General Chemistry I Chem 110 Dr Laila Mohammed










![Temperature Scales � Fahrenheit °F →°F = [ (9/5) × °C] + 32 � Temperature Scales � Fahrenheit °F →°F = [ (9/5) × °C] + 32 �](https://slidetodoc.com/presentation_image_h/dfbba92c5ee83b37ba9f3a51981830df/image-25.jpg)


- Slides: 31

General Chemistry I Chem 110 Dr. Laila Mohammed Al-Harbi Assistant professor Contact Info: Lalhrbi@kau. edu. sa Web Site: http: //lalhrbi. kau. edu. sa

Technicalities Class hours: • SMW 8: 00 am to 8: 50 am (AA) � SMW 10: 00 am to 10: 50 am (AE) � Locations: ◦ Science tower 07 room 173 first floor ◦ phone 6400000 ext. 23024 ◦ email Lalhrbi@kau. edu. sa ◦ web site: http: //lalhrbi. kau. edu. sa � Exam schedule: � 1 st exam : from lecture 1 -11 ( Chapters 1 -4) = 30 marks � 2 nd exam: from lecture 12 -24 (Chapters 5, 7 -9) = 30 marks � Final exam: from lecture 1 -33 = 40 marks � (Chapters 1, 2, 3, 4, 5, 7, 8, 9, 10, 14, 15, 24, 25) � Home works = Bonus marks •


CHEM 110 1 Course No. Course Title Chem 110 General Chemistry I No. of Units Th. Pr. Credit 3 3 Pre-requisites - Course Objectives: The course aims to introduce students to basic knowledge and principle in chemistry. Course Description : It provides an introduction to the general principles of chemistry for students planning a professional career in chemistry, a related science, the health professions, or engineering. By the end of this course the student will be able to understand the following: Significant figures, scientific notation and units, stoichiometry, atomic structure & periodic table, chemical bonding, gases, ionic equilibrium, basic principles of organic and basic principles of biochemistry. Main text books: § Chemistry, by Chang, 10 th. ed. , 2007, Mc. Graw-Hill. § Chemistry, by Steven S. Zumdahl, 6 th ed. , Houghton Mifflin College Div. Subsidiary books : Chemistry, by Mortimer, 6 th ed. , Wadsworth Inc.

CHEM 110 Main text book : § Chemistry, by Chang, 10 th. ed. , 2007, Mc. Graw. Hill.






Chapter 1 The study of change � 1 -7 Measurement v v SI units Mass and weight Volume Temperature scales Ø Homework Page 33 1. 17, 1. 19, 1. 22, 1. 23, 1. 25, 1. 26.

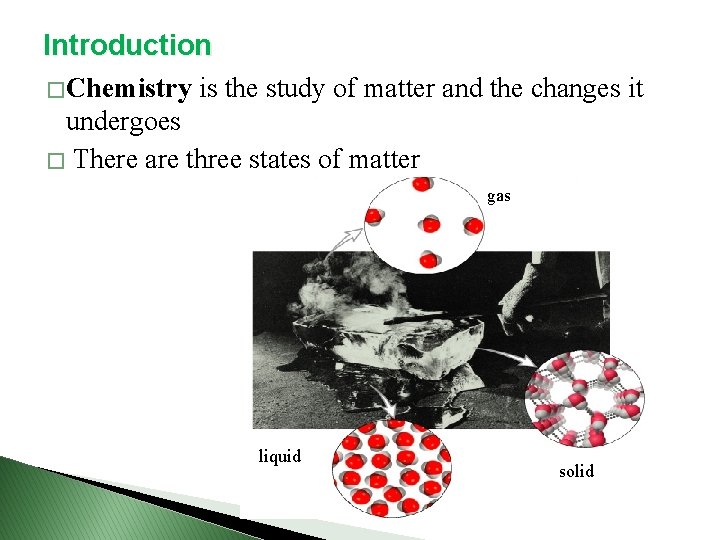
Introduction � Chemistry is the study of matter and the changes it undergoes � There are three states of matter gas liquid solid

