Electron Configuration H Chemistry Mrs Cool Electron Configuration


- Slides: 29

Electron Configuration H. Chemistry Mrs. Cool

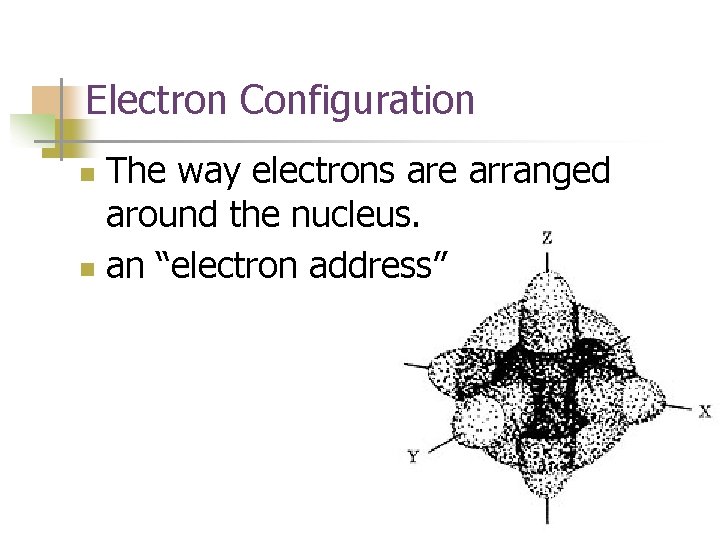
Electron Configuration The way electrons are arranged around the nucleus. n an “electron address” n

Quantum Mechanical Model n n 1920’s Werner Heisenberg (Uncertainty Principle) Louis de Broglie (electron has wave properties) Erwin Schrodinger (mathematical equations using probability, quantum numbers)

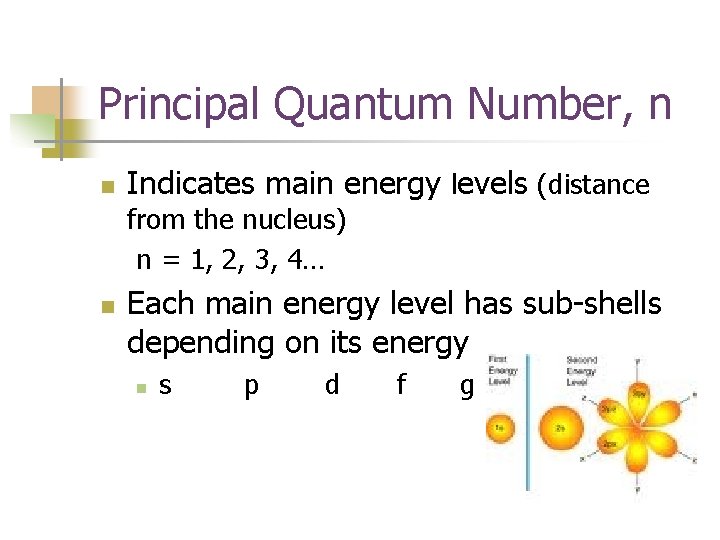
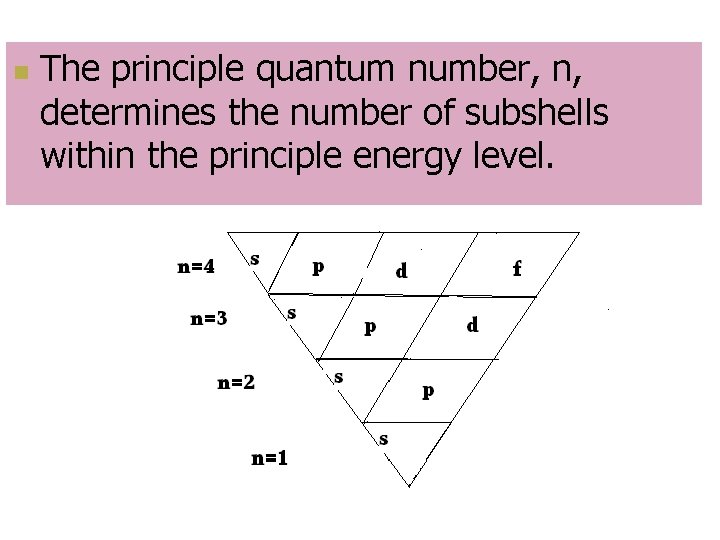
Principal Quantum Number, n n Indicates main energy levels (distance from the nucleus) n = 1, 2, 3, 4… n Each main energy level has sub-shells depending on its energy n s p d f g

