GENERAL CHEMISTRY CHEM 110 REVISION CHAPTERS 14 15



![or Basicity p. H is inversely proportional to [H+] or Basicity p. H is inversely proportional to [H+]](https://slidetodoc.com/presentation_image_h2/c07a543eec40aa22200e10e7ba0a9a81/image-22.jpg)
![[1] Calculate the concentration of H+ ions in a 0. 54 M Na. OH [1] Calculate the concentration of H+ ions in a 0. 54 M Na. OH](https://slidetodoc.com/presentation_image_h2/c07a543eec40aa22200e10e7ba0a9a81/image-23.jpg)
![[4] Calculate the concentration of H+ ions in a 0. 8 M KOH solution? [4] Calculate the concentration of H+ ions in a 0. 8 M KOH solution?](https://slidetodoc.com/presentation_image_h2/c07a543eec40aa22200e10e7ba0a9a81/image-24.jpg)
![[7] The H+ in concentration of a rainwater is 2. 5× 10 -6 M. [7] The H+ in concentration of a rainwater is 2. 5× 10 -6 M.](https://slidetodoc.com/presentation_image_h2/c07a543eec40aa22200e10e7ba0a9a81/image-25.jpg)
- Slides: 25

GENERAL CHEMISTRY CHEM 110 REVISION CHAPTERS 14 &15

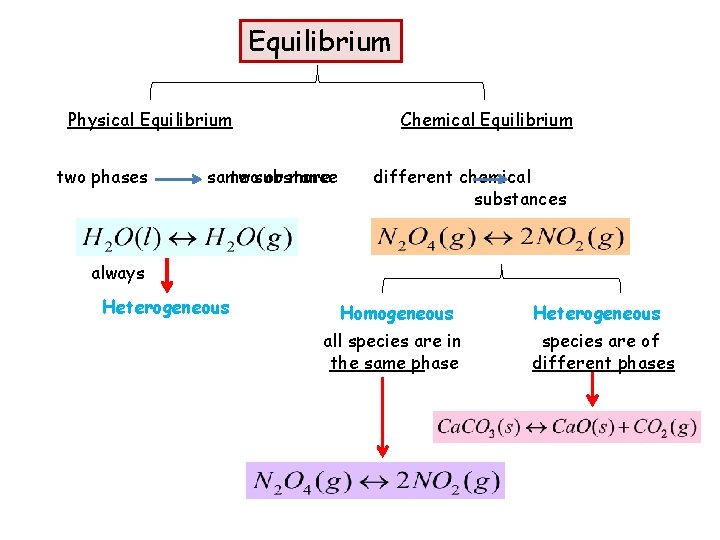
Equilibrium Physical Equilibrium two phases Chemical Equilibrium same twosubstance or more different chemical substances always Heterogeneous Homogeneous all species are in the same phase Heterogeneous species are of different phases

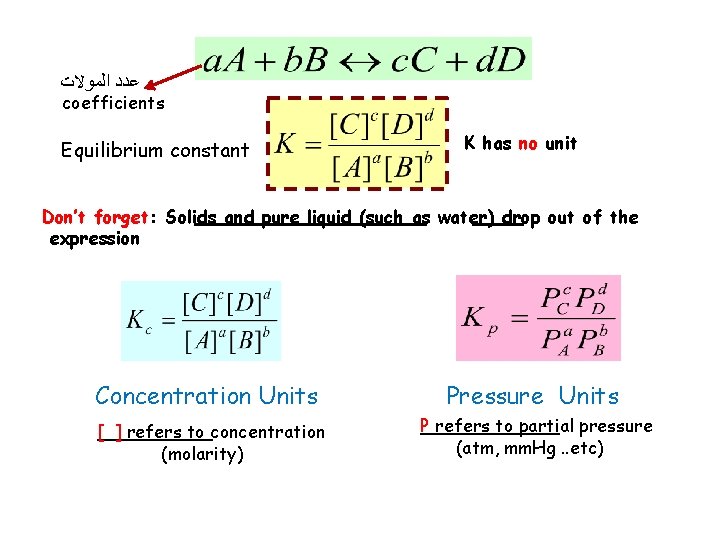
ﻋﺪﺩ ﺍﻟﻤﻮﻻﺕ coefficients Equilibrium constant K has no unit Don’t forget: Solids and pure liquid (such as water) drop out of the expression Concentration Units Pressure Units [ ] refers to concentration (molarity) P refers to partial pressure (atm, mm. Hg. . etc)

