Chemical Change Chapter 2 Dr Suzan A Khayyat





- Slides: 50

Chemical Change Chapter 2 Dr. Suzan A. Khayyat 1

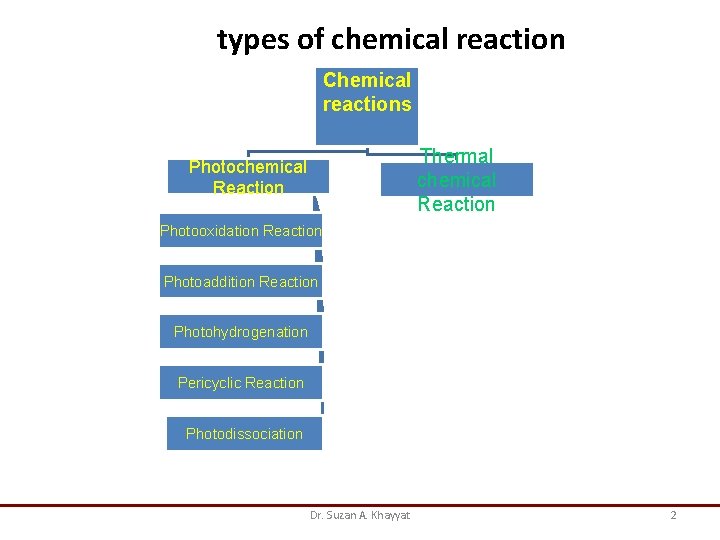
types of chemical reaction Chemical reactions Thermal chemical Reaction Photooxidation Reaction Photoaddition Reaction Photohydrogenation Pericyclic Reaction Photodissociation Dr. Suzan A. Khayyat 2

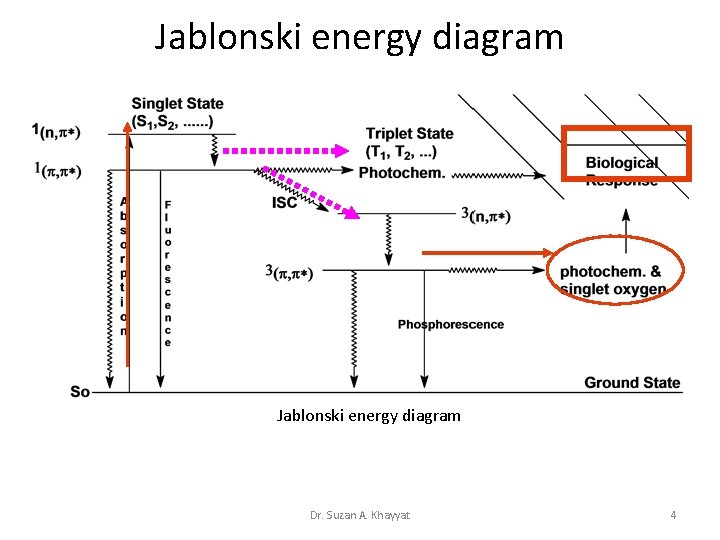
• The Jablonski Diagram • The energy gained by a molecule when it absorbs a photon causes an electron to be promoted to a higher electronic energy level. Figure 3 illustrates the principal photophysical radiative and non-radiative processes displayed by organic molecules in solution. The symbols So, S 1, T 2, etc. , refer to the ground electronic state (So), first excited singlet state (S 1), second excited triplet state (T 2), and so on. The horizontal lines represent the vibrational levels of each electronic state. Straight arrows indicate radiative transitions, and curly arrows indicate nonradiative transitions. The boxes detail the electronic spins in each orbital, with electrons shown as up and down arrows, to distinguish their spin. • Note that all transitions from one electronic state to another originate from the lowest vibrational level of the initial electronic state. For example, fluorescence occurs only from S 1, because the higher singlet states (S 2, etc. ) decay so rapidly by internal conversion that fluorescence from these states cannot compete. Dr. Suzan A. Khayyat 3

Jablonski energy diagram Dr. Suzan A. Khayyat 4

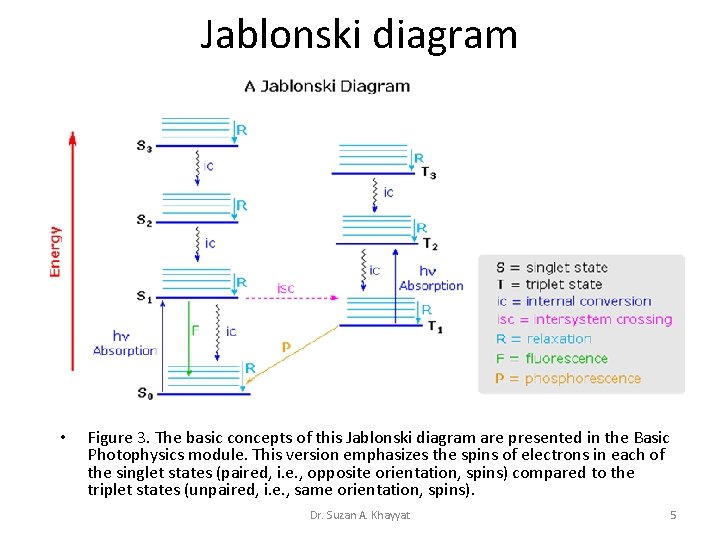
Jablonski diagram • Figure 3. The basic concepts of this Jablonski diagram are presented in the Basic Photophysics module. This version emphasizes the spins of electrons in each of the singlet states (paired, i. e. , opposite orientation, spins) compared to the triplet states (unpaired, i. e. , same orientation, spins). Dr. Suzan A. Khayyat 5

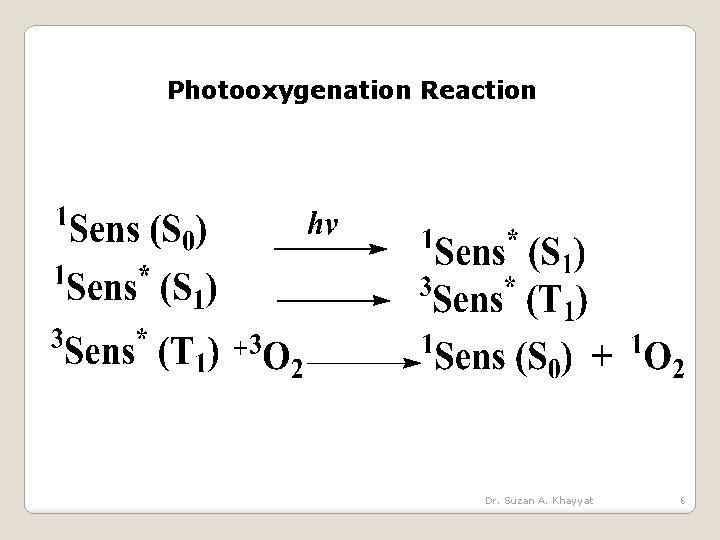
Photooxygenation Reaction Dr. Suzan A. Khayyat 6