n The principle quantum number, n, determines the number of subshells within the principle energy level.

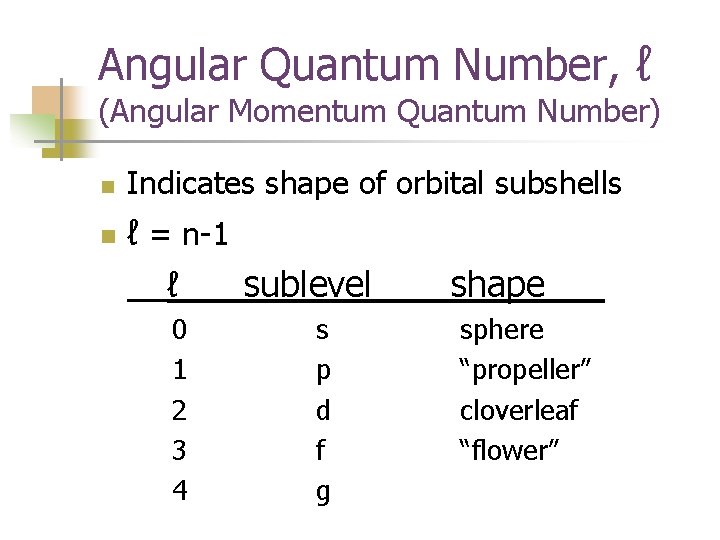
Angular Quantum Number, ℓ (Angular Momentum Quantum Number) n n Indicates shape of orbital subshells ℓ = n-1 ℓ sublevel____shape___ 0 1 2 3 4 s p d f g sphere “propeller” cloverleaf “flower”

Orbital n n The space where there is a high probability that it is occupied by a pair of electrons. Orbitals are solutions of Schrodinger’s equations.

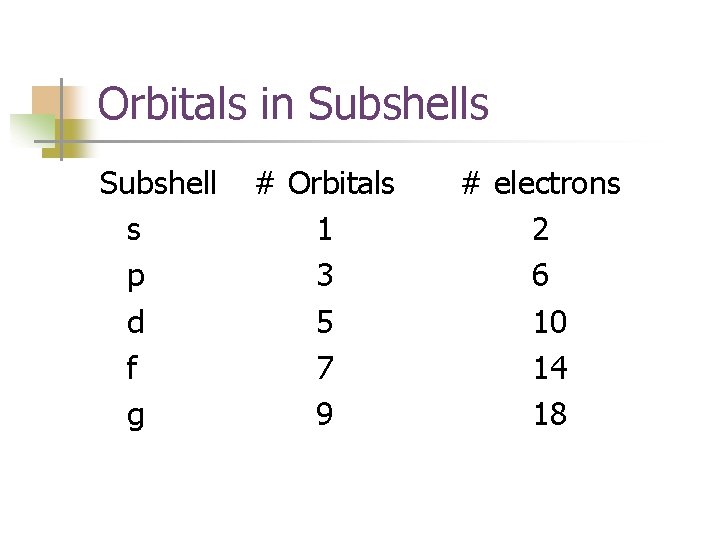
Orbitals in Subshells Subshell s p d f g # Orbitals 1 3 5 7 9 # electrons 2 6 10 14 18

Magnetic Quantum Number mℓ n n This tells you how the orbital is oriented in space. Its values are - ℓ … 0…+ ℓ n n n Since the s orbital (a sphere) can have only 1 orientation, the only value for its mℓ =0 A p orbital can fall on the x, y, or z axis, so it has 3 values for mℓ : -1, 0, 1 There are 5 possiblities for the d orbitals orientation, so there are 5 mℓ values.

