Electron Configurations part III Short hand notation Valence


















![Question 4 a. b. c. d. [Ar] 4 s 2 3 d 10 4 Question 4 a. b. c. d. [Ar] 4 s 2 3 d 10 4](https://slidetodoc.com/presentation_image_h2/40aeb8ea0da74aac638060d16621f9ba/image-28.jpg)


- Slides: 31

Electron Configurations - part III - Short hand notation - Valence electrons

Essential questions Is there a way to write electron configuration even easier? n I’ve heard the valence electrons are the important – what are they and why? n

Valence Electrons n Valence electrons are the electron’s located in the highest energy level n These are the electrons that determine the chemical properties of an element n And these are the electrons involved in chemical reactions and bonding!

Valence Electrons n Valence electrons = electrons in ALL the subshells with the highest principal energy shell (outermost shell) n Core electrons = in lower energy shells n Atoms will try to either gain or lose electrons in order to get to a full or empty outer most shell 4

Shorthand Notation n. A way of abbreviating long electron configurations n Since we are only concerned about the outermost or valence electrons, we can skip to the closest noble gas and then finish the configuration

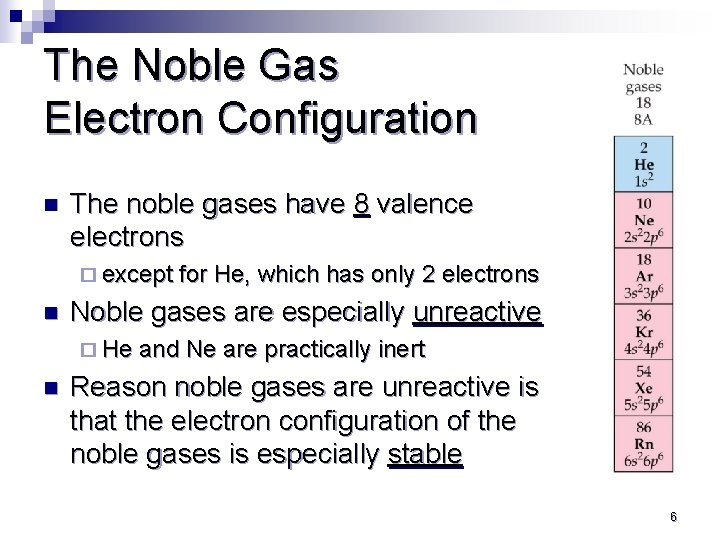
The Noble Gas Electron Configuration n The noble gases have 8 valence electrons ¨ except for He, which has only 2 electrons n Noble gases are especially unreactive ¨ He and Ne are practically inert n Reason noble gases are unreactive is that the electron configuration of the noble gases is especially stable 6

Shorthand Notation n n Step 1: It’s the Showcase Showdown! Find the closest noble gas to the atom (or ion), WITHOUT GOING OVER the number of electrons in the atom (or ion). Write the noble gas in brackets [ ]. Step 2: Find where to resume by finding the next energy level Step 3: Resume the configuration until it’s finished. Step 4: Check by counting the total number of electrons!

Shorthand Notation n Let’s try chlorine ¨Step 1 ¨Step 2 ¨Step 3 ¨Step 4

Energy Levels n=1 n=2 n=3 n=4

Let’s Practice Work with a partner to write the electron configurations for the first 20 elements on your blank periodic table. n The first 2 has been done for you n You can use the noble gas notation for elements after Neon n

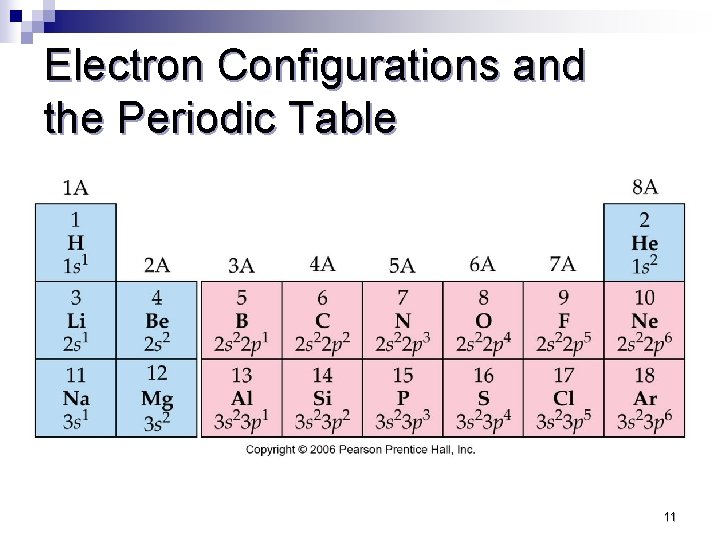
Electron Configurations and the Periodic Table 11

Take aways for shorthand Electron configurations 1. 2. The number of protons equals the atomic number which equals number of electrons The number of the highest energy level occupied by electrons equals the period number of the element.