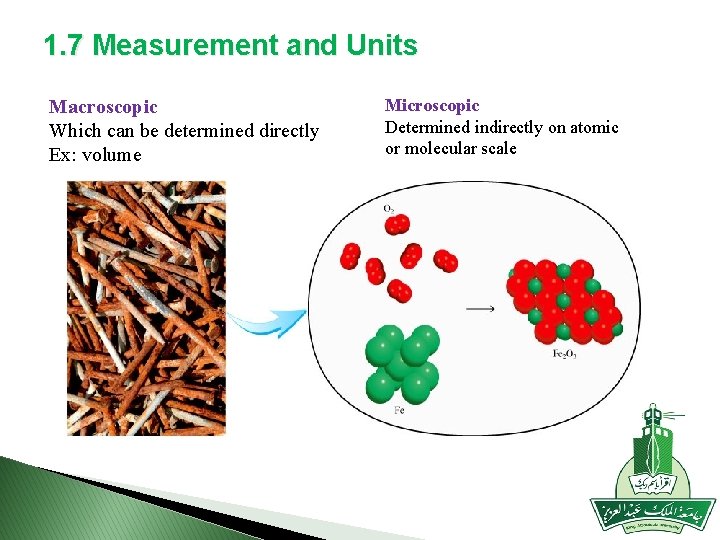
1. 7 Measurement and Units Macroscopic Which can be determined directly Ex: volume Microscopic Determined indirectly on atomic or molecular scale

Extensive and Intensive Properties An extensive property of a material depends upon how much matter is being considered. • mass • length • volume An intensive property of a material does not depend upon how much matter is being considered. • density • temperature • color

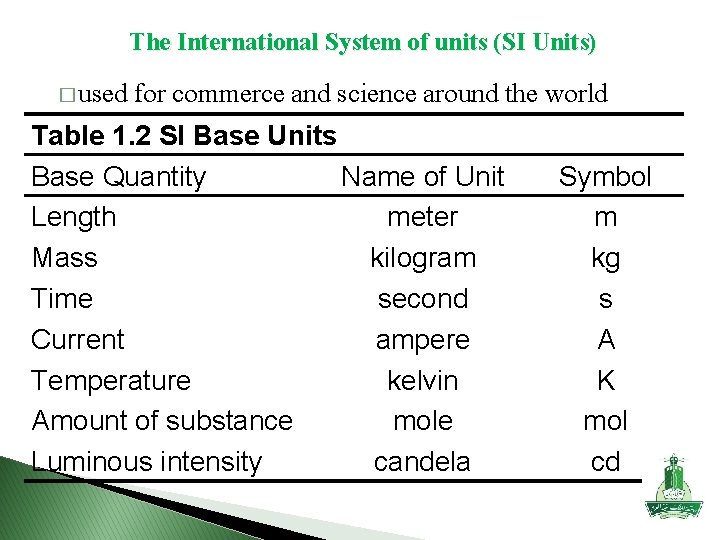
The International System of units (SI Units) � used for commerce and science around the world Table 1. 2 SI Base Units Base Quantity Name of Unit Length meter Mass kilogram Time second Current ampere Temperature kelvin Amount of substance mole Luminous intensity candela Symbol m kg s A K mol cd