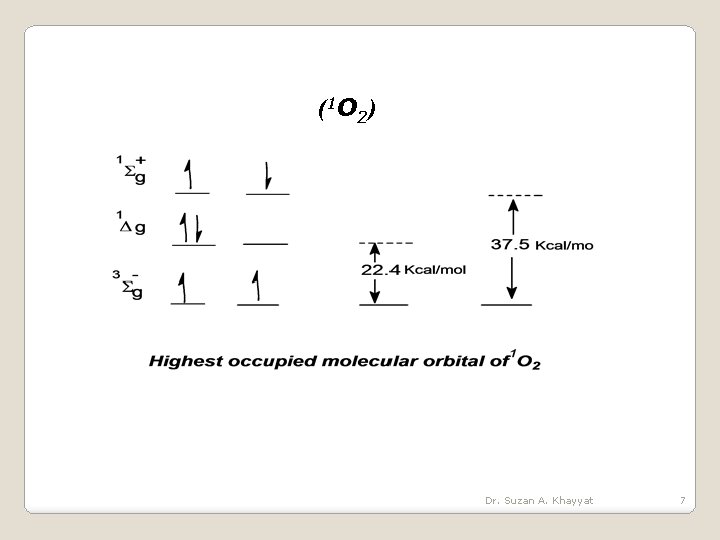
(1 O 2 ) Dr. Suzan A. Khayyat 7

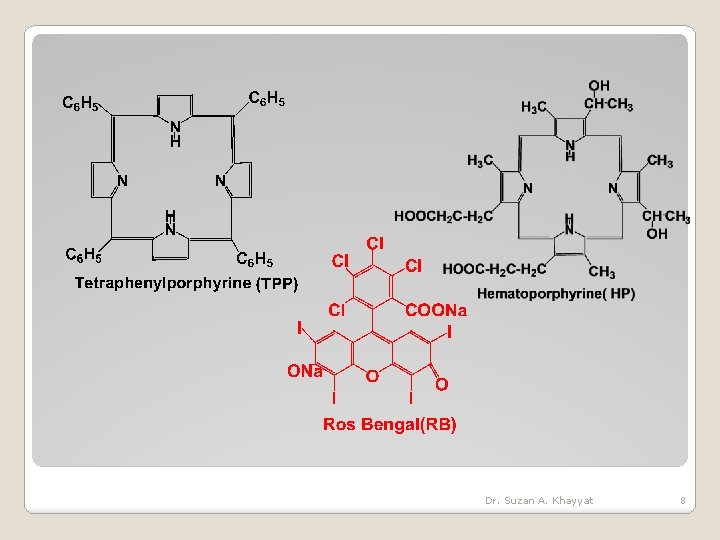
Dr. Suzan A. Khayyat 8

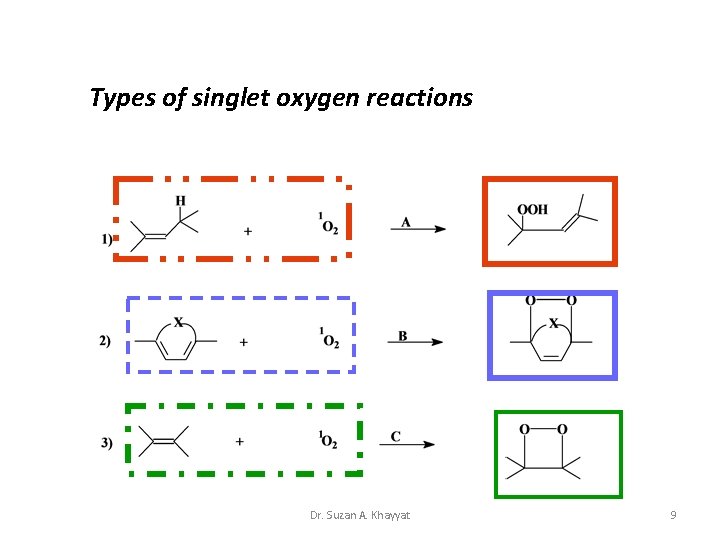
Types of singlet oxygen reactions Dr. Suzan A. Khayyat 9

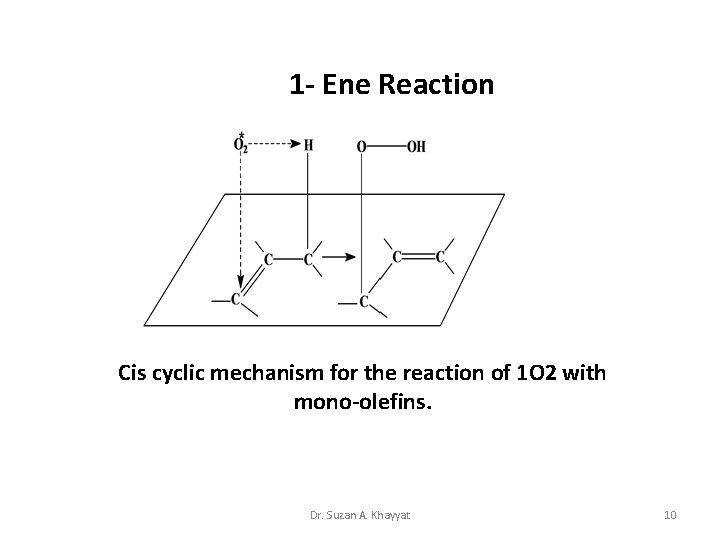
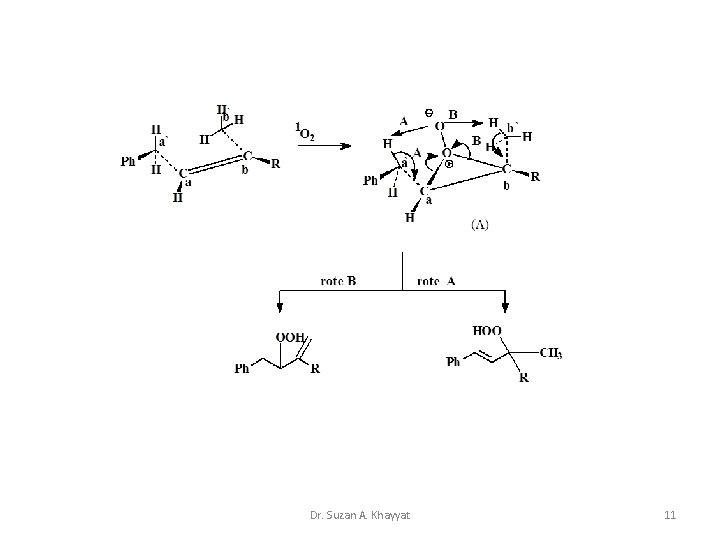
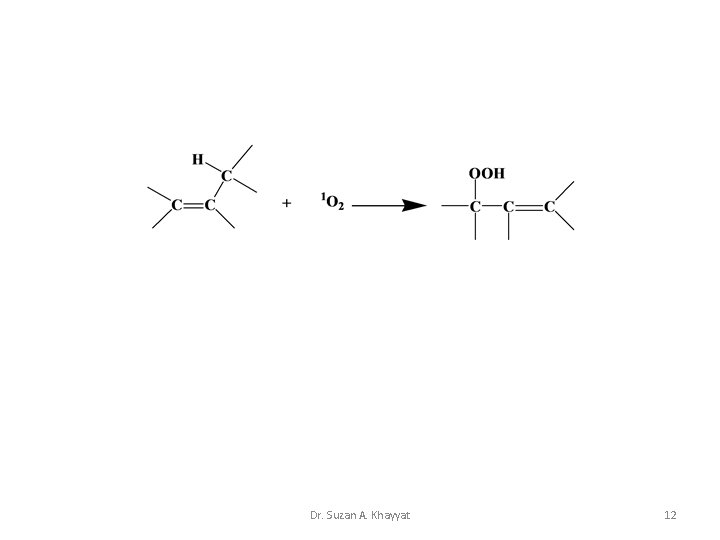
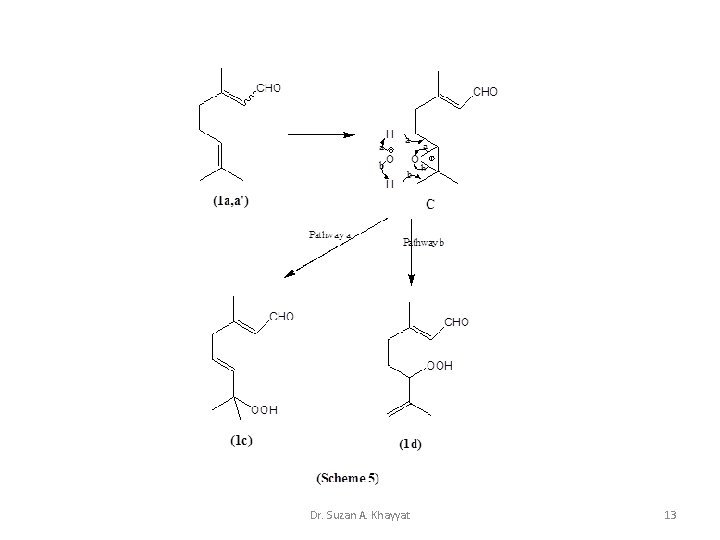
1 - Ene Reaction Cis cyclic mechanism for the reaction of 1 O 2 with mono-olefins. Dr. Suzan A. Khayyat 10