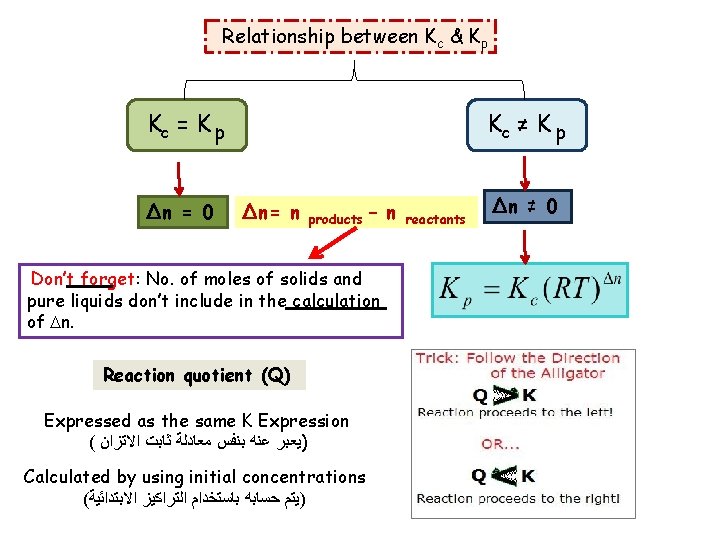
Relationship between Kc & Kp Kc = K p ∆n = 0 Kc ≠ K p ∆n= n products – n Don’t forget: No. of moles of solids and pure liquids don’t include in the calculation of n. Reaction quotient (Q) Expressed as the same K Expression ( )ﻳﻌﺒﺮ ﻋﻨﻪ ﺑﻨﻔﺲ ﻣﻌﺎﺩﻟﺔ ﺛﺎﺑﺖ ﺍﻻﺗﺰﺍﻥ Calculated by using initial concentrations ( )ﻳﺘﻢ ﺣﺴﺎﺑﻪ ﺑﺎﺳﺘﺨﺪﺍﻡ ﺍﻟﺘﺮﺍﻛﻴﺰ ﺍﻻﺑﺘﺪﺍﺋﻴﺔ reactants ∆n ≠ 0

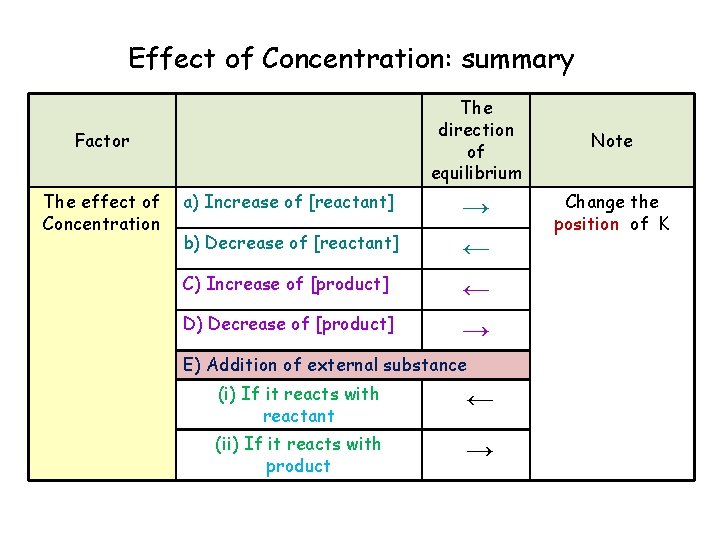
Effect of Concentration: summary The direction of equilibrium Factor The effect of Concentration a) Increase of [reactant] b) Decrease of [reactant] C) Increase of [product] D) Decrease of [product] → ← ← → E) Addition of external substance (i) If it reacts with reactant ← (ii) If it reacts with product → Note Change the position of K

Effect of Pressure (Only gases): summary* The direction of equilibrium Factor The effect of Pressure 1. Δn > 0 (a) Increase of total pressure ← → (b) Decrease of total pressure Note Change the position of K 2. Δn < 0 (a) Increase of total pressure → ← (b) Decrease of total pressure 3. Δn =0 No effect * Volume effects reverse the impact of pressure Pressure (P) Inversely ﻋﻜﺴﻴﺔ Volume (V) Directly ﻃﺮﺩﻳﺔ mole No. (n)