m

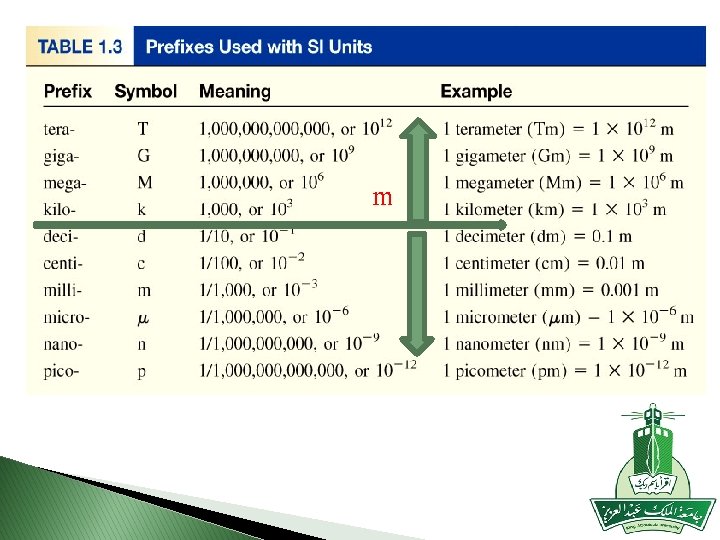
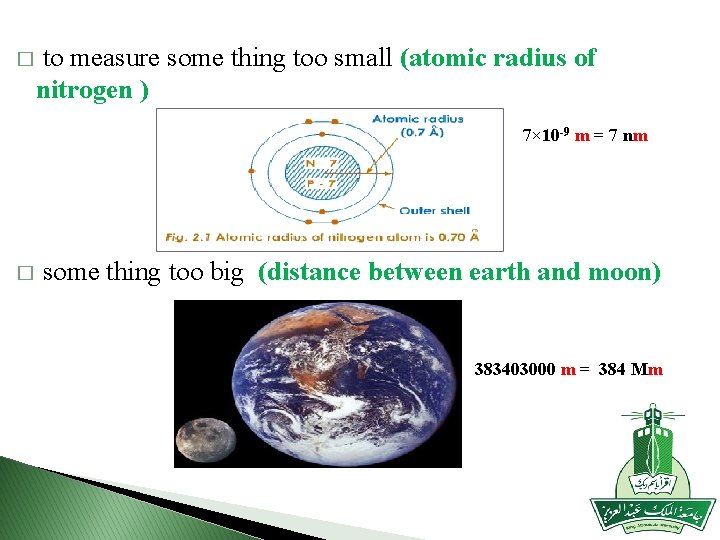
� to measure some thing too small (atomic radius of nitrogen ) 7× 10 -9 m = 7 nm � some thing too big (distance between earth and moon) 383403000 m = 384 Mm

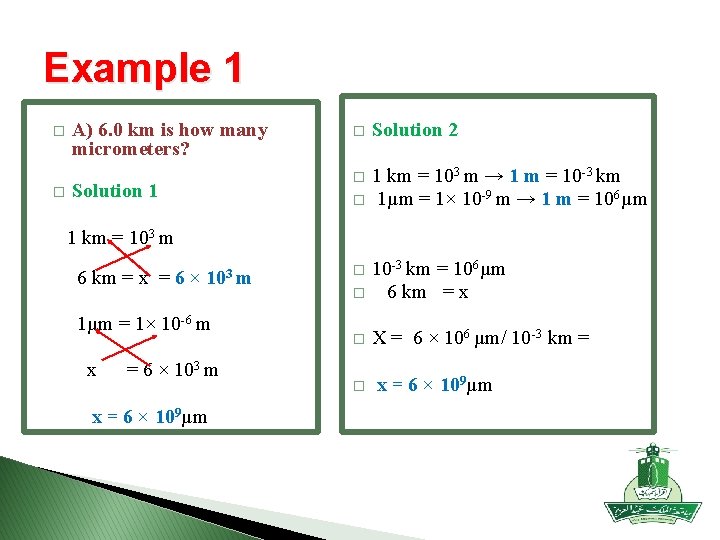
Example 1 � � A) 6. 0 km is how many micrometers? Solution 1 � Solution 2 � 1 km = 103 m → 1 m = 10 -3 km 1µm = 1× 10 -9 m → 1 m = 106µm � 1 km = 103 m 6 km = x = 6 × 103 m 1µm = 1× 10 -6 m x = 6 × 103 m x = 6 × 109µm � 10 -3 km = 106µm 6 km = x � X = 6 × 106 µm/ 10 -3 km = � x = 6 × 109µm �

