CHEM 110 Dr Suzan A Khayyat CHEM 110









































- Slides: 54

CHEM 110 Dr. Suzan A. Khayyat


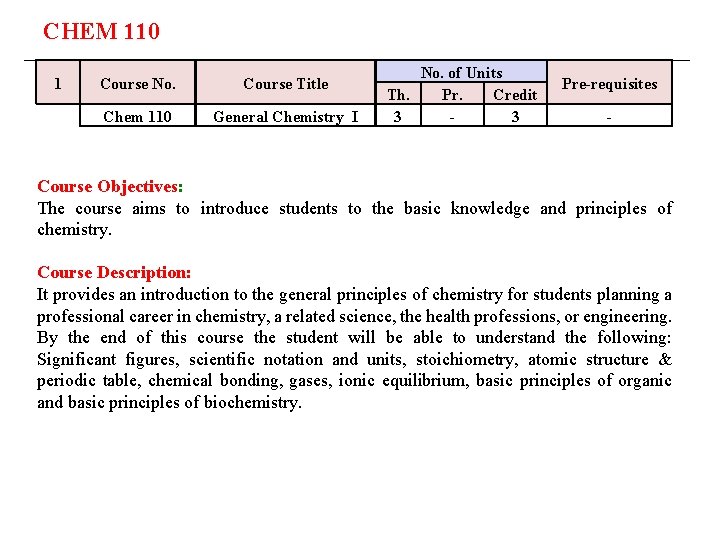
CHEM 110 1 Course No. Course Title Chem 110 General Chemistry I No. of Units Th. Pr. Credit 3 3 Pre-requisites - Course Objectives: The course aims to introduce students to the basic knowledge and principles of chemistry. Course Description: It provides an introduction to the general principles of chemistry for students planning a professional career in chemistry, a related science, the health professions, or engineering. By the end of this course the student will be able to understand the following: Significant figures, scientific notation and units, stoichiometry, atomic structure & periodic table, chemical bonding, gases, ionic equilibrium, basic principles of organic and basic principles of biochemistry.

Chemistry – 10 th Edition by Raymond Chang Main text book: § Chemistry, by Chang, 10 th. ed. , 2007, Mc. Graw-Hill. Or any updated versions (Chapters: 1, 2, 3, 4, 5, 7, 8, 9, 14, 15, 24, 25)





Chapter 1 The Study of Change

Chemistry Is the study of matter and the changes it undergoes

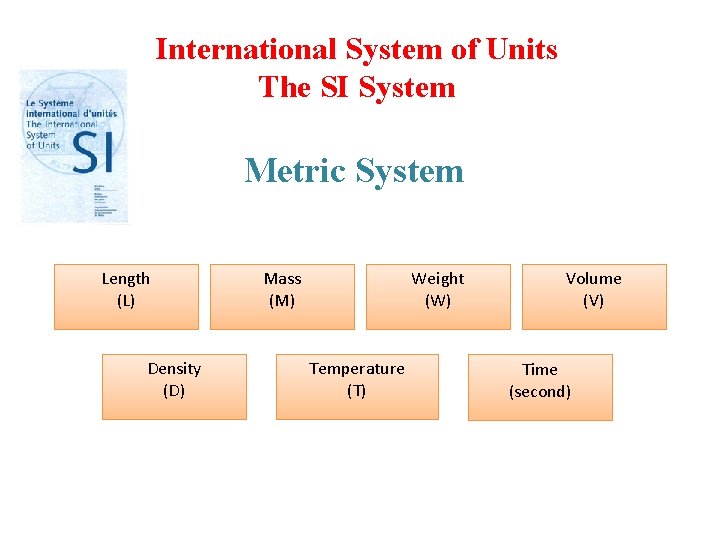
1. 7 Measurement (p. 15 -20) • SI units. • Mass and Weight • Volume • Density • Temperature Scales. • Table 1. 2 & Table 1. 3 • Examples 1. 1 -1. 2 -1. 3

International System of Units The SI System Metric System Length (L) Density (D) Mass (M) Weight (W) Temperature (T) Volume (V) Time (second)

