7 85 The electron configuration of a neutral





![Electron configurations of cations and anions (representative elements) Na [Ne]3 s 1 Na+ [Ne] Electron configurations of cations and anions (representative elements) Na [Ne]3 s 1 Na+ [Ne]](https://slidetodoc.com/presentation_image/1f576cbdc9f9a8fc2d178406917b94c2/image-12.jpg)
![Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or](https://slidetodoc.com/presentation_image/1f576cbdc9f9a8fc2d178406917b94c2/image-14.jpg)
- Slides: 15

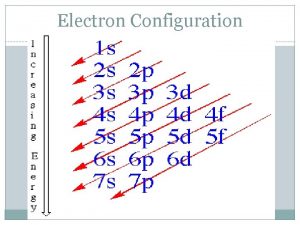
7. 85 The electron configuration of a neutral atom is 1 s 22 p 63 s 2. Write a complete set of quantum numbers for each of the electrons. Name the element. 1 s: 2 p: 3 s: n=1, l=0, ml=0, ms= +1/2 or -1/2 n=2, l=1, ml=-1, 0, and 1, ms= +1/2, or -1/2 n=3, l=0, ms= +1/2 or -1/2 Mg 10/29/2020 1

7. 104 Only a fraction of the electrical energy supplied to a tungsten lightbulb is converted to visible light. The rest of the energy shows up as infrared radiation (heat). A 75 -W lightbulb converts 15. 0% of the energy supplied to it into visible light (assume the wavelength to be 550 nm). How many photons are emitted by the lightbulb per second? (1 W = 1 J/s) 1 photon = 3. 62 x 10 -19 J 11. 25 J/(3. 62 x 10 -19 J photon-1)= 3. 1 x 1019 photons 10/29/2020 2

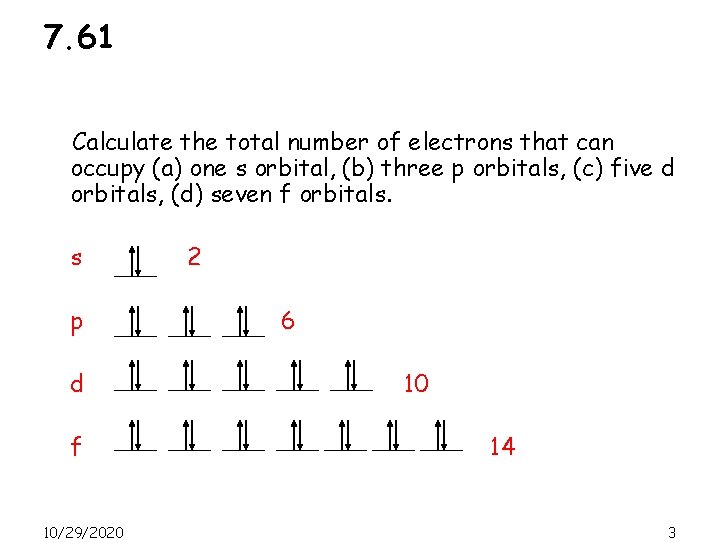
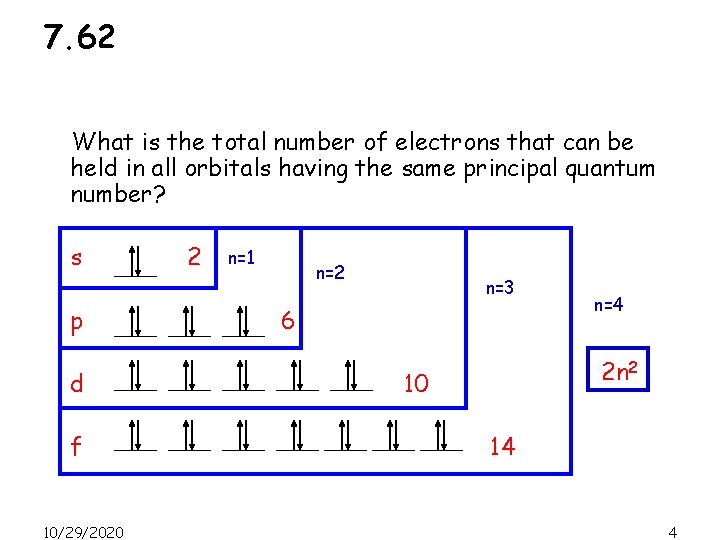
7. 61 Calculate the total number of electrons that can occupy (a) one s orbital, (b) three p orbitals, (c) five d orbitals, (d) seven f orbitals. s p d f 10/29/2020 2 6 10 14 3