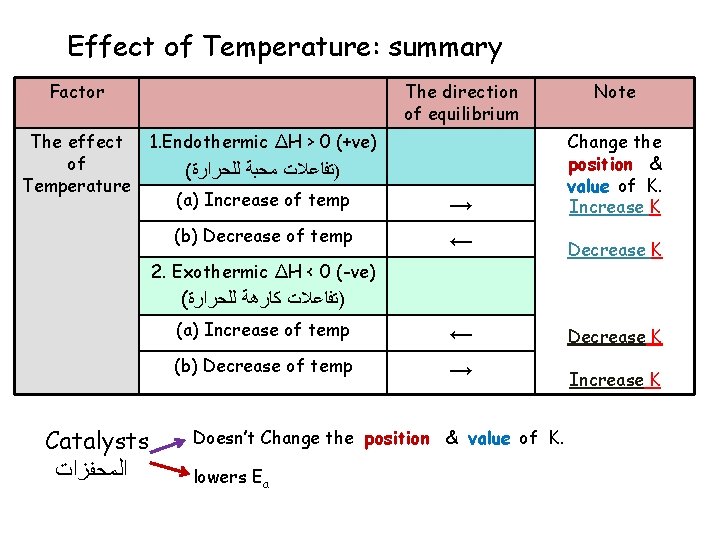
Effect of Temperature: summary Factor The effect of Temperature The direction of equilibrium 1. Endothermic ΔH > 0 (+ve) Note (a) Increase of temp → Change the position & value of K. Increase K (b) Decrease of temp ← Decrease K ( )ﺗﻔﺎﻋﻼﺕ ﻣﺤﺒﺔ ﻟﻠﺤﺮﺍﺭﺓ 2. Exothermic ΔH < 0 (-ve) ( )ﺗﻔﺎﻋﻼﺕ ﻛﺎﺭﻫﺔ ﻟﻠﺤﺮﺍﺭﺓ (a) Increase of temp (b) Decrease of temp Catalysts ﺍﻟﻤﺤﻔﺰﺍﺕ ← → Doesn’t Change the position & value of K. lowers Ea Decrease K Increase K

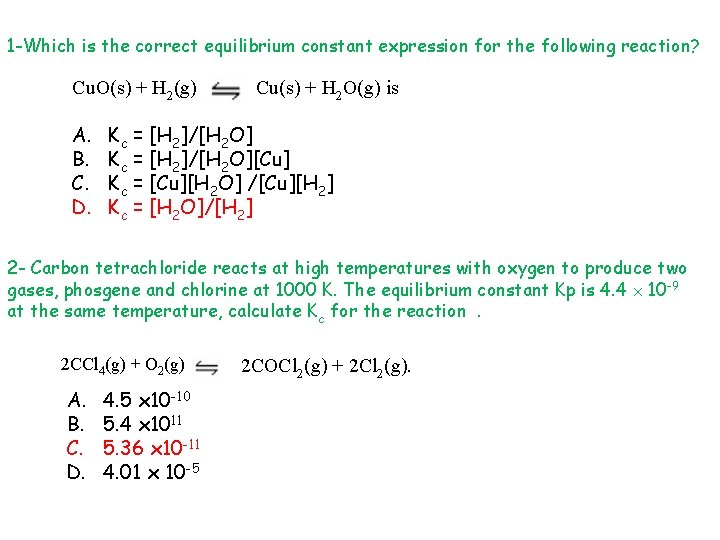
1 -Which is the correct equilibrium constant expression for the following reaction? Cu. O(s) + H 2(g) A. B. C. D. Cu(s) + H 2 O(g) is Kc = [H 2]/[H 2 O][Cu] Kc = [Cu][H 2 O] /[Cu][H 2] Kc = [H 2 O]/[H 2] 2 - Carbon tetrachloride reacts at high temperatures with oxygen to produce two gases, phosgene and chlorine at 1000 K. The equilibrium constant Kp is 4. 4 10 -9 at the same temperature, calculate Kc for the reaction. 2 CCl 4(g) + O 2(g) A. B. C. D. 4. 5 x 10 -10 5. 4 x 1011 5. 36 x 10 -11 4. 01 x 10 -5 2 COCl 2(g) + 2 Cl 2(g).

