The Structure of the Atom The atom is








- Slides: 18

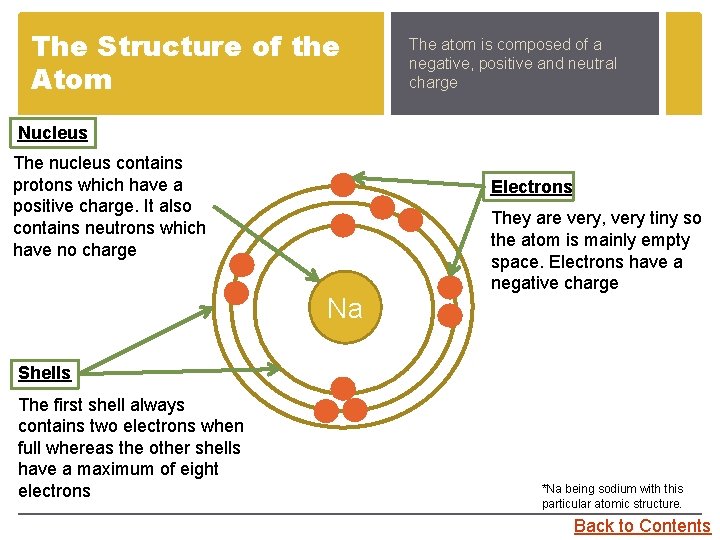
The Structure of the Atom The atom is composed of a negative, positive and neutral charge Nucleus The nucleus contains protons which have a positive charge. It also contains neutrons which have no charge Electrons Na They are very, very tiny so the atom is mainly empty space. Electrons have a negative charge Shells The first shell always contains two electrons when full whereas the other shells have a maximum of eight electrons *Na being sodium with this particular atomic structure. Back to Contents

How atoms combine Ionic bonding Back to Contents

Scientists found that elements in Group 8 were very nonreactive. They also noticed that those in Groups 1, 2, 6 and 7 were extremely reactive. They also noticed that metallic substances had several properties that were very different from other elements. They could not at first understand why. Eventually they discovered that it had to do with Prepared by JGL 8/21/2009 WHY DO COMPOUNDS FORM IN THE FIRST PLACE? ELECTRON CONFIGURATIONS and STABILITY 3

Scientists’ research showed that in compounds, elements will combine so that the valence or outermost electrons will have the same electron configuration as the nearest noble gas (in Group 8) Prepared by JGL 8/21/2009 ELECTRON CONFIGURATION AND STABILITY 4

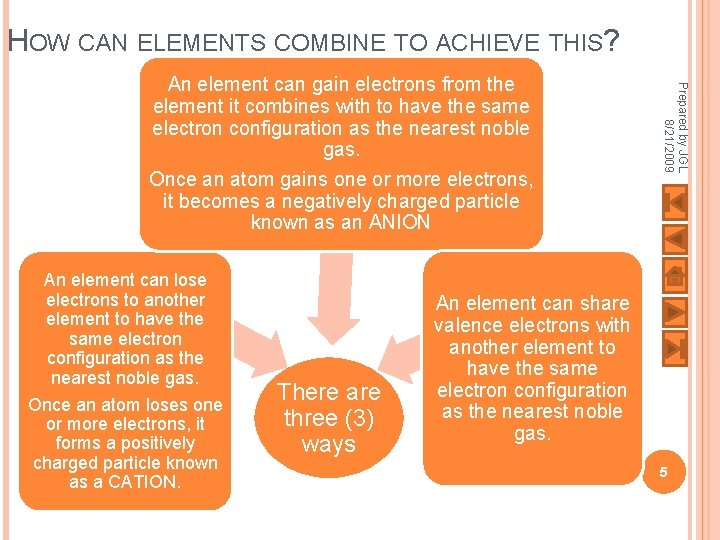
HOW CAN ELEMENTS COMBINE TO ACHIEVE THIS? An element can lose electrons to another element to have the same electron configuration as the nearest noble gas. Once an atom loses one or more electrons, it forms a positively charged particle known as a CATION. There are three (3) ways Prepared by JGL 8/21/2009 An element can gain electrons from the element it combines with to have the same electron configuration as the nearest noble gas. Once an atom gains one or more electrons, it becomes a negatively charged particle known as an ANION An element can share valence electrons with another element to have the same electron configuration as the nearest noble gas. 5