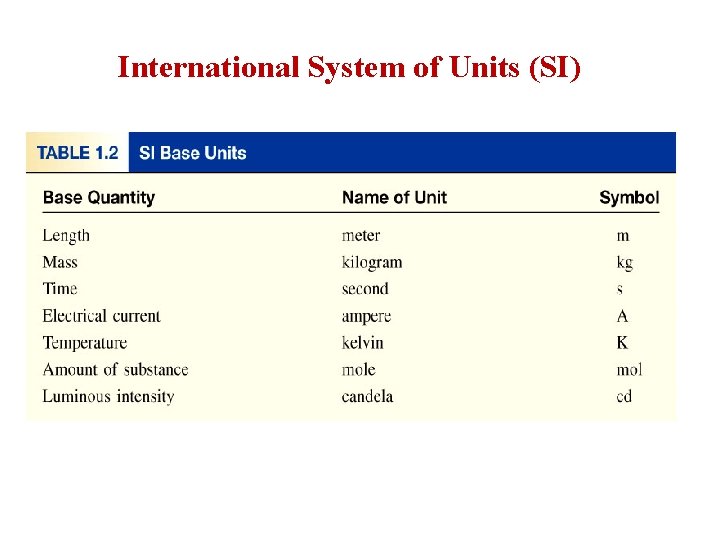
International System of Units (SI)



p 103 10 -12 n 10 -9 103 10 -6 103 m 103 c unit d 103 10 -2 10 -1 1 K 103 ÷ x ﺍﻟﺘﺤﻮﻳﻼﺕ ﻣﺴﻄﺮﺓ Perfixes Scale 103 M 106 103 G 109 103 T 1012

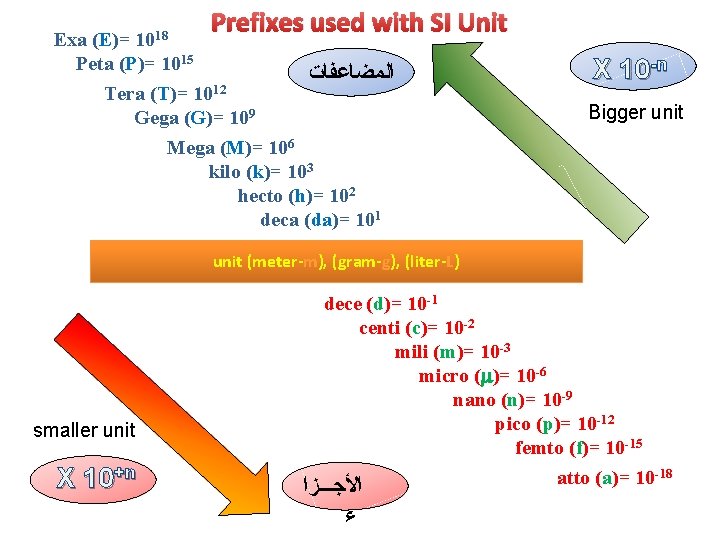
Exa (E)= 1018 Prefixes used with SI Unit Peta (P)= 1015 Tera (T)= 1012 Gega (G)= 109 ﺍﻟﻤﻀﺎﻋﻔﺎﺕ X 10 -n Bigger unit Mega (M)= 106 kilo (k)= 103 hecto (h)= 102 deca (da)= 101 unit (meter-m), (gram-g), (liter-L) smaller unit X 10+n dece (d)= 10 -1 centi (c)= 10 -2 mili (m)= 10 -3 micro (m)= 10 -6 nano (n)= 10 -9 pico (p)= 10 -12 femto (f)= 10 -15 atto (a)= 10 -18 ﺍﻷﺠـــﺰﺍ ﺀ

Example The SI prefixes giga- and micro represent, respectively: (a) 10 -9 and 10 -6 (b) 106 and 10 -3 (c) 103 and 10 -3 (d) 109 and 10 -6


Which of the following is the smallest distance? (a) 21 m → 21 m (b) 2. 1 x 102 cm → 2. 1 m (c) 21 mm→ 0. 021 m (d) 2. 1 x 104 pm → 2. 1 x 10 -8 m Put all of them in the same unit

