GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION
![In all aqueous solutions at 25ºC, the product of [H 3 O+] and [OH−] In all aqueous solutions at 25ºC, the product of [H 3 O+] and [OH−]](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-12.jpg)
![p. OH = −log[OH−] Slide 16 - 14 General Chemistry: Chapter 16 Copyright © p. OH = −log[OH−] Slide 16 - 14 General Chemistry: Chapter 16 Copyright ©](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-14.jpg)
![KW = [H 3 O+][OH−] −log. KW = −(log[H 3 O+]+log[OH−]) p. KW = KW = [H 3 O+][OH−] −log. KW = −(log[H 3 O+]+log[OH−]) p. KW =](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-15.jpg)
![FIGURE 16 -4 Relating [H 3 O+], p. H, [OH−], and p. OH Slide FIGURE 16 -4 Relating [H 3 O+], p. H, [OH−], and p. OH Slide](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-17.jpg)


![Complex ions FIGURE 16 -11 The Lewis structure of [Al(H 2 O)6]3+ and a Complex ions FIGURE 16 -11 The Lewis structure of [Al(H 2 O)6]3+ and a](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-71.jpg)
![FIGURE 16 -12 Hydrolysis of [Al(H 2 O)6]3+ to produce H 3 O+ Slide FIGURE 16 -12 Hydrolysis of [Al(H 2 O)6]3+ to produce H 3 O+ Slide](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-72.jpg)

- Slides: 74

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA Acids and Bases BISSONNETTE 16 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 16 - 1 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

Acids and Bases Slide 16 - 2 CONTENTS 16 -1 Acids, Bases, and Conjugate Acid -Base Pairs 16 -2 Self Ionization of Water and the p. H Scale 16 -3 Ionization of Acids and Bases in Water 16 -4 Strong Acids and Strong Bases 16 -5 Weak Acids and Weak Bases 16 -6 Polyprotic Acids 16 -7 Simultaneous or Consecutive Acid -Base Reactions: A General Approach General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

Acids and Bases CONTENTS 16 -8 Ions as Acids and Bases 16 -9 Qualitative Aspects of Acid-Base Reactions 16 -10 Molecular Structure and Acid Base Behavior 16 -11 Lewis Acids and Bases Slide 16 - 3 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

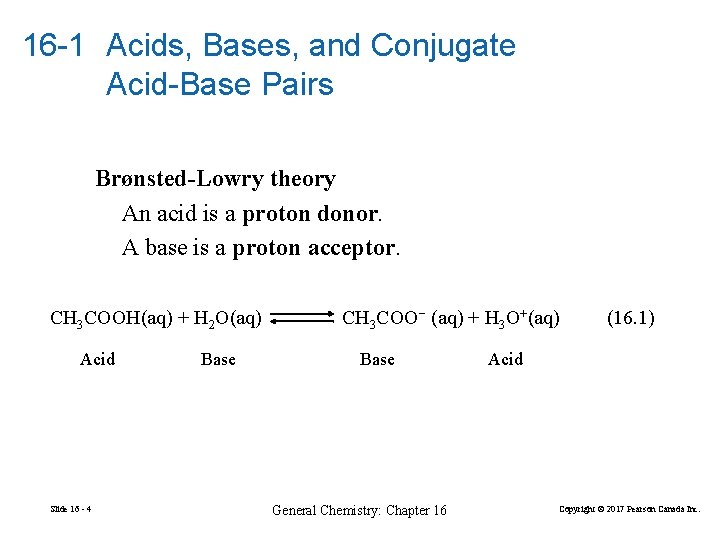
16 -1 Acids, Bases, and Conjugate Acid-Base Pairs Brønsted-Lowry theory An acid is a proton donor. A base is a proton acceptor. CH 3 COOH(aq) + H 2 O(aq) Acid Slide 16 - 4 Base CH 3 COO− (aq) + H 3 O+(aq) Base General Chemistry: Chapter 16 (16. 1) Acid Copyright © 2017 Pearson Canada Inc.

