GENERAL CHEMISTRY CHEM 110 REVISION 1 2 3















- Slides: 30

GENERAL CHEMISTRY CHEM 110 REVISION

1 2. 3 Which of the following is not an SI base unit? A) kilometer * B) kilogram C) second D) kelvin Which of the following SI base units is not commonly used in chemistry? A) kilogram B) kelvin C) candela * D) mole Which of the following prefixes means 1/1000? A) kilo B) deci C) centi D) milli *

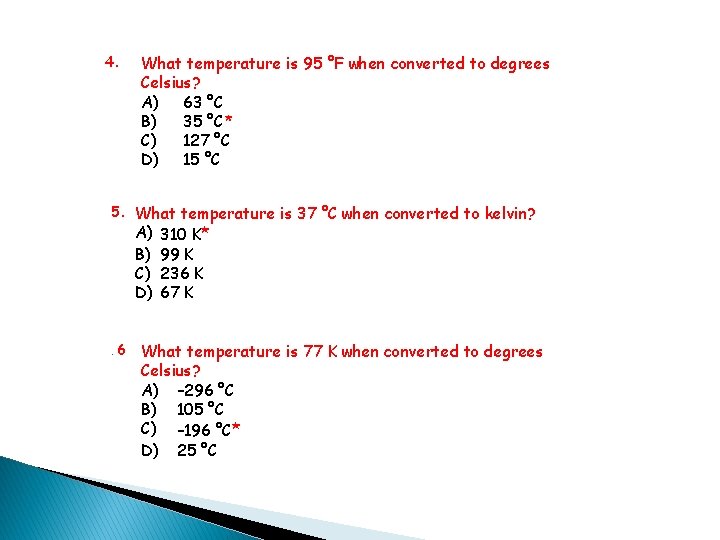
4. What temperature is 95 °F when converted to degrees Celsius? A) 63 °C B) 35 °C* C) 127 °C D) 15 °C 5. What temperature is 37 °C when converted to kelvin? A) B) C) D) . 6 310 K* 99 K 236 K 67 K What temperature is 77 K when converted to degrees Celsius? A) – 296 °C B) 105 °C C) – 196 °C* D) 25 °C

7. 8. What is 22. 6 m when converted to decimeters? A) 0. 226 dm B) 2. 26 dm C) 226 dm * D) 2. 26 x 10– 3 dm What is 25. 4 mg when converted to kilograms? A) 2540 kg B) 2. 54 x 10– 5 kg * C) 2. 54 kg D) 2. 54 x 104 kg ﻣﺎﻫﻲ ﺍﻟﻘﺮﺍﺀﺓ ﺍﻟﺮﻗﻤﻴﺔ ﻟﺪﺭﺟﺔ ﺍﻟﺤﺮﺍﺭﺓ ﻋﻠﻰ ﺍﻟﺘﺮﻣﻮﻣﺘﺮ ﺍﻟﻤﺌﻮﻱ ﺍﻟﺘﻲ ﺗﺘﺴﺎﻭﻯ ﻣﻊ ﺩﺭﺟﺔ ﺍﻟﺤﺮﺍﺭﺓ ﻋﻠﻰ ﺍﻟﺘﺮﻣﻮﻣﺘﺮ ﺍﻟﻔﻬﺮﻳﻨﻬﺎﻳﺘﻲ 9. At what temperature does the numerical reading on a Celsius thermometer equal that on a Fahrenheit thermometer? A) 0 °C B) – 40 °C * C) 100 °C D) – 32 °C

10. A. B. C. D. E. The SI prefixes giga and micro represent, respectively: 10 -9 and 10 -6. 106 and 10 -3. 103 and 10 -3. 109 and 10 -6. * 10 -9 and 10 -3 11. Lithium is the least dens metal known (density= 0. 53 g/cm 3 ). What is the volume occupied by 1. 2 x 103 g of lithium? A. B. C. D. 0. 0875 cm 3 11. 4 cm 3 0. 44 x 10 -3 cm 3 2. 26 x 103 cm 3 *

