the limiting reagent, we could determine the amount of the product!! n Third: after we know the excess reagent, we could determine how much excess of it is left at the end of the reaction!!! 87

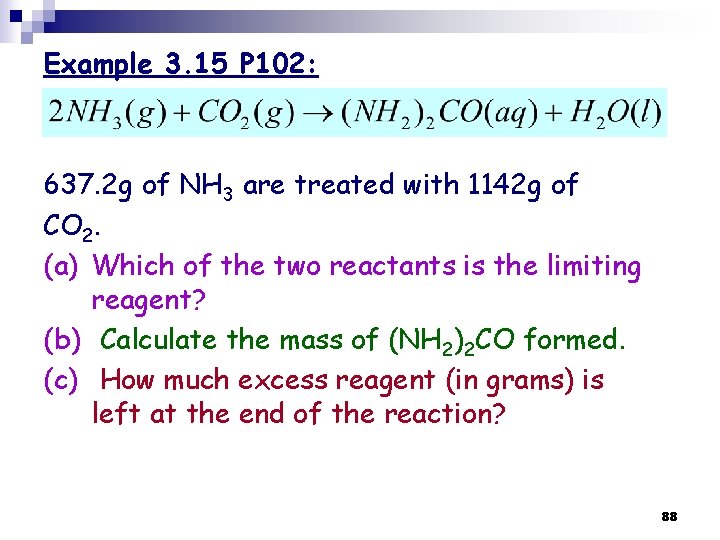
Example 3. 15 P 102: 637. 2 g of NH 3 are treated with 1142 g of CO 2. (a) Which of the two reactants is the limiting reagent? (b) Calculate the mass of (NH 2)2 CO formed. (c) How much excess reagent (in grams) is left at the end of the reaction? 88


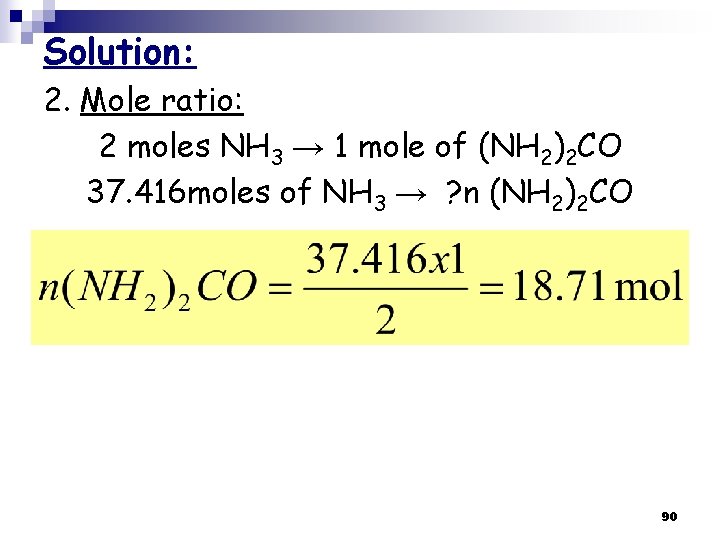
Solution: 2. Mole ratio: 2 moles NH 3 → 1 mole of (NH 2)2 CO 37. 416 moles of NH 3 → ? n (NH 2)2 CO 90

