The Atom Lesson 4 Electron Configurations 1 Principal



![Shorthand practice Write the shorthand electron configuration of: P [Ne] 3 s 2 3 Shorthand practice Write the shorthand electron configuration of: P [Ne] 3 s 2 3](https://slidetodoc.com/presentation_image_h/028a1bb8afe91bc60f44483710d125cb/image-14.jpg)
- Slides: 22

The Atom Lesson 4 Electron Configurations 1

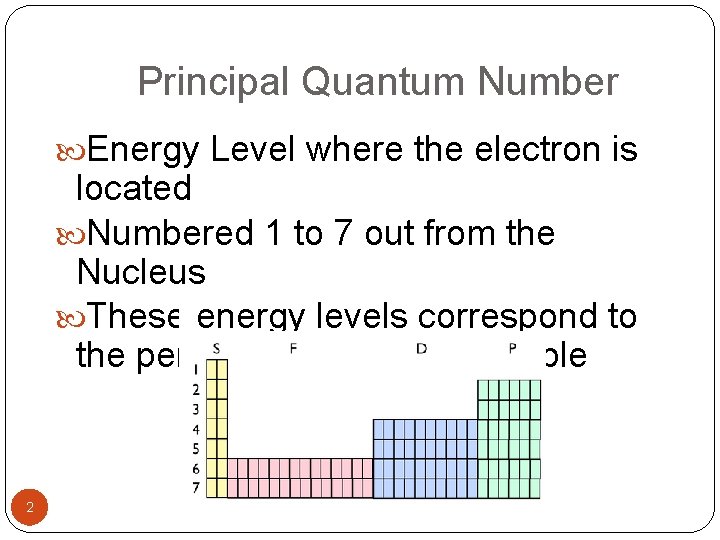
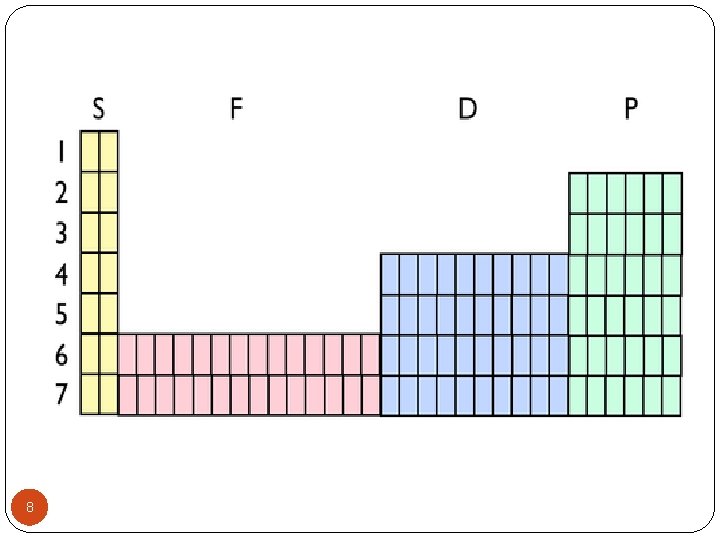
Principal Quantum Number Energy Level where the electron is located Numbered 1 to 7 out from the Nucleus These energy levels correspond to the periods on the periodic table 2