Prepared by JGL 8/21/2009 YOU MAY WELL BE ASKING WHAT DOES THIS MEAN? 6

Prepared by JGL 8/21/2009 LET’S TAKE A LOOK AT SOME EXAMPLES TO UNDERSTAND THIS CONCEPT MORE FULLY 7

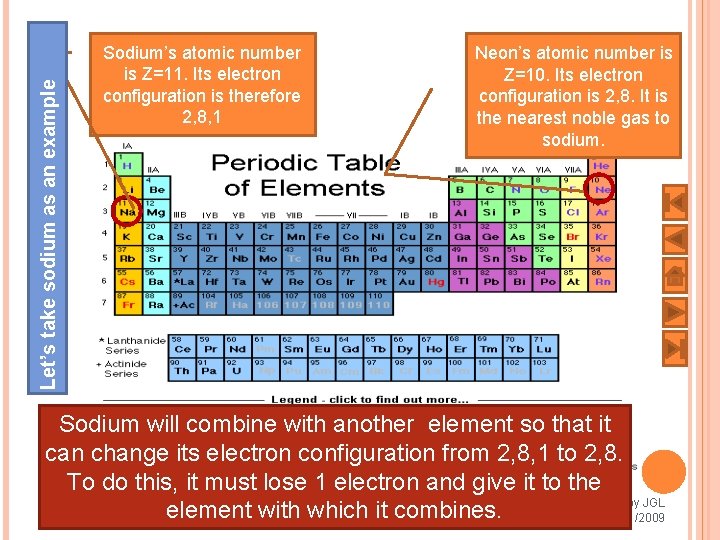
Let’s take sodium as an example Sodium’s atomic number is Z=11. Its electron configuration is therefore 2, 8, 1 Neon’s atomic number is Z=10. Its electron configuration is 2, 8. It is the nearest noble gas to sodium. Sodium will combine with another element so that it can change its electron configuration from 2, 8, 1 to 2, 8. To do this, it must lose 1 electron and give it to the Prepared by JGL 8 element with which it combines. 8/21/2009

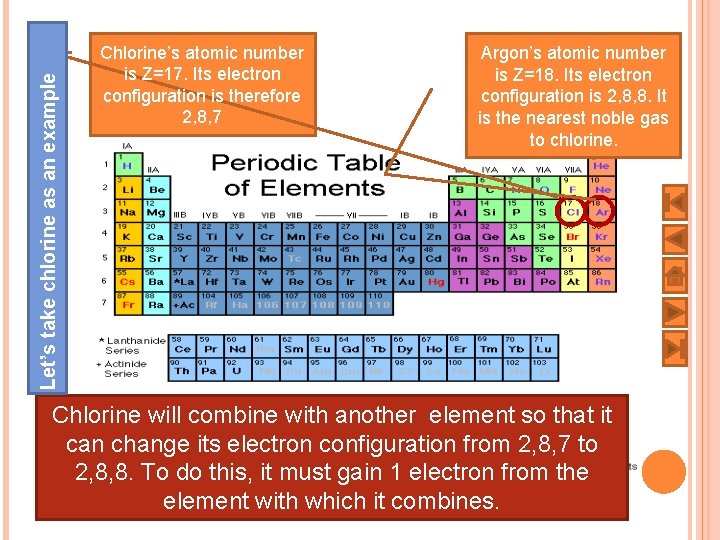
Let’s take chlorine as an example Chlorine’s atomic number is Z=17. Its electron configuration is therefore 2, 8, 7 Argon’s atomic number is Z=18. Its electron configuration is 2, 8, 8. It is the nearest noble gas to chlorine. Chlorine will combine with another element so that it can change its electron configuration from 2, 8, 7 to 2, 8, 8. To do this, it must gain 1 electron from the element with which it combines. 9

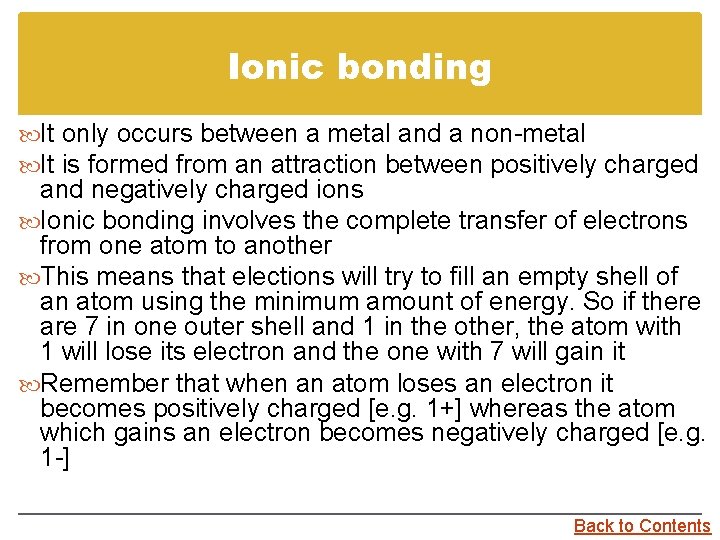
Ionic bonding It only occurs between a metal and a non-metal It is formed from an attraction between positively charged and negatively charged ions Ionic bonding involves the complete transfer of electrons from one atom to another This means that elections will try to fill an empty shell of an atom using the minimum amount of energy. So if there are 7 in one outer shell and 1 in the other, the atom with 1 will lose its electron and the one with 7 will gain it Remember that when an atom loses an electron it becomes positively charged [e. g. 1+] whereas the atom which gains an electron becomes negatively charged [e. g. 1 -] Back to Contents