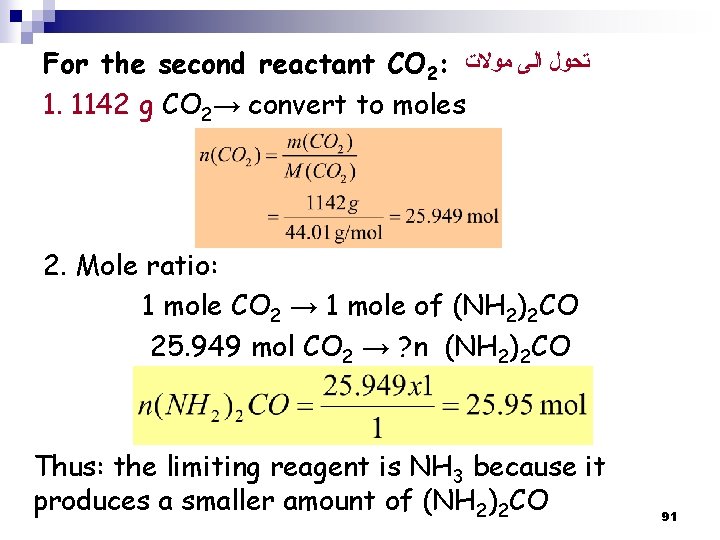
For the second reactant CO 2: ﺗﺤﻮﻝ ﺍﻟﻰ ﻣﻮﻻﺕ 1. 1142 g CO 2→ convert to moles 2. Mole ratio: 1 mole CO 2 → 1 mole of (NH 2)2 CO 25. 949 mol CO 2 → ? n (NH 2)2 CO Thus: the limiting reagent is NH 3 because it produces a smaller amount of (NH 2)2 CO 91

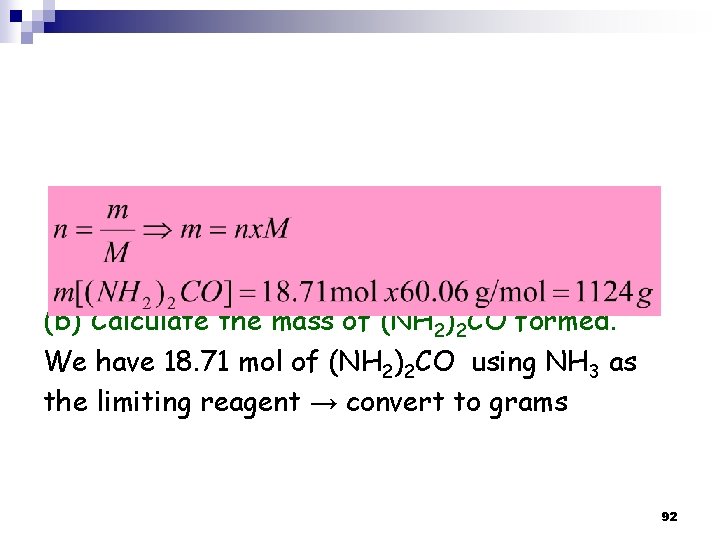
(b) Calculate the mass of (NH 2)2 CO formed. We have 18. 71 mol of (NH 2)2 CO using NH 3 as the limiting reagent → convert to grams 92

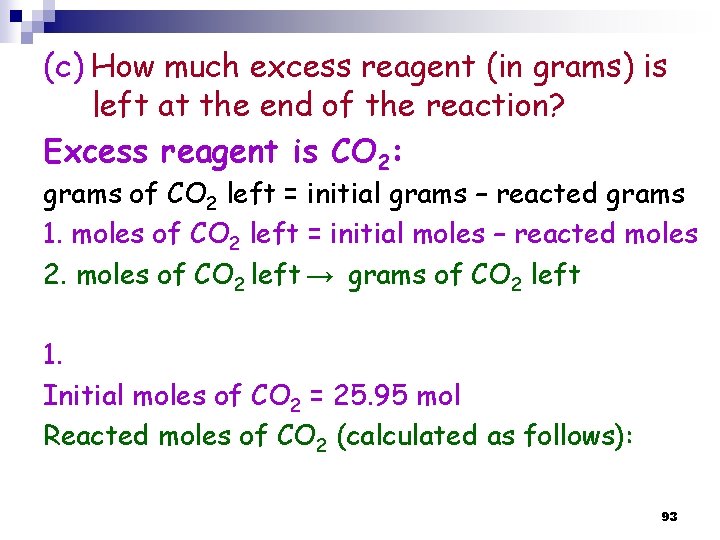
(c) How much excess reagent (in grams) is left at the end of the reaction? Excess reagent is CO 2: grams of CO 2 left = initial grams – reacted grams 1. moles of CO 2 left = initial moles – reacted moles 2. moles of CO 2 left → grams of CO 2 left 1. Initial moles of CO 2 = 25. 95 mol Reacted moles of CO 2 (calculated as follows): 93