Dr. Suzan A. Khayyat 11

Dr. Suzan A. Khayyat 12

Dr. Suzan A. Khayyat 13

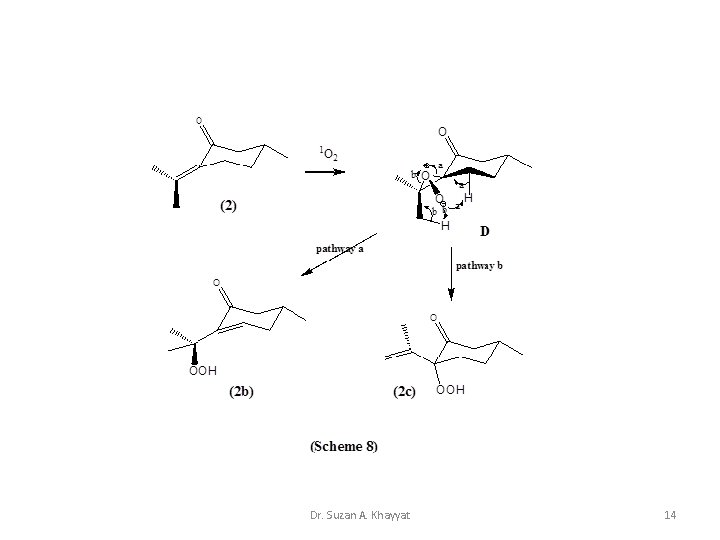
Dr. Suzan A. Khayyat 14

Dr. Suzan A. Khayyat 15

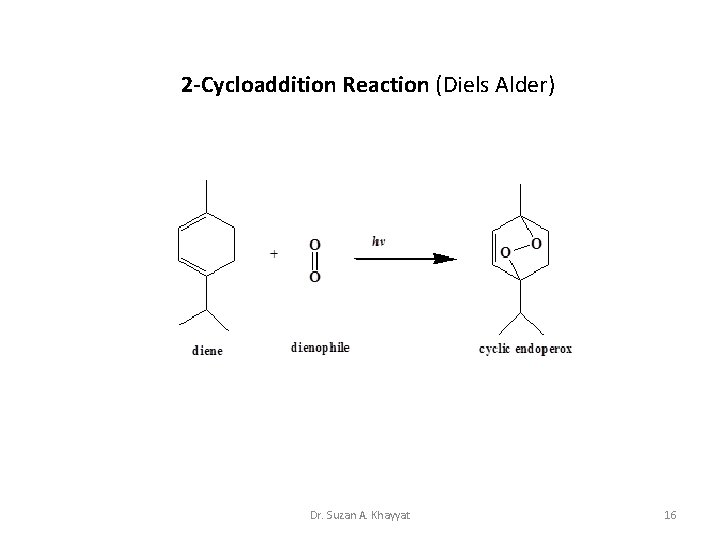
2 -Cycloaddition Reaction (Diels Alder) Dr. Suzan A. Khayyat 16

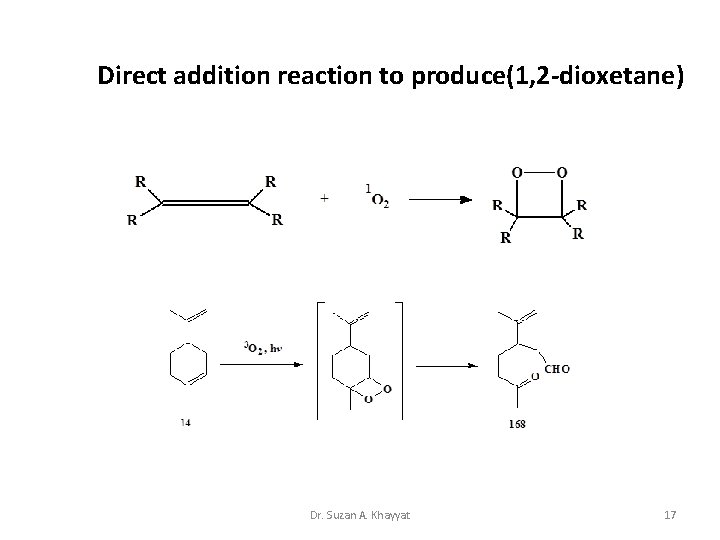
Direct addition reaction to produce(1, 2 -dioxetane) Dr. Suzan A. Khayyat 17

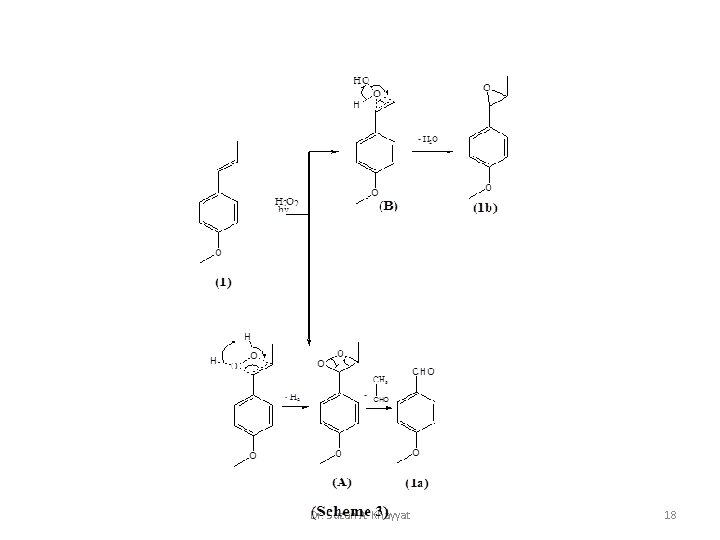
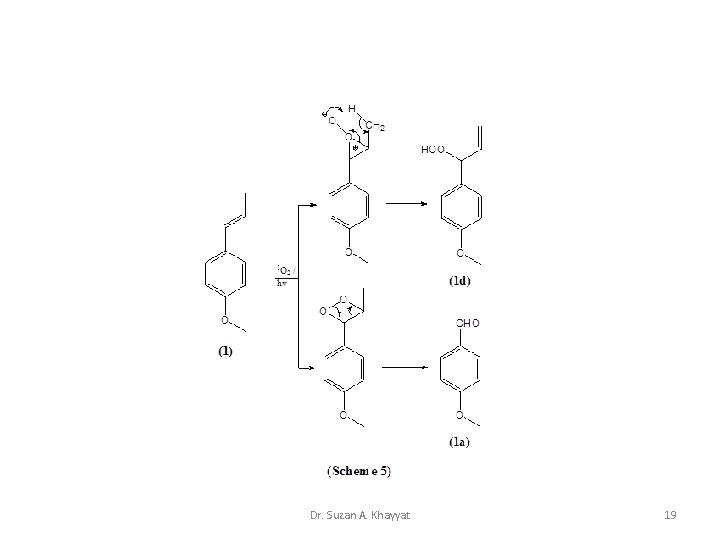
Dr. Suzan A. Khayyat 18

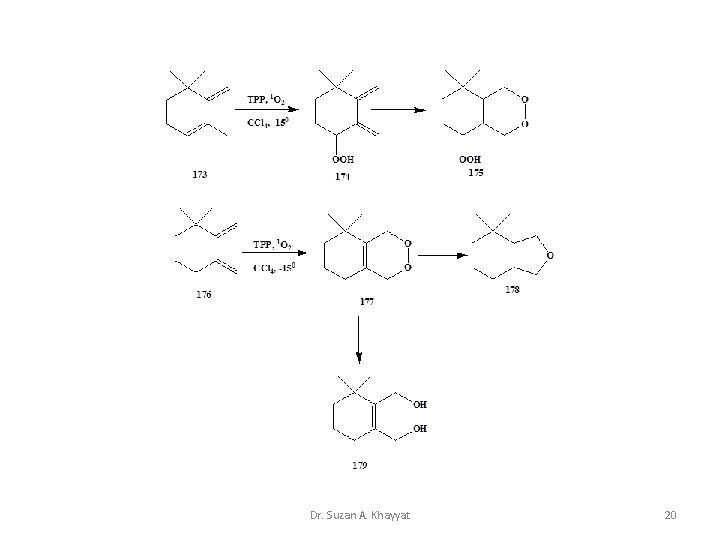
Dr. Suzan A. Khayyat 19

Dr. Suzan A. Khayyat 20

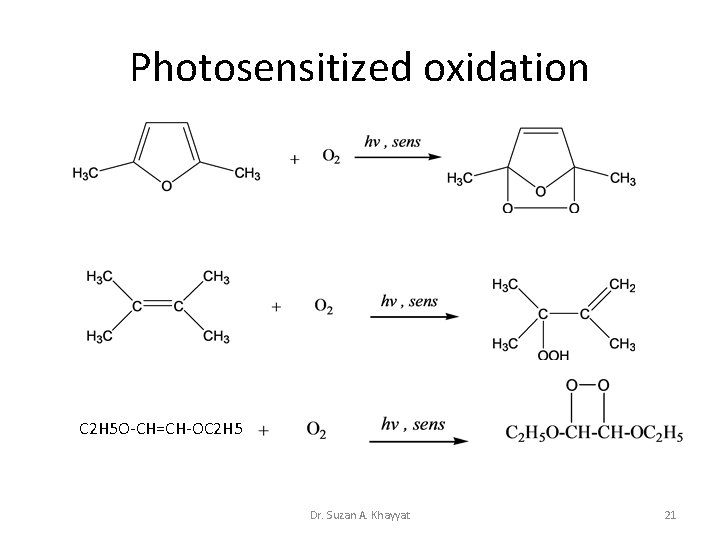
Photosensitized oxidation C 2 H 5 O-CH=CH-OC 2 H 5 Dr. Suzan A. Khayyat 21