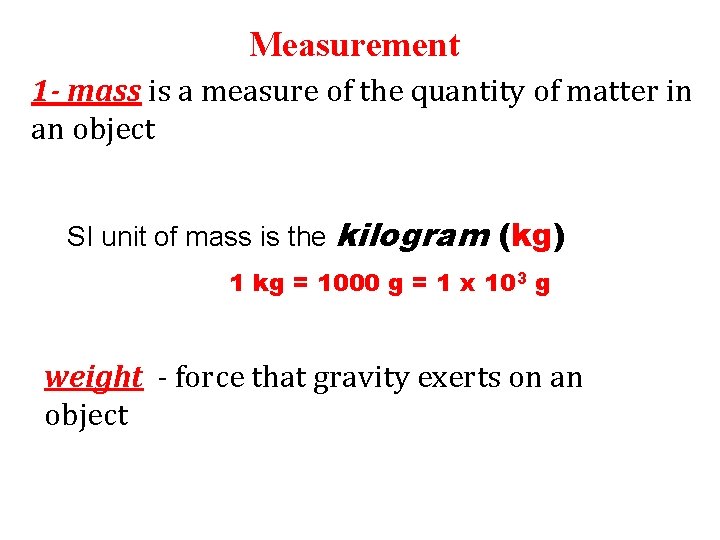
Measurement 1 - mass is a measure of the quantity of matter in an object SI unit of mass is the kilogram (kg) 1 kg = 1000 g = 1 x 103 g weight - force that gravity exerts on an object

The Mass of an object doesn't change when an object's location changes. Weight, on the other hand does change with location. weight = c x mass A 1 kg bar will weigh on earth, c = 1. 0 1 kg on earth on moon, c ~ 0. 1 kg on moon

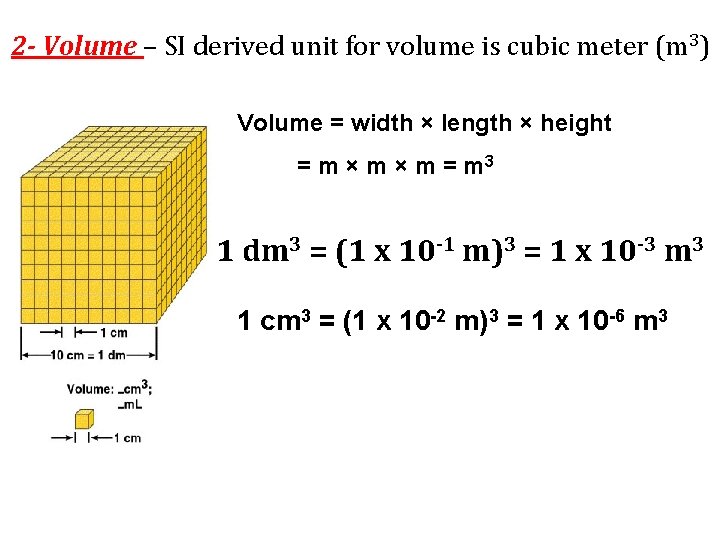
2 - Volume – SI derived unit for volume is cubic meter (m 3) Volume = width × length × height = m × m = m 3 1 dm 3 = (1 x 10 -1 m)3 = 1 x 10 -3 m 3 1 cm 3 = (1 x 10 -2 m)3 = 1 x 10 -6 m 3

