Electron Configuration Take notes on the appropriate page


- Slides: 13

Electron Configuration Take notes on the appropriate page of your packet

Electrons • Bohr: placed electrons in energy levels – Energy levels aren’t perfect circles – Within each energy level we can give a more specific location of the electron • Kind of like a street address • Electron configuration: the “exact” location of an electron at a given time

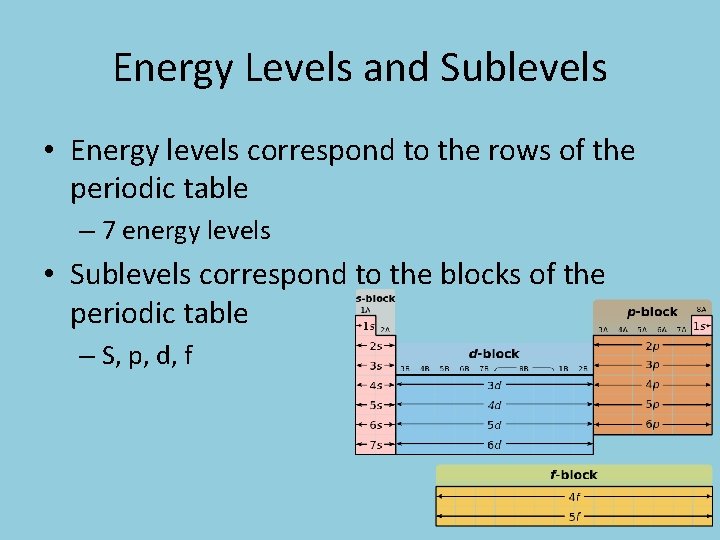
Energy Levels and Sublevels • Energy levels correspond to the rows of the periodic table – 7 energy levels • Sublevels correspond to the blocks of the periodic table – S, p, d, f

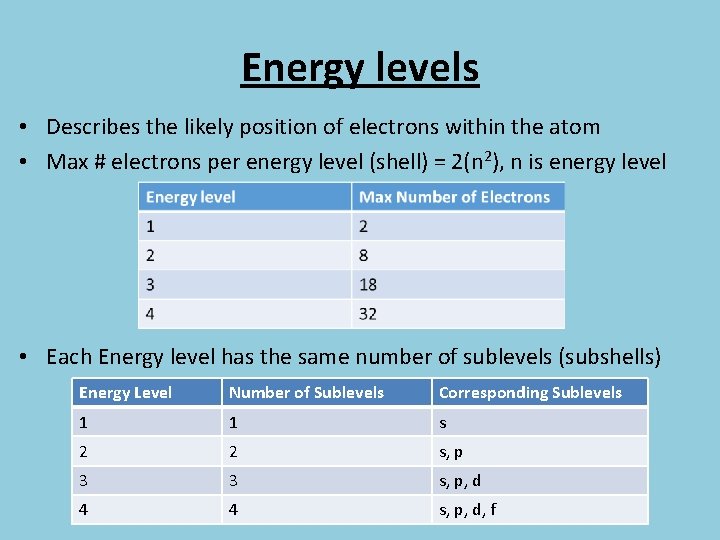
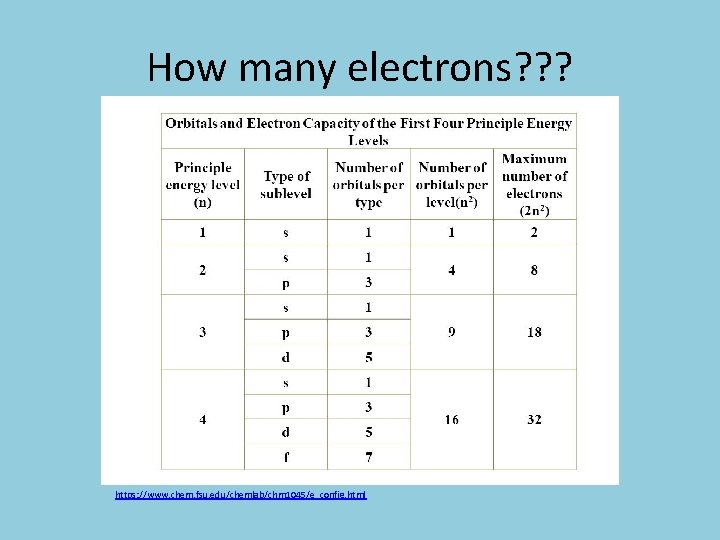
Energy levels • Describes the likely position of electrons within the atom • Max # electrons per energy level (shell) = 2(n 2), n is energy level • Each Energy level has the same number of sublevels (subshells) Energy Level Number of Sublevels Corresponding Sublevels 1 1 s 2 2 s, p 3 3 s, p, d 4 4 s, p, d, f

