Ions Electron Configurations and Sizes Energy Effects in





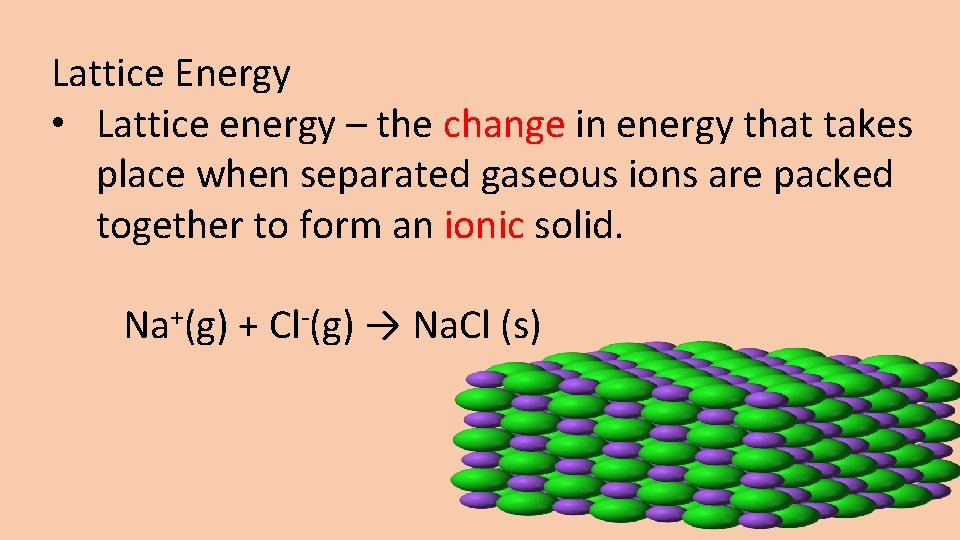




- Slides: 16

Ions: Electron Configurations and Sizes Energy Effects in Binary Ionic Compounds

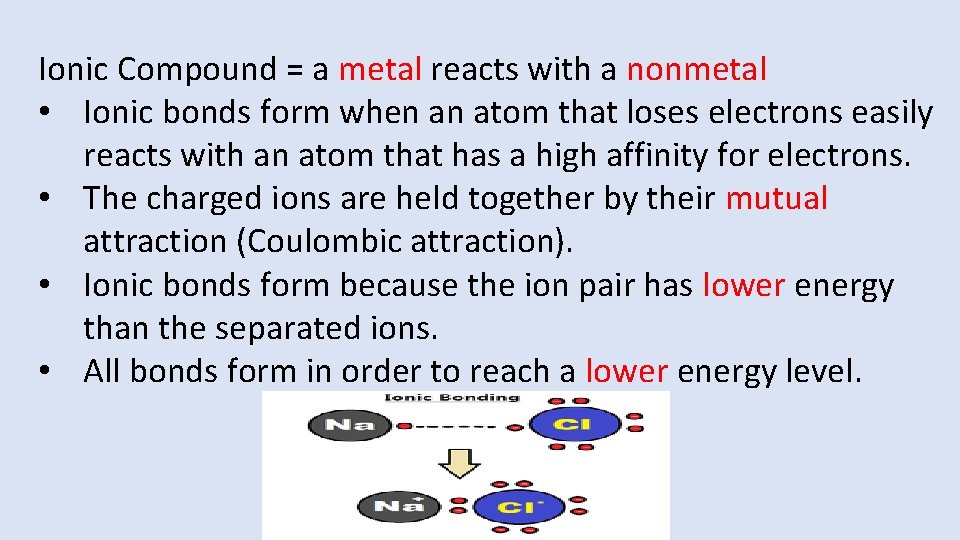
Ionic Compound = a metal reacts with a nonmetal • Ionic bonds form when an atom that loses electrons easily reacts with an atom that has a high affinity for electrons. • The charged ions are held together by their mutual attraction (Coulombic attraction). • Ionic bonds form because the ion pair has lower energy than the separated ions. • All bonds form in order to reach a lower energy level.