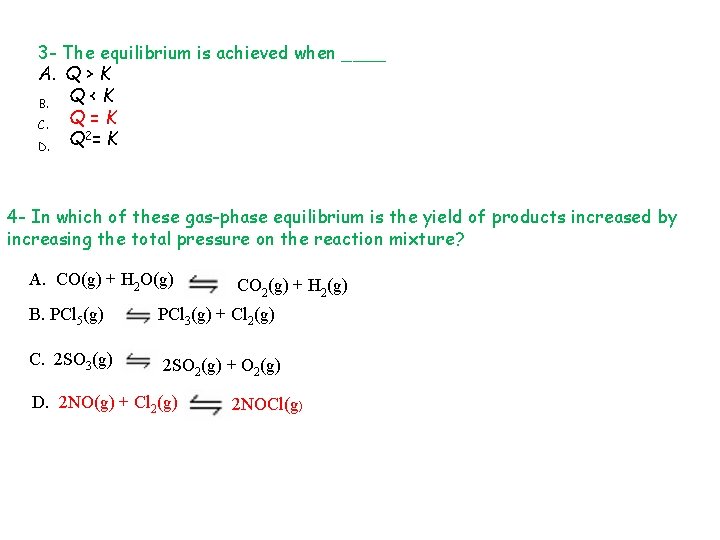
3 - The equilibrium is achieved when ____ A. Q > K Q<K B. Q=K C. Q 2= K D. 4 - In which of these gas-phase equilibrium is the yield of products increased by increasing the total pressure on the reaction mixture? A. CO(g) + H 2 O(g) CO 2(g) + H 2(g) B. PCl 5(g) PCl 3(g) + Cl 2(g) C. 2 SO 3(g) 2 SO 2(g) + O 2(g) D. 2 NO(g) + Cl 2(g) 2 NOCl(g)

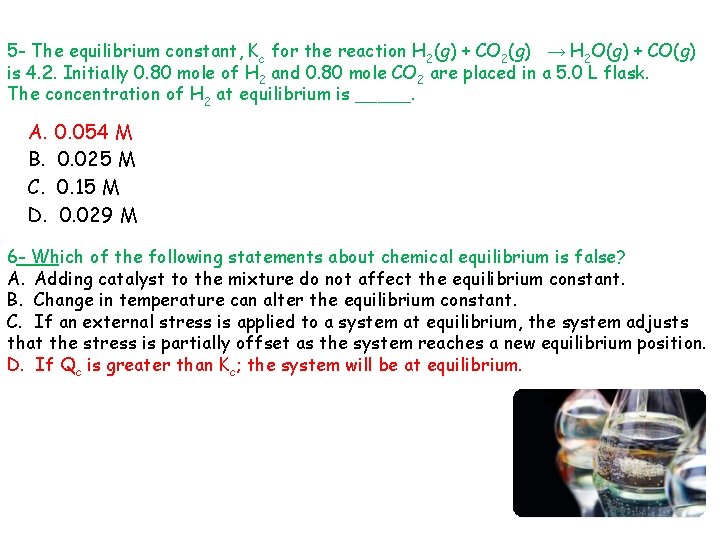
5 - The equilibrium constant, Kc for the reaction H 2(g) + CO 2(g) → H 2 O(g) + CO(g) is 4. 2. Initially 0. 80 mole of H 2 and 0. 80 mole CO 2 are placed in a 5. 0 L flask. The concentration of H 2 at equilibrium is _____. A. B. C. D. 0. 054 M 0. 025 M 0. 15 M 0. 029 M 6 - Which of the following statements about chemical equilibrium is false? A. Adding catalyst to the mixture do not affect the equilibrium constant. B. Change in temperature can alter the equilibrium constant. C. If an external stress is applied to a system at equilibrium, the system adjusts that the stress is partially offset as the system reaches a new equilibrium position. D. If Qc is greater than Kc; the system will be at equilibrium.