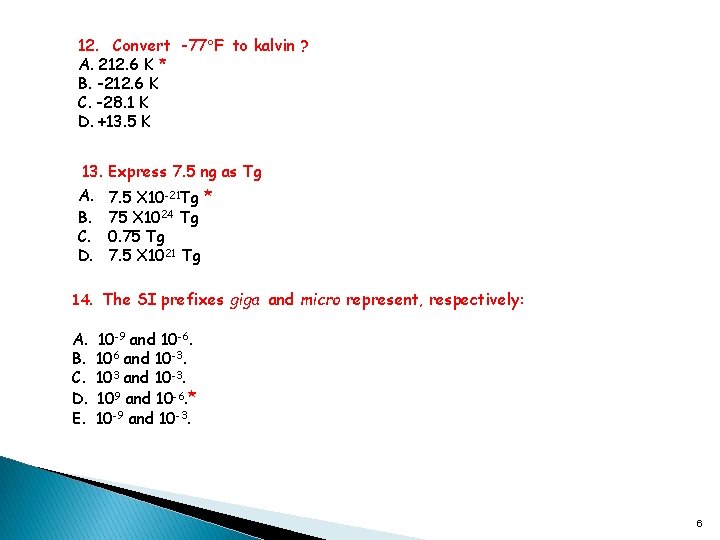
12. Convert -77 F to kalvin ? A. 212. 6 K * B. -212. 6 K C. -28. 1 K D. +13. 5 K 13. Express 7. 5 ng as Tg A. 7. 5 X 10 -21 Tg * B. 75 X 1024 Tg C. 0. 75 Tg D. 7. 5 X 1021 Tg 14. The SI prefixes giga and micro represent, respectively: A. B. C. D. E. 10 -9 and 10 -6. 106 and 10 -3. 103 and 10 -3. 109 and 10 -6. * 10 -9 and 10 -3. 6

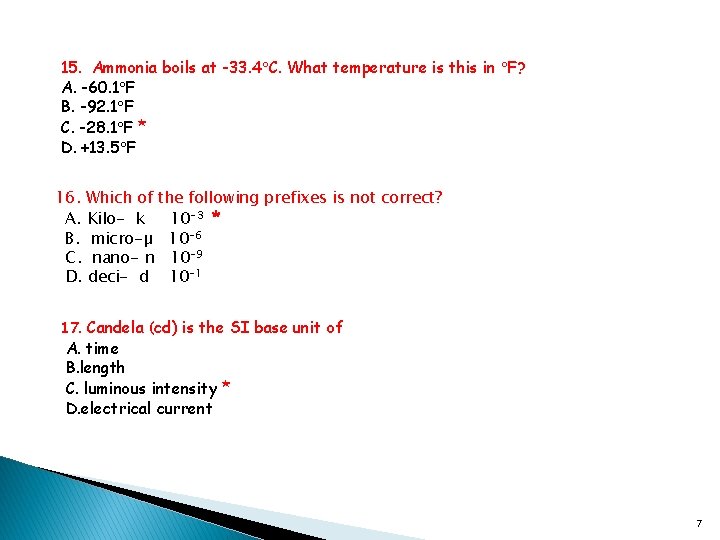
15. Ammonia boils at -33. 4 C. What temperature is this in F? A. -60. 1 F B. -92. 1 F C. -28. 1 F * D. +13. 5 F 16. Which of the following prefixes is not correct? A. Kilo- k 10 -3 * B. micro-µ 10 -6 C. nano- n 10 -9 D. deci- d 10 -1 17. Candela (cd) is the SI base unit of A. time B. length C. luminous intensity * D. electrical current 7

18. Express 5500 nm as picometers. A. 5. 5 10 -6 pm B. 55. 0 pm C. 550 pm D. 5. 5 106 pm * 19. The SI prefix giga represents: A. 10 -6 B. 106 C. 103 D. 109 * 20 Which of the following prefixes means 106 ? A. B. C. D. Mega * nano Tera milli 8

21. A piece of iron (Fe) metal weighing 194. 3 g is placed in a graduated cylinder containing 242. 0 m. L of water. The volume of water now reads 260. 5 m. L. From these data calculate the density of iron. A. 10. 5 g/cm 3 * B. 1. 25 g/cm 3 C. 0. 746 g/cm 3 D. 21. 0 g/cm 3 22. 23 Which of the following SI base units is used to measure Electrical Current? A. candela B. kelvin C. Ampere * D. mole Which of the following prefixes means 1/100? A. kilo B. deci C. Centi * D. milli 9

24 Which of the following SI base units is not commonly used in chemistry? A. B. C. D. kilogram kelvin ampere * mole 25. The diameter of an atom is approximately 1 10 -7 mm. What is this diameter when expressed in nanometers? A. 1 10 -18 nm B. 1 10 -15 nm C. 1 10 -9 nm D. 1 10 -1 nm* 26. Express 75 Tg as pg A. 7. 5 pg B. 75 X 1024 pg * C. 0. 75 pg D. 75 X 10 -24 pg 10