Magnetic Spin Quantum Number ms n n n Since each orbital can hold 2 electrons, there must be a way to distinguish between the two. We do this by saying one spins “clockwise” and one spins “counter-clockwise”. The numeric designations are +½ and -½

Quantum Numbers & Electron Configuration n n In all, 4 quantum numbers are needed to describe the probable location of any electron in an atom. Sometimes quantum numbers aren’t the most convenient way to picture electrons in an atom, so we also use a more “visual” way…

Three rules are used to build the electron configuration: Aufbau principle n Pauli Exclusion Principle n Hund’s Rule n

Aufbau Principle Electrons occupy orbitals of lower energy first. n This means that the first 2 electrons of any atom will always be (most probably) in the 1 s orbital. n

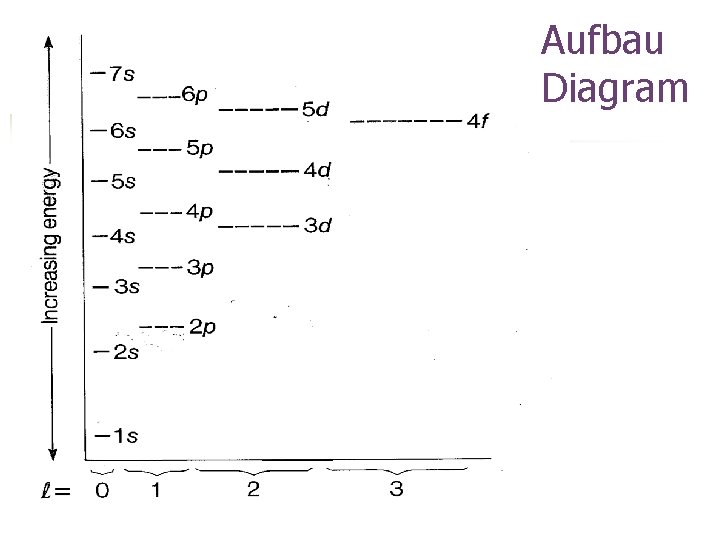
Aufbau Diagram

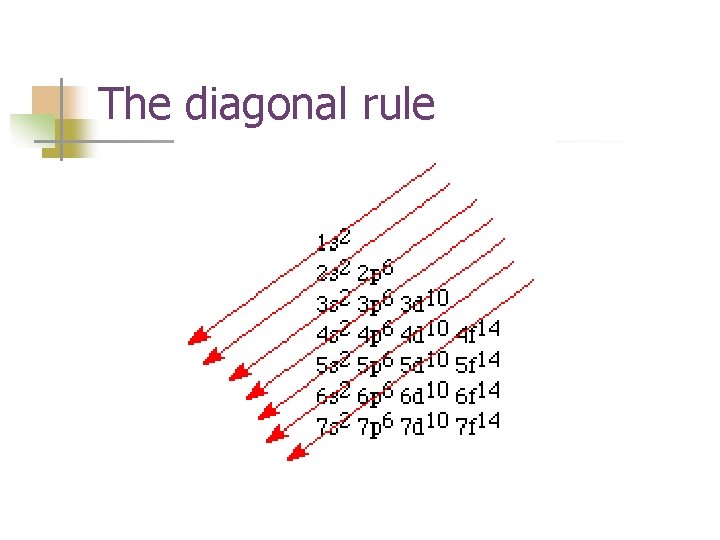
The diagonal rule

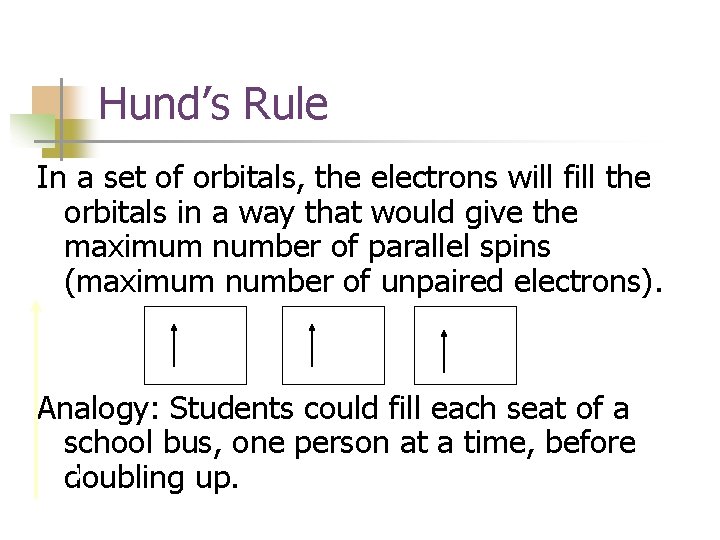
Hund’s Rule In a set of orbitals, the electrons will fill the orbitals in a way that would give the maximum number of parallel spins (maximum number of unpaired electrons). Analogy: Students could fill each seat of a school bus, one person at a time, before doubling up.

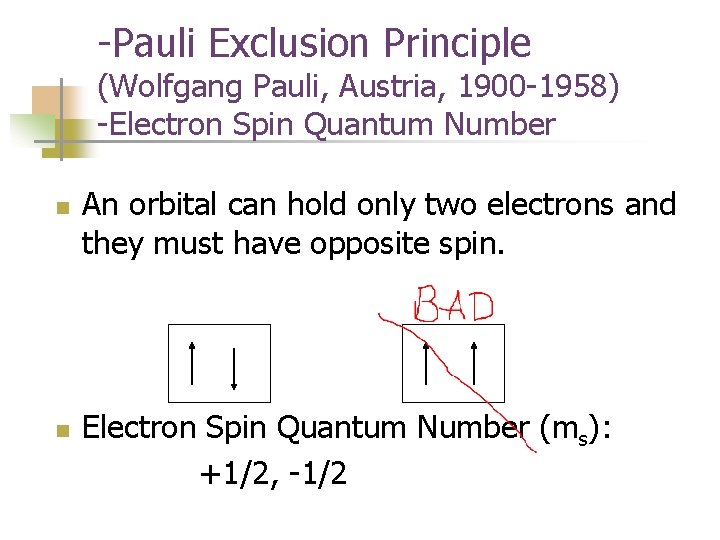
-Pauli Exclusion Principle (Wolfgang Pauli, Austria, 1900 -1958) -Electron Spin Quantum Number n n An orbital can hold only two electrons and they must have opposite spin. Electron Spin Quantum Number (ms): +1/2, -1/2