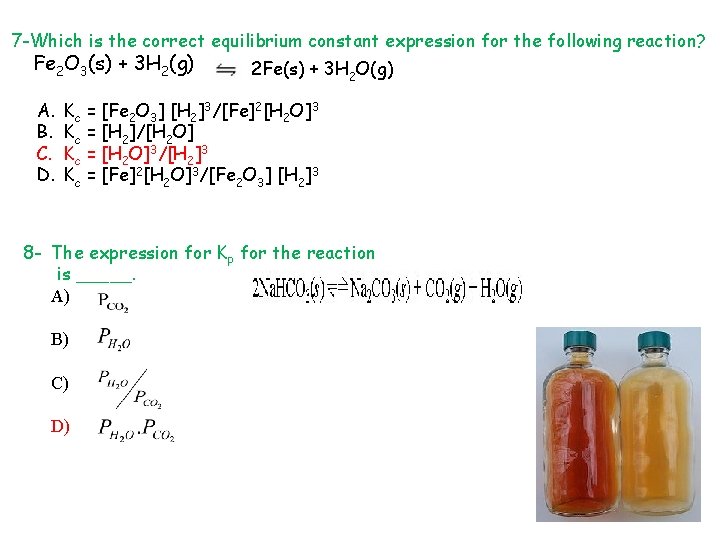
7 -Which is the correct equilibrium constant expression for the following reaction? Fe 2 O 3(s) + 3 H 2(g) A. B. C. D. 2 Fe(s) + 3 H 2 O(g) Kc = [Fe 2 O 3] [H 2]3/[Fe]2[H 2 O]3 Kc = [H 2]/[H 2 O] Kc = [H 2 O]3/[H 2]3 Kc = [Fe]2[H 2 O]3/[Fe 2 O 3] [H 2]3 8 - The expression for Kp for the reaction is _____. A) B) C) D)

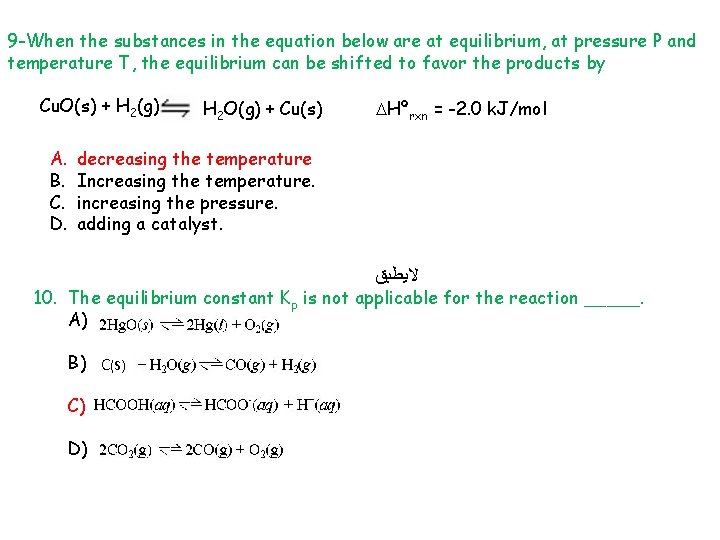
9 -When the substances in the equation below are at equilibrium, at pressure P and temperature T, the equilibrium can be shifted to favor the products by Cu. O(s) + H 2(g) A. B. C. D. H 2 O(g) + Cu(s) Hºrxn = -2. 0 k. J/mol decreasing the temperature Increasing the temperature. increasing the pressure. adding a catalyst. ﻻﻳﻄﺒﻖ 10. The equilibrium constant Kp is not applicable for the reaction _____. A) B) C(S) C) D)

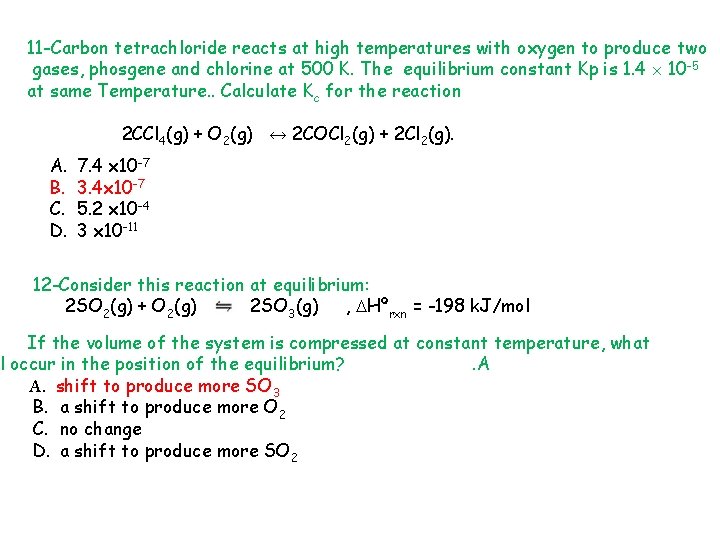
11 -Carbon tetrachloride reacts at high temperatures with oxygen to produce two gases, phosgene and chlorine at 500 K. The equilibrium constant Kp is 1. 4 10 -5 at same Temperature. . Calculate Kc for the reaction 2 CCl 4(g) + O 2(g) ↔ 2 COCl 2(g) + 2 Cl 2(g). A. B. C. D. 7. 4 x 10 -7 3. 4 x 10 -7 5. 2 x 10 -4 3 x 10 -11 12 -Consider this reaction at equilibrium: 2 SO 2(g) + O 2(g) 2 SO 3(g) , Hºrxn = -198 k. J/mol If the volume of the system is compressed at constant temperature, what l occur in the position of the equilibrium? . A A. shift to produce more SO 3 B. a shift to produce more O 2 C. no change D. a shift to produce more SO 2