7. 62 What is the total number of electrons that can be held in all orbitals having the same principal quantum number? s p d f 10/29/2020 2 n=1 n=2 n=3 6 n=4 2 n 2 10 14 4

7. 57 Discuss the similarities and differences between a 1 s and a 2 s orbital. Both have a spherical shape 2 s orbital is larger 1 s orbital is lower in energy Harder to remove a 1 s electron 10/29/2020 5

7. 81 The atomic number of an element is 73. Is this element diamagnetic or paramagnetic? paramagnetic If an element has an odd number of electrons, it is paramagnetic. If an element has an even number of electrons, it may be either paramagnetic or diamagnetic. 10/29/2020 6

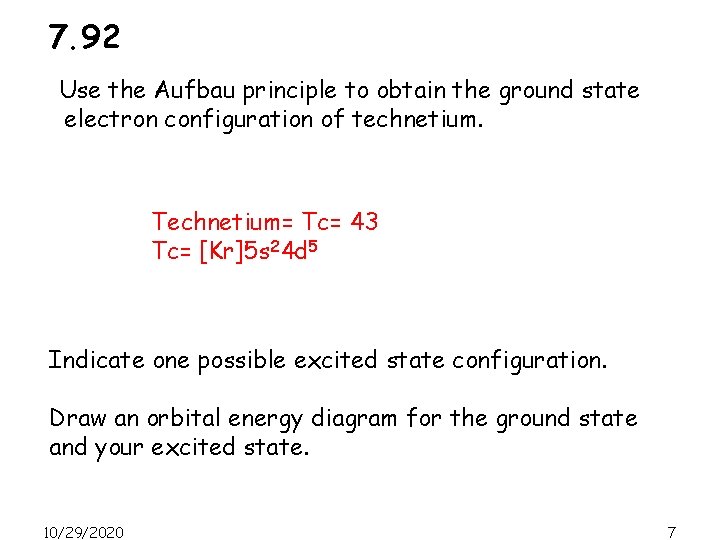
7. 92 Use the Aufbau principle to obtain the ground state electron configuration of technetium. Technetium= Tc= 43 Tc= [Kr]5 s 24 d 5 Indicate one possible excited state configuration. Draw an orbital energy diagram for the ground state and your excited state. 10/29/2020 7

Periodic Relationships Among the Elements Chapter 8

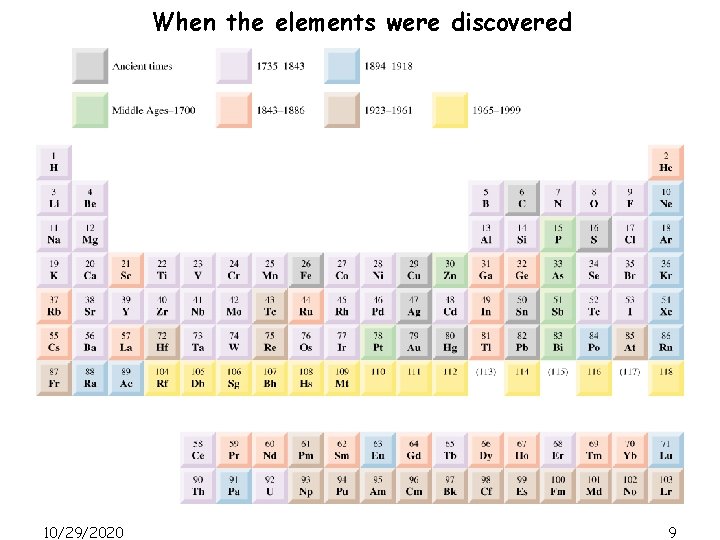
When the elements were discovered 10/29/2020 9