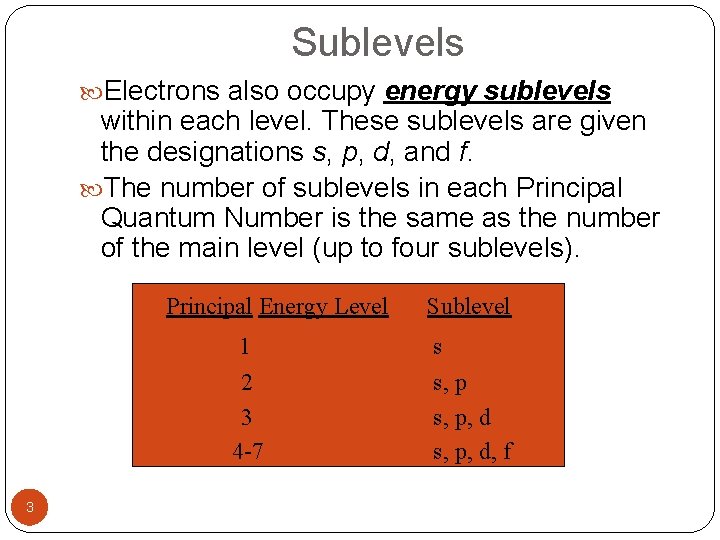
Sublevels Electrons also occupy energy sublevels within each level. These sublevels are given the designations s, p, d, and f. The number of sublevels in each Principal Quantum Number is the same as the number of the main level (up to four sublevels). Principal Energy Level 1 2 3 4 -7 3 Sublevel s s, p, d, f

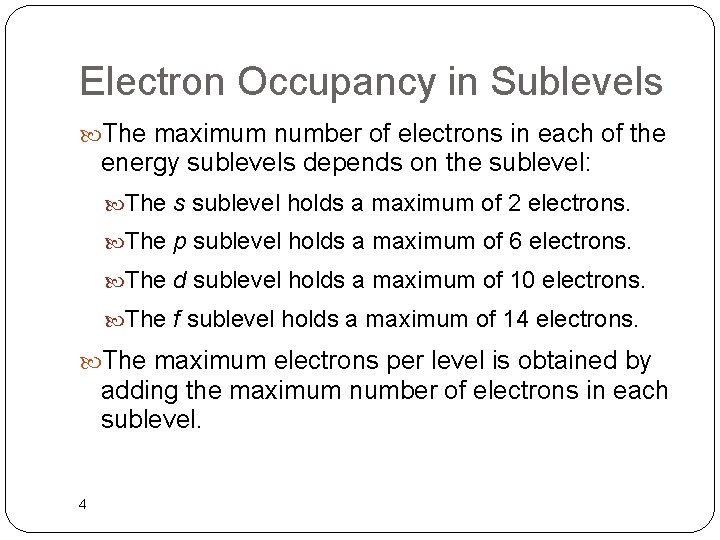
Electron Occupancy in Sublevels The maximum number of electrons in each of the energy sublevels depends on the sublevel: The s sublevel holds a maximum of 2 electrons. The p sublevel holds a maximum of 6 electrons. The d sublevel holds a maximum of 10 electrons. The f sublevel holds a maximum of 14 electrons. The maximum electrons per level is obtained by adding the maximum number of electrons in each sublevel. 4

Aufbau Principle Gives the order in which atomic sublevels are filled Electrons occupy the sublevels of lowest energy first The Periodic Table is a guide for the Aufbau Principle, going from left to right as you move down the periodic table Each element represents one electron, each period (row) represents one energy level. 5

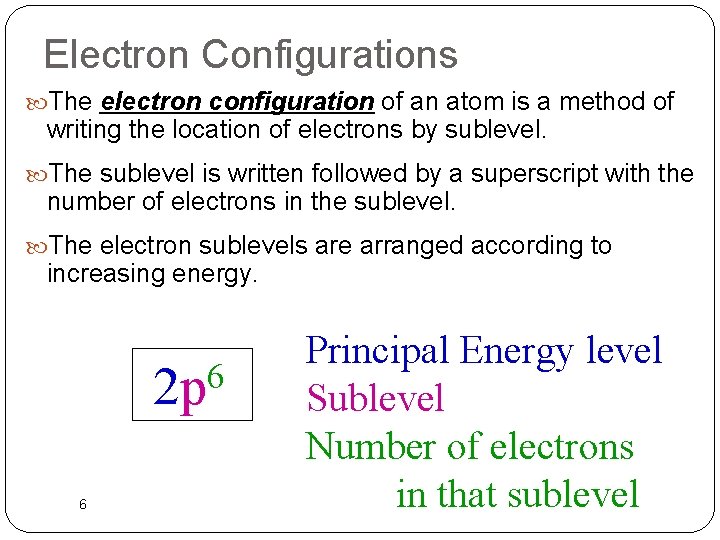
Electron Configurations The electron configuration of an atom is a method of writing the location of electrons by sublevel. The sublevel is written followed by a superscript with the number of electrons in the sublevel. The electron sublevels are arranged according to increasing energy. 6 2 p 6 Principal Energy level Sublevel Number of electrons in that sublevel