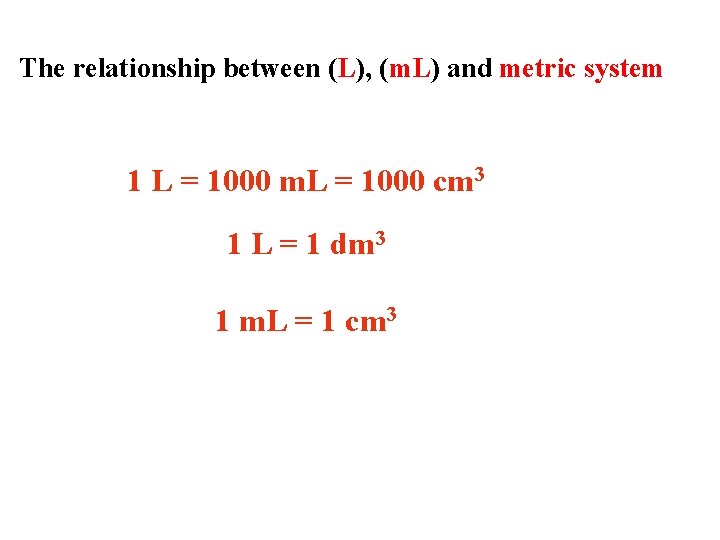
The relationship between (L), (m. L) and metric system 1 L = 1000 m. L = 1000 cm 3 1 L = 1 dm 3 1 m. L = 1 cm 3

Examples Convert the followings to m 3 A. 2. 5 x 104 cm 3 B. 1. 20 x 10 -3 dm 3 Convert the followings to m. L A. 800 L B. 3. 09 cm 3

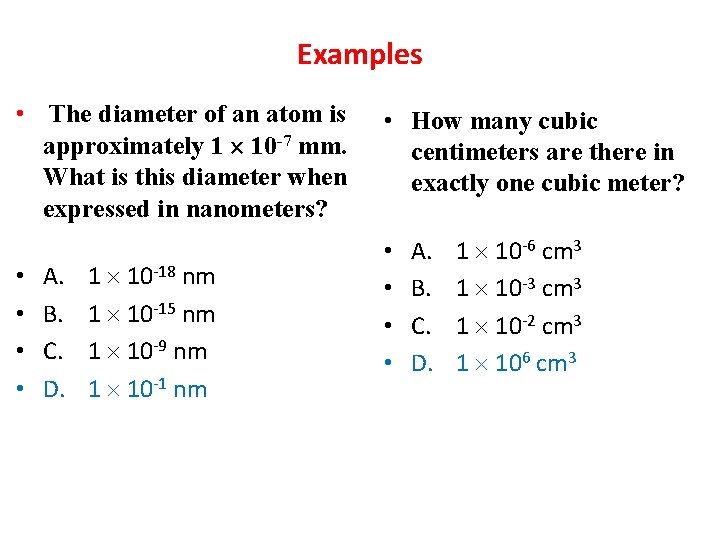
Examples • The diameter of an atom is approximately 1 10 -7 mm. What is this diameter when expressed in nanometers? • • A. B. C. D. 1 10 -18 nm 1 10 -15 nm 1 10 -9 nm 1 10 -1 nm • How many cubic centimeters are there in exactly one cubic meter? • • A. B. C. D. 1 10 -6 cm 3 1 10 -3 cm 3 1 10 -2 cm 3 1 106 cm 3

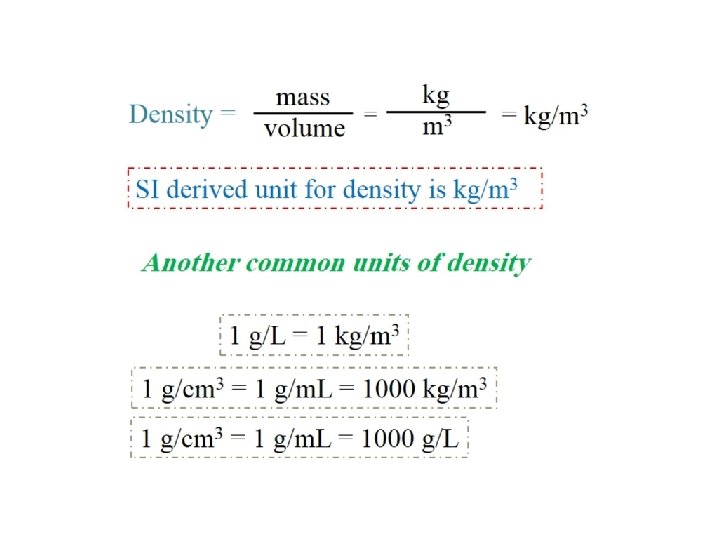
3 - Density – SI derived unit for density is kg/m 3 mass density = volume m d = V 1 g/cm 3 = 1 g/m. L = 1000 kg/m 3 1 g/m. L = 1× 10 -3 g/L m d v