27. The SI unit of time is the A. hour B. Second * C. minute D. ampere 28 The following procedure was used to determine the volume of a flask. The flask was weighted dry and then filled with water. If the masses of empty flask and filled flask were 56. 12 g and 87. 39 g, respectively, and the density of water is 0. 9976 g/cm 3, Calculate the volume of the flask in cm 3 ? A. B. C. D. 56. 255 cm 3 87. 6 cm 3 31. 345 cm 3 * 143. 855 29. Express 2200 nm as picometers. A. 2. 2 X 10 -7 pm B. 22. 0 pm C. 220 pm D. 2. 2 X 106 pm * 11

30. The SI prefixes kilo and milli represent, respectively: A. 10 -9 and 10 -6 B. 106 and 10 -3 C. 103 and 10 -3 * D. 109 and 10 -6 E. 10 -9 and 10 -3 31. The SI prefixes Tara and nano represent, respectively: A. 10 -9 and 10 -6 B. 106 and 10 -3 C. 103 and 10 -3 D. 1012 and 10 -9 * 32. The density of octane is 0. 702 g/cm 3. what is the mass of 65 m. L of octane? A. 45. 6 g * B. 92. 6 g C. 22. 5 g D. 110 g 12

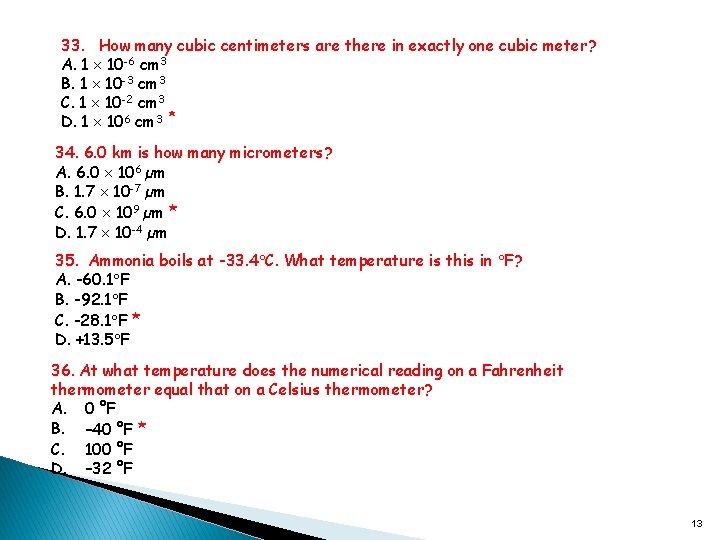
33. How many cubic centimeters are there in exactly one cubic meter? A. 1 10 -6 cm 3 B. 1 10 -3 cm 3 C. 1 10 -2 cm 3 D. 1 106 cm 3 * 34. 6. 0 km is how many micrometers? A. 6. 0 106 µm B. 1. 7 10 -7 µm C. 6. 0 109 µm * D. 1. 7 10 -4 µm 35. Ammonia boils at -33. 4 C. What temperature is this in F? A. -60. 1 F B. -92. 1 F C. -28. 1 F * D. +13. 5 F 36. At what temperature does the numerical reading on a Fahrenheit thermometer equal that on a Celsius thermometer? A. 0 °F B. – 40 °F * C. 100 °F D. – 32 °F 13

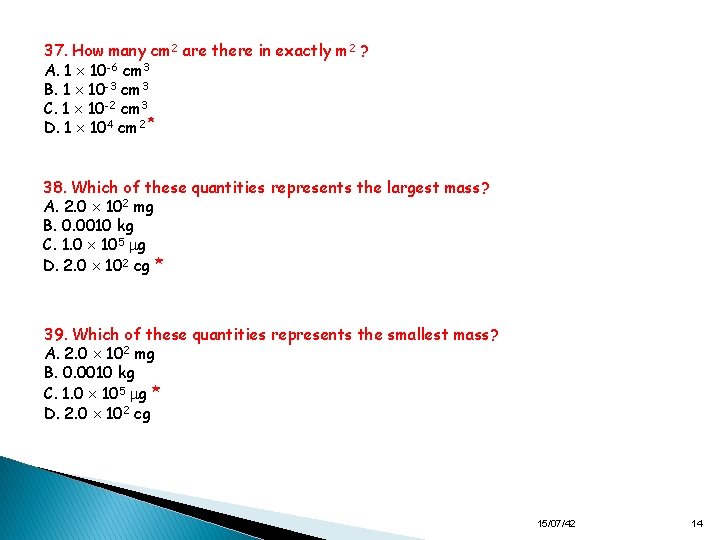
37. How many cm 2 are there in exactly m 2 ? A. 1 10 -6 cm 3 B. 1 10 -3 cm 3 C. 1 10 -2 cm 3 D. 1 104 cm 2 * 38. Which of these quantities represents the largest mass? A. 2. 0 102 mg B. 0. 0010 kg C. 1. 0 105 g D. 2. 0 102 cg * 39. Which of these quantities represents the smallest mass? A. 2. 0 102 mg B. 0. 0010 kg C. 1. 0 105 g * D. 2. 0 102 cg 15/07/42 14