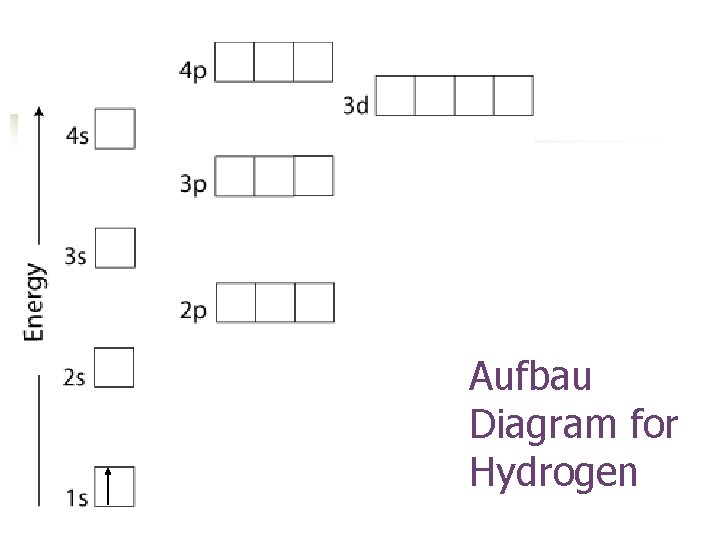
Aufbau Diagram for Hydrogen

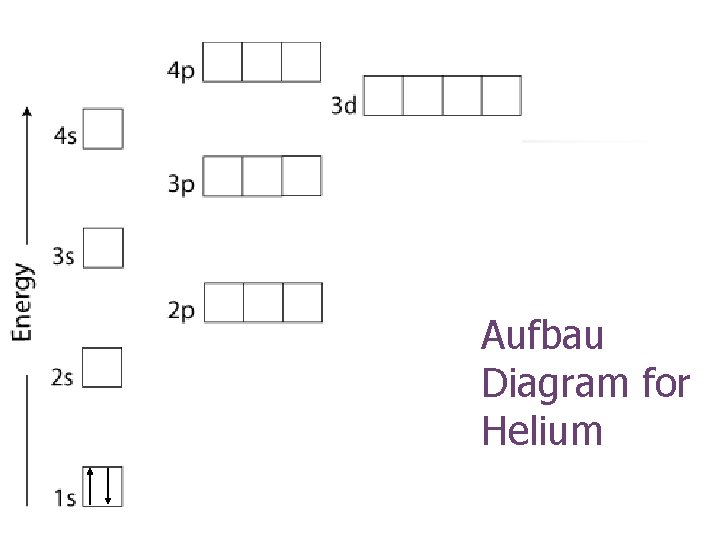
Aufbau Diagram for Helium

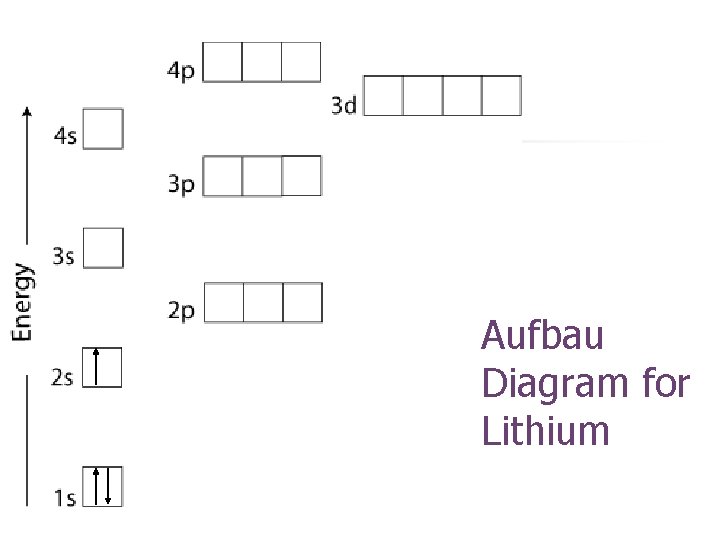
Aufbau Diagram for Lithium

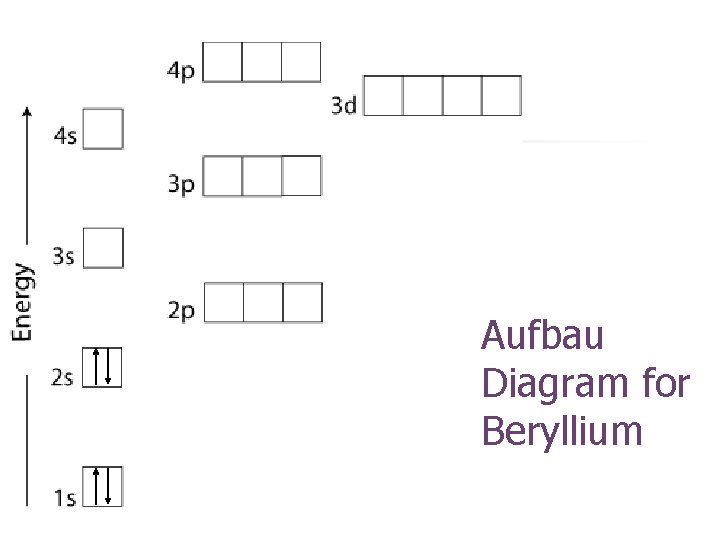