8

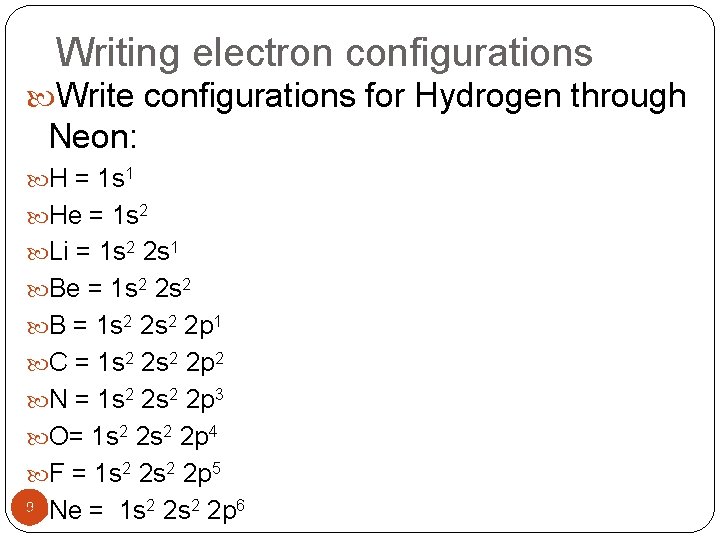
Writing electron configurations Write configurations for Hydrogen through Neon: H = 1 s 1 He = 1 s 2 Li = 1 s 2 2 s 1 Be = 1 s 2 2 s 2 B = 1 s 2 2 p 1 C = 1 s 2 2 p 2 N = 1 s 2 2 p 3 O= 1 s 2 2 p 4 F = 1 s 2 2 p 5 Ne = 1 s 2 2 p 6 9

Writing electron configurations Write configurations for Ni, Br, Sr Ni= 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 8 Br= 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 5 Sr= 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 10

Valence Electrons = electrons in an atom’s 11 outermost principal energy level (furthest from nucleus). When an atom undergoes a chemical reaction, only the outermost electrons are involved. These electrons are generally further from the nucleus are of the highest energy and determine the chemical properties of an element--they are the “most important” electrons to chemists. Each element can have a maximum of 8

Shorthand e- configurations Since the valence electrons are the “important” electrons, we use a shorthand system to show an element’s valence electrons All noble gases (group 18) have 8 valence electrons (except for helium) and therefore have a very stable configuration (most atoms want 8 valence electrons) 12

Electron Configuration Shorthand Write configurations for K and Ar K = 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 Ar= 1 s 2 2 p 6 3 s 2 3 p 6 Write configuration for K using shorthand K= [Ar] 4 s 1 13
![Shorthand practice Write the shorthand electron configuration of P Ne 3 s 2 3 Shorthand practice Write the shorthand electron configuration of: P [Ne] 3 s 2 3](https://slidetodoc.com/presentation_image_h/028a1bb8afe91bc60f44483710d125cb/image-14.jpg)
Shorthand practice Write the shorthand electron configuration of: P [Ne] 3 s 2 3 p 3 Br [Ar]4 s 23 d 104 p 5 Ca [Ar] 4 s 2 V [Ar]4 s 23 d 3 14