Take aways for shorthand Electron configurations 3. The number of electrons in the highest used energy level (valence level) equals the roman numeral group number. 4. We can break up the periodic table in blocks that correspond to the ending configuration orbitals.

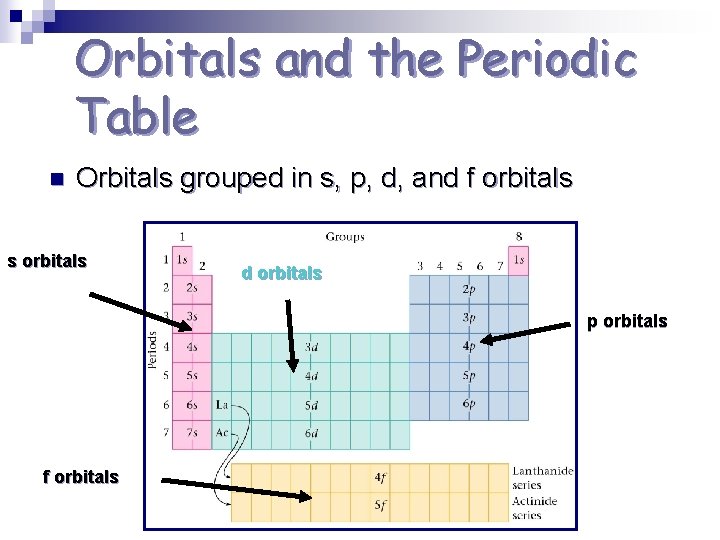
Orbitals and the Periodic Table n Orbitals grouped in s, p, d, and f orbitals s orbitals d orbitals p orbitals f orbitals

What about valence electrons for transition metals? The general method for counting valence electrons is generally not useful for the dblock transition metals. n Therefore, we will focus on s and p block elements. n

Valence Electrons n Go back and write the number of valence electrons for each of first 20 elements you wrote the configuration for in the upper left corner of the box and circle it. Li

Ions Can you predict if the atoms will want to gain or lose electrons for fail to react chemically? n All atoms want to have full outermost energy levels n Go back and write the ion most likely to form in the upper right corner of each box. n See any trends? n

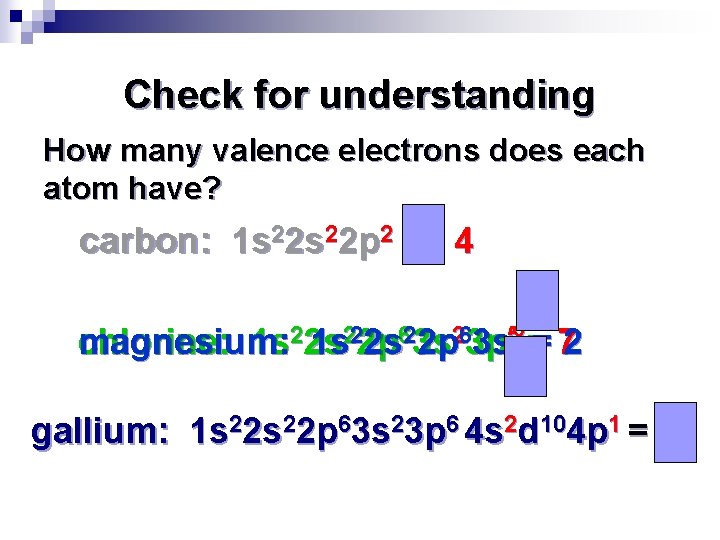
Check for understanding How many valence electrons does each atom have? carbon: 1 s 22 p 2 = 4 magnesium: chlorine: 1 s 22 s 1 s 222 p 2 s 623 s 2 p 263 p 3 s 52 == 72 gallium: 1 s 22 p 63 s 23 p 6 4 s 2 d 104 p 1 = 3