Aufbau Diagram for Beryllium

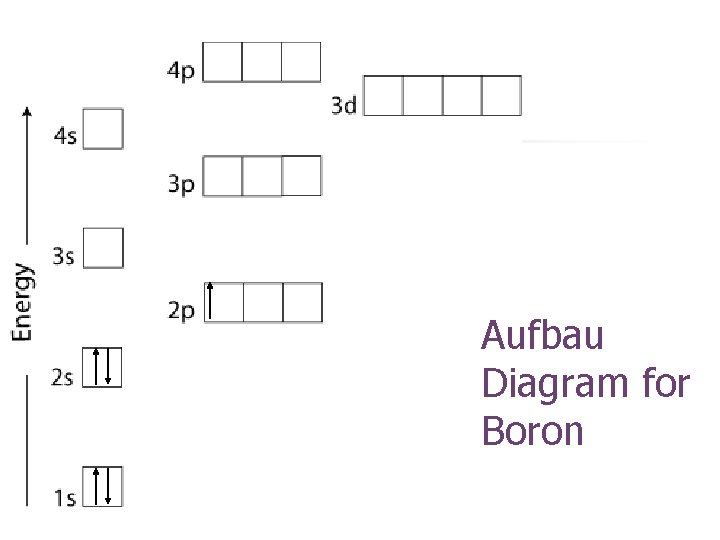
Aufbau Diagram for Boron

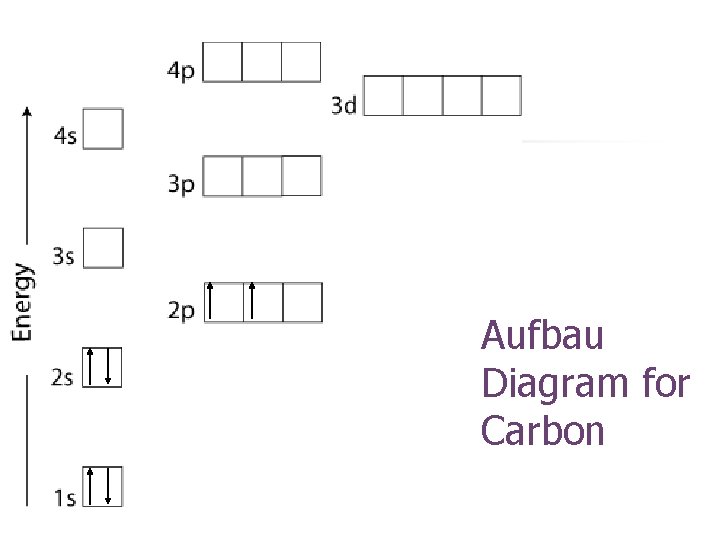
Aufbau Diagram for Carbon

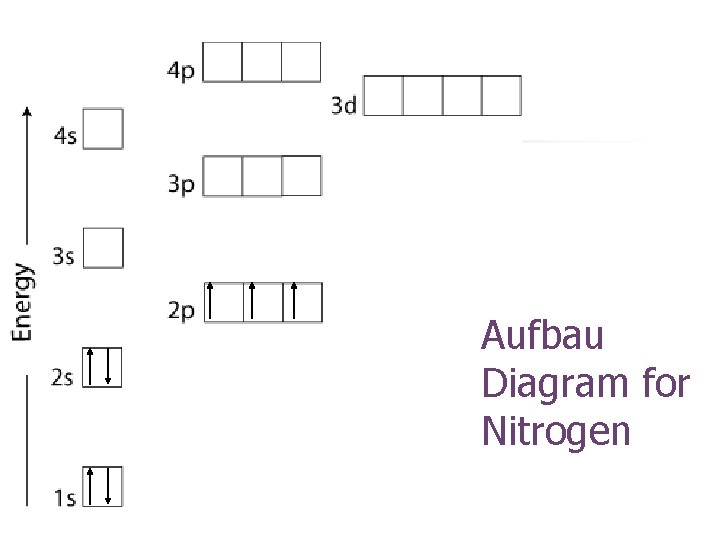
Aufbau Diagram for Nitrogen