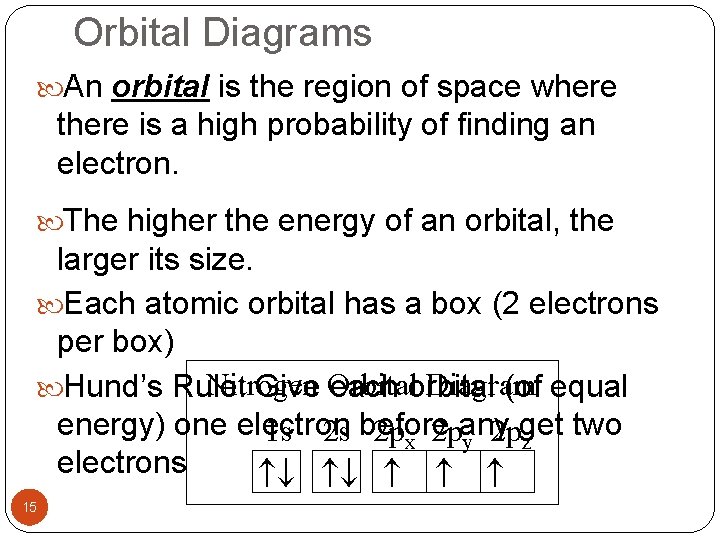
Orbital Diagrams An orbital is the region of space where there is a high probability of finding an electron. The higher the energy of an orbital, the larger its size. Each atomic orbital has a box (2 electrons per box) Nitrogen Diagram Hund’s Rule: Give Orbital each orbital (of equal energy) one electron get two 1 s 2 s before 2 px 2 pany 2 p y z electrons 15

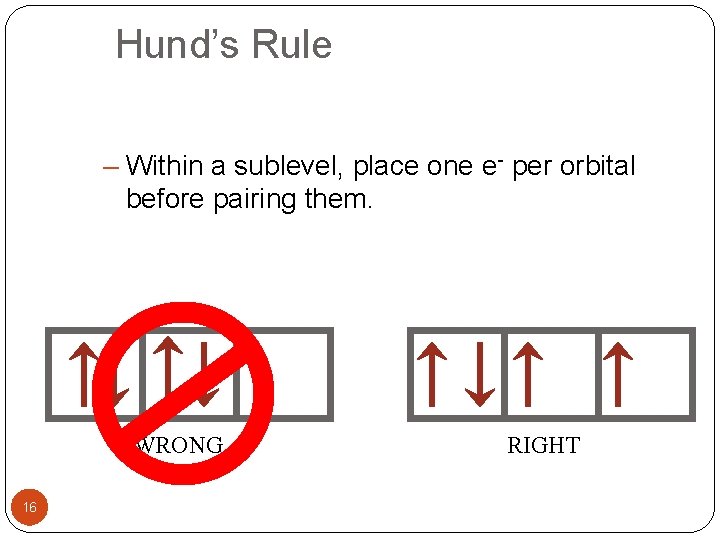
Hund’s Rule – Within a sublevel, place one e- per orbital before pairing them. WRONG 16 RIGHT

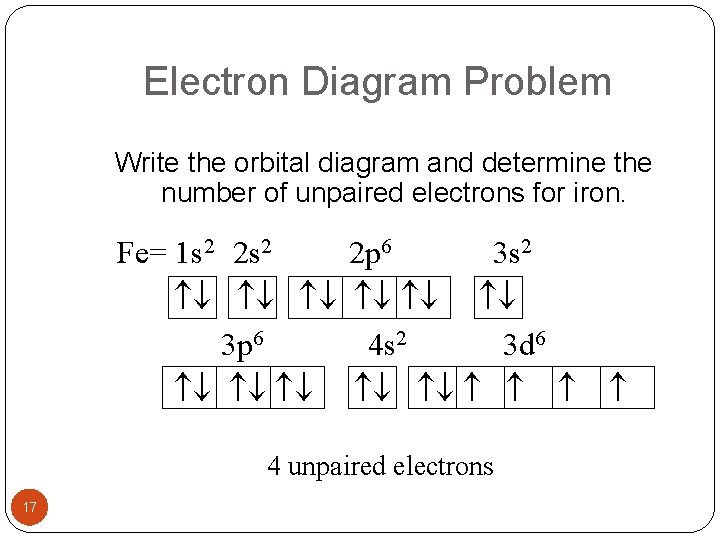
Electron Diagram Problem Write the orbital diagram and determine the number of unpaired electrons for iron. Fe= 1 s 2 2 s 2 3 p 6 2 p 6 3 s 2 4 s 2 3 d 6 4 unpaired electrons 17