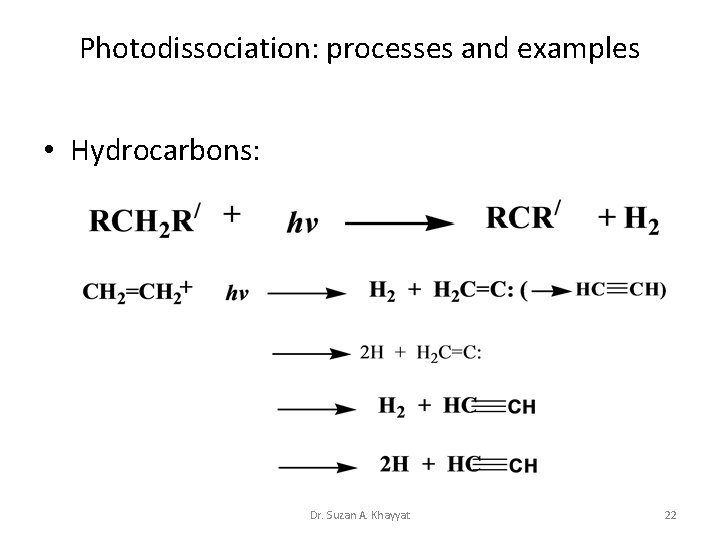
Photodissociation: processes and examples • Hydrocarbons: Dr. Suzan A. Khayyat 22

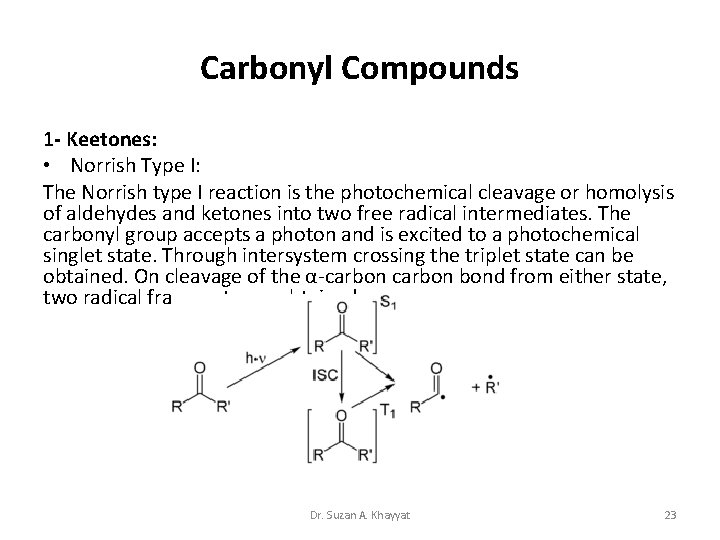
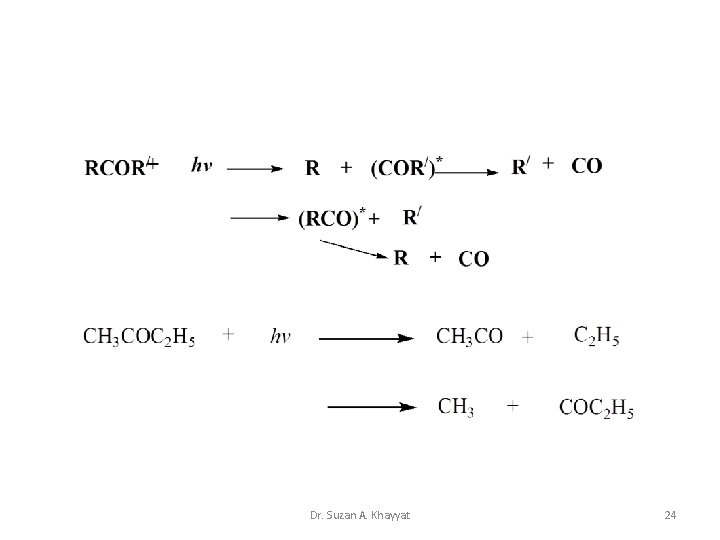
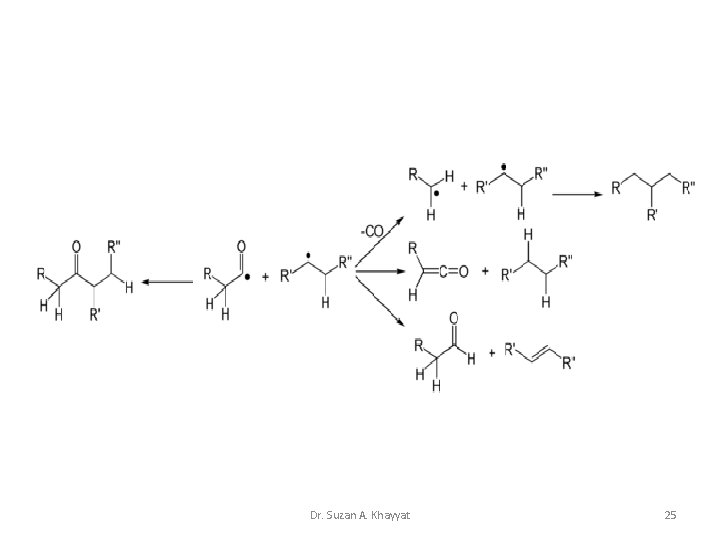
Carbonyl Compounds 1 - Keetones: • Norrish Type I: The Norrish type I reaction is the photochemical cleavage or homolysis of aldehydes and ketones into two free radical intermediates. The carbonyl group accepts a photon and is excited to a photochemical singlet state. Through intersystem crossing the triplet state can be obtained. On cleavage of the α-carbon bond from either state, two radical fragments are obtained. Dr. Suzan A. Khayyat 23

Dr. Suzan A. Khayyat 24

Dr. Suzan A. Khayyat 25

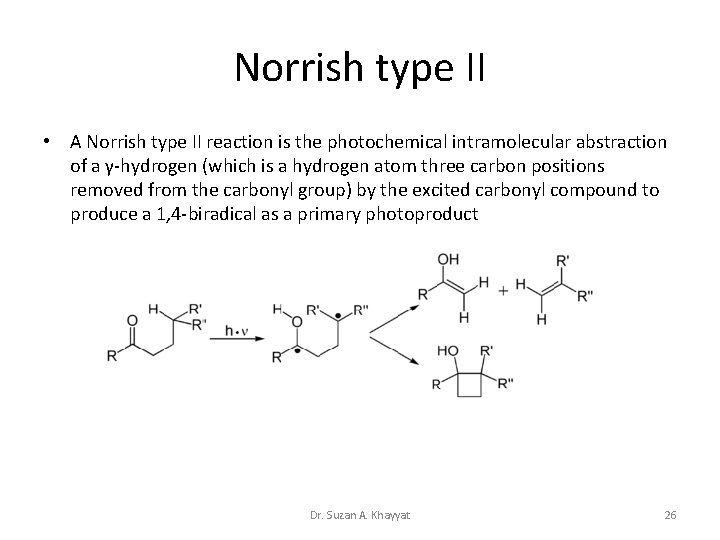
Norrish type II • A Norrish type II reaction is the photochemical intramolecular abstraction of a γ-hydrogen (which is a hydrogen atom three carbon positions removed from the carbonyl group) by the excited carbonyl compound to produce a 1, 4 -biradical as a primary photoproduct Dr. Suzan A. Khayyat 26