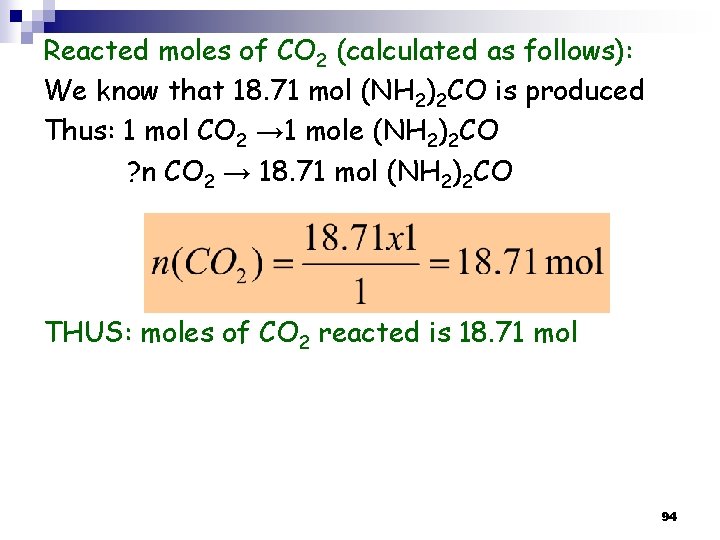
Reacted moles of CO 2 (calculated as follows): We know that 18. 71 mol (NH 2)2 CO is produced Thus: 1 mol CO 2 → 1 mole (NH 2)2 CO ? n CO 2 → 18. 71 mol (NH 2)2 CO THUS: moles of CO 2 reacted is 18. 71 mol 94

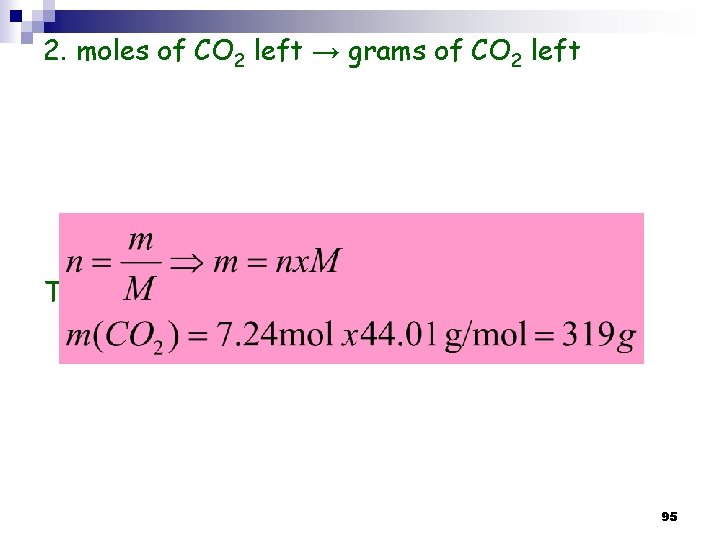
2. moles of CO 2 left → grams of CO 2 left Thus: the mass of CO 2 remaining (left) = 319 g 95

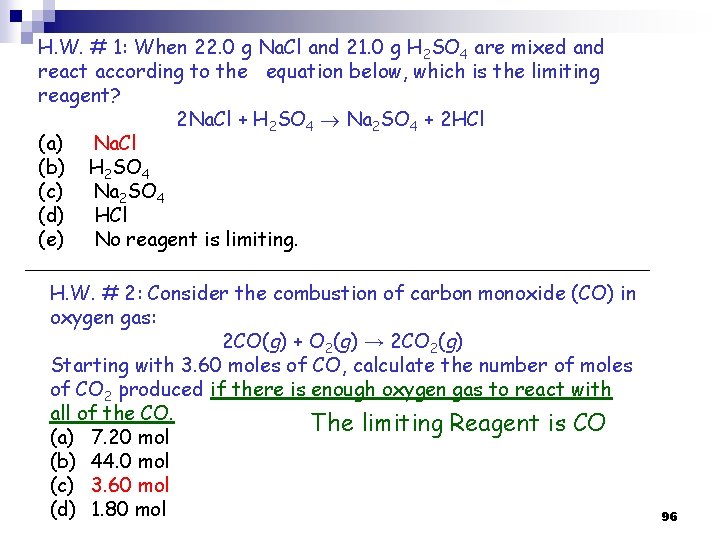
H. W. # 1: When 22. 0 g Na. Cl and 21. 0 g H 2 SO 4 are mixed and react according to the equation below, which is the limiting reagent? 2 Na. Cl + H 2 SO 4 Na 2 SO 4 + 2 HCl (a) Na. Cl (b) H 2 SO 4 (c) Na 2 SO 4 (d) HCl (e) No reagent is limiting. H. W. # 2: Consider the combustion of carbon monoxide (CO) in oxygen gas: 2 CO(g) + O 2(g) → 2 CO 2(g) Starting with 3. 60 moles of CO, calculate the number of moles of CO 2 produced if there is enough oxygen gas to react with all of the CO. The limiting Reagent is CO (a) 7. 20 mol (b) 44. 0 mol (c) 3. 60 mol (d) 1. 80 mol 96