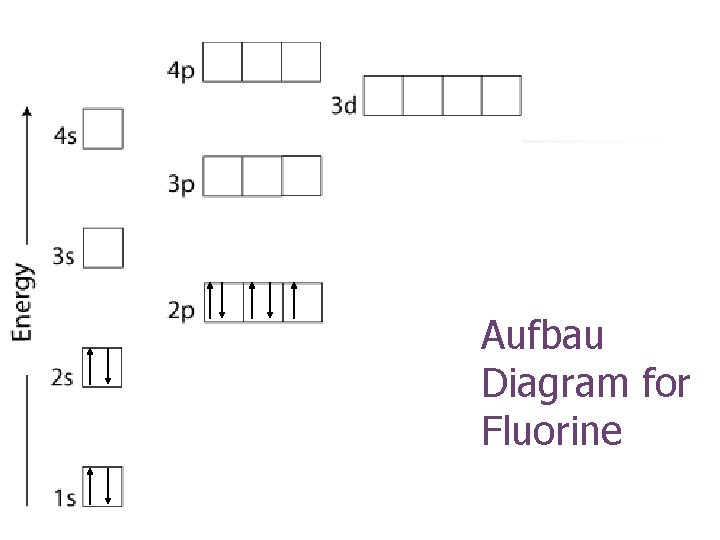
Aufbau Diagram for Fluorine

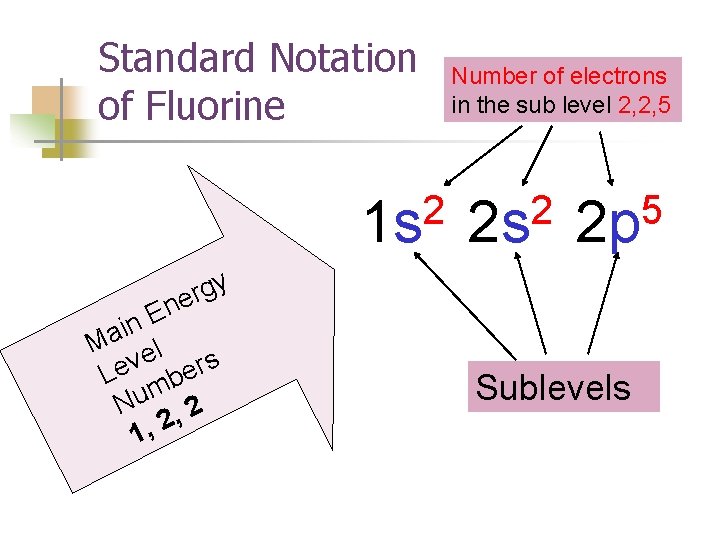
Standard Notation of Fluorine 2 1 s y g r e n E n i Ma el v s r e e L b m Nu , 2 2 , 1 Number of electrons in the sub level 2, 2, 5 2 2 s 5 2 p Sublevels