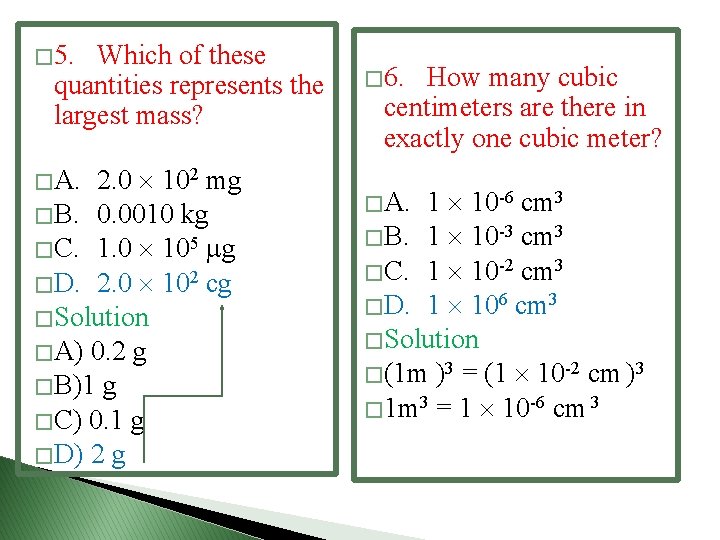
� Examples 2 � Examples 3 � The diameter of an atom is approximately 1 10 -7 mm. What is this diameter when expressed in nanometers? � A. 1 10 -18 nm � B. 1 10 -15 nm � C. 1 10 -9 nm � D. 1 10 -1 nm � = 1× 10 -7 × 106 = � 1 × 10 -1 nm = 0. 1 nm � Which of these quantities represents the largest mass? � A. 2. 0 102 mg � B. 0. 0010 kg � C. 1. 0 105 g � D. 2. 0 102 cg � Put all of them in the same unit

Mass and Weight � Mass is the measure of the amount of matter in an object. SI unit of mass is the kilogram (kg) 1 kg = 1000 g = 1 x 103 g � Weight is the measurement of the pull of gravity on an object. weight = c x mass The Mass of an object doesn't change when an object's location changes. Weight, on the other hand does change The weight of man on earth is 50 pounds. with location. is 8. 25 pounds on moon � Chemist are interested primarily in mass �

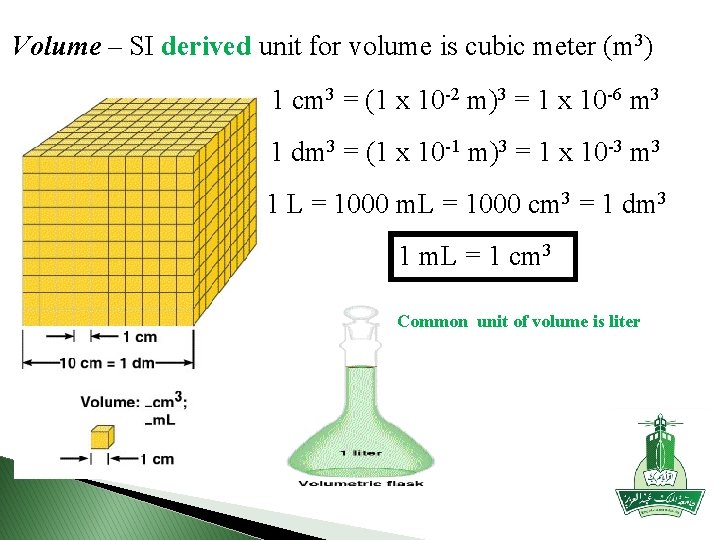
Volume – SI derived unit for volume is cubic meter (m 3) 1 cm 3 = (1 x 10 -2 m)3 = 1 x 10 -6 m 3 1 dm 3 = (1 x 10 -1 m)3 = 1 x 10 -3 m 3 1 L = 1000 m. L = 1000 cm 3 = 1 dm 3 1 m. L = 1 cm 3 Common unit of volume is liter

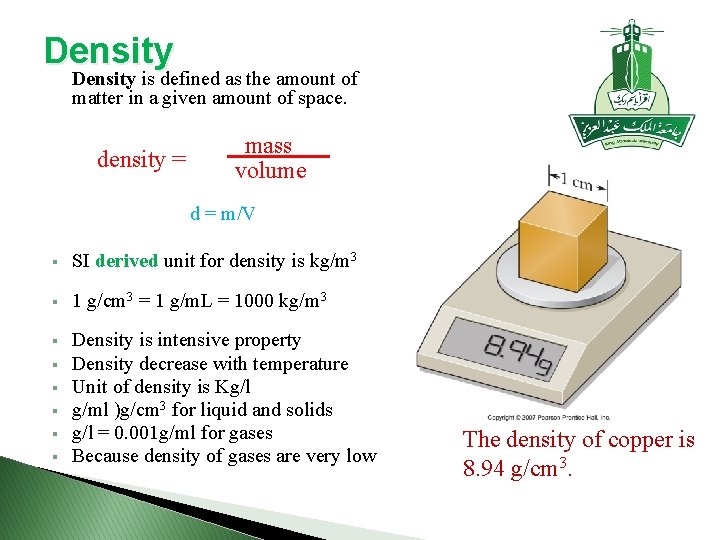
Density is defined as the amount of matter in a given amount of space. density = mass volume d = m/V § SI derived unit for density is kg/m 3 § 1 g/cm 3 = 1 g/m. L = 1000 kg/m 3 § Density is intensive property Density decrease with temperature Unit of density is Kg/l g/ml )g/cm 3 for liquid and solids g/l = 0. 001 g/ml for gases Because density of gases are very low § § § The density of copper is 8. 94 g/cm 3.

