Electron Configuration Theoretical Chemistry Unit 3 1 Electron










- Slides: 16

Electron Configuration Theoretical Chemistry Unit 3 1

Electron Configuration “Address” of an electron (probable location of an electron in an atom) Energy Level Sublevel Orbital Shape Spin 2

Energy Levels Whole numbers (n = 1, 2, 3…) Describes distance of an electron from the nucleus Describes size of the electron cloud 2 n 2 is used to calculate the maximum number of electrons in an energy level 3

Sublevels Found in each energy level Energy level # = # of sublevels 1 st energy level has one sublevel 2 nd energy level has two sublevels Identified with letters (s, p, d, f) 4 th energy level has 4 4

Sublevels (cont’d) Maximum number of electrons s sublevel can hold 1 pair of e(2) p sublevel can hold 3 pairs of e (6) d sublevel can hold 5 pairs of e (10) f sublevel can hold 7 pairs of 5

Orbital Shapes Space that a pair of electrons occupies is called an orbital Orbital shape is based on sublevel s sublevel = sphere p sublevel = peanut d sublevel = double-peanut f sublevel = flower 6

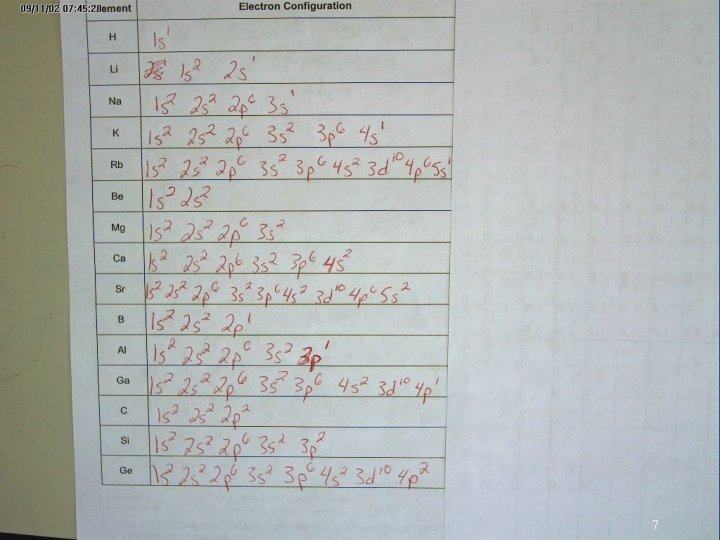
7

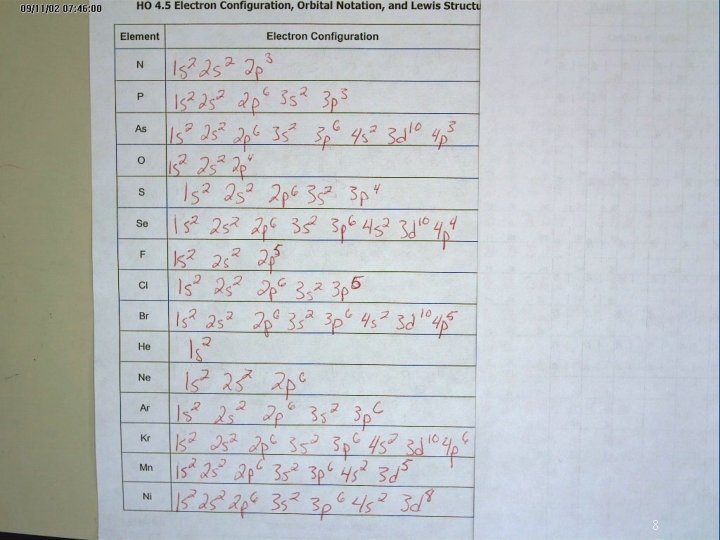
8

Spin Each orbital holds 2 electrons (1 pair) Electrons in an orbital must have opposite spins (1 clockwise, 1 counterclockwise) 9

Rules for Electron Configuration Aufbau Principle – building up principle, electrons enter orbitals at the lowest energy level first Pauli Exclusion Principle – no two electrons can have the same location at the same time with the same spin 10

Electron Dot Diagrams AKA Lewis Dot Structures Show all valence e for an element Valence e are found at an 5 8 element’s highest energy 7 1 B level X 4 2 3 6 r 11

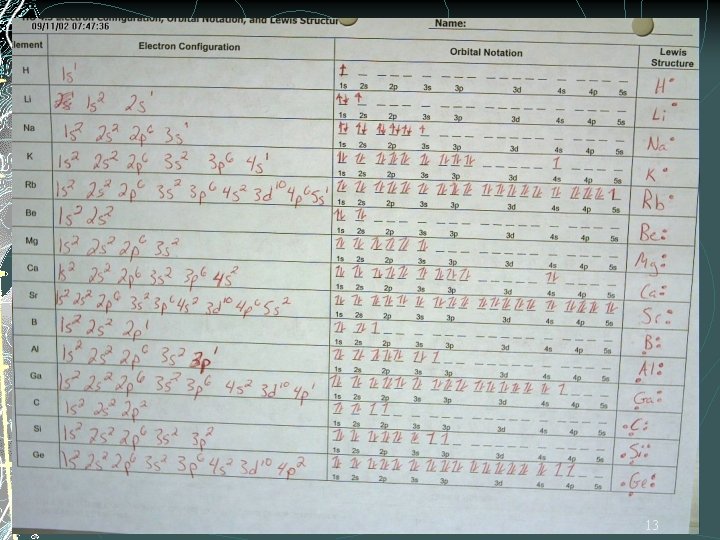
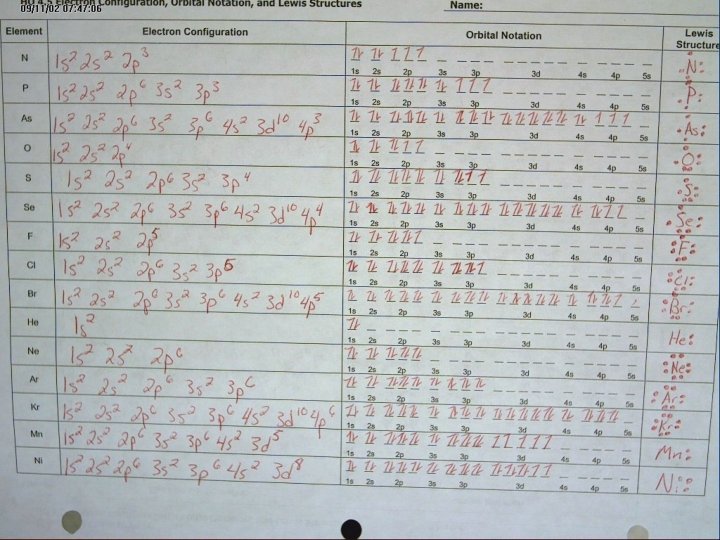
What element does this configuration represent? 1 s 2 2 p 6 3 s 2 How many pairs of electrons can each sublevel hold? What’s the difference between the 1 s and 2 s sublevels? What are the 4 orbital shapes? 12

13

14

Electron Configuration Short hand Start with the element symbol for of the Noble Gas from the previous period (Row) Then write the electron configuration of the last period only. Ex. (Titanium) l [Ar] 4 s 2 3 d 2 15

16