40. Convert -77 F to kalvin ? A. 212. 6 K * B. -212. 6 K C. -28. 1 K D. +13. 5 K 41. Which of the following prefixes is not correct A. decid 10 * B. kilo. K 103 C. Pico p 10 -12 D. micro. M 10 -6 42. A lead sphere has a mass of 1. 2 x 104 g, and its volume is 1. 05 x 103 cm 3. Calculate the density of lead ? A. 0. 0875 g/cm 3 B. 11. 4 g/cm 3 * C. 1. 26 x 107 g/cm 3 D. 0. 8 g/cm 3 15

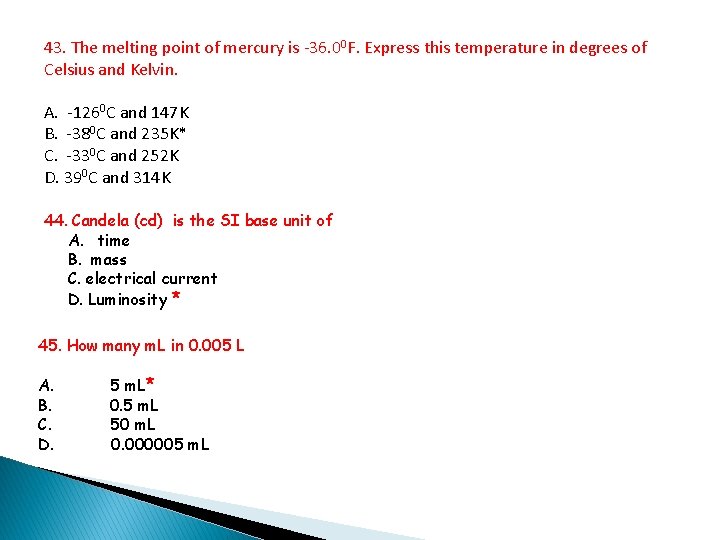
43. The melting point of mercury is -36. 00 F. Express this temperature in degrees of Celsius and Kelvin. A. -1260 C and 147 K B. -380 C and 235 K* C. -330 C and 252 K D. 390 C and 314 K 44. Candela (cd) is the SI base unit of A. time B. mass C. electrical current D. Luminosity * 45. How many m. L in 0. 005 L A. B. C. D. 5 m. L* 0. 5 m. L 50 m. L 0. 000005 m. L

1. An anion is defined as A. a charged atom or group of atoms with a net negative charge. * B. a stable atom. C. a group of stable atoms. D. an atom or group of atoms with a net positive charge. 2. Atoms of the same element with different mass numbers are called A. ions. B. neutrons. C. allotropes. D. chemical families. E. isotopes. * 3. How many neutrons are there in an atom of lead A. 82 B. 126* C. 208 D. 290 E. none of them 82 Pb whose mass number is 208?

4. An atom of the isotope sulfur-31 consists of how many protons, neutrons, and electrons? (p = proton, n = neutron, e = electron) A. 15 p, 16 n, 15 e B. 16 p, 15 n, 16 e * C. 16 p, 31 n, 16 e D. 32 p, 31 n, 32 e E. 16 p, 16 n, 15 e 5. A magnesium ion, Mg 2+, has A. 12 protons and 13 electrons. B. 24 protons and 26 electrons. C. 12 protons and 10 electrons. * D. 24 protons and 22 electrons. E. 12 protons and 14 electrons. 6. A sulfide ion, S 2 - , has: A. 16 protons and 16 electrons B. 32 protons and 16 electrons C. 16 protons and 14 electrons D. 16 protons and 18 electrons * E. 32 protons and 18 electrons