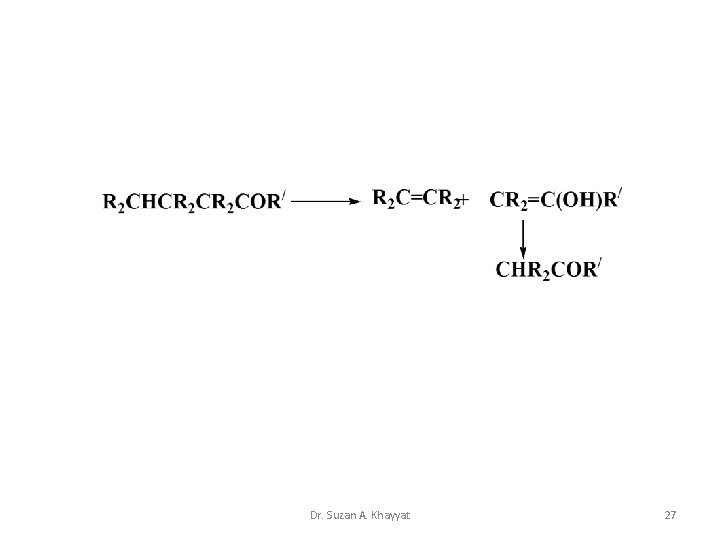
Dr. Suzan A. Khayyat 27

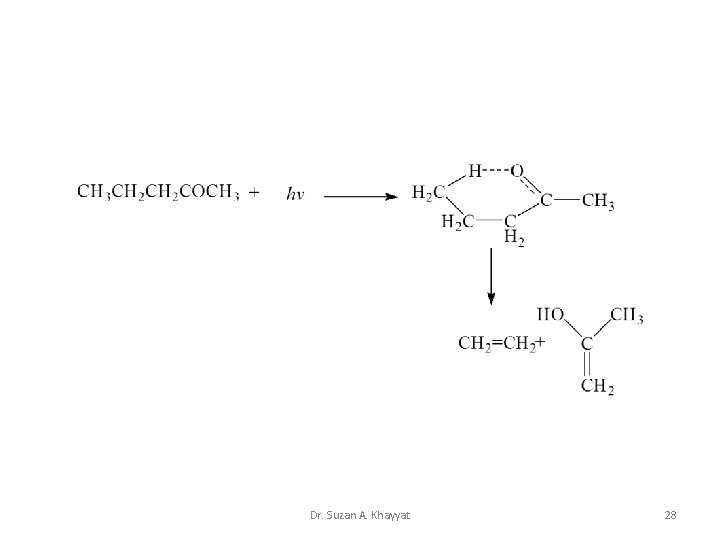
Dr. Suzan A. Khayyat 28

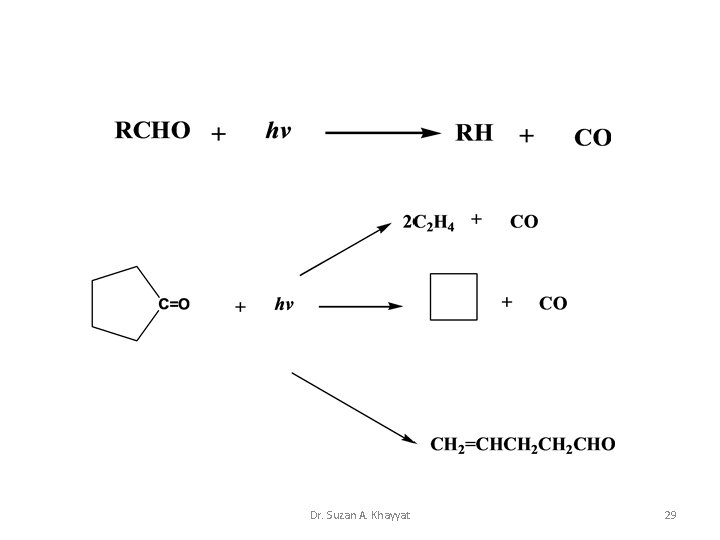
Dr. Suzan A. Khayyat 29

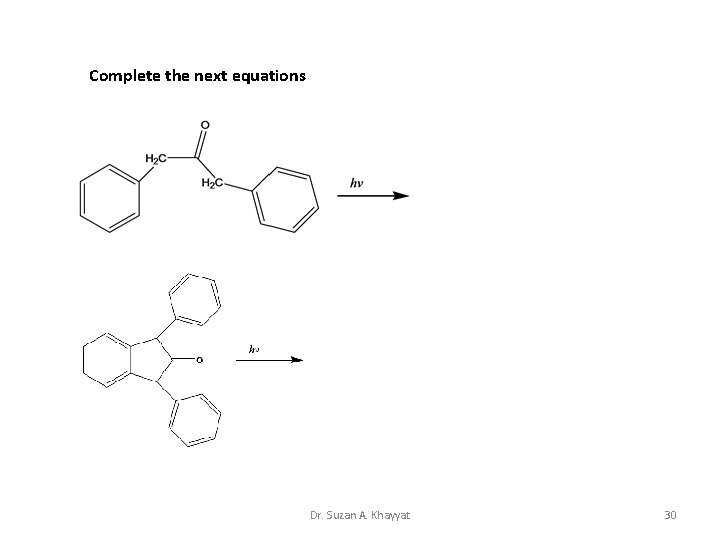
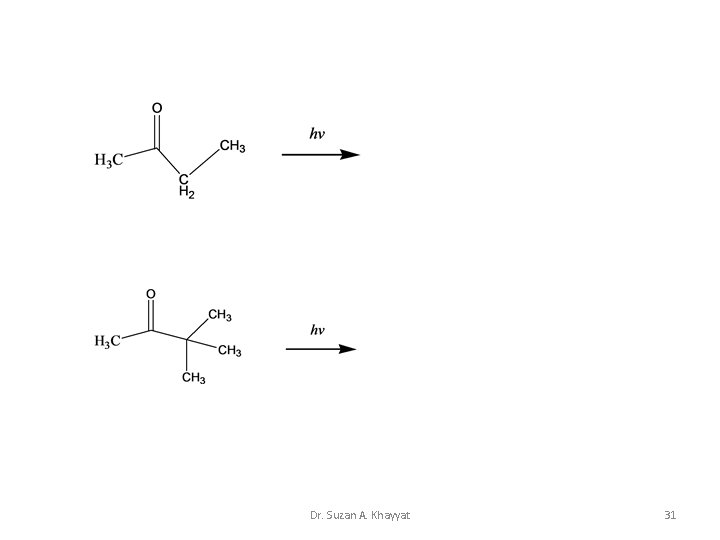
Complete the next equations Dr. Suzan A. Khayyat 30