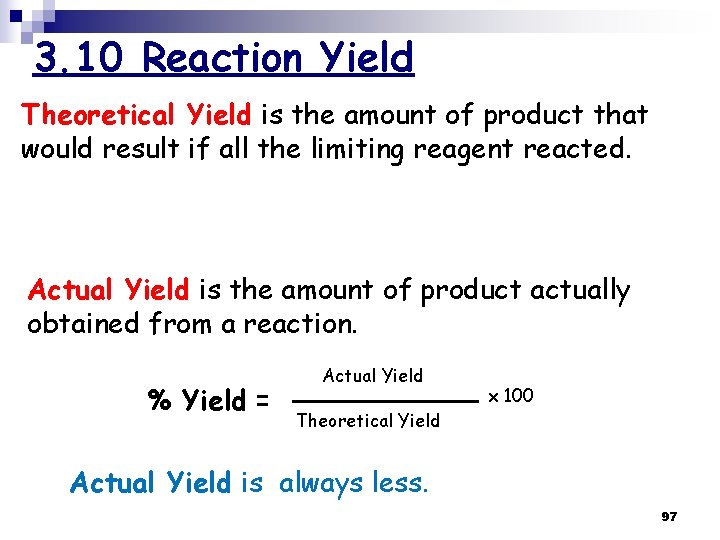
3. 10 Reaction Yield Theoretical Yield is the amount of product that would result if all the limiting reagent reacted. Actual Yield is the amount of product actually obtained from a reaction. % Yield = Actual Yield x 100 Theoretical Yield Actual Yield is always less. 97

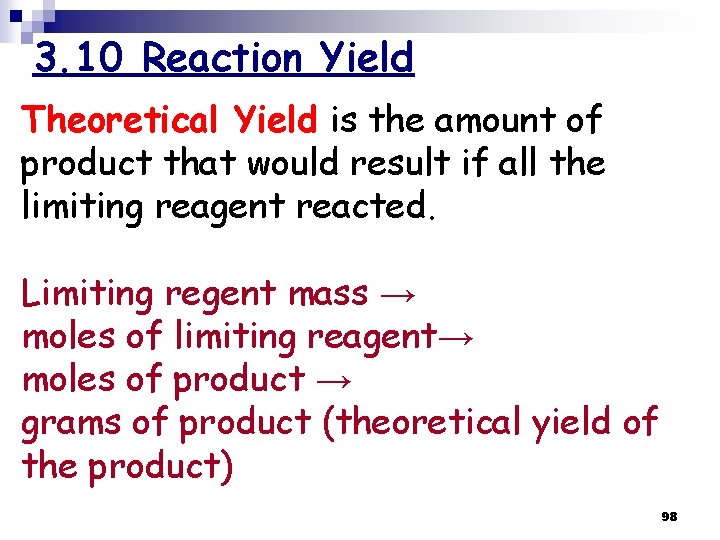
3. 10 Reaction Yield Theoretical Yield is the amount of product that would result if all the limiting reagent reacted. Limiting regent mass → moles of limiting reagent→ moles of product → grams of product (theoretical yield of the product) 98