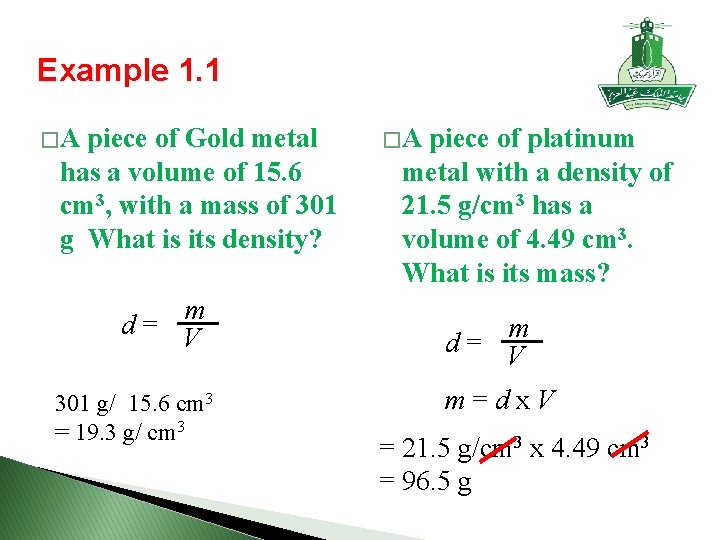
Example 1. 1 � A piece of Gold metal has a volume of 15. 6 cm 3, with a mass of 301 g What is its density? m d = V 301 g/ 15. 6 cm 3 = 19. 3 g/ cm 3 � A piece of platinum metal with a density of 21. 5 g/cm 3 has a volume of 4. 49 cm 3. What is its mass? m d = V m = d x V = 21. 5 g/cm 3 x 4. 49 cm 3 = 96. 5 g

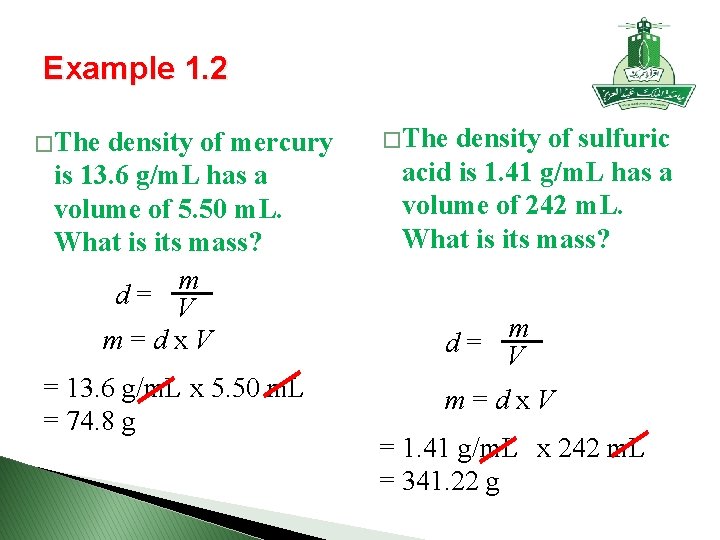
Example 1. 2 � The density of mercury is 13. 6 g/m. L has a volume of 5. 50 m. L. What is its mass? m d = V m = d x V = 13. 6 g/m. L x 5. 50 m. L = 74. 8 g � The density of sulfuric acid is 1. 41 g/m. L has a volume of 242 m. L. What is its mass? m d = V m = d x V = 1. 41 g/m. L x 242 m. L = 341. 22 g
![Temperature Scales Fahrenheit F F 95 C 32 Temperature Scales � Fahrenheit °F →°F = [ (9/5) × °C] + 32 �](https://slidetodoc.com/presentation_image_h/dfbba92c5ee83b37ba9f3a51981830df/image-25.jpg)
Temperature Scales � Fahrenheit °F →°F = [ (9/5) × °C] + 32 � Celsius °C → °C = (5/9) (°F - 32) � Kelvin ° K → ° K = °C + 273. 15