• When a nonmetal and a group A (1) metal react to form a binary ionic compound, the ions form so that the valence electron configuration of the nonmetal is completed and the valence orbitals of the metal are emptied to give both noble gas configurations.

Ions form to get noble gas configurations: -exceptions in Group A metals Sn 2+ and Sn 4+ Pb 2+ and Pb 4+ Bi 3+ and Bi 5+ Tl+ and Tl 3+ • Metals with d electrons will lose their highest numerical energy level electrons before losing their inner d electrons.

Size of Ions • Positive ions (cations) are smaller than their parent atoms since they are losing electrons. (More protons than electrons = greater nuclear pull) • Negative ions (anions) are larger than their parent atoms since they are gaining electrons. (Fewer protons than electrons = lower nuclear pull) Think: Monster Ants and miniature cats • Ion size increases going down a group.

Isoelectronic ions -ions containing the same number of electrons 2+ 2+ 3+ O , F , Na , Mg , Al all have the Ne configuration. They are isoelectronic. ***For an isoelectronic series, size decreases as Z (atomic number) increases.
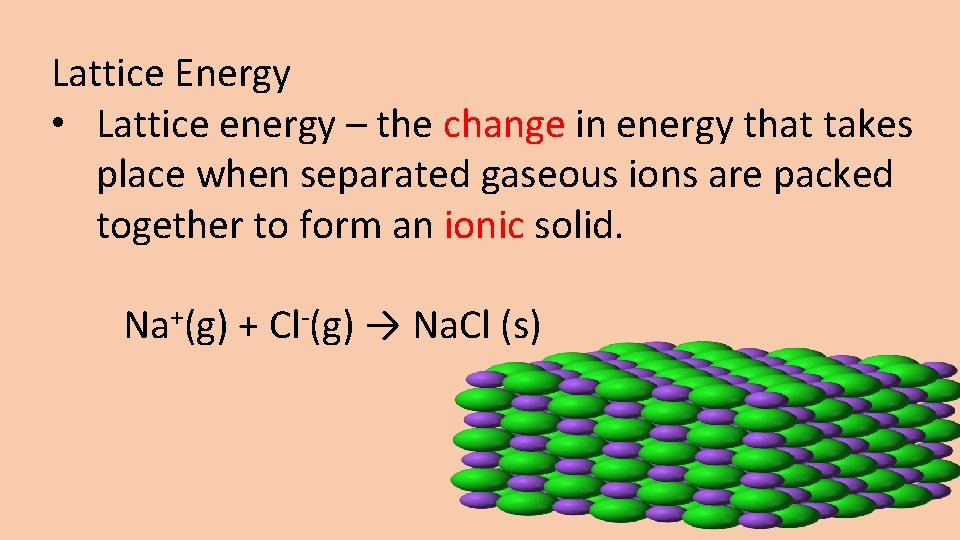
Lattice Energy • Lattice energy – the change in energy that takes place when separated gaseous ions are packed together to form an ionic solid. Na+(g) + Cl-(g) → Na. Cl (s)

• If exothermic, the sign will be negative and the ionic solid will be the stable form. • We can use a variety of steps to determine the heat of formation for an ionic solid from its elements. • This is called the Born-Haber cycle. • See examples on pages 314 -316.


• Since the ions will have opposite charges, lattice energy will be negative (exothermic). • The attractive force between a pair of oppositely charged ions increases with increased charge on the ions or with decreased ionic sizes.