13 -When the following reaction is at equilibrium, which of these relationships is true at equilibrium? 2 NO(g) + Cl 2(g) 2 NOCl(g) A. B. C. D. [NO]2[Cl 2]2 = [NOCl]2 PNOCl / P NO PCl [NO]2[Cl 2] = Kc[NOCl]2 P 2 NO / P 2 NOCl 14 - Kc is 3. 8 10 -5 at 1000 K for the equilibrium I 2(g) ↔ 2 I(g) Starting with 0. 0456 moles of I 2 in a 2. 30 L flask, at 1000 K. The equilibrium concentration of I 2 is: A. B. C. D. 1. 0954 M 0. 0194 M 0. 12 M 0. 081 M

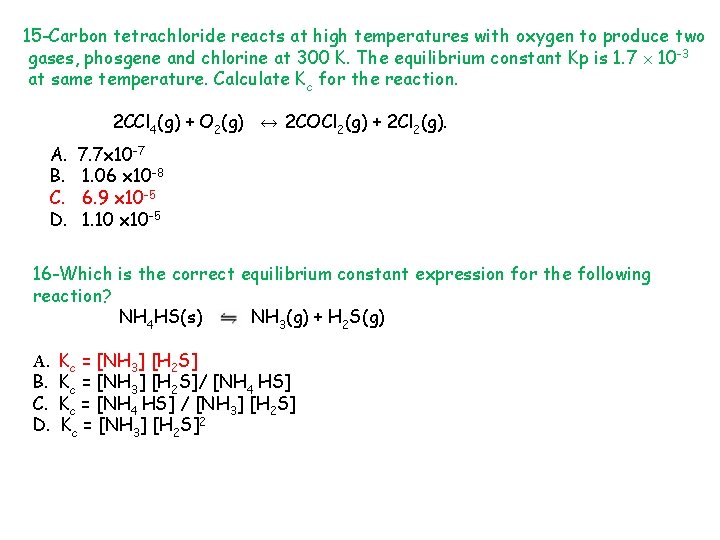
15 -Carbon tetrachloride reacts at high temperatures with oxygen to produce two gases, phosgene and chlorine at 300 K. The equilibrium constant Kp is 1. 7 10 -3 at same temperature. Calculate Kc for the reaction. 2 CCl 4(g) + O 2(g) ↔ 2 COCl 2(g) + 2 Cl 2(g). A. B. C. D. 7. 7 x 10 -7 1. 06 x 10 -8 6. 9 x 10 -5 1. 10 x 10 -5 16 -Which is the correct equilibrium constant expression for the following reaction? NH 4 HS(s) NH 3(g) + H 2 S(g) A. B. C. D. Kc = [NH 3] [H 2 S]/ [NH 4 HS] Kc = [NH 4 HS] / [NH 3] [H 2 S] Kc = [NH 3] [H 2 S]2

17 -For the equilibrium reaction 2 SO 2(g) + O 2(g) ↔ 2 SO 3(g), Hºrxn = -198 k. J/mol. Which one of these factors would cause the equilibrium constant to increase? A. B. C. D. Add SO 2 gas. Remove O 2 gas. Decrease the temperature. Increase the temperature. 18 -The reaction in which increased pressure has no affect on the equilibrium reaction is A. B. C. D. N 2(g)+3 H 2(g)↔ 2 NH 3(g) 2 H 2+CO(g)↔CH 3 OH(l) CO(g)+H 2 O(g)↔CO 2 (g)+H 2(g) Ca. CO 3(s)↔Ca. O(s)+CO 2 (g)

