The Electron Configuration OUTCOME QUESTIONS C 12 2


![Cr: [Ar] 4 s 2 3 d 4 4 s 3 d Actual configurations: Cr: [Ar] 4 s 2 3 d 4 4 s 3 d Actual configurations:](https://slidetodoc.com/presentation_image/f7850d80fc7b1d7ba117f019e5ea5e6f/image-18.jpg)

- Slides: 19

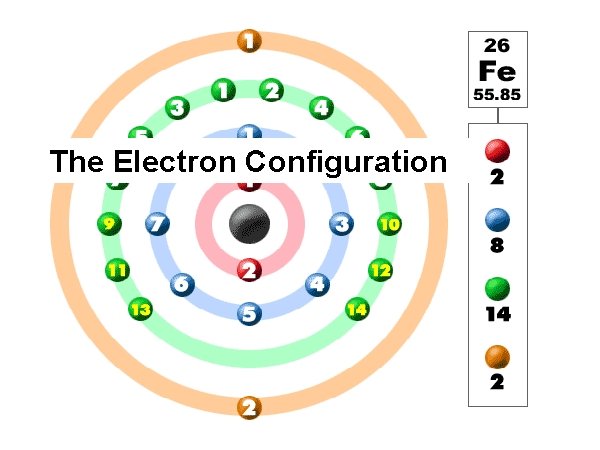
The Electron Configuration

OUTCOME QUESTION(S): C 12 -2 -06 ELECTRON CONFIGURATION • Relate the electron configuration of an element to its valence electron(s) and its position on the Periodic Table. Include: quantum energy level, shapes, and orbitals. • Write the electron configuration for a variety of atoms and ions using the 3 configuration laws. Include: shorthand notation and valence configuration Vocabulary & Concepts Pauli Exclusion Principle Aufbau Principle Hund Rule

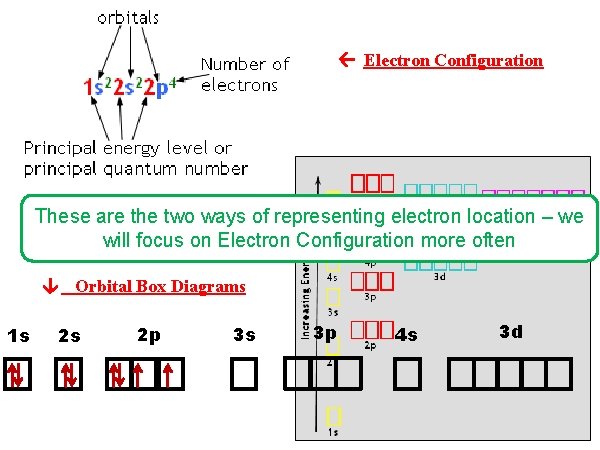
Electron Configuration These are the two ways of representing electron location – we will focus on Electron Configuration more often 1 s Orbital Box Diagrams 2 s 2 p 3 s 3 p 4 s 3 d

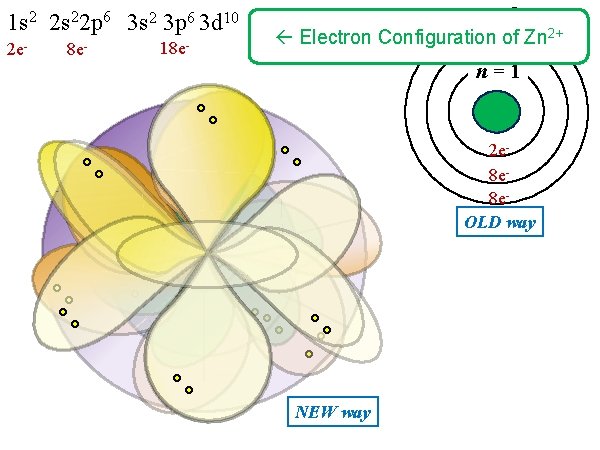
1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 2 e- 8 e- 18 e- n = 3 Electron Configuration of Zn 2+ n = 2 n = 1 2 e 8 e 8 e. OLD way NEW way