A piece of platinum metal with a density of 21. 5 g/cm 3 has a volume of 4. 49 cm 3. What is its mass? m d = V

Common unit of volume is liter (L) and milliliter (m. L) 1 L = 1000 m. L = 1000 cm 3 = 1 dm 3 The relationship between (L), (m. L) and metric system 1 m. L = 1 cm 3 1 L = 1 dm 3

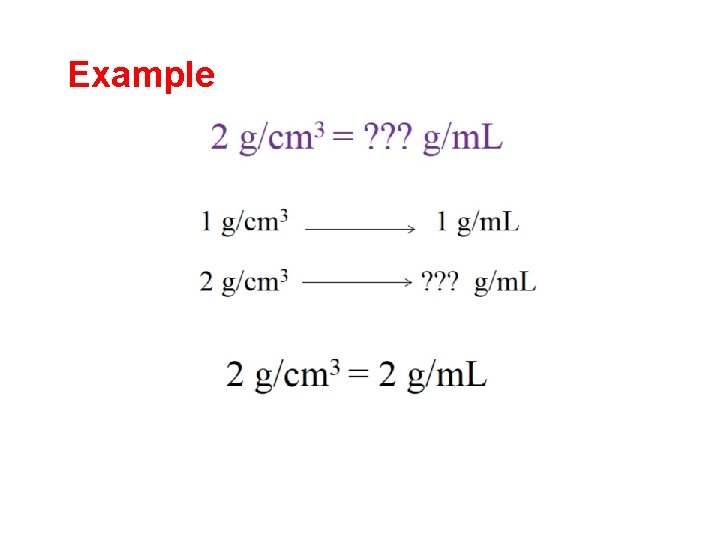
Example



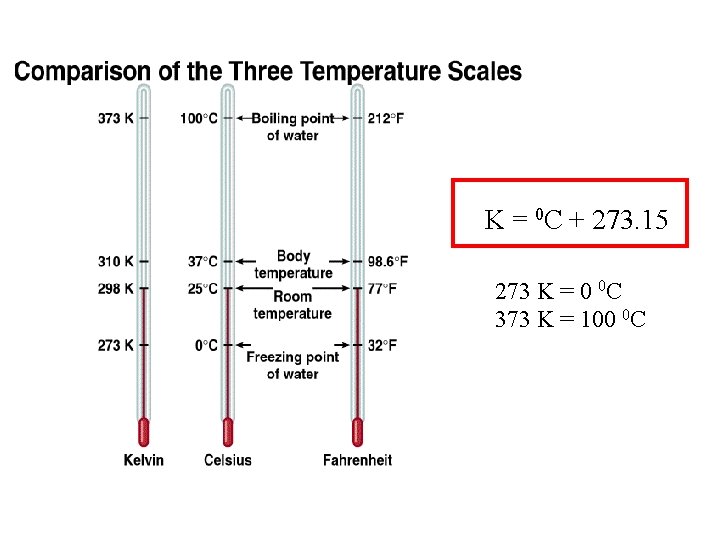
4 -Temperature Scales There are three temperature scales, their units are: 1. Degrees Celsius 0 C 2. Degrees Fahrenheit 0 F 3. Kelvin K Kelvin is the SI Base Unit of Temperature

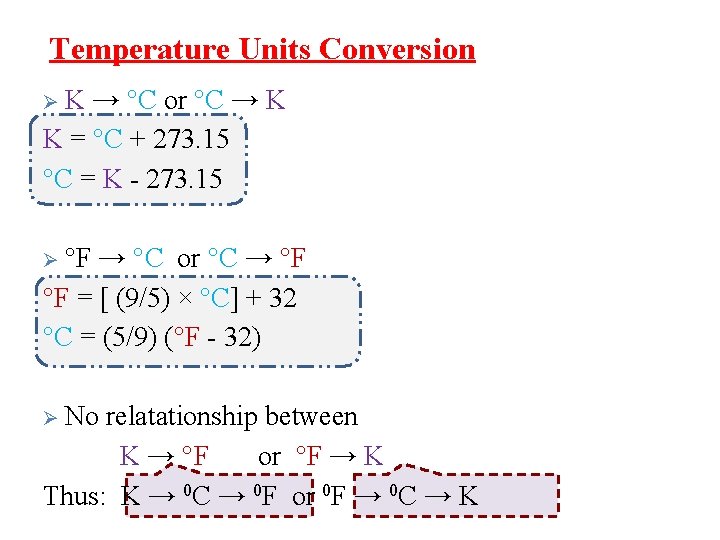
K = 0 C + 273. 15 273 K = 0 0 C 373 K = 100 0 C