Dr. Suzan A. Khayyat 31

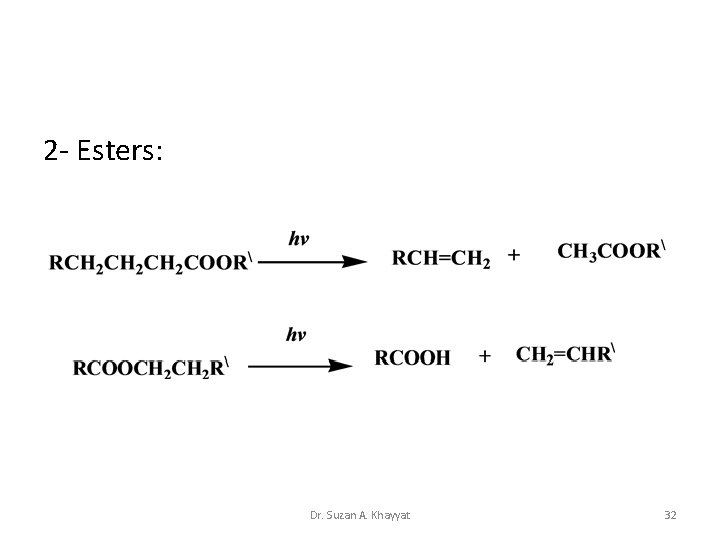
2 - Esters: Dr. Suzan A. Khayyat 32

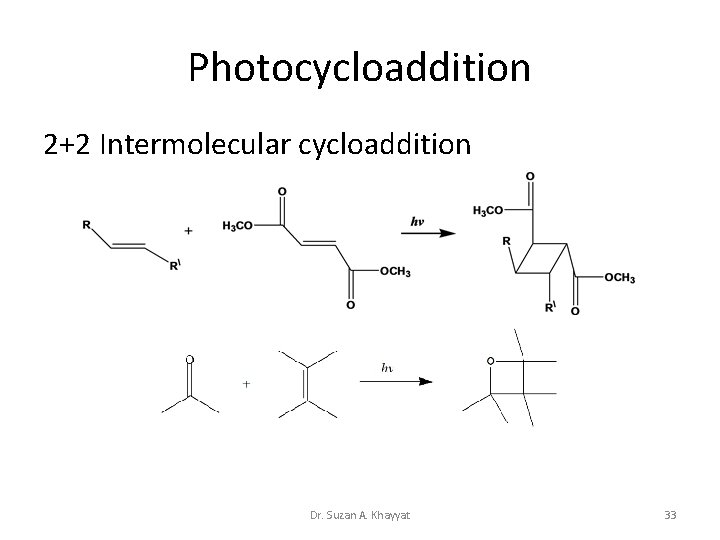
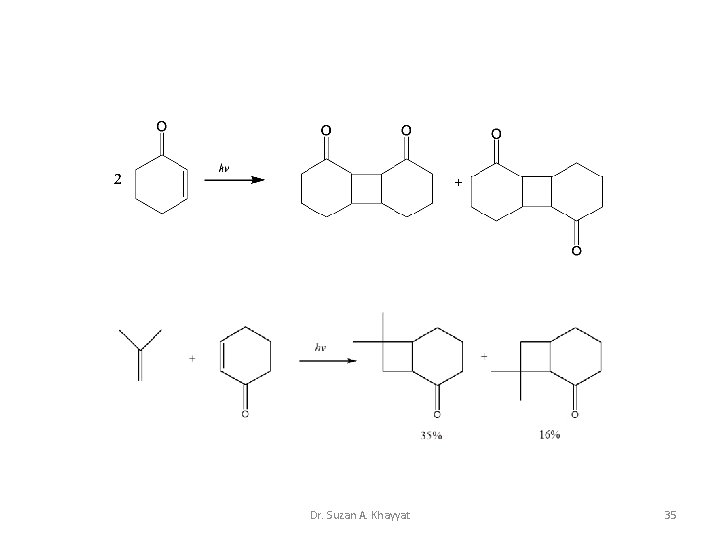
Photocycloaddition 2+2 Intermolecular cycloaddition Dr. Suzan A. Khayyat 33

Dr. Suzan A. Khayyat 34

Dr. Suzan A. Khayyat 35

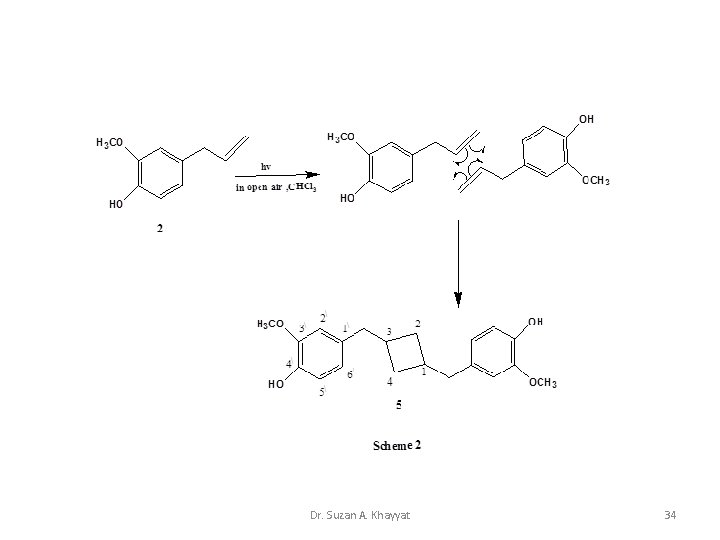
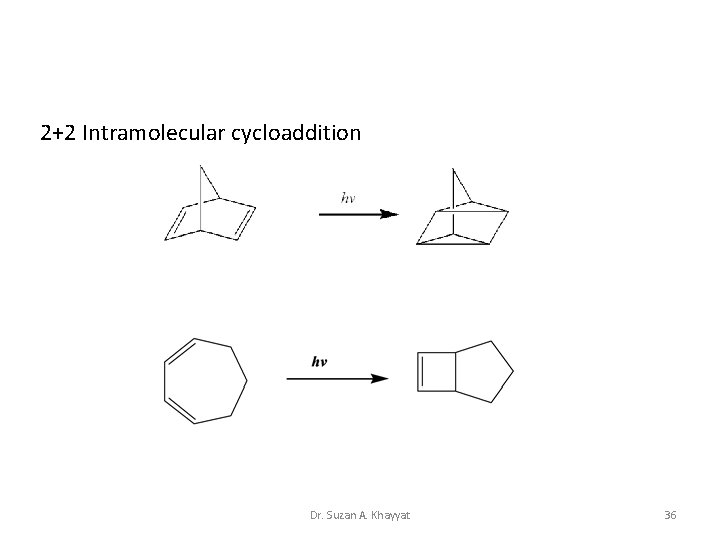
2+2 Intramolecular cycloaddition Dr. Suzan A. Khayyat 36

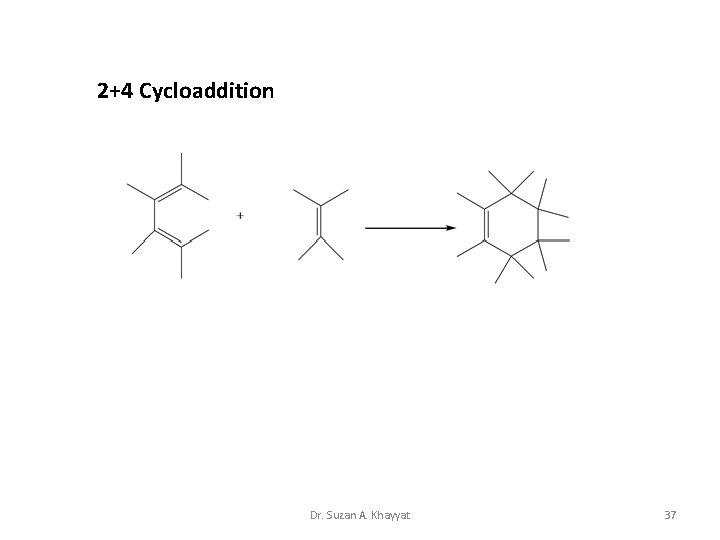
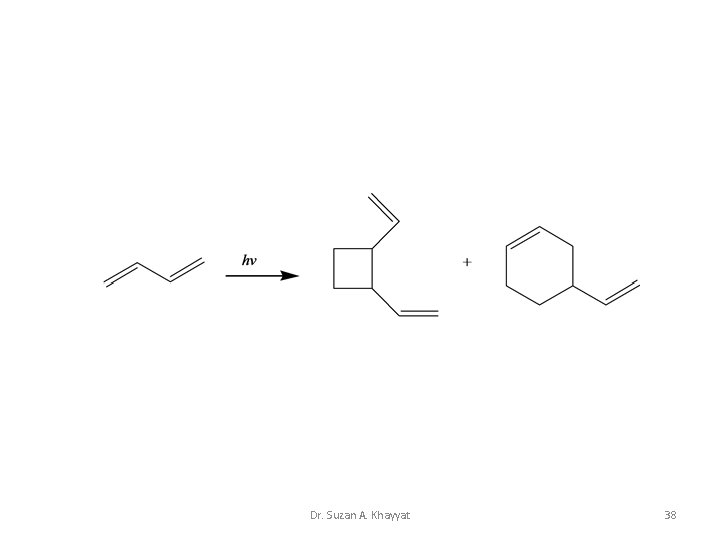
2+4 Cycloaddition Dr. Suzan A. Khayyat 37