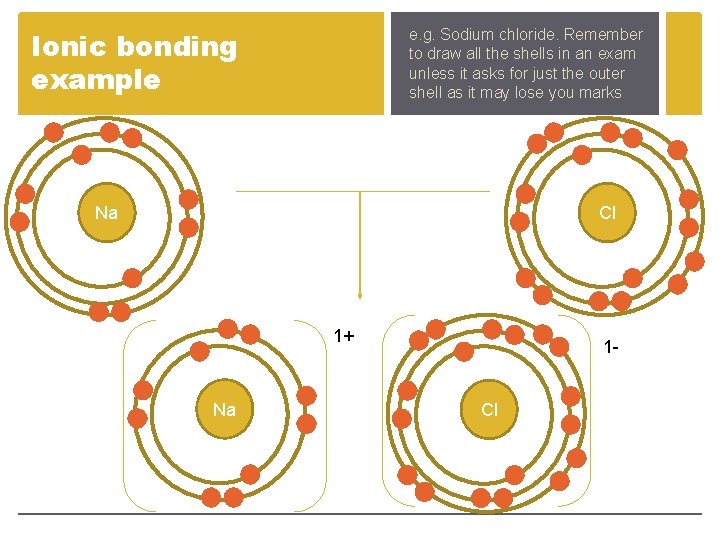
Ionic bonding example e. g. Sodium chloride. Remember to draw all the shells in an exam unless it asks for just the outer shell as it may lose you marks Na Cl 1+ Na 1 Cl

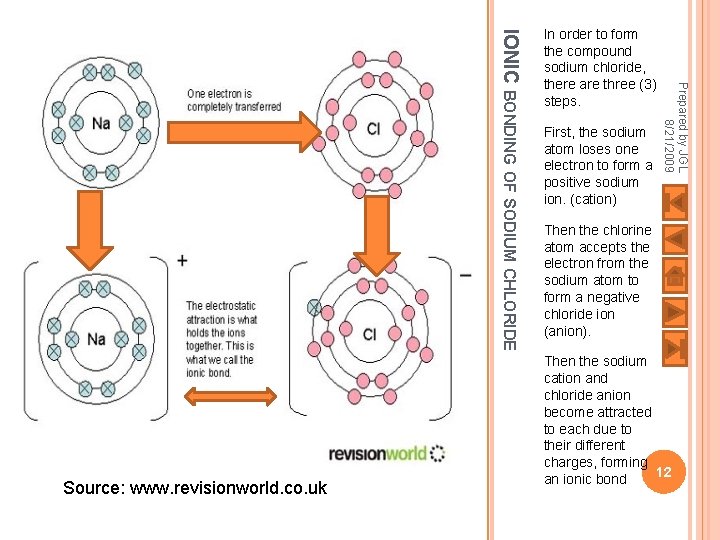
First, the sodium atom loses one electron to form a positive sodium ion. (cation) Prepared by JGL 8/21/2009 IONIC BONDING OF SODIUM CHLORIDE Source: www. revisionworld. co. uk In order to form the compound sodium chloride, there are three (3) steps. Then the chlorine atom accepts the electron from the sodium atom to form a negative chloride ion (anion). Then the sodium cation and chloride anion become attracted to each due to their different charges, forming 12 an ionic bond

An ion is an atom or molecule where the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. Prepared by JGL 8/21/2009 IONS DEFINED 13