Example 1. 3: (a) Convert 224 0 C → 0 F? °F = [(9 /5 ) × °C ] + 32 [°F = (9 /5 ) × 224 °C] + 32 = 435 0 F (b) Convert -452 0 F → 0 C ? °C = (5 /9 ) (°F - 32 ) °C = (5 /9 ) (-452 - 32 ) = -269 0 C (c) Convert -38. 9 0 C → K ? ° K = -38. 9 °C + 273. 15 = 234. 3 K

Temperature Units Conversion Ø K → °C or °C → K K = °C + 273. 15 °C = K - 273. 15 Ø °F → °C or °C → °F = [ (9/5) × °C] + 32 °C = (5/9) (°F - 32) Ø No relatationship between K → °F or °F → K Thus: K → 0 C → 0 F or 0 F → 0 C → K

1 2. 3 17/04/42 Which of the following is not an SI base unit? A) kilometer B) kilogram C) second D) Kelvin Which of the following SI base units is not commonly used in chemistry? A) kilogram B) kelvin C) candela D) mole Which of the following prefixes means 1/1000? A) kilo B) deci C) centi D) milli 41

4. What temperature is 95 °F when converted to degrees Celsius? A) B) C) D) 5. . 17/04/42 6 63 °C 35 °C 127 °C 15 °C What temperature is 37 °C when converted to kelvin? A) 310 K B) 99 K C) 236 K D) 67 K What temperature is 77 K when converted to degrees Celsius? A) B) C) D) – 296 °C 105 °C – 196 °C 25 °C 42

7. Lithium is the least dens metal known (density= 0. 53 g/cm 3 ). What is the volume occupied by 1. 2 x 103 g of lithium? A. B. C. D. 0. 0875 cm 3 11. 4 cm 3 0. 44 x 10 -3 cm 3 2. 26 x 103 cm 3 8. Convert -77 F to kalvin ? A. 212. 6 K B. -212. 6 K C. -28. 1 K D. +13. 5 K 9 Express 7. 5 ng as Tg A. 7. 5 X 10 -21 Tg B. 75 X 1024 Tg C. 0. 75 Tg D. 7. 5 X 1021 Tg 17/04/42 43

10 11 12 17/04/42 What is 22. 6 m when converted to decimeters? A) 0. 226 dm B) 2. 26 dm C) 226 dm D) 2. 26 x 10– 3 dm What is 25. 4 mg when converted to kilograms? A) 2540 kg B) 2. 54 x 10– 5 kg C) 2. 54 kg D) 2. 54 x 104 kg At what temperature does the numerical reading on a Celsius thermometer equal that on a Fahrenheit thermometer? A) B) C) D) 0 °C – 40 °C 100 °C – 32 °C 44

13. A. B. C. D. E. The SI prefixes giga and micro represent, respectively: 10 -9 and 10 -6. 106 and 10 -3. 103 and 10 -3. 109 and 10 -6. 10 -9 and 10 -3. 14. Ammonia boils at -33. 4 C. What temperature is this in F? A. -60. 1 F B. -92. 1 F C. -28. 1 F D. +13. 5 F 15. Which of the following prefixes is not correct? A. Kilok 10 -3 B. micro- µ 10 -6 C. nano- n 10 -9 D. deci- d 10 -1 17/04/42 45