19 -Consider the reaction 2 NO(g)+O 2(g)↔ 2 NO 2(g) At 4300 C, an equilibrium mixture consist of 0. 020 mole of O 2, 0. 040 mole of NO, and 0. 96 mole of NO 2. calculate Kp for the reaction, given that the total pressure is 0. 20 atm. A. 1. 5× 10 -5 B. 150 C. 1. 32× 103 D. 1. 5× 105 148512. 83 =1. 5× 105 20 -Consider the reaction H 2(g)+Cl 2(g)↔ 2 HCl(g) At 2800 0 C, an equilibrium mixture consist of 0. 57 mole of H 2, 3. 39 mole of HCl, and 0. 1 mole of Cl 2. calculate Kp for the reaction, given that the total pressure is 2. 00 atm. A. 203 B. 2. 03 C. 20. 3 D. 2. 03× 103

21 -For the following reaction at equilibrium in a reaction vessel, which one of these stresses would cause the Br 2 concentration to increase. 2 NOBr(g)↔ 2 NO(g)+Br 2(g) ∆H 0=70 KJ/mol A-Lowering the temperature B-Removing some NOBr C-Increasing the temperature D- Increasing the pressure of the system 22 -The substances in the equation below are at equilibrium. The equilibrium can be shifted to favor the products by: Cu. O(s)+H 2(g)↔H 2 O(g)+Cu(s) A-decreasing the temperature B-Removing some Cu. O C-Increasing the temperature D- adding a catalyst ∆H 0= -2. 0 KJ/mol

23. The equation relating Kp and Kc is _____. A) B) C) D) Kp = Kc (RT) n Kp = Kc RT n Kc = Kp (RT) n 24. Kp will be equal to Kc if _____. A. B. C. D. n = 1 n = 0 RT = 0 n = ¥ 25. Kp = Kc for the reaction _____. A) B) C) D)

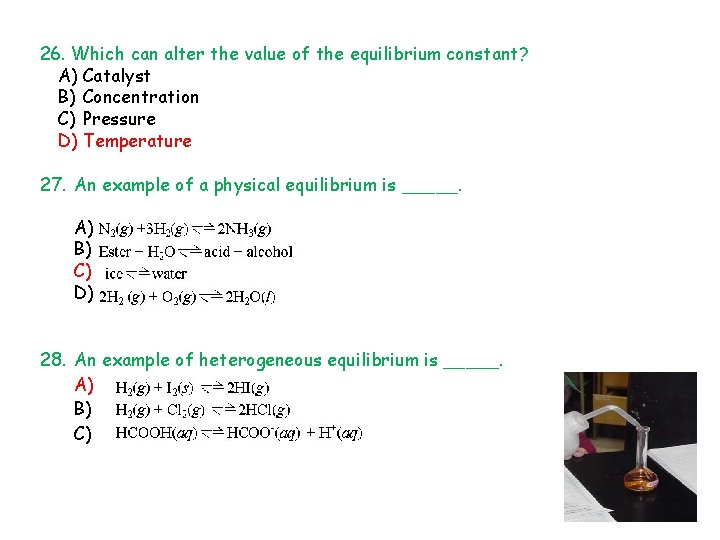
26. Which can alter the value of the equilibrium constant? A) Catalyst B) Concentration C) Pressure D) Temperature 27. An example of a physical equilibrium is _____. A) B) C) D) 28. An example of heterogeneous equilibrium is _____. A) B) C)