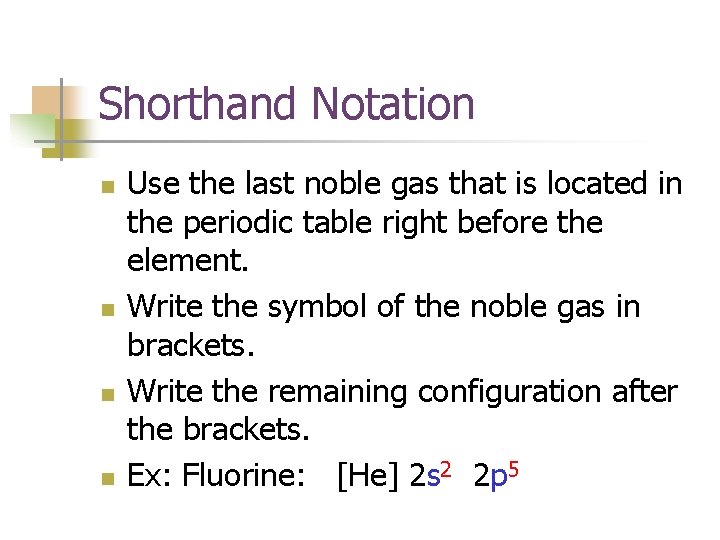
Shorthand Notation n n Use the last noble gas that is located in the periodic table right before the element. Write the symbol of the noble gas in brackets. Write the remaining configuration after the brackets. Ex: Fluorine: [He] 2 s 2 2 p 5

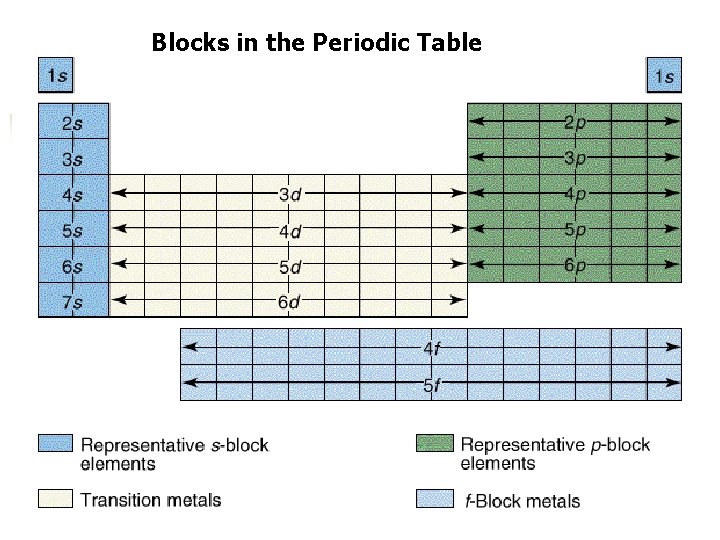
Blocks in the Periodic Table

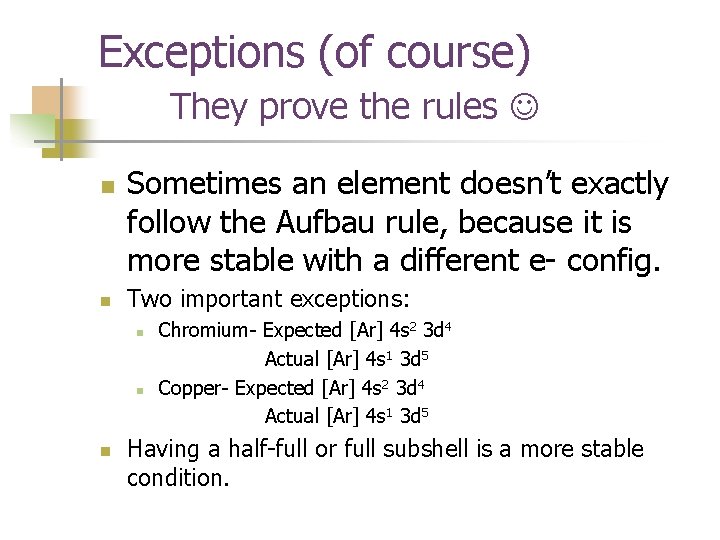
Exceptions (of course) They prove the rules n n Sometimes an element doesn’t exactly follow the Aufbau rule, because it is more stable with a different e- config. Two important exceptions: n n n Chromium- Expected [Ar] 4 s 2 3 d 4 Actual [Ar] 4 s 1 3 d 5 Copper- Expected [Ar] 4 s 2 3 d 4 Actual [Ar] 4 s 1 3 d 5 Having a half-full or full subshell is a more stable condition.