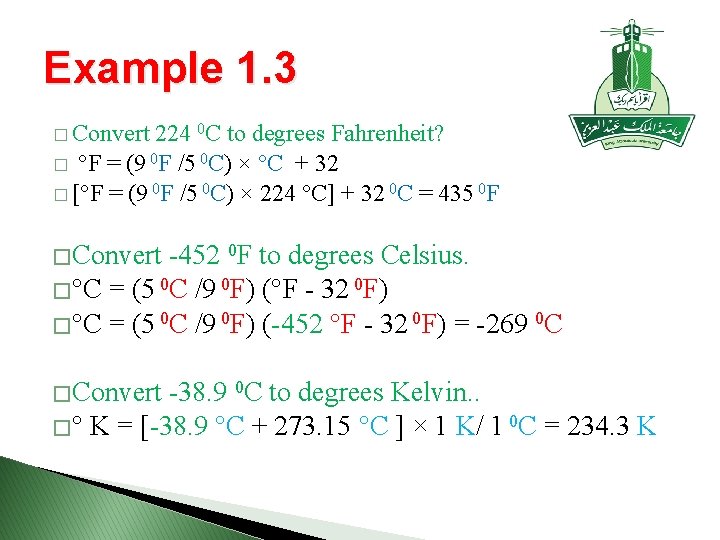
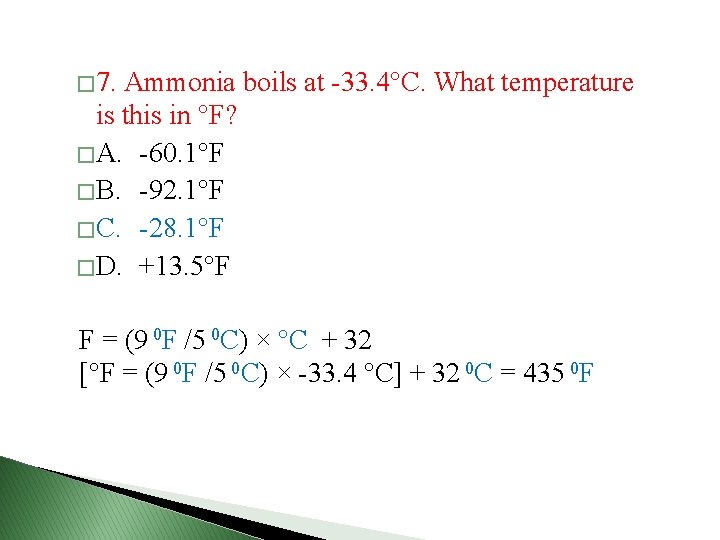
Example 1. 3 � Convert 224 0 C to degrees Fahrenheit? � °F = (9 0 F /5 0 C) × °C + 32 � [°F = (9 0 F /5 0 C) × 224 °C] + 32 0 C = 435 0 F � Convert -452 0 F to degrees Celsius. � °C = (5 0 C /9 0 F) (°F - 32 0 F) � °C = (5 0 C /9 0 F) (-452 °F - 32 0 F) = -269 0 C � Convert -38. 9 0 C to degrees Kelvin. . � ° K = [-38. 9 °C + 273. 15 °C ] × 1 K/ 1 0 C = 234. 3 K

Answers of problems: Chapter 1 � 1. 17 � SI Unit � 1. 18 Prefix number � length meter (m) � mega- 106 � volume cubic meter (m 3) � kilo- 103 � mass kilogram (kg) � deci- 10 -1 � time second (s) � centi- 10 -2 � temperature Kelvin (K) � milli- 10 -3 � micro- 10 -6 � nano- 10 -9 � pico- 10 -12

Test bank � 1. The SI unit of time is the � A. hour � B. second � C. minute � D. ampere � 2. The diameter of an atom is approximately 1 10 -7 mm. What is this diameter when expressed in nanometers? � A. 1 10 -18 nm � B. 1 10 -15 nm � C. 1 10 -9 nm � D. 1 10 -1 nm