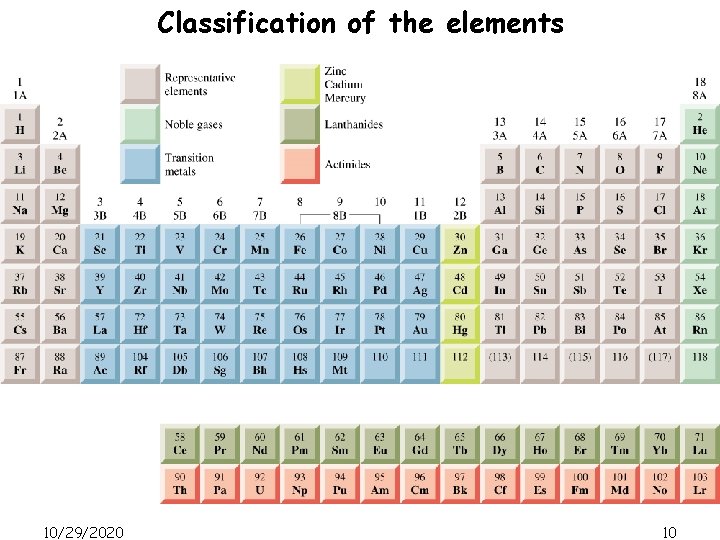
Classification of the elements 10/29/2020 10

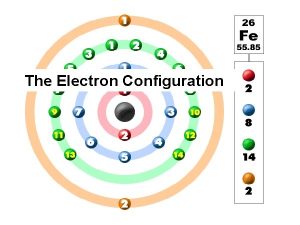
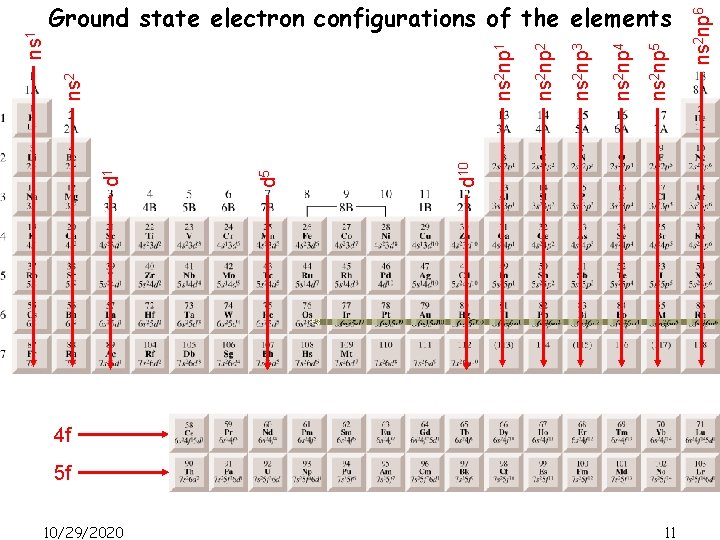
4 f 5 f 10/29/2020 11 ns 2 np 6 ns 2 np 5 ns 2 np 4 ns 2 np 3 ns 2 np 2 ns 2 np 1 d 10 d 5 d 1 ns 2 ns 1 Ground state electron configurations of the elements
![Electron configurations of cations and anions representative elements Na Ne3 s 1 Na Ne Electron configurations of cations and anions (representative elements) Na [Ne]3 s 1 Na+ [Ne]](https://slidetodoc.com/presentation_image/1f576cbdc9f9a8fc2d178406917b94c2/image-12.jpg)
Electron configurations of cations and anions (representative elements) Na [Ne]3 s 1 Na+ [Ne] Ca [Ar]4 s 2 Ca 2+ [Ar] Al [Ne]3 s 23 p 1 Al 3+ [Ne] Atoms lose electrons so that cation has a noble-gas outer electron configuration. H 1 s 1 Atoms gain electrons so that anion has a noble-gas outer electron configuration. 10/29/2020 H- 1 s 2 or [He] F 1 s 22 p 5 O 1 s 22 p 4 F- 1 s 22 p 6 or [Ne] N 1 s 22 p 3 N 3 - 1 s 22 p 6 or [Ne] O 2 - 1 s 22 p 6 or [Ne] 12