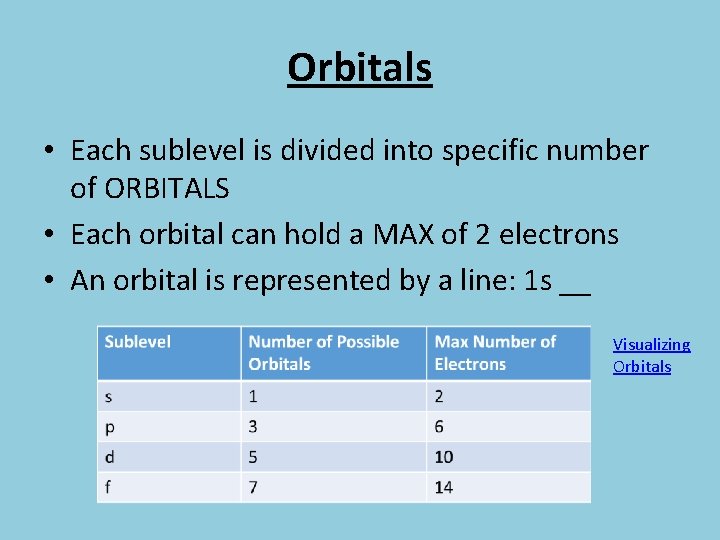
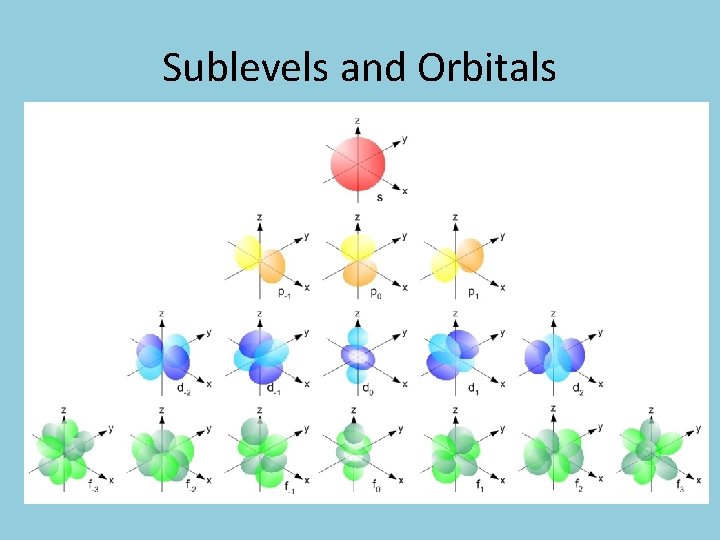
Orbitals • Each sublevel is divided into specific number of ORBITALS • Each orbital can hold a MAX of 2 electrons • An orbital is represented by a line: 1 s __ Visualizing Orbitals

Sublevels and Orbitals

How many electrons? ? ? https: //www. chem. fsu. edu/chemlab/chm 1045/e_config. html

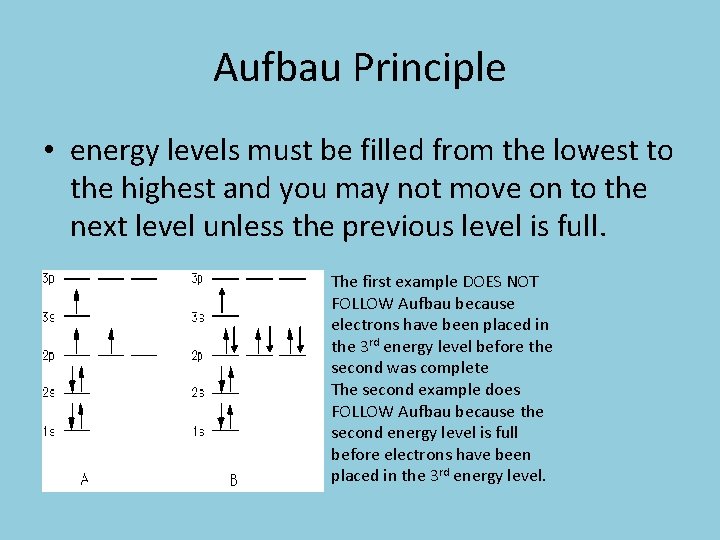
Aufbau Principle • energy levels must be filled from the lowest to the highest and you may not move on to the next level unless the previous level is full. The first example DOES NOT FOLLOW Aufbau because electrons have been placed in the 3 rd energy level before the second was complete The second example does FOLLOW Aufbau because the second energy level is full before electrons have been placed in the 3 rd energy level.

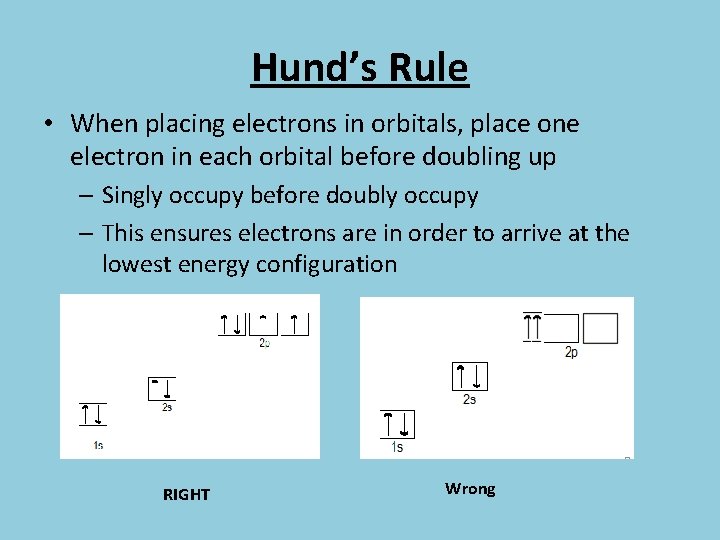
Hund’s Rule • When placing electrons in orbitals, place one electron in each orbital before doubling up – Singly occupy before doubly occupy – This ensures electrons are in order to arrive at the lowest energy configuration RIGHT Wrong