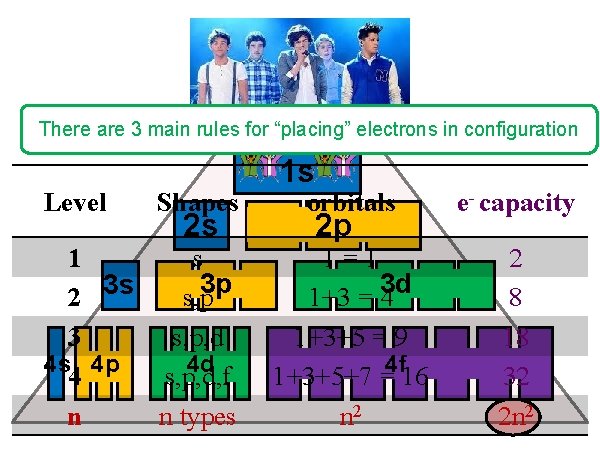
NUCLEUS There are 3 main rules for “placing” electrons in configuration Level Shapes 2 s 1 s 3 p 2 3 s s, p 3 s, p, d 4 s 4 p 4 d 4 s, p, d, f n n types 1 s orbitals e- capacity 1=1 1+3 = 43 d 1+3+5 = 9 4 f 1+3+5+7 = 16 n 2 2 8 18 32 2 n 2 2 p

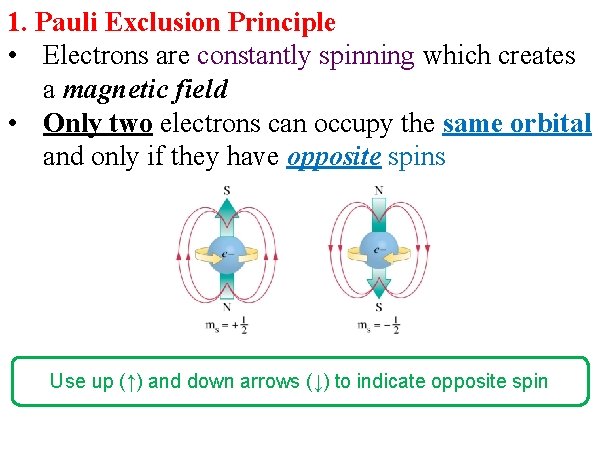
1. Pauli Exclusion Principle • Electrons are constantly spinning which creates a magnetic field • Only two electrons can occupy the same orbital and only if they have opposite spins Use up (↑) and down arrows (↓) to indicate opposite spin

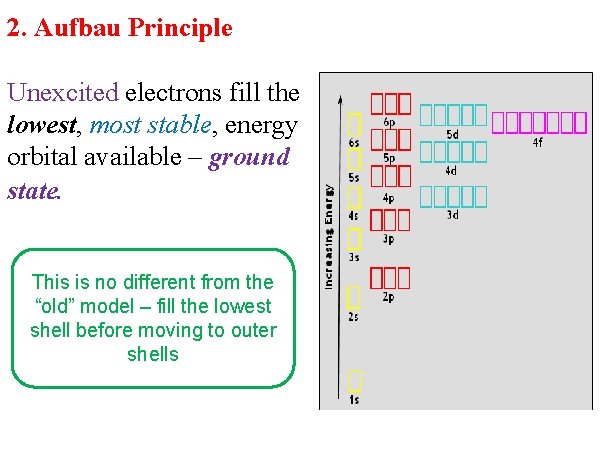
2. Aufbau Principle Unexcited electrons fill the lowest, most stable, energy orbital available – ground state. This is no different from the “old” model – fill the lowest shell before moving to outer shells