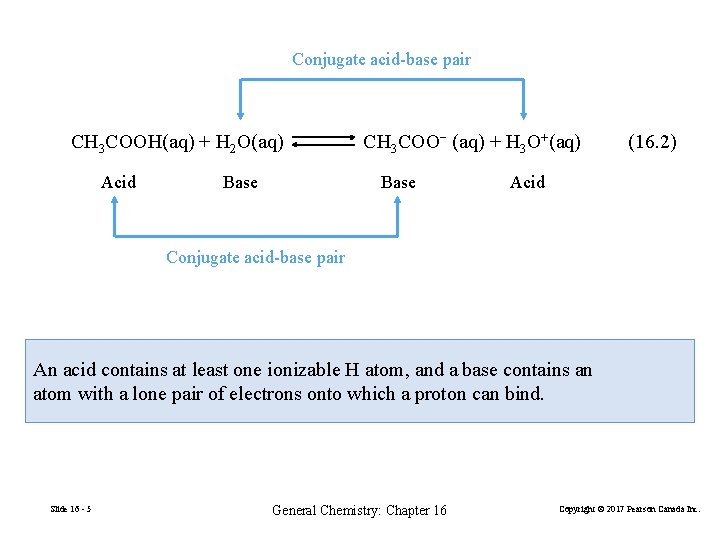
Conjugate acid-base pair CH 3 COOH(aq) + H 2 O(aq) Acid Base CH 3 COO− (aq) + H 3 O+(aq) Base (16. 2) Acid Conjugate acid-base pair An acid contains at least one ionizable H atom, and a base contains an atom with a lone pair of electrons onto which a proton can bind. Slide 16 - 5 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

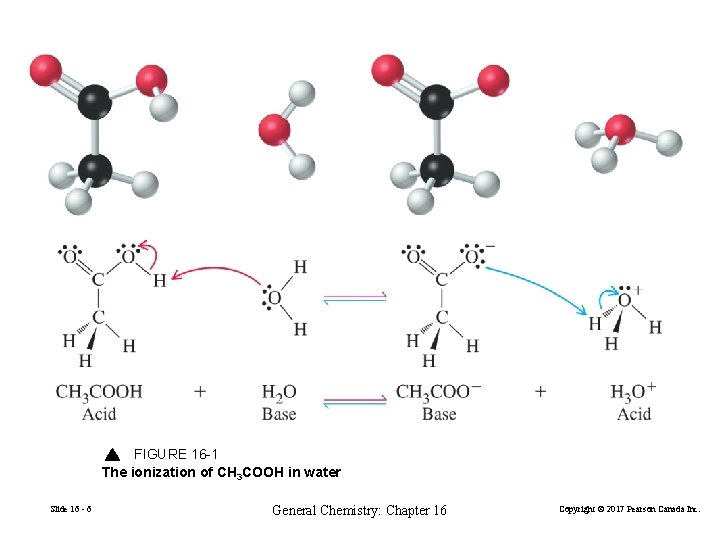
FIGURE 16 -1 The ionization of CH 3 COOH in water Slide 16 - 6 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

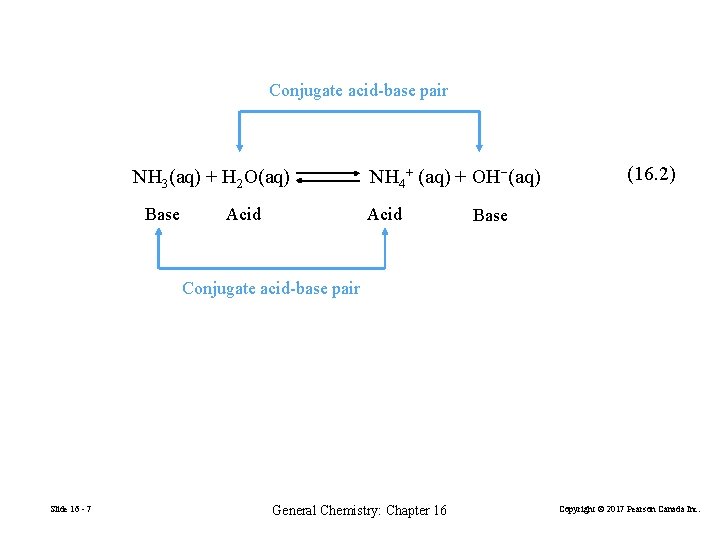
Conjugate acid-base pair NH 3(aq) + H 2 O(aq) Base Acid NH 4+ (aq) + OH−(aq) Acid (16. 2) Base Conjugate acid-base pair Slide 16 - 7 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