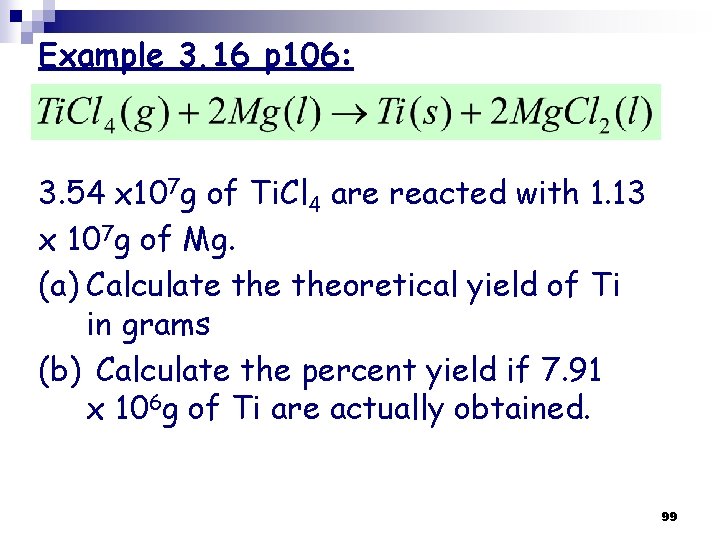
Example 3. 16 p 106: 3. 54 x 107 g of Ti. Cl 4 are reacted with 1. 13 x 107 g of Mg. (a) Calculate theoretical yield of Ti in grams (b) Calculate the percent yield if 7. 91 x 106 g of Ti are actually obtained. 99

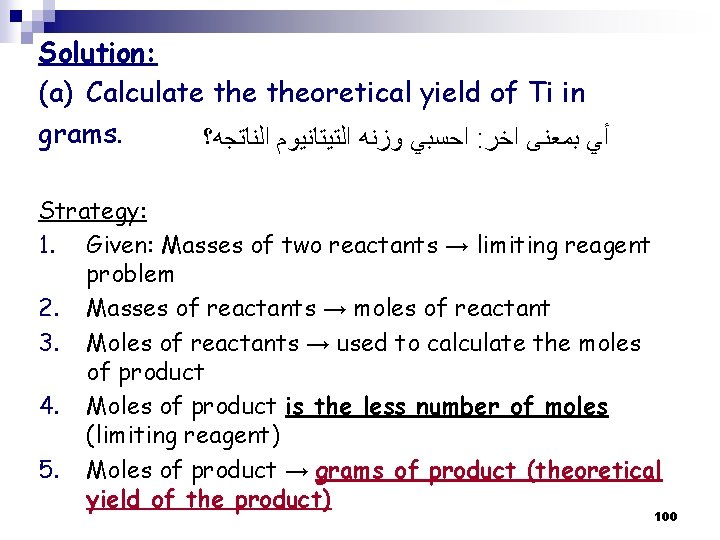
Solution: (a) Calculate theoretical yield of Ti in grams. ﺍﺣﺴﺒﻲ ﻭﺯﻧﻪ ﺍﻟﺘﻴﺘﺎﻧﻴﻮﻡ ﺍﻟﻨﺎﺗﺠﻪ؟ : ﺃﻲ ﺑﻤﻌﻨﻰ ﺍﺧﺮ Strategy: 1. Given: Masses of two reactants → limiting reagent problem 2. Masses of reactants → moles of reactant 3. Moles of reactants → used to calculate the moles of product 4. Moles of product is the less number of moles (limiting reagent) 5. Moles of product → grams of product (theoretical yield of the product) 100