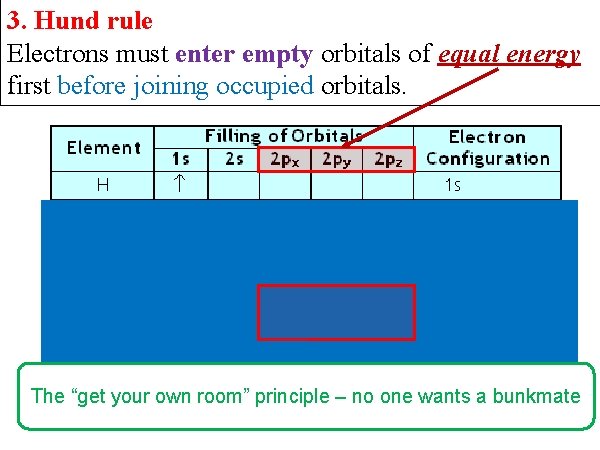
3. Hund rule Electrons must enter empty orbitals of equal energy first before joining occupied orbitals. The “get your own room” principle – no one wants a bunkmate

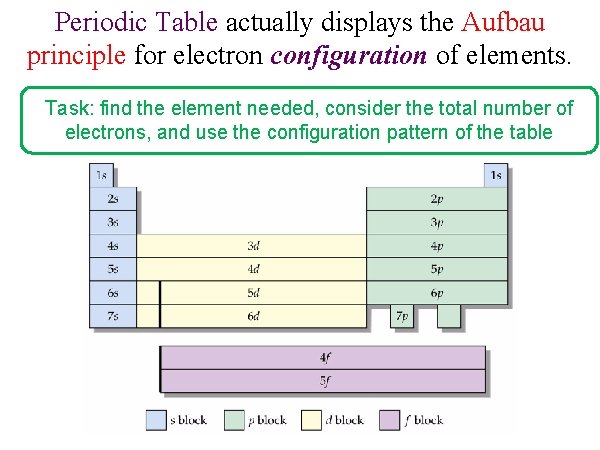
Periodic Table actually displays the Aufbau principle for electron configuration of elements. Task: find the element needed, consider the total number of electrons, and use the configuration pattern of the table

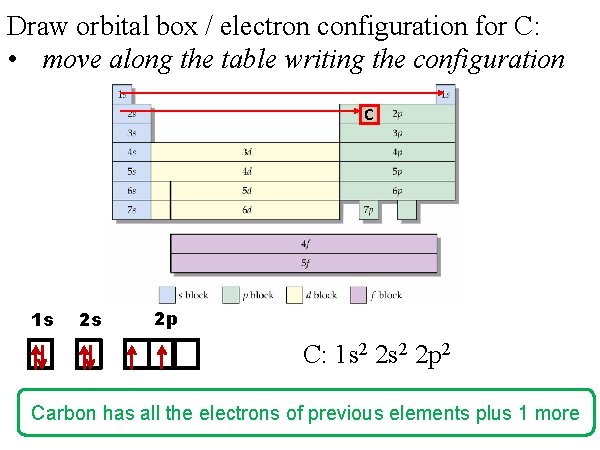
Draw orbital box / electron configuration for C: • move along the table writing the configuration C 1 s 2 s 2 p C: 1 s 2 2 p 2 Carbon has all the electrons of previous elements plus 1 more

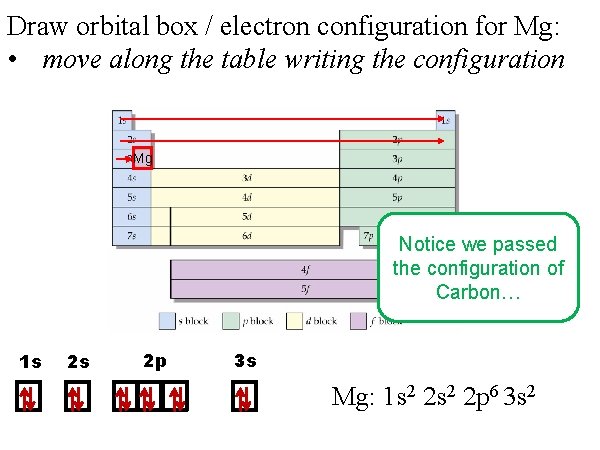
Draw orbital box / electron configuration for Mg: • move along the table writing the configuration Mg Notice we passed the configuration of Carbon… 1 s 2 s 2 p 3 s Mg: 1 s 2 2 p 6 3 s 2