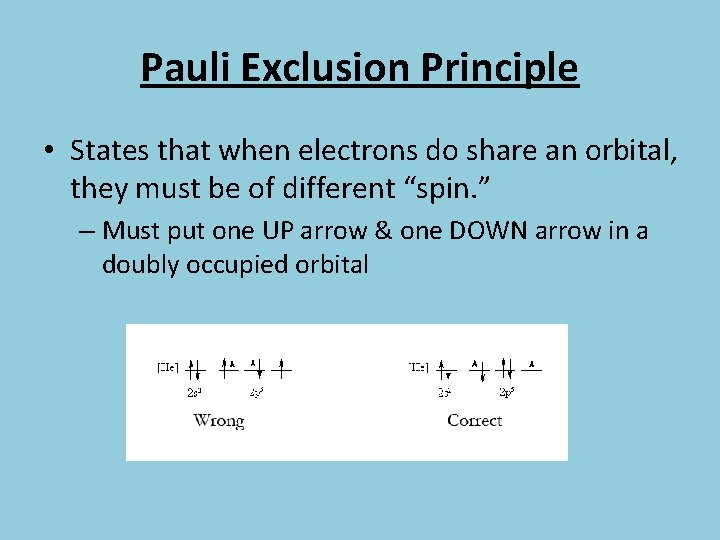
Pauli Exclusion Principle • States that when electrons do share an orbital, they must be of different “spin. ” – Must put one UP arrow & one DOWN arrow in a doubly occupied orbital

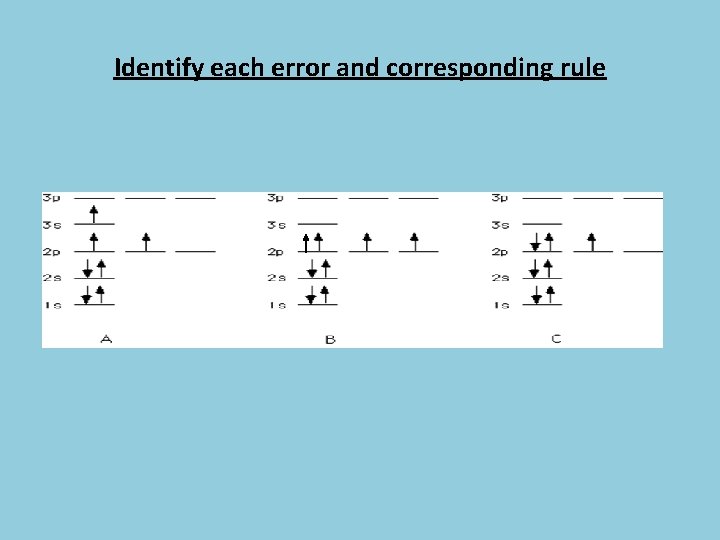
Identify each error and corresponding rule

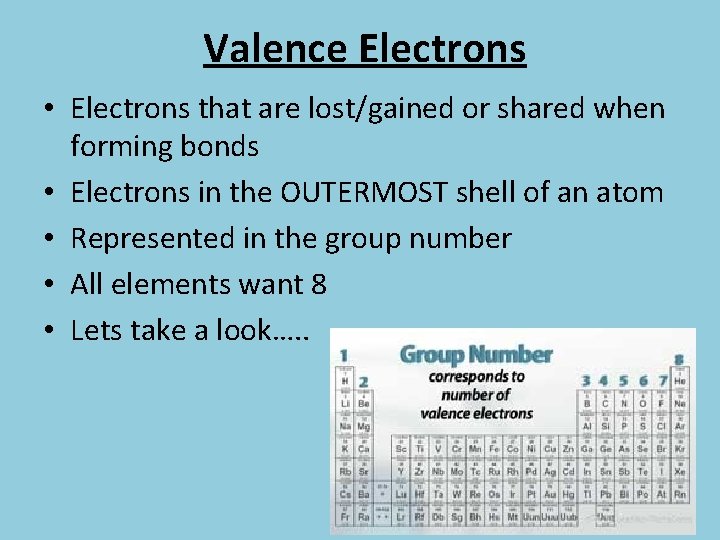
Valence Electrons • Electrons that are lost/gained or shared when forming bonds • Electrons in the OUTERMOST shell of an atom • Represented in the group number • All elements want 8 • Lets take a look…. .

Lewis Dot structure • 1 st: determine the elements number of valence electrons • 2 nd: place dots around the symbol to represent the valence electrons • Ex: • You try: Aluminum Chlorine