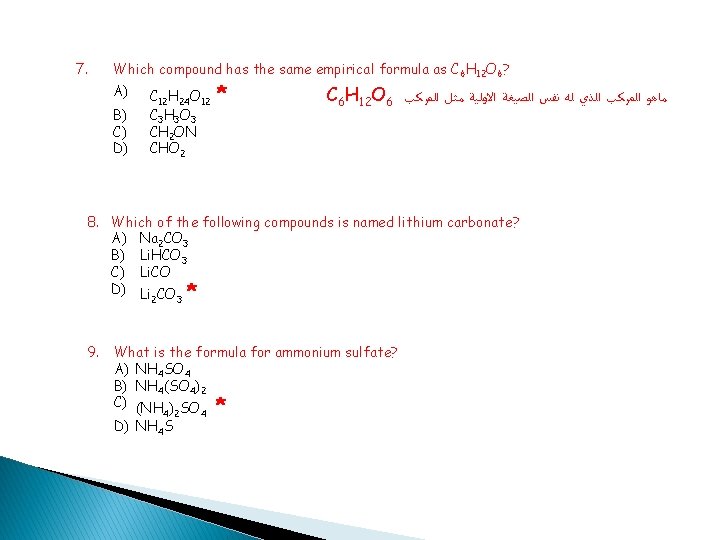
7. Which compound has the same empirical formula as C 6 H 12 O 6? A) B) C) D) C 12 H 24 O 12 C 3 H 3 O 3 CH 2 ON CHO 2 * C 6 H 12 O 6 ﻣﺎﻫﻮ ﺍﻟﻤﺮﻛﺐ ﺍﻟﺬﻱ ﻟﻪ ﻧﻔﺲ ﺍﻟﺼﻴﻐﺔ ﺍﻻﻭﻟﻴﺔ ﻣﺜﻞ ﺍﻟﻤﺮﻛﺐ 8. Which of the following compounds is named lithium carbonate? A) Na 2 CO 3 B) Li. HCO 3 C) Li. CO D) Li CO * 9. What is the formula for ammonium sulfate? A) NH 4 SO 4 B) NH 4(SO 4)2 C) (NH ) SO * 2 3 4 2 D) NH 4 S 4

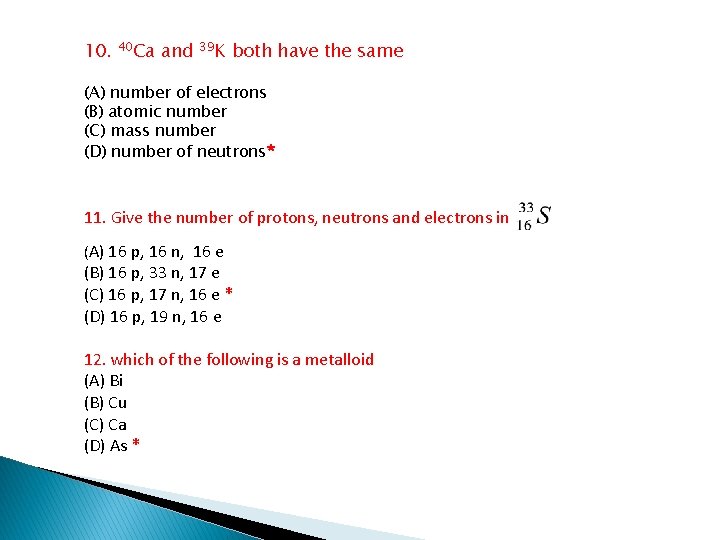
10. 40 Ca and 39 K both have the same (A) number of electrons (B) atomic number (C) mass number (D) number of neutrons* 11. Give the number of protons, neutrons and electrons in (A) 16 p, 16 n, 16 e (B) 16 p, 33 n, 17 e (C) 16 p, 17 n, 16 e * (D) 16 p, 19 n, 16 e 12. which of the following is a metalloid (A) Bi (B) Cu (C) Ca (D) As *

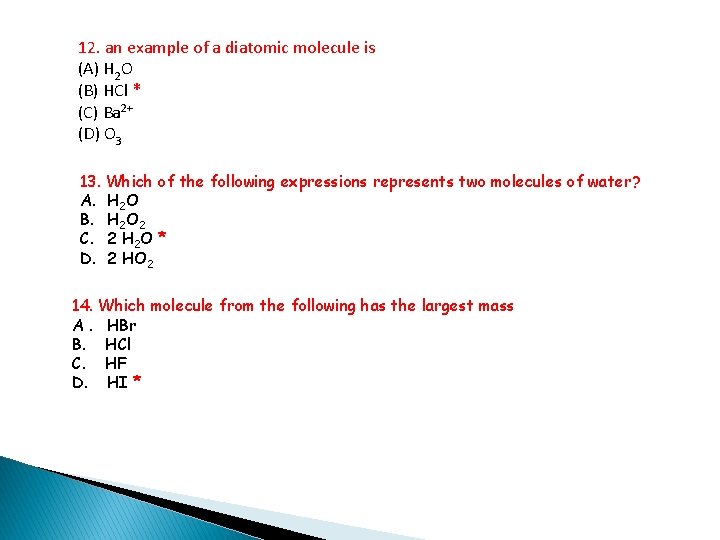
12. an example of a diatomic molecule is (A) H 2 O (B) HCl * (C) Ba 2+ (D) O 3 13. Which of the following expressions represents two molecules of water? A. H 2 O B. H 2 O 2 C. 2 H 2 O * D. 2 HO 2 14. Which molecule from the following has the largest mass A. HBr B. HCl C. HF D. HI *