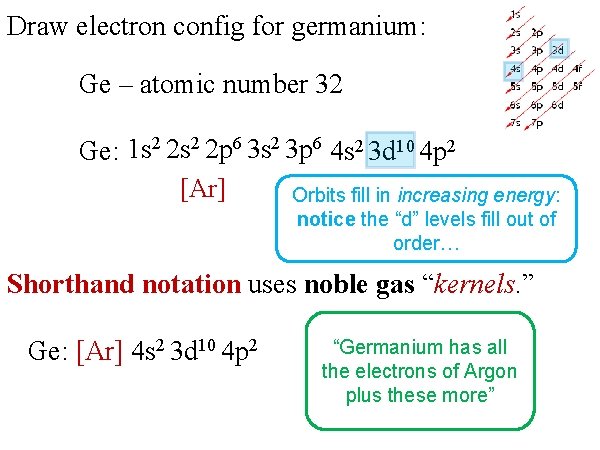
Draw electron config for germanium: Ge – atomic number 32 Ge: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 2 [Ar] Orbits fill in increasing energy: notice the “d” levels fill out of order… Shorthand notation uses noble gas “kernels. ” Ge: [Ar] 4 s 2 3 d 10 4 p 2 “Germanium has all the electrons of Argon plus these more”

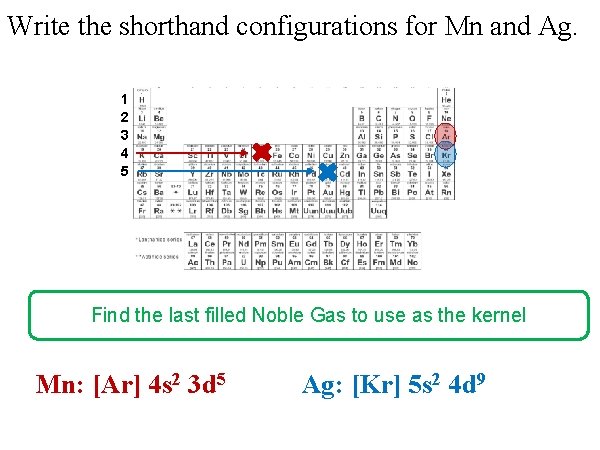
Write the shorthand configurations for Mn and Ag. 1 2 3 4 5 Find the last filled Noble Gas to use as the kernel Mn: [Ar] 4 s 2 3 d 5 Ag: [Kr] 5 s 2 4 d 9

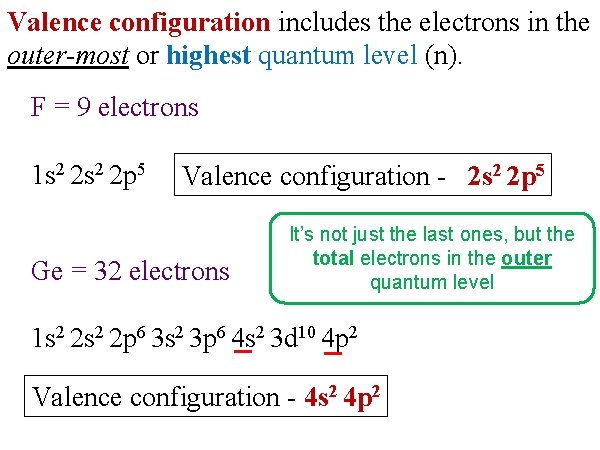
Valence configuration includes the electrons in the outer-most or highest quantum level (n). F = 9 electrons 1 s 2 2 p 5 Valence configuration - 2 s 2 2 p 5 Ge = 32 electrons It’s not just the last ones, but the total electrons in the outer quantum level 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 2 Valence configuration - 4 s 2 4 p 2