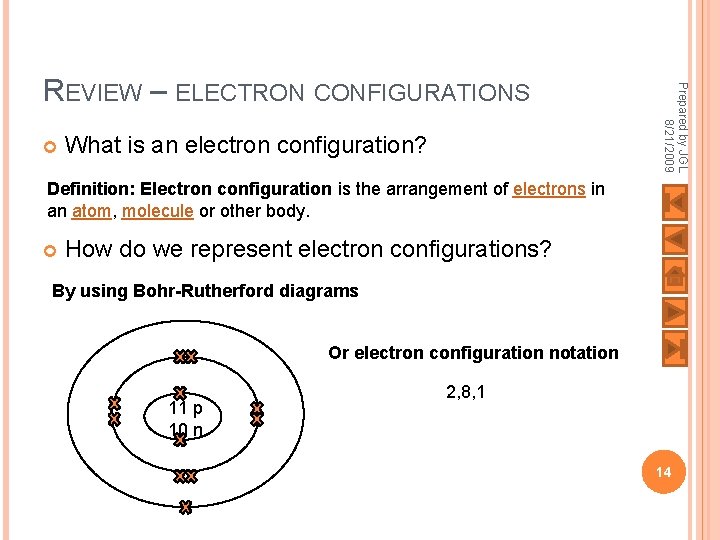
What is an electron configuration? Prepared by JGL 8/21/2009 REVIEW – ELECTRON CONFIGURATIONS Definition: Electron configuration is the arrangement of electrons in an atom, molecule or other body. How do we represent electron configurations? By using Bohr-Rutherford diagrams Or electron configuration notation 11 p 10 n 2, 8, 1 14

REMEMBER – “CONTRAST” MEANS “LOOK AT THE DIFFERENCES” Fluorine Neon Element symbol F Group 17 Atomic Number Z = 9 Mass number A = 19 Electron configuration: 2, 7 Bohr-Rutherford diagram 9 p 10 n Prepared by JGL 8/21/2009 LET’S CONTRAST –FLOURINE AND NEON Element symbol Ne Group 18 Atomic Number Z = 10 Mass number A = 20 Electron configuration: 2, 8 Bohr-Rutherford diagram 10 p 10 n 15

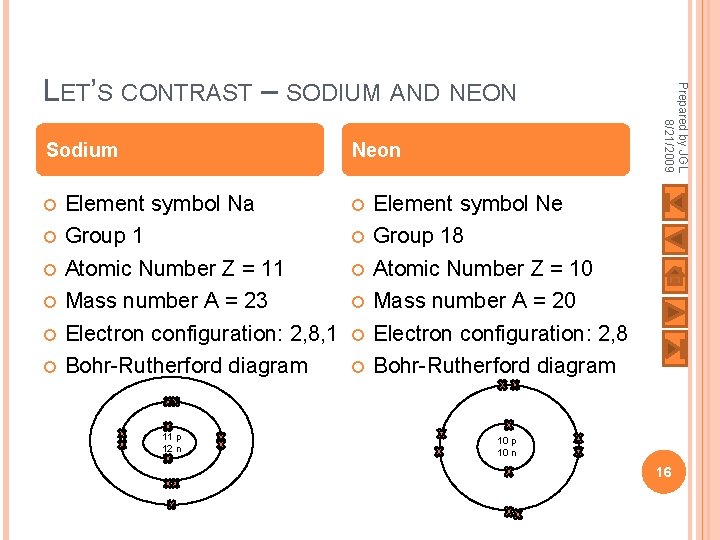
Sodium Neon Element symbol Na Group 1 Atomic Number Z = 11 Mass number A = 23 Electron configuration: 2, 8, 1 Bohr-Rutherford diagram 11 p 12 n Prepared by JGL 8/21/2009 LET’S CONTRAST – SODIUM AND NEON Element symbol Ne Group 18 Atomic Number Z = 10 Mass number A = 20 Electron configuration: 2, 8 Bohr-Rutherford diagram 10 p 10 n 16

Remember – “Compare” means “Look at Similarities F and Ne have the same number of electron shells Scientists found that when elements from Group 1 and Group 7 combine, they lose or gain an electron to have the same number of electrons as the nearest Noble Gas. i. e. F and Na form ions that are ISO-ELECTRONIC with Ne Differences Prepared by JGL 8/21/2009 COMPARE AND CONTRAST ALL 3 ELEMENTS Different atomic numbers (Z) and therefore protons Different mass numbers (A) and therefore different neutrons F needs to gain 1 electron to have the same number of electrons as Ne Na needs to lose 1 electron to have the same number of electrons as Ne 17

IN GENERAL 1. 2. 3. To become ISOELECTRONIC with the nearest Noble Gas (either within the same Period or the Period just above) Group 1 elements lose 1 e. Group 2 elements lose 2 e. Group 3 elements lose 3 e- Groups 15, 16 and 17 1. 2. 3. This only happens when combining or reacting with another element(s) from Groups 15, 16 or 17 To become ISO-ELECTRONIC with the nearest Noble Gas (either within the same Period or the Period just above) Group 15 elements gain 3 e. Group 16 elements gain 2 e. Group 17 elements gain 1 e- Prepared by JGL 8/21/2009 Groups 1, 2 and 3 This only happens when combining or reacting with another element(s) from Groups 1, 2 or 3 18