15. A. B. C. D. 16. A. B. C. D. Which of these compounds is a binary compound? Na. Cl * Mg. SO 4 Na. OH HCN Which of these compounds is a ternary compound? Na. Cl H 2 O Na. OH * Mg. Br 2 17. the species S 2 -, F-, and Cl- are all A. cations B. anions * C. isotopes D. Halogens

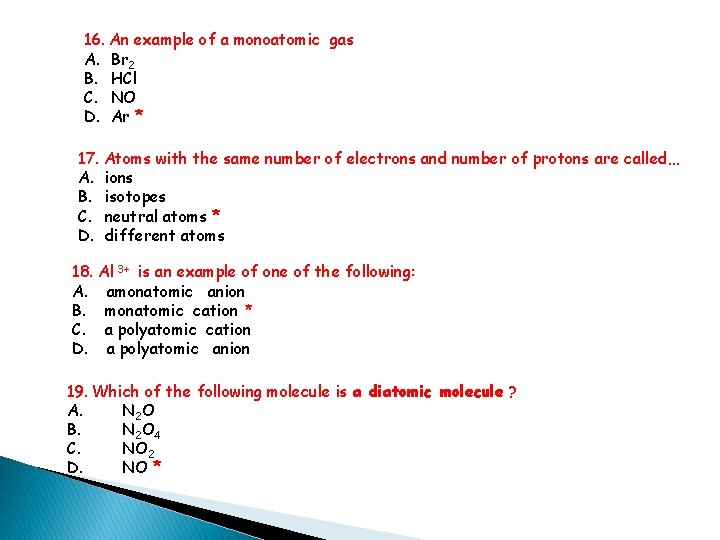
16. An example of a monoatomic gas A. Br 2 B. HCl C. NO D. Ar * 17. Atoms with the same number of electrons and number of protons are called… A. ions B. isotopes C. neutral atoms * D. different atoms 18. Al 3+ is an example of one of the following: A. amonatomic anion B. monatomic cation * C. a polyatomic cation D. a polyatomic anion 19. Which of the following molecule is a diatomic molecule ? A. N 2 O B. N 2 O 4 C. NO 2 D. NO *

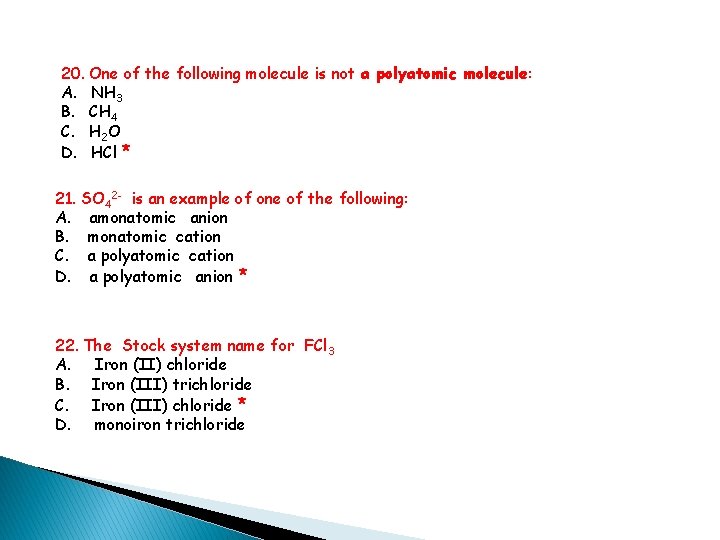
20. One of the following molecule is not a polyatomic molecule: A. NH 3 B. CH 4 C. H 2 O D. HCl * 21. SO 42 - is an example of one of the following: A. amonatomic anion B. monatomic cation C. a polyatomic cation D. a polyatomic anion * 22. The Stock system name for FCl 3 A. Iron (II) chloride B. Iron (III) trichloride C. Iron (III) chloride * D. monoiron trichloride