16. Candela (cd) is the SI base unit of A. time B. length C. luminous intensity D. electrical current 17. Express 5500 nm as picometers. A. 5. 5 10 -6 pm B. 55. 0 pm C. 550 pm D. 5. 5 106 pm 18. The SI prefix giga represents: A. 10 -6 B. 106 C. 103 D. 109 19 Which of the following prefixes means 106 ? A. Mega B. nano C. Tera D. milli 17/04/42 46

20. A piece of iron (Fe) metal weighing 194. 3 g is placed in a graduated cylinder containing 242. 0 m. L of water. The volume of water now reads 260. 5 m. L. From these data calculate the density of iron. A. 10. 5 g/cm 3 B. 1. 25 g/cm 3 C. 0. 746 g/cm 3 D. 21. 0 g/cm 3 21 22 Which of the following SI base units is used to measure Electrical Current? Acandela. Bkelvin. CAmpere Which. of the following prefixes means 1/100? A. Dmole kilo B. . deci C. Centi D. milli 17/04/42 47

23 Which of the following SI base units is not commonly used in chemistry? A. kilogram B. kelvin C. ampere D. mole 24. The diameter of an atom is approximately 1 10 -7 mm. What is this diameter when expressed in nanometers? A. 1 10 -18 nm B. 1 10 -15 nm C. 1 10 -9 nm D. 1 10 -1 nm 25. Express 75 Tg as pg A. 7. 5 pg B. 75 X 1024 pg C. 0. 75 pg D. 75 X 10 -24 pg 17/04/42 48

26. The SI unit of time is the A. hour B. Second C. minute D. ampere 27 The following procedure was used to determine the volume of a flask. The flask was weighted dry and then filled with water. If the masses of empty flask and filled flask were 56. 12 g and 87. 39 g, respectively, and the density of water is 0. 9976 g/cm 3, Calculate the volume of the flask in cm 3 ? A. B. C. D. 56. 255 cm 3 87. 6 cm 3 31. 345 cm 3 143. 855 28. Express 2200 nm as picometers A. 2. 2 X 10 -7 pm B. 22. 0 pm C. 220 pm D. 2. 2 X 106 pm 17/04/42 49

29. 30. A. B. C. D. The SI prefixes kilo and milli represent, respectively: A. 10 -9 and 10 -6 B. 106 and 10 -3 C. 103 and 10 -3 D. 109 and 10 -6 E. 10 -9 and 10 -3 The SI prefixes Tara and nano represent, respectively: 10 -9 and 10 -6 106 and 10 -3 103 and 10 -3 1012 and 10 -9 31. The density of octane is 0. 702 g/cm 3. what is the mass of 65 m. L of octane? A. 45. 6 g B. 92. 6 g C. 22. 5 g D. 110 g 17/04/42 50

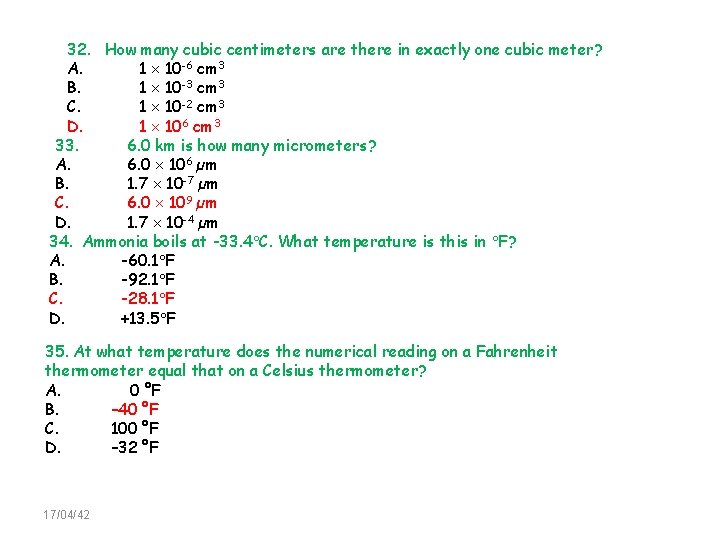
32. How many cubic centimeters are there in exactly one cubic meter? A. 1 10 -6 cm 3 B. 1 10 -3 cm 3 C. 1 10 -2 cm 3 D. 1 106 cm 3 33. 6. 0 km is how many micrometers? A. 6. 0 106 µm B. 1. 7 10 -7 µm C. 6. 0 109 µm D. 1. 7 10 -4 µm 34. Ammonia boils at -33. 4 C. What temperature is this in F? A. -60. 1 F B. -92. 1 F C. -28. 1 F D. +13. 5 F 35. At what temperature does the numerical reading on a Fahrenheit thermometer equal that on a Celsius thermometer? A. 0 °F B. – 40 °F C. 100 °F D. – 32 °F 17/04/42 51