Dr. Suzan A. Khayyat 38

Dr. Suzan A. Khayyat 39

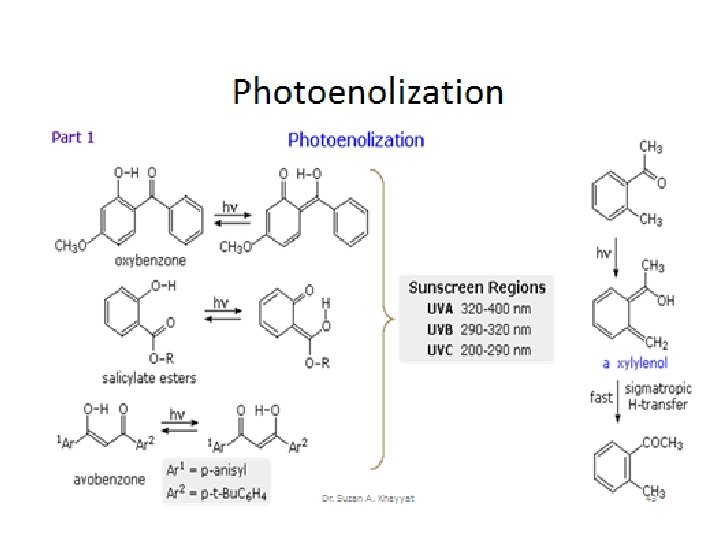
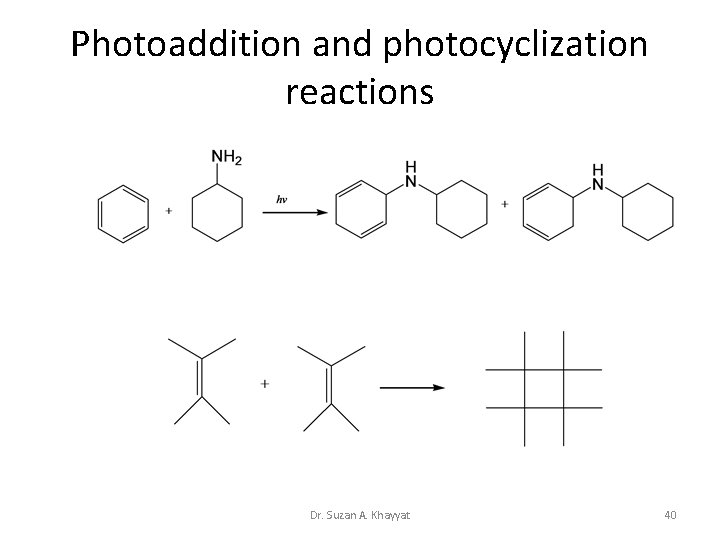
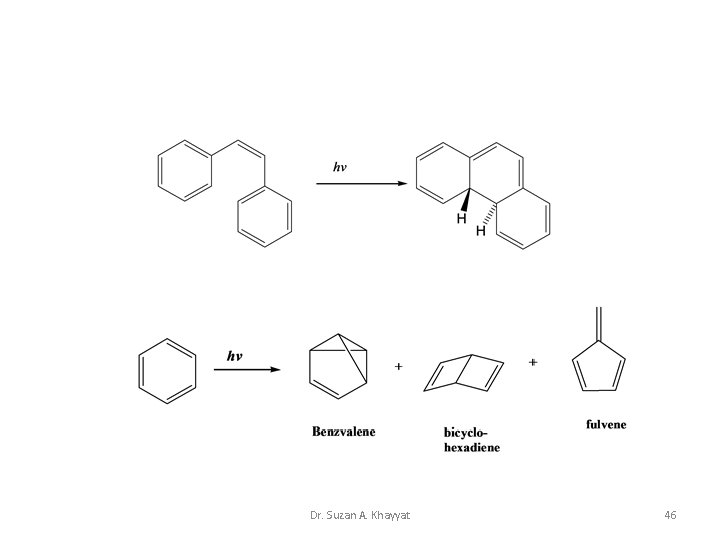
Photoaddition and photocyclization reactions Dr. Suzan A. Khayyat 40

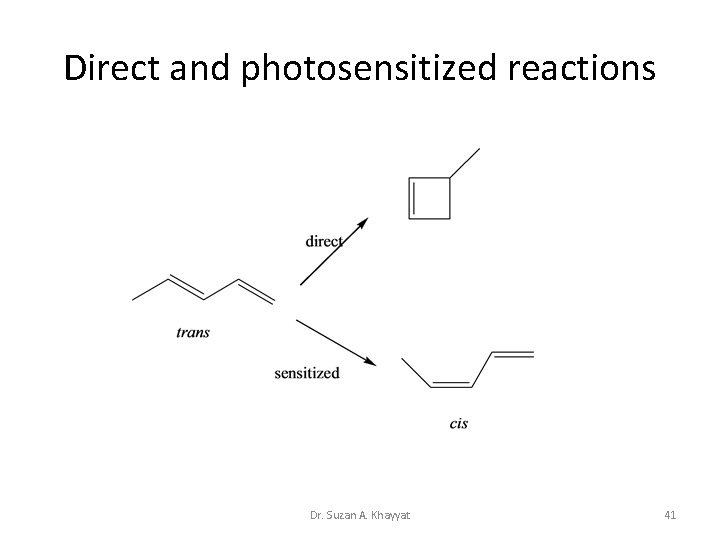
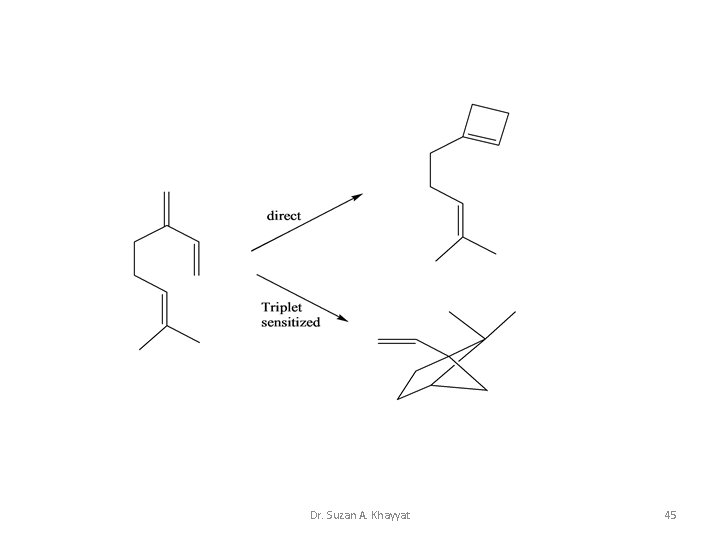
Direct and photosensitized reactions Dr. Suzan A. Khayyat 41

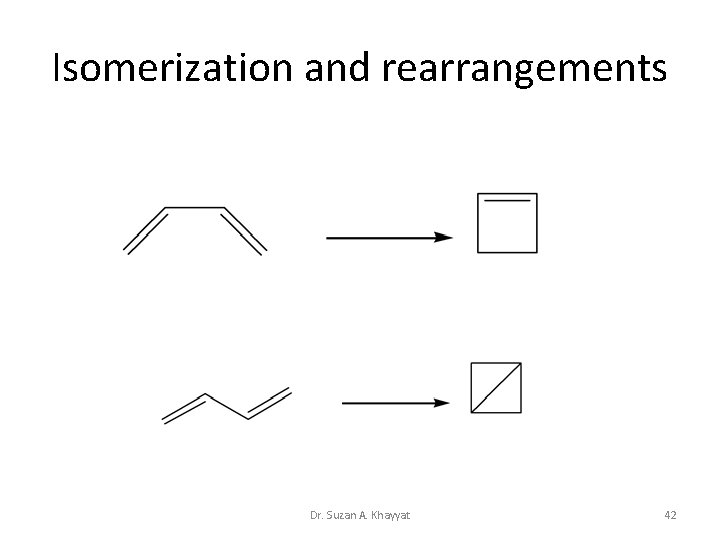
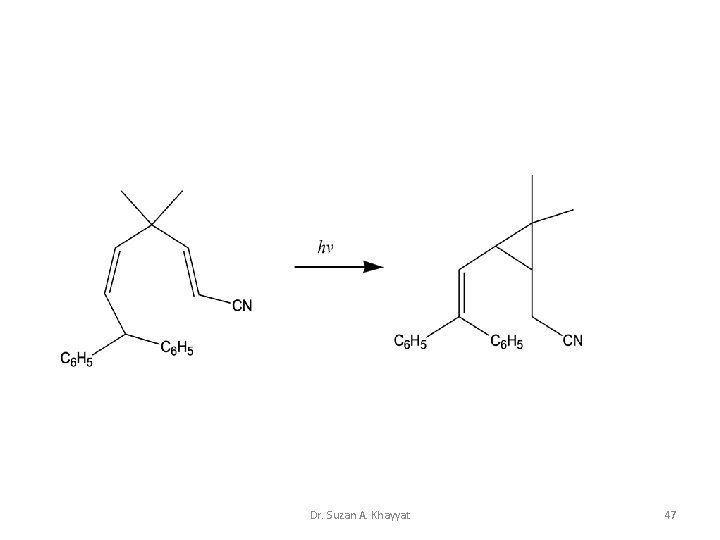
Isomerization and rearrangements Dr. Suzan A. Khayyat 42