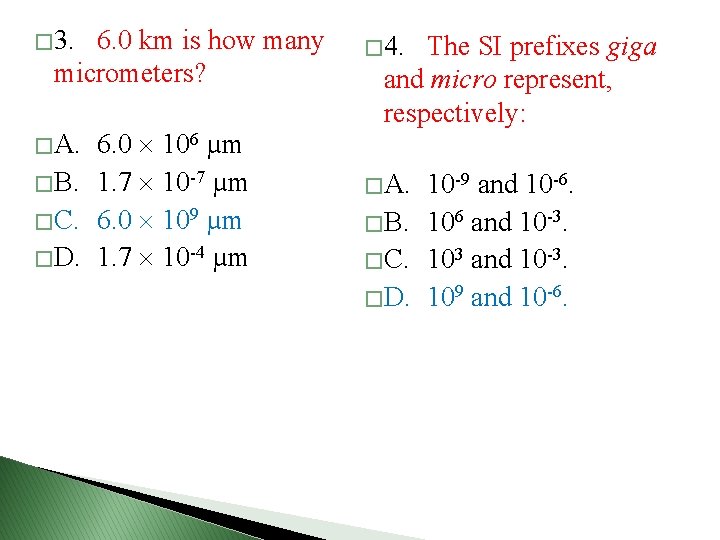
� 3. 6. 0 km is how many micrometers? 6. 0 106 µm � B. 1. 7 10 -7 µm � C. 6. 0 109 µm � D. 1. 7 10 -4 µm � 4. The SI prefixes giga and micro represent, respectively: � A. 10 -9 and 10 -6. � B. 106 and 10 -3. � C. 103 and 10 -3. � D. 109 and 10 -6.

� 5. Which of these quantities represents the largest mass? � A. 2. 0 102 mg � B. 0. 0010 kg � C. 1. 0 105 g � D. 2. 0 102 cg � Solution � A) 0. 2 g � B)1 g � C) 0. 1 g � D) 2 g � 6. How many cubic centimeters are there in exactly one cubic meter? � A. 1 10 -6 cm 3 � B. 1 10 -3 cm 3 � C. 1 10 -2 cm 3 � D. 1 106 cm 3 � Solution � (1 m )3 = (1 10 -2 cm )3 � 1 m 3 = 1 10 -6 cm 3

� 7. Ammonia boils at -33. 4 C. What temperature is this in F? � A. -60. 1 F � B. -92. 1 F � C. -28. 1 F � D. +13. 5 F F = (9 0 F /5 0 C) × °C + 32 [°F = (9 0 F /5 0 C) × -33. 4 °C] + 32 0 C = 435 0 F
 Numero niss
Numero niss 110 000 110 111 000 111
110 000 110 111 000 111 Laila henzele
Laila henzele Laila knio
Laila knio Laila hussein
Laila hussein Laila heid
Laila heid Where is the pelvic girdle located
Where is the pelvic girdle located Kushari
Kushari Laila medin
Laila medin Laila loste
Laila loste Tanner staging female
Tanner staging female Layla and majnun sparknotes
Layla and majnun sparknotes How to find one mole of a compound
How to find one mole of a compound Rahaf mohammed video
Rahaf mohammed video Dr nadia mohammed
Dr nadia mohammed Mohammed mona md
Mohammed mona md Source
Source Dr samah mohammed
Dr samah mohammed Dr mohammed tarrabain
Dr mohammed tarrabain Mohammed al kamali
Mohammed al kamali Dr mohammed ahmed
Dr mohammed ahmed Gedicht prophet mohammed
Gedicht prophet mohammed Dr mohammed ahmed
Dr mohammed ahmed Mohammed yusuf (boko haram)
Mohammed yusuf (boko haram) Dr mohammed shaker
Dr mohammed shaker Aleem mohammed
Aleem mohammed Dr mohammed tarrabain
Dr mohammed tarrabain Epcardium
Epcardium Pneumococci
Pneumococci Mohammed aledhari
Mohammed aledhari Dr samah mohammed
Dr samah mohammed Mohammed airaj
Mohammed airaj