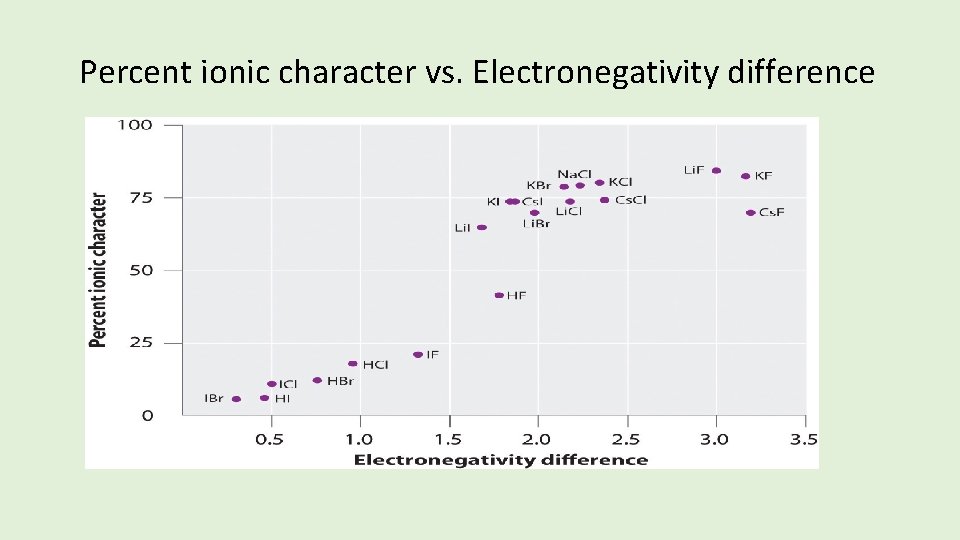
• There are probably no totally ionic bonds. • Percent ionic character in binary ionic compounds cam be calculated. • Percent ionic character increases with electronegativity difference. • Compounds with more than 50% ionic character are considered to be ionic (electronegativity difference of about 1. 7).

Percent ionic character vs. Electronegativity difference

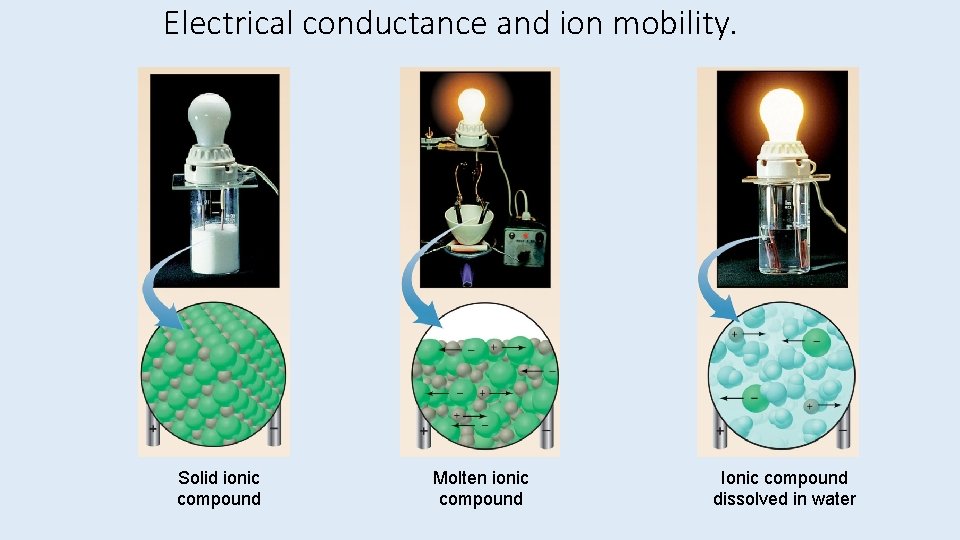
Properties of Ionic Compounds • Ionic compounds tend to be hard, rigid, and brittle, with high melting points. • Ionic compounds do not conduct electricity in the solid state. • In the solid state, the ions are fixed in place in the lattice and do not move. • Ionic compounds conduct electricity when melted or dissolved. • In the liquid state or in solution, the ions are free to move and carry a current.