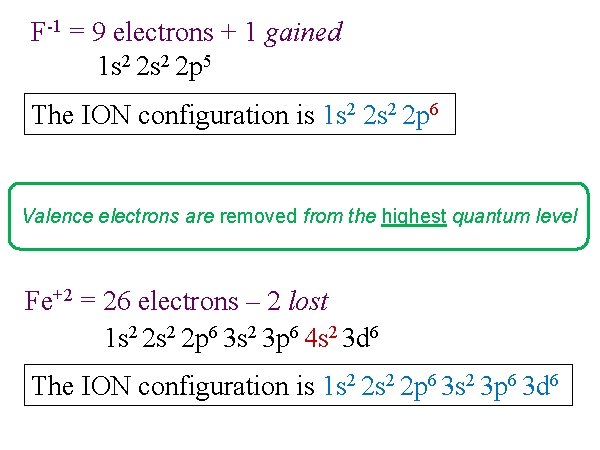
F-1 = 9 electrons + 1 gained 1 s 2 2 p 5 The ION configuration is 1 s 2 2 p 6 Valence electrons are removed from the highest quantum level Fe+2 = 26 electrons – 2 lost 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 6 The ION configuration is 1 s 2 2 p 6 3 s 2 3 p 6 3 d 6

Some exceptions to the rule:

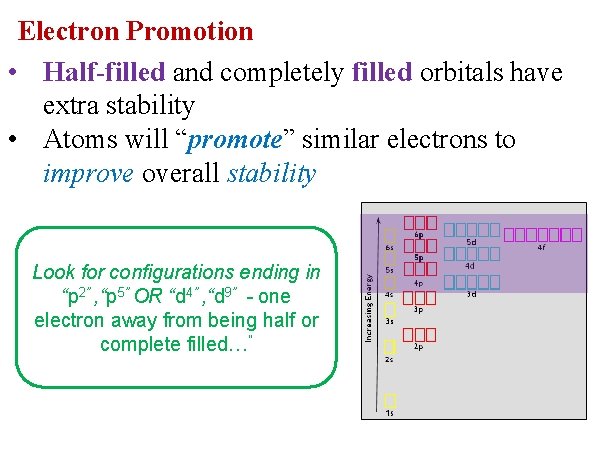
Electron Promotion • Half-filled and completely filled orbitals have extra stability • Atoms will “promote” similar electrons to improve overall stability Look for configurations ending in “p 2” , “p 5” OR “d 4” , “d 9” - one electron away from being half or complete filled…”
![Cr Ar 4 s 2 3 d 4 4 s 3 d Actual configurations Cr: [Ar] 4 s 2 3 d 4 4 s 3 d Actual configurations:](https://slidetodoc.com/presentation_image/f7850d80fc7b1d7ba117f019e5ea5e6f/image-18.jpg)
Cr: [Ar] 4 s 2 3 d 4 4 s 3 d Actual configurations: Cr: [Ar] 4 s 1 3 d 5 Cu: [Ar] 4 s 2 3 d 9 4 s 3 d Cu: [Ar] 4 s 1 3 d 10 *Promotion accounts for multiple ionization states (Fe +2, Fe+3…) Think of this as moving a CFO into a CEO at a company – you don’t move the mailperson…

CAN YOU / HAVE YOU? C 12 -2 -06 ELECTRON CONFIGURATION • Relate the electron configuration of an element to its valence electron(s) and its position on the Periodic Table. Include: quantum energy level, shapes, and orbitals. • Write the electron configuration for a variety of atoms and ions using the 3 configuration laws. Include: shorthand notation and valence configuration Vocabulary & Concepts Pauli Exclusion Principle Aufbau Principle Hund Rule