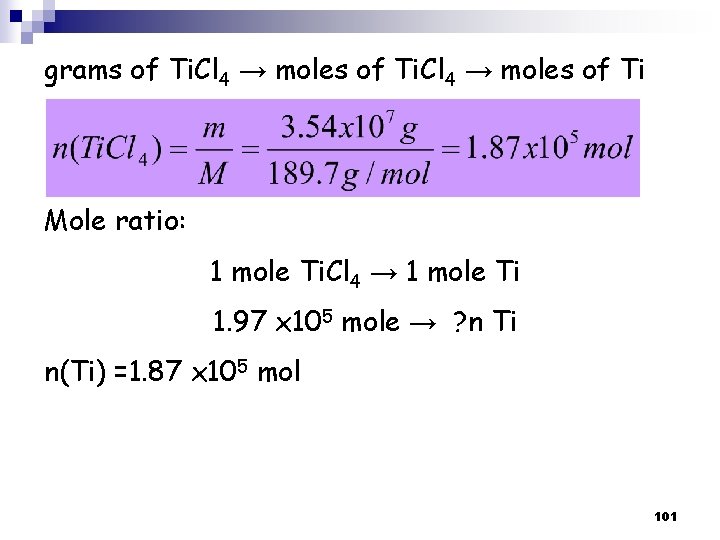
grams of Ti. Cl 4 → moles of Ti Mole ratio: 1 mole Ti. Cl 4 → 1 mole Ti 1. 97 x 105 mole → ? n Ti n(Ti) =1. 87 x 105 mol 101
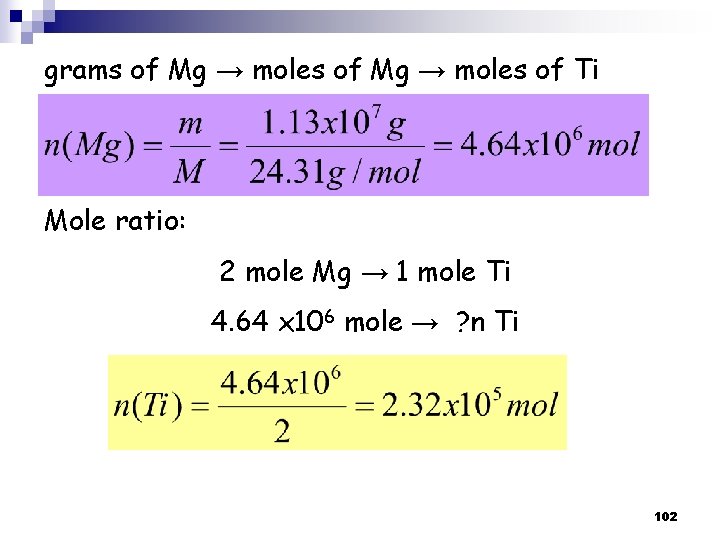
grams of Mg → moles of Ti Mole ratio: 2 mole Mg → 1 mole Ti 4. 64 x 106 mole → ? n Ti 102

n(Ti) is the less number of moles (limiting reagent) = 1. 87 x 105 mol Moles of Ti → grams of Ti Thus: mass of Ti = theoretical yield of Ti = m =n. M 103

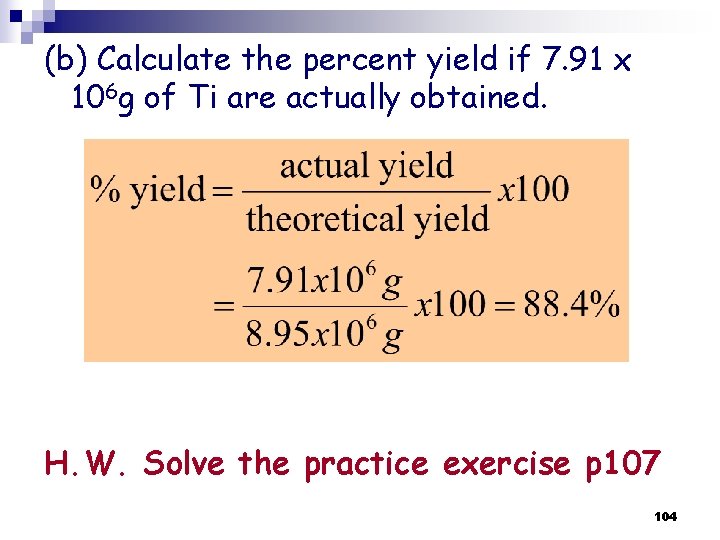
(b) Calculate the percent yield if 7. 91 x 106 g of Ti are actually obtained. H. W. Solve the practice exercise p 107 104