23. A. B. C. D. Which of these ions is a monoatomic ions ? Na+ * SO 42 NO 3 NH 4+ 24. A. B. C. D. Which of these compounds is a ternary compound? Na. Cl H 2 O Na. OH * Mg. Br 2 25. A. B. C. D. Which of these compounds is a binary compound? Na. Cl * Mg. SO 4 Na. OH HCN

26. A. B. C. D. Which of these compounds is a ternary compound? Na. Cl H 2 O Na. OH * Mg. Br 2 27. molecules consist of the same element with different numbers of atoms and chemical structure are called … A. ions. B. neutrons. C. allotropes * D. isotopes. 28. A magnesium ion, 12 Mg 2+, has A. 12 protons and 13 electrons. B. 24 protons and 26 electrons. C. 12 protons and 10 electrons. * D. 24 protons and 22 electrons.

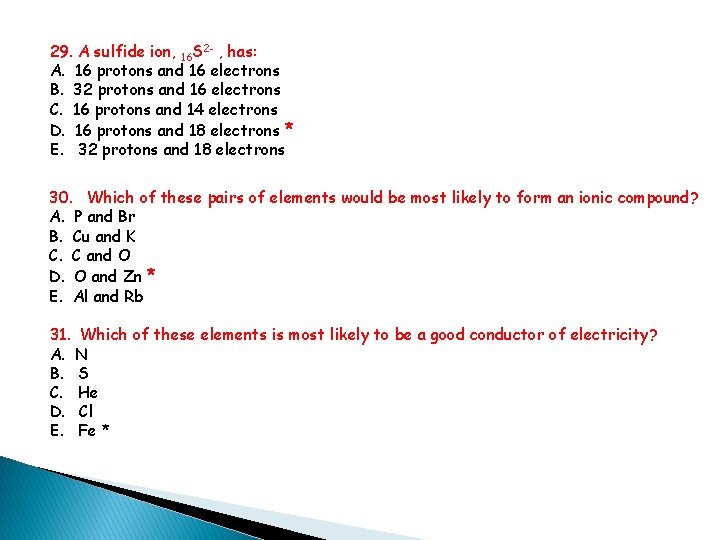
29. A sulfide ion, 16 S 2 - , has: A. 16 protons and 16 electrons B. 32 protons and 16 electrons C. 16 protons and 14 electrons D. 16 protons and 18 electrons * E. 32 protons and 18 electrons 30. Which of these pairs of elements would be most likely to form an ionic compound? A. P and Br B. Cu and K C. C and O D. O and Zn * E. Al and Rb 31. Which of these elements is most likely to be a good conductor of electricity? A. N B. S C. He D. Cl E. Fe *

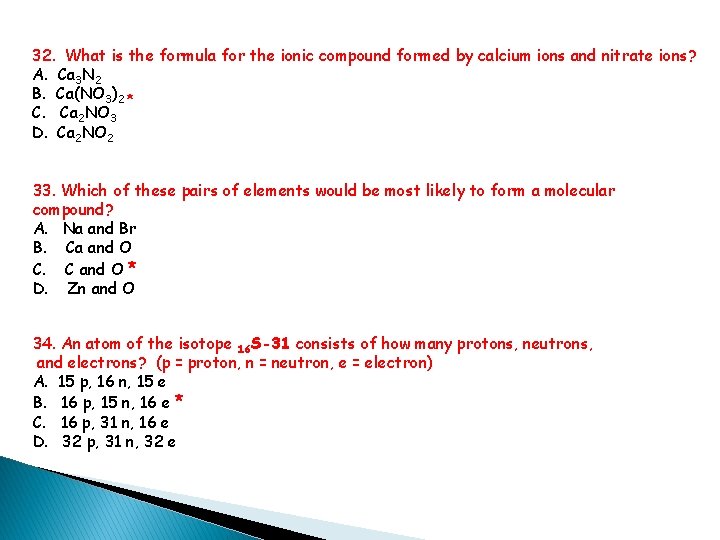
32. What is the formula for the ionic compound formed by calcium ions and nitrate ions? A. Ca 3 N 2 B. Ca(NO 3)2 * C. Ca 2 NO 3 D. Ca 2 NO 2 33. Which of these pairs of elements would be most likely to form a molecular compound? A. Na and Br B. Ca and O C. C and O * D. Zn and O 34. An atom of the isotope 16 S-31 consists of how many protons, neutrons, and electrons? (p = proton, n = neutron, e = electron) A. 15 p, 16 n, 15 e B. 16 p, 15 n, 16 e * C. 16 p, 31 n, 16 e D. 32 p, 31 n, 32 e