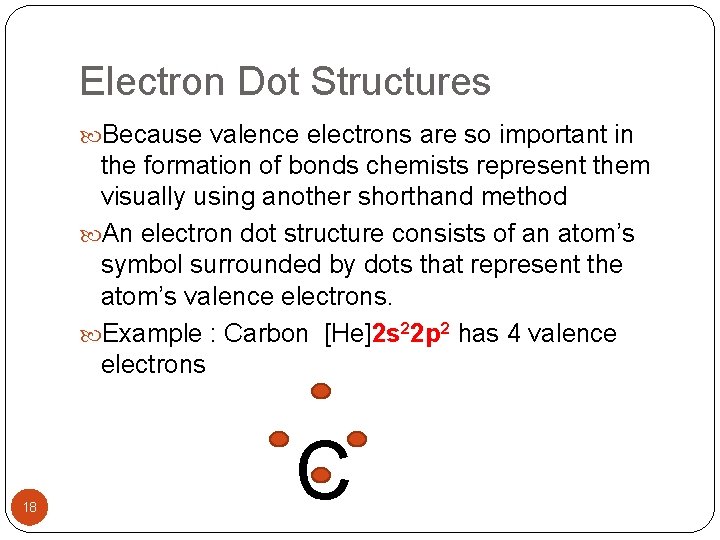
Electron Dot Structures Because valence electrons are so important in the formation of bonds chemists represent them visually using another shorthand method An electron dot structure consists of an atom’s symbol surrounded by dots that represent the atom’s valence electrons. Example : Carbon [He]2 s 22 p 2 has 4 valence electrons 18 C

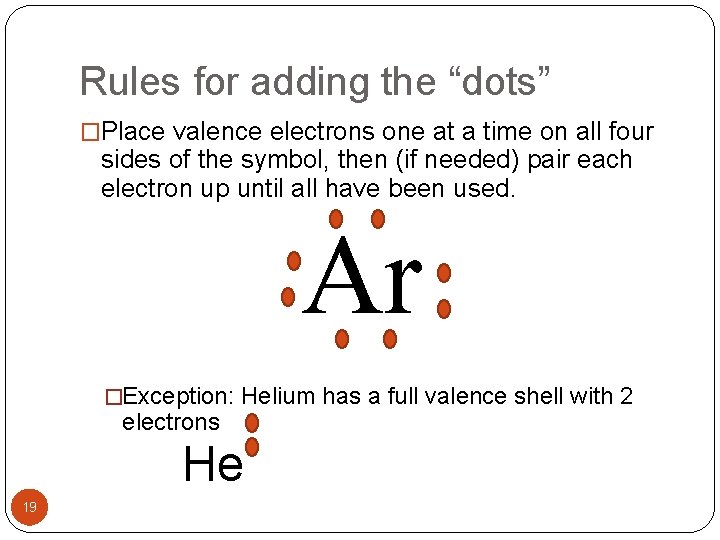
Rules for adding the “dots” �Place valence electrons one at a time on all four sides of the symbol, then (if needed) pair each electron up until all have been used. Ar �Exception: Helium has a full valence shell with 2 electrons He 19