Practice more n Let’s COMPLETE WORKSHEET 5 -6 together

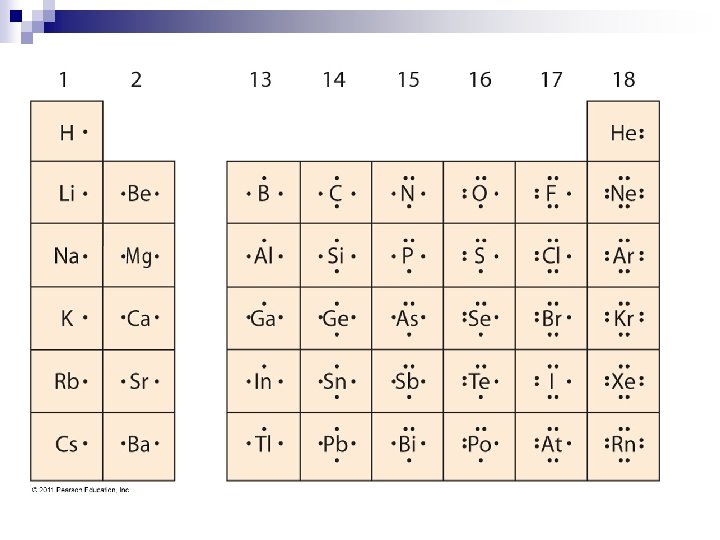
Electron Dot Notation

Electron Dot Diagrams • If you want to see how atoms of one element will react, it is handy to have an easier way to represent the atoms and the electrons in their outer energy levels. • An electric dot diagram is the symbol for the element surrounded by as many dots as there are valence electrons. • # of dots = # of valence electrons

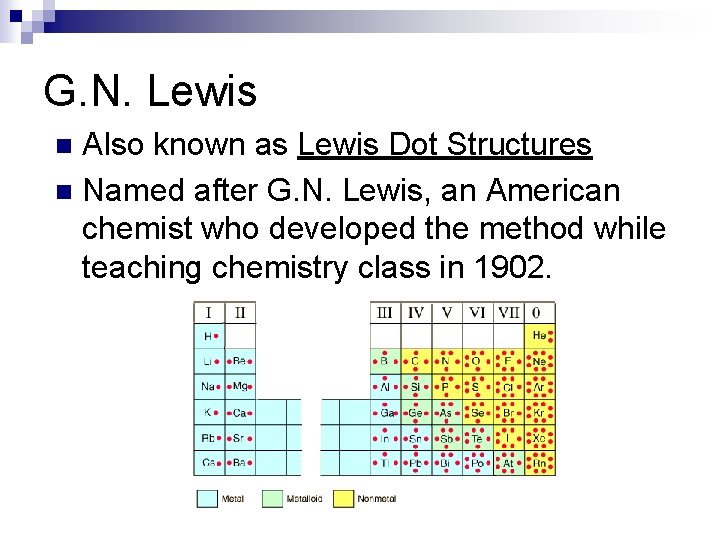
G. N. Lewis Also known as Lewis Dot Structures n Named after G. N. Lewis, an American chemist who developed the method while teaching chemistry class in 1902. n

How to write them N • Start by writing one dot to the right of the element symbol, then work your way around clockwise adding dots to the bottom, left, and top. • Add a fifth dot to the right to make a pair. Continue in this manner until you reach eight dots to complete the level.

Check for understanding 1. Electrons are now known to swarm around the nucleus of an atom in a configuration known as the _______. A. electron circle B. electron cloud C. electron configuration D. electron swarm The answer is B. The “cloud” includes all the regions where an electron might be found.

Question 2 What information can you learn from this diagram? It tells you that nitrogen contains five electrons in its outer energy level.

Question 3 The _______ an energy level is from the nucleus, the ______ electrons it can hold. A. closer, more B. closer, less C. farther, less D. farther, more The answer is D. The farthest shells contain the greatest number of electrons.

Question 4 a. Write the shorthand electron configuration for arsenic (atomic number 33) b. What block is it in? c. How many valence electrons does it have? d. Write the electron dot diagram.
![Question 4 a b c d Ar 4 s 2 3 d 10 4 Question 4 a. b. c. d. [Ar] 4 s 2 3 d 10 4](https://slidetodoc.com/presentation_image_h2/40aeb8ea0da74aac638060d16621f9ba/image-28.jpg)
Question 4 a. b. c. d. [Ar] 4 s 2 3 d 10 4 p 3 p-block 3 valence electrons

Work on your own n Write the electron dot diagrams for the first 20 elements on the periodic table in your packets.


Jeopardy Review Game n http: //www. superteachertools. com/jeopard yx/jeopardy-review-gameconvert. php? gamefile=. . /jeopardy/userga mes/Nov 201148/jeopardy 1322703493. txt