Dr. Suzan A. Khayyat 45

Dr. Suzan A. Khayyat 46

Dr. Suzan A. Khayyat 47

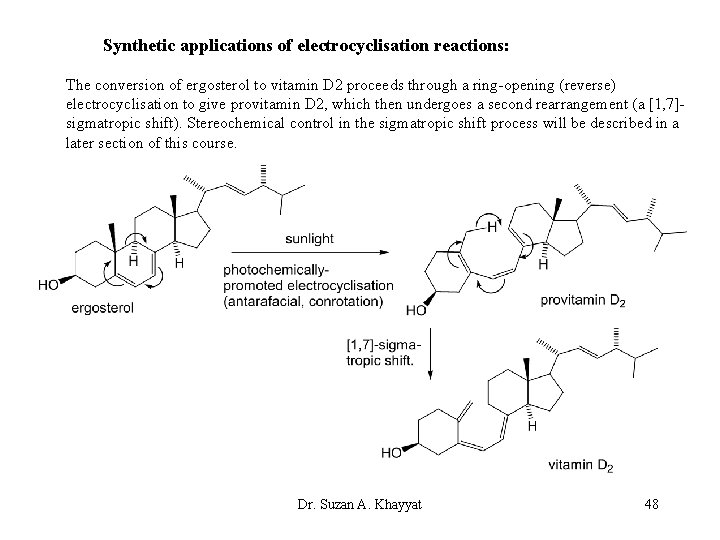
Synthetic applications of electrocyclisation reactions: The conversion of ergosterol to vitamin D 2 proceeds through a ring-opening (reverse) electrocyclisation to give provitamin D 2, which then undergoes a second rearrangement (a [1, 7]sigmatropic shift). Stereochemical control in the sigmatropic shift process will be described in a later section of this course. Dr. Suzan A. Khayyat 48

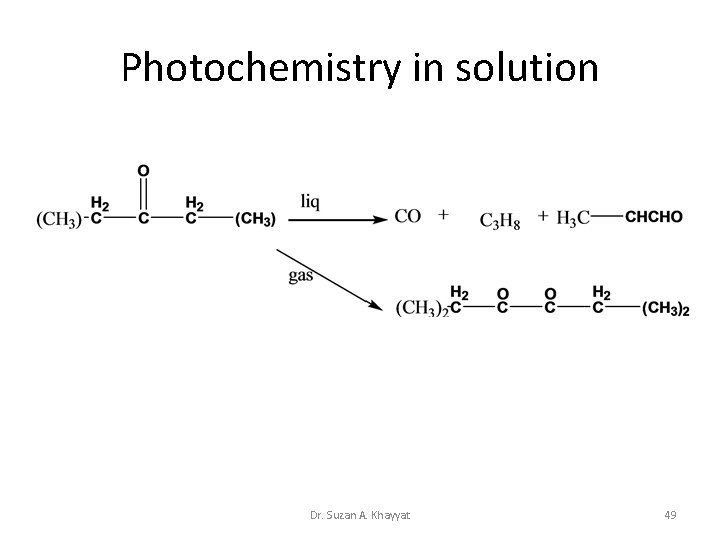
Photochemistry in solution Dr. Suzan A. Khayyat 49

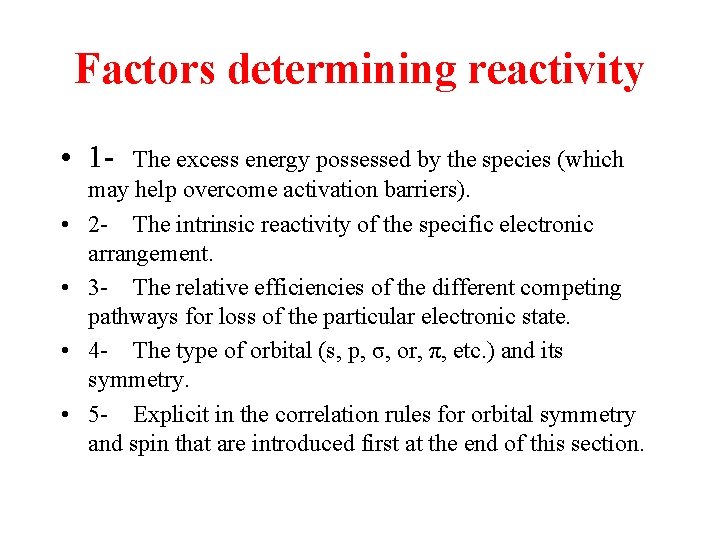
Factors determining reactivity • 1 • • The excess energy possessed by the species (which may help overcome activation barriers). 2 - The intrinsic reactivity of the specific electronic arrangement. 3 - The relative efficiencies of the different competing pathways for loss of the particular electronic state. 4 - The type of orbital (s, p, σ, or, π, etc. ) and its symmetry. 5 - Explicit in the correlation rules for orbital symmetry and spin that are introduced first at the end of this section.
 O ch
O ch Suzan van der lee
Suzan van der lee Suzan alteri
Suzan alteri Triage sieve and sort
Triage sieve and sort Suzan fischbein
Suzan fischbein Examples of physical changes
Examples of physical changes What is physical change and chemical change
What is physical change and chemical change Differences between chemical and physical changes
Differences between chemical and physical changes Physical change
Physical change Spare change physical versus chemical change
Spare change physical versus chemical change Whats a chemical change
Whats a chemical change When does a physical change occur study jams
When does a physical change occur study jams Physical or chemical change
Physical or chemical change Chopping wood chemical or physical change
Chopping wood chemical or physical change Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Love chemical formula
Love chemical formula Are kc and kp equal
Are kc and kp equal Chapter 15 energy and chemical change
Chapter 15 energy and chemical change Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chemical reactions study guide
Chapter 9 chemical reactions study guide Representative metal
Representative metal Is tearing paper a physical or chemical change
Is tearing paper a physical or chemical change Undesirable change
Undesirable change Is mixing kool aid a chemical change
Is mixing kool aid a chemical change Aluminum foil is cut in half physical or chemical change
Aluminum foil is cut in half physical or chemical change Separating sand from gravel physical or chemical change
Separating sand from gravel physical or chemical change When does puberty end
When does puberty end Blue color is physical or chemical
Blue color is physical or chemical The color of a sunset physical or chemical
The color of a sunset physical or chemical Physcial change
Physcial change Separating sand from gravel chemical or physical
Separating sand from gravel chemical or physical Is grating cheese a physical change
Is grating cheese a physical change Is sharpening a pencil a physical or chemical change
Is sharpening a pencil a physical or chemical change Observing chemical change
Observing chemical change Is milk becoming sour a chemical change
Is milk becoming sour a chemical change Quantitative chemistry grade 11
Quantitative chemistry grade 11 Chemical change grade 10
Chemical change grade 10 Is salt dissolving in water a chemical change or physical
Is salt dissolving in water a chemical change or physical Is the small intestine a physical or chemical change
Is the small intestine a physical or chemical change 5 general types of chemical reactions
5 general types of chemical reactions Example of a chemical change
Example of a chemical change Change in temperature chemical reaction example
Change in temperature chemical reaction example Is salt dissolving in water a chemical change or physical
Is salt dissolving in water a chemical change or physical Wood rots physical or chemical change
Wood rots physical or chemical change Indicators of chemical change
Indicators of chemical change Chemical change indicators
Chemical change indicators Whats the difference between a chemical and physical change
Whats the difference between a chemical and physical change Chemical vs physical change
Chemical vs physical change