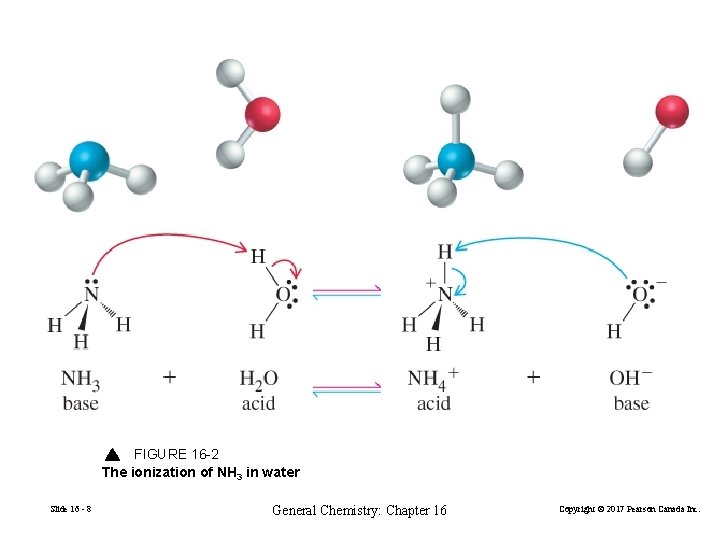
FIGURE 16 -2 The ionization of NH 3 in water Slide 16 - 8 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

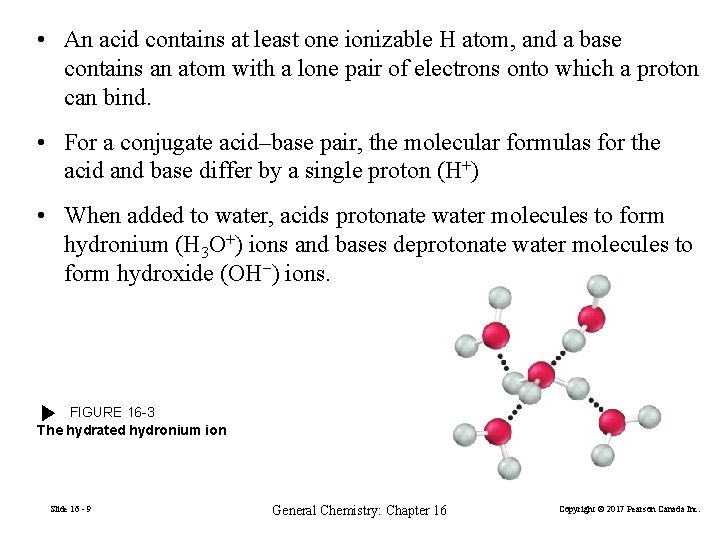
• An acid contains at least one ionizable H atom, and a base contains an atom with a lone pair of electrons onto which a proton can bind. • For a conjugate acid–base pair, the molecular formulas for the acid and base differ by a single proton (H+) • When added to water, acids protonate water molecules to form hydronium (H 3 O+) ions and bases deprotonate water molecules to form hydroxide (OH−) ions. FIGURE 16 -3 The hydrated hydronium ion Slide 16 - 9 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

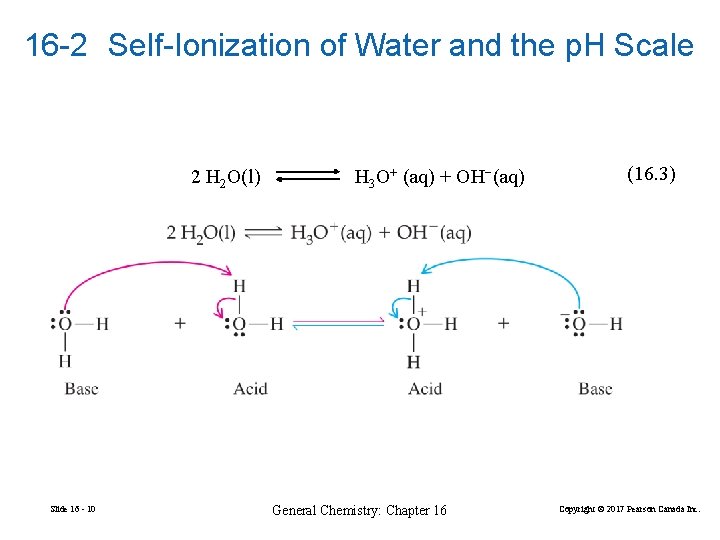
16 -2 Self-Ionization of Water and the p. H Scale 2 H 2 O(l) Slide 16 - 10 H 3 O+ (aq) + OH−(aq) General Chemistry: Chapter 16 (16. 3) Copyright © 2017 Pearson Canada Inc.