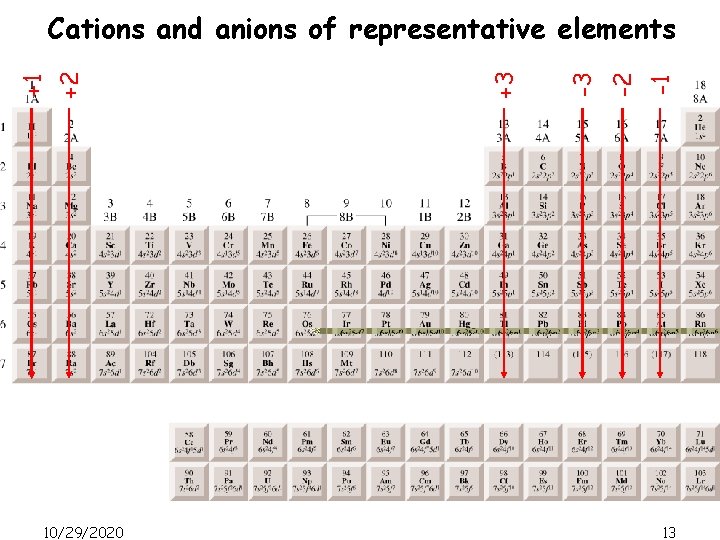
10/29/2020 -1 -2 -3 +3 +2 +1 Cations and anions of representative elements 13
![Na Ne Al 3 Ne O 2 1 s 22 p 6 or Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or](https://slidetodoc.com/presentation_image/1f576cbdc9f9a8fc2d178406917b94c2/image-14.jpg)
Na+: [Ne] Al 3+: [Ne] O 2 -: 1 s 22 p 6 or [Ne] F-: 1 s 22 p 6 or [Ne] N 3 -: 1 s 22 p 6 or [Ne] Na+, Al 3+, F-, O 2 -, and N 3 - are all isoelectronic with Ne What neutral atom is isoelectronic with H- ? H-: 1 s 2 10/29/2020 same electron configuration as He 14
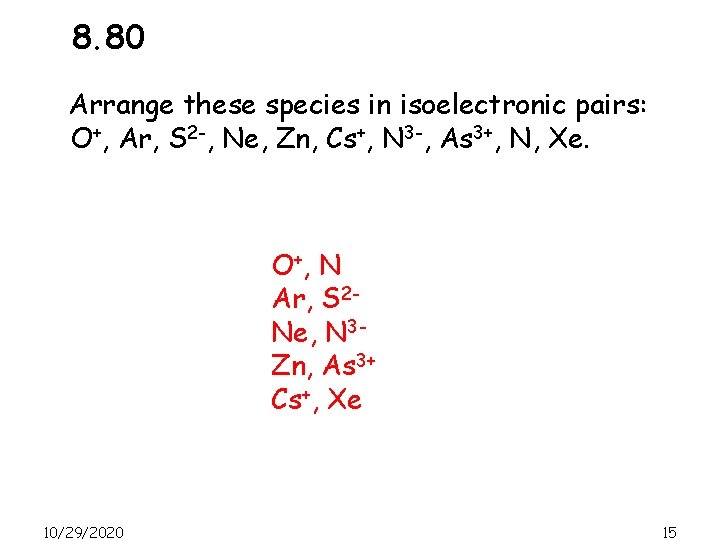
8. 80 Arrange these species in isoelectronic pairs: O+, Ar, S 2 -, Ne, Zn, Cs+, N 3 -, As 3+, N, Xe. O+, N Ar, S 2 Ne, N 3 Zn, As 3+ Cs+, Xe 10/29/2020 15
 How to read electron configuration
How to read electron configuration Electron configuration vs noble gas configuration
Electron configuration vs noble gas configuration What is absolute configuration
What is absolute configuration Planar chirality
Planar chirality Absolute configuration vs relative configuration
Absolute configuration vs relative configuration Electron configuration chapter 4
Electron configuration chapter 4 Germanium electron configuration
Germanium electron configuration Write the electronic configuration of 12mg
Write the electronic configuration of 12mg Electronic configuration rule in chemistry
Electronic configuration rule in chemistry Electron song
Electron song 1s 22 s22 p63 s23 p64
1s 22 s22 p63 s23 p64 Electron configuration hotel
Electron configuration hotel Bond length of benzene
Bond length of benzene As33 electron configuration
As33 electron configuration Stability and electron configuration ch 4
Stability and electron configuration ch 4 Electron configuration for oxygen
Electron configuration for oxygen