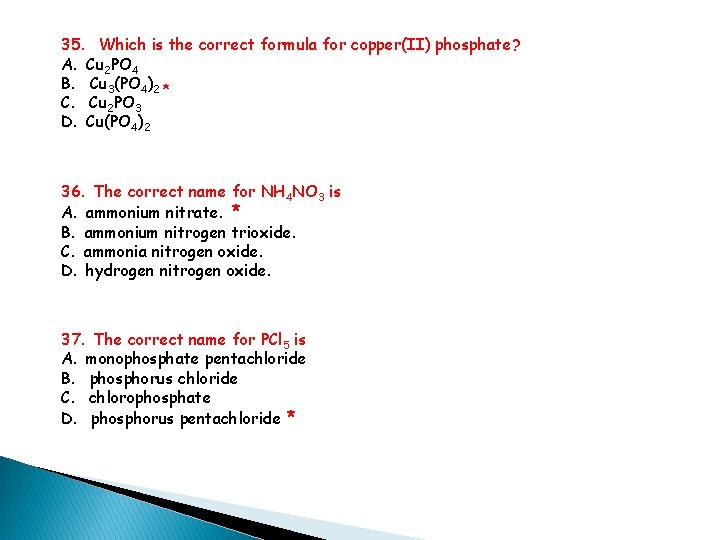
35. Which is the correct formula for copper(II) phosphate? A. Cu 2 PO 4 B. Cu 3(PO 4)2 * C. Cu 2 PO 3 D. Cu(PO 4)2 36. The correct name for NH 4 NO 3 is A. ammonium nitrate. * B. ammonium nitrogen trioxide. C. ammonia nitrogen oxide. D. hydrogen nitrogen oxide. 37. The correct name for PCl 5 is A. monophosphate pentachloride B. phosphorus chloride C. chlorophosphate D. phosphorus pentachloride *

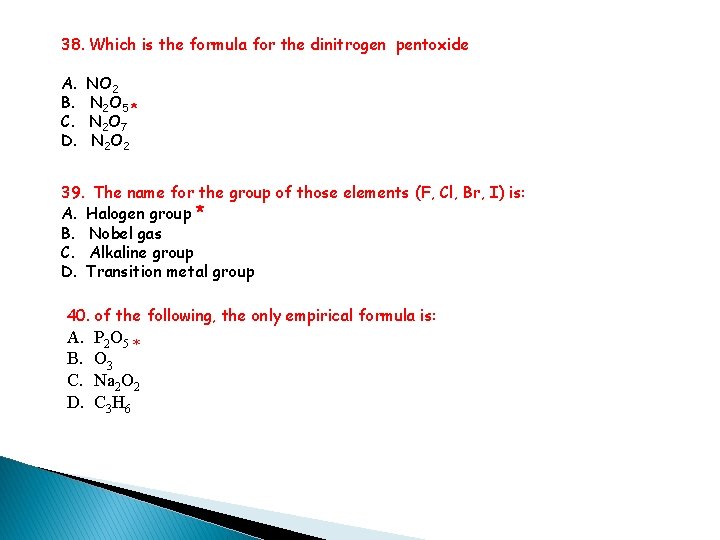
38. Which is the formula for the dinitrogen pentoxide A. NO 2 B. N 2 O 5 * C. N 2 O 7 D. N 2 O 2 39. The name for the group of those elements (F, Cl, Br, I) is: A. Halogen group * B. Nobel gas C. Alkaline group D. Transition metal group 40. of the following, the only empirical formula is: A. B. C. D. P 2 O 5 * O 3 Na 2 O 2 C 3 H 6
 Vignette mutuelle 110/110
Vignette mutuelle 110/110 110-000-110 & 111-000-111
110-000-110 & 111-000-111 Passive voice revision
Passive voice revision How to find one mole of a compound
How to find one mole of a compound General revision of assessments and property classification
General revision of assessments and property classification Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 Klorpentan
Klorpentan General chemistry 11th edition
General chemistry 11th edition General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? Iannonechem
Iannonechem Unit 7 ap chemistry
Unit 7 ap chemistry Types of intermolecular forces
Types of intermolecular forces Chem 130 final exam
Chem 130 final exam Organic chemistry prefix
Organic chemistry prefix Principles of kmt
Principles of kmt January 2018 regents chemistry
January 2018 regents chemistry Ap chemistry absorbance
Ap chemistry absorbance Chem 1020
Chem 1020 Ion induced dipole examples
Ion induced dipole examples