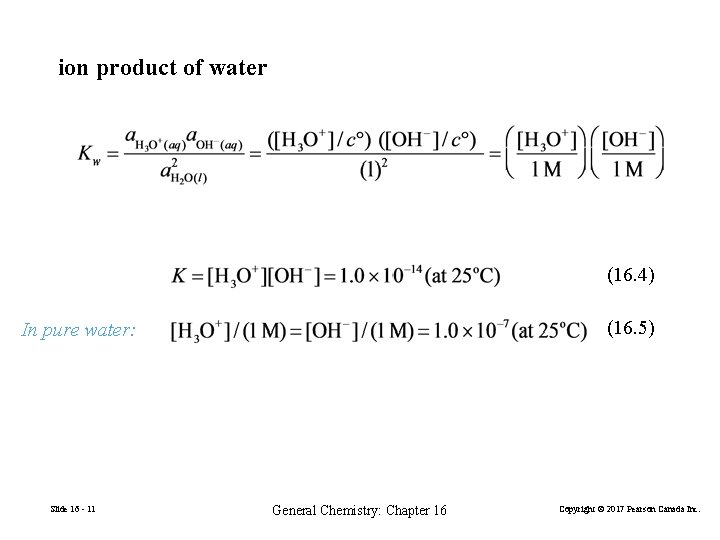
ion product of water (16. 4) (16. 5) In pure water: Slide 16 - 11 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.
![In all aqueous solutions at 25ºC the product of H 3 O and OH In all aqueous solutions at 25ºC, the product of [H 3 O+] and [OH−]](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-12.jpg)
In all aqueous solutions at 25ºC, the product of [H 3 O+] and [OH−] always equals 1. 0× 10− 14. The self-ionization of water is partially suppressed by the addition of acid or base to water. Slide 16 - 12 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

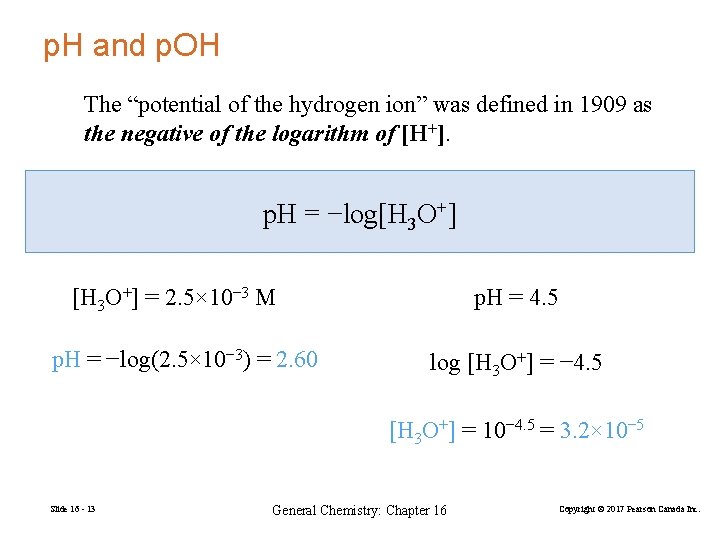
p. H and p. OH The “potential of the hydrogen ion” was defined in 1909 as the negative of the logarithm of [H+]. p. H = −log[H 3 O+] = 2. 5× 10− 3 M p. H = −log(2. 5× 10− 3) = 2. 60 p. H = 4. 5 log [H 3 O+] = − 4. 5 [H 3 O+] = 10− 4. 5 = 3. 2× 10− 5 Slide 16 - 13 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.
![p OH logOH Slide 16 14 General Chemistry Chapter 16 Copyright p. OH = −log[OH−] Slide 16 - 14 General Chemistry: Chapter 16 Copyright ©](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-14.jpg)
p. OH = −log[OH−] Slide 16 - 14 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.
![KW H 3 OOH log KW logH 3 OlogOH p KW KW = [H 3 O+][OH−] −log. KW = −(log[H 3 O+]+log[OH−]) p. KW =](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-15.jpg)
KW = [H 3 O+][OH−] −log. KW = −(log[H 3 O+]+log[OH−]) p. KW = −(log[H 3 O+]+log[OH−]) = −log[H 3 O+] −log[OH−] = p. H + p. OH KW = 1. 0× 10− 14 p. KW = 14 p. H + p. OH = 14 (at 25ºC) Slide 16 - 15 General Chemistry: Chapter 16 (16. 8) Copyright © 2017 Pearson Canada Inc.