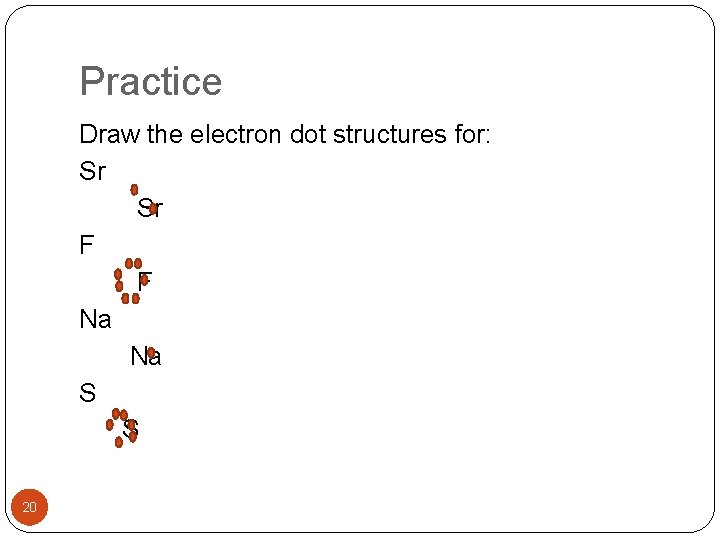
Practice Draw the electron dot structures for: Sr Sr F F Na Na S S 20

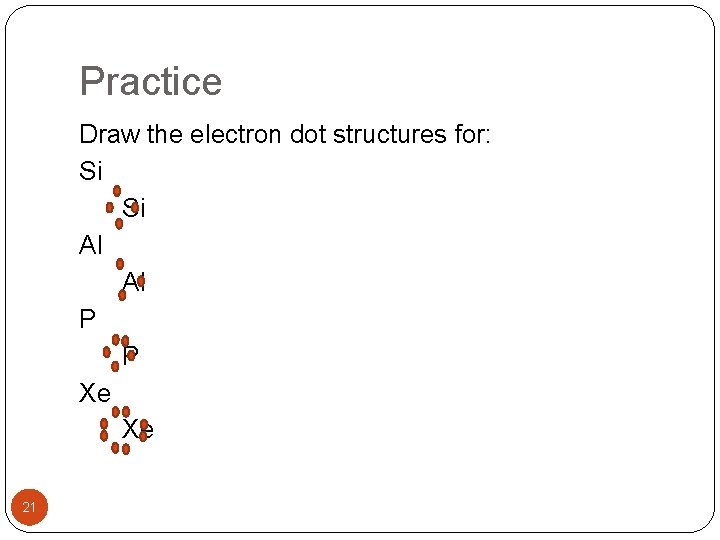
Practice Draw the electron dot structures for: Si Si Al Al P P Xe Xe 21

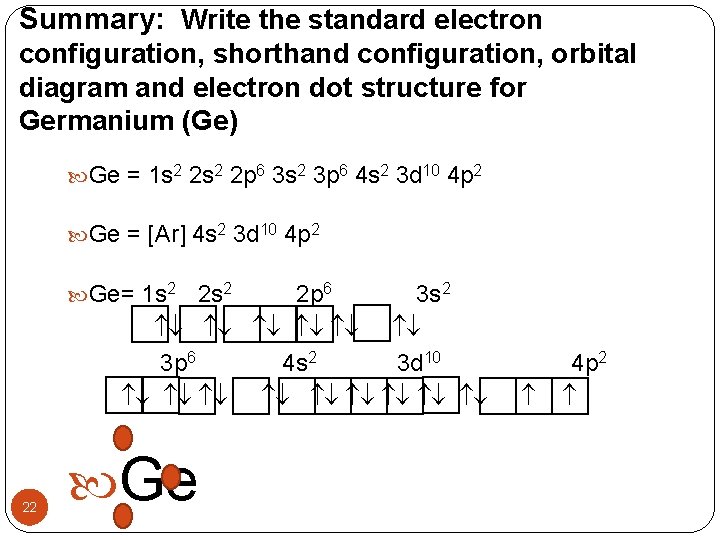
Summary: Write the standard electron configuration, shorthand configuration, orbital diagram and electron dot structure for Germanium (Ge) Ge = 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 2 Ge = [Ar] 4 s 2 3 d 10 4 p 2 Ge= 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 22 Ge 4 p 2