36. How many cm 2 are there in exactly m 2 ? A. 1 10 -6 cm 3 B. 1 10 -3 cm 3 C. 1 10 -2 cm 3 D. 1 104 cm 2 37. A. B. C. D. 38. A. B. C. D. Which of these quantities represents the largest mass? 2. 0 102 mg 0. 0010 kg 1. 0 105 g 2. 0 102 cg Which of these quantities represents the smallest mass? 2. 0 102 mg 0. 0010 kg 1. 0 105 g 2. 0 102 cg 52

39. Convert -77 F to kalvin ? A. 212. 6 K B. -212. 6 K C. -28. 1 K D. +13. 5 K 40. Which of the following prefixes is not correct A. decid 10 B. kilo. K 103 C. Pico p 10 -12 D. micro. M 10 -6 41. A lead sphere has a mass of 1. 2 x 104 g, and its volume is 1. 05 x 103 cm 3. Calculate the density of lead ? A. B. C. D. 0. 0875 g/cm 3 11. 4 g/cm 3 1. 26 x 107 g/cm 3 0. 8 g/cm 3 53

 Dr khayyat
Dr khayyat Suzan fischbein
Suzan fischbein Suzan van der lee
Suzan van der lee Suzan alteri
Suzan alteri Naru triage
Naru triage Où se trouve le numéro d'affiliation mutuelle vignette ?
Où se trouve le numéro d'affiliation mutuelle vignette ? 011 101 110
011 101 110 Beer’s law plot
Beer’s law plot Chem 301 gas law simulator
Chem 301 gas law simulator Chemistry law hazard pay
Chemistry law hazard pay Chem moles
Chem moles Dyno chem
Dyno chem Beraderm
Beraderm Iannone chem moodle
Iannone chem moodle Chem
Chem Www.chem.purdue/gchelp/atoms/elements.html
Www.chem.purdue/gchelp/atoms/elements.html Photoelectron spectroscopy ap chemistry
Photoelectron spectroscopy ap chemistry Ap chemistry equilibrium review
Ap chemistry equilibrium review Chem
Chem Gen chem cheat sheet
Gen chem cheat sheet E-chem-portal
E-chem-portal Nss chemistry
Nss chemistry Energy quanta
Energy quanta Molecular attraction
Molecular attraction Chem
Chem Ethyl methyl chart
Ethyl methyl chart Chem connections india
Chem connections india Ap chem equilibrium
Ap chem equilibrium Chem
Chem Induction chemistry
Induction chemistry Chem
Chem Trigonal planar vs trigonal pyramidal
Trigonal planar vs trigonal pyramidal Guan chem chen
Guan chem chen Chem 30 equilibrium
Chem 30 equilibrium Chapter 19 acids bases and salts answer key
Chapter 19 acids bases and salts answer key Pdbe chem
Pdbe chem Chem.fsu.edu
Chem.fsu.edu Gen chem review for ochem
Gen chem review for ochem Gen chem
Gen chem Chem lab
Chem lab Ap chemistry thermochemistry frq
Ap chemistry thermochemistry frq Chem
Chem Significant figure rules
Significant figure rules Sừng bên tủy sống
Sừng bên tủy sống Chem 494
Chem 494 Kinetics ap chemistry
Kinetics ap chemistry Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Chem
Chem Chem commun impact factor
Chem commun impact factor Amy gottfried umich
Amy gottfried umich Kmt chem
Kmt chem Hkale chem
Hkale chem Chem 1020
Chem 1020 Gen chem
Gen chem Chem
Chem