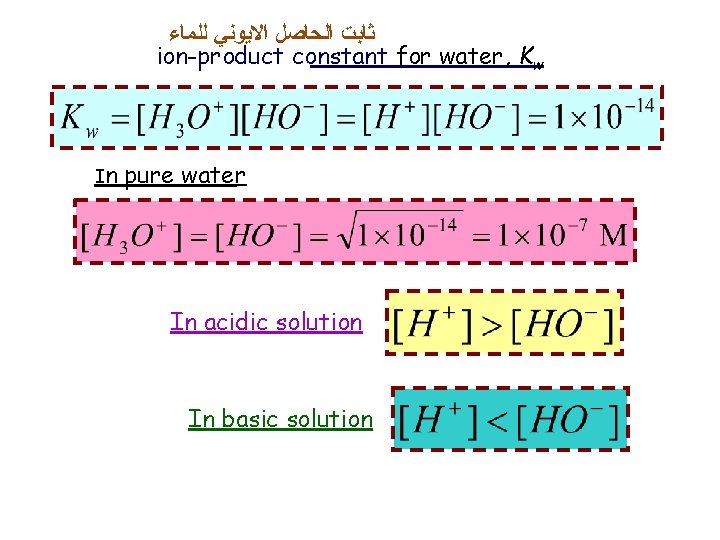
ﺛﺎﺑﺖ ﺍﻟﺤﺎﺻﻞ ﺍﻻﻳﻮﻧﻲ ﻟﻠﻤﺎﺀ ion-product constant for water, Kw In pure water In acidic solution In basic solution
![or Basicity p H is inversely proportional to H or Basicity p. H is inversely proportional to [H+]](https://slidetodoc.com/presentation_image_h2/c07a543eec40aa22200e10e7ba0a9a81/image-22.jpg)
or Basicity p. H is inversely proportional to [H+]
![1 Calculate the concentration of H ions in a 0 54 M Na OH [1] Calculate the concentration of H+ ions in a 0. 54 M Na. OH](https://slidetodoc.com/presentation_image_h2/c07a543eec40aa22200e10e7ba0a9a81/image-23.jpg)
[1] Calculate the concentration of H+ ions in a 0. 54 M Na. OH solution? A. B. C. D. [H+] = 8. 5 x 10 -14 M [H+] = 1. 05 x 10 -11 M [H+] = 3. 02 x 10 -4 M [H+] = 1. 85 x 10 -14 M [2] The H+ in concentration of a blood sample is 1. 5× 10 -7 M. What is the p. H of the blood? A. 6. 82 B. 1. 35 C. 13 D. 7 [3] The p. H of a 1. 0 10– 7 M aqueous HCl solution will be _____. A. 7. 2 B. 7. 0 C. 6. 8 D. 4. 2
![4 Calculate the concentration of H ions in a 0 8 M KOH solution [4] Calculate the concentration of H+ ions in a 0. 8 M KOH solution?](https://slidetodoc.com/presentation_image_h2/c07a543eec40aa22200e10e7ba0a9a81/image-24.jpg)
[4] Calculate the concentration of H+ ions in a 0. 8 M KOH solution? A. B. C. D. [H+] = 1. 25 x 10 -14 M [H+] = 1. 05 x 10 -14 M [H+] = 1. 8 x 10 -11 M [H+] = 3. 02 x 10 -4 M [5] Which of the following statements is true for a 1. 0 M solution of a strong acid HA? A. [A–] > [H+] B. [HA] = 1. 0 M C. p. H = 1 D. p. H = 0 [6] Calculate the concentration of H A. B. C. D. [H+] = 2. 5 x 10 -14 M [H+] = 8 x 10 -11 M [H+] = 5 x 10 -14 M [H+] = 4. 2 x 10 -4 M + ions in a 0. 2 M KOH solution?
![7 The H in concentration of a rainwater is 2 5 10 6 M [7] The H+ in concentration of a rainwater is 2. 5× 10 -6 M.](https://slidetodoc.com/presentation_image_h2/c07a543eec40aa22200e10e7ba0a9a81/image-25.jpg)
[7] The H+ in concentration of a rainwater is 2. 5× 10 -6 M. What is the p. H of the rainwater? A. B. C. D. 4. 82 1. 35 5. 6 7. 2 [8] Calculate the concentration of H+ ions in a 0. 3 M Na. OH solution? A. B. C. D. [H+] = 1. 6 x 10 -14 M [H+] = 9. 1 x 10 -2 M [H+] = 3. 33 x 10 -14 M [H+] = 3. 02 x 10 -4 M [9] Calculate the H A. B. C. D. 4. 0 x 10 -2 M 250 M 0. 38 M 4. 0 x 10 -3 M + ions concentration in lemon juice having a p. H of 2. 4
 Où se trouve le numéro d'affiliation mutuelle vignette ?
Où se trouve le numéro d'affiliation mutuelle vignette ? 011 101 110
011 101 110 Active revision vs passive revision
Active revision vs passive revision Chemistry 110
Chemistry 110 Analytical chemistry chapter 1
Analytical chemistry chapter 1 Phy 1214
Phy 1214 General revision of assessments and property classification
General revision of assessments and property classification Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 General chemistry ders notları
General chemistry ders notları General chemistry
General chemistry General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry Planos en cinematografia
Planos en cinematografia Where did general lee surrender to general grant?
Where did general lee surrender to general grant? Iannonechem
Iannonechem Unit 7 ap chemistry
Unit 7 ap chemistry Concept of imfa
Concept of imfa