End of Chapter 3 105
 Vignette mutuelle 110/110
Vignette mutuelle 110/110 110-000-110 & 111-000-111
110-000-110 & 111-000-111 Chemistry 110
Chemistry 110 Head tilt chin lift and jaw thrust maneuver is used for
Head tilt chin lift and jaw thrust maneuver is used for Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry ders notları
General chemistry ders notları General chemistry 11th edition
General chemistry 11th edition General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry Exchange energy
Exchange energy General chemistry
General chemistry General chemistry
General chemistry Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? Iannone chem moodle
Iannone chem moodle Ap chem unit 7
Ap chem unit 7 Imf chem
Imf chem Chem 130 final exam
Chem 130 final exam Eth myth prop
Eth myth prop Principles of kmt
Principles of kmt January 2016 chemistry regents answers
January 2016 chemistry regents answers Absorbance chemistry
Absorbance chemistry Chem 1020
Chem 1020 Chem ufl
Chem ufl Chemistry law hazard pay
Chemistry law hazard pay Gen chem review for ochem
Gen chem review for ochem Chem 109
Chem 109 Chemfeed
Chemfeed E-chem-portal
E-chem-portal Amy gottfried umich
Amy gottfried umich Environics chempro x
Environics chempro x Chem 253
Chem 253 Chapter 19 acids and bases worksheet answers
Chapter 19 acids and bases worksheet answers The formula for the hydronium ion is _____. ho3 h3o+ h+ oh-
The formula for the hydronium ion is _____. ho3 h3o+ h+ oh- Specific heat chem worksheet 16-1
Specific heat chem worksheet 16-1 Spectroscopy ap chem
Spectroscopy ap chem Ap chem kinetics
Ap chem kinetics Photoelectron spectroscopy ap chemistry
Photoelectron spectroscopy ap chemistry Ap chem equilibrium review
Ap chem equilibrium review Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Entropy ap chem
Entropy ap chem 2017 ap chem exam
2017 ap chem exam Ario organic chem
Ario organic chem Chem quiz.net
Chem quiz.net Model chem lab
Model chem lab Www.chem.purdue/gchelp/atoms/elements.html
Www.chem.purdue/gchelp/atoms/elements.html Bó thon bó chêm
Bó thon bó chêm Piz4 ap
Piz4 ap Chem pharma impex
Chem pharma impex Chem connections india
Chem connections india Chem 30 data booklet
Chem 30 data booklet Chem 200
Chem 200 Ap chem equilibrium
Ap chem equilibrium Electrochemistry ap chemistry
Electrochemistry ap chemistry Determining molecular polarity
Determining molecular polarity Dyno chem
Dyno chem Gen chem
Gen chem Chemical equilibrum
Chemical equilibrum Chem 433
Chem 433 Chem
Chem Gen chem
Gen chem Brinclhof
Brinclhof Chem moles
Chem moles Pdbe chem
Pdbe chem Chem
Chem Chem
Chem Nss chemistry
Nss chemistry Chem 401
Chem 401 Tera chem
Tera chem Energy quanta
Energy quanta Chem
Chem Chem
Chem Lei luo build
Lei luo build Guan chem chen
Guan chem chen Chem 101
Chem 101 Chem 4 kids.com
Chem 4 kids.com Geminal and vicinal coupling constants
Geminal and vicinal coupling constants Chem 494
Chem 494 Chem part 3
Chem part 3 Chem
Chem Chem
Chem Cpa chem
Cpa chem Chemsheets as 1040 answers
Chemsheets as 1040 answers Molecular geometry
Molecular geometry Application of hess law
Application of hess law Chem
Chem Crystalline vs amorphous
Crystalline vs amorphous Significant figures
Significant figures Types of equations chem worksheet 10-3
Types of equations chem worksheet 10-3 Cationic polymerization
Cationic polymerization Chem book
Chem book Zelinsky reaction
Zelinsky reaction Chem
Chem Chem pub
Chem pub Chem lab
Chem lab Chem
Chem Chem libretext
Chem libretext Chem
Chem Chem libretext
Chem libretext Pub chem
Pub chem