Acidic, Basic, and Neutral Solutions Slide 16 - 16 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.
![FIGURE 16 4 Relating H 3 O p H OH and p OH Slide FIGURE 16 -4 Relating [H 3 O+], p. H, [OH−], and p. OH Slide](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-17.jpg)
FIGURE 16 -4 Relating [H 3 O+], p. H, [OH−], and p. OH Slide 16 - 17 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

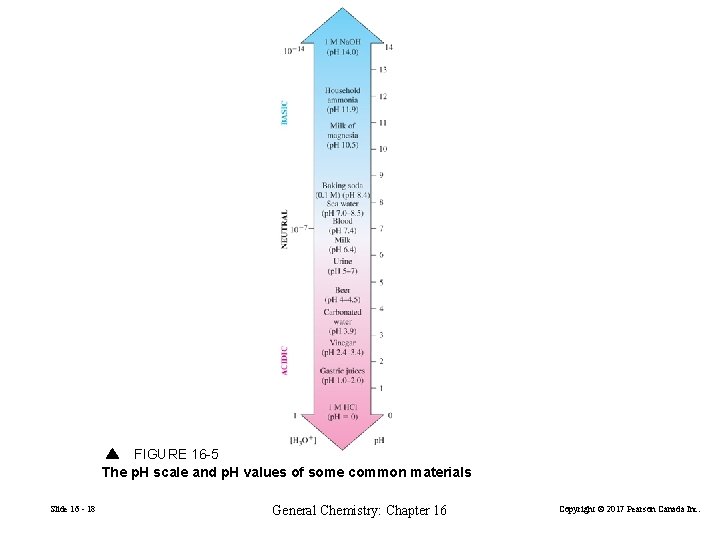
FIGURE 16 -5 The p. H scale and p. H values of some common materials Slide 16 - 18 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

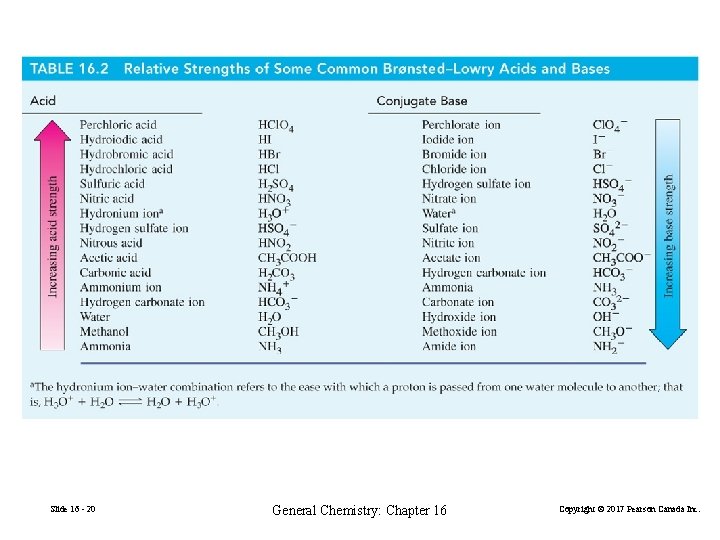
16 -3 Ionization of Acids and Bases in Water FIGURE 16 -6 Strong and weak acids compared Slide 16 - 19 Thymol Blue Indicator p. H < 1. 2 < p. H < 2. 8 < p. H General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

Slide 16 - 20 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

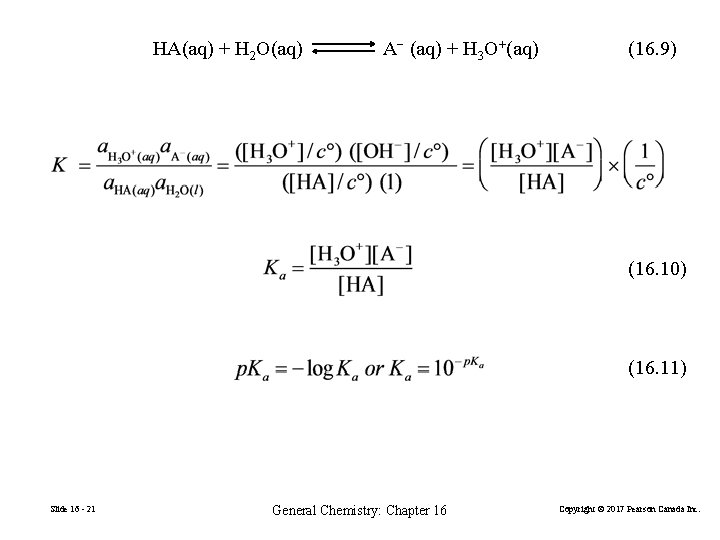
HA(aq) + H 2 O(aq) A− (aq) + H 3 O+(aq) (16. 9) (16. 10) (16. 11) Slide 16 - 21 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