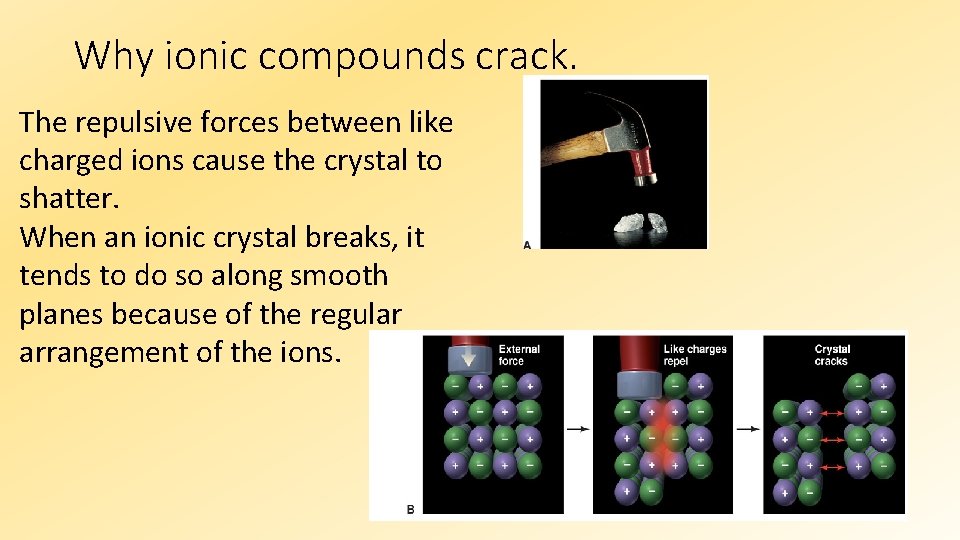
Why ionic compounds crack. The repulsive forces between like charged ions cause the crystal to shatter. When an ionic crystal breaks, it tends to do so along smooth planes because of the regular arrangement of the ions.

Electrical conductance and ion mobility. Solid ionic compound Molten ionic compound Ionic compound dissolved in water

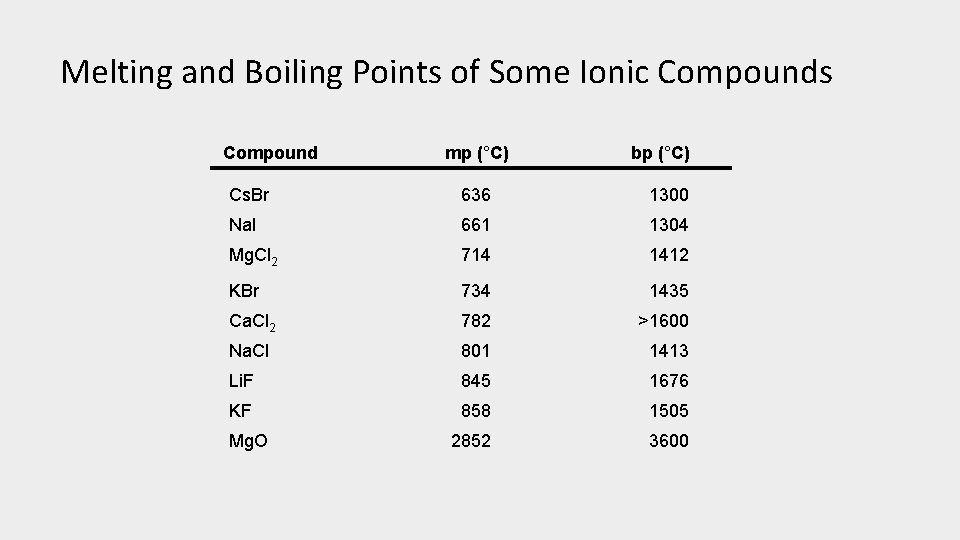
Melting and Boiling Points of Some Ionic Compounds Compound mp (°C) bp (°C) Cs. Br 636 1300 Na. I 661 1304 Mg. Cl 2 714 1412 KBr 734 1435 Ca. Cl 2 782 >1600 Na. Cl 801 1413 Li. F 845 1676 KF 858 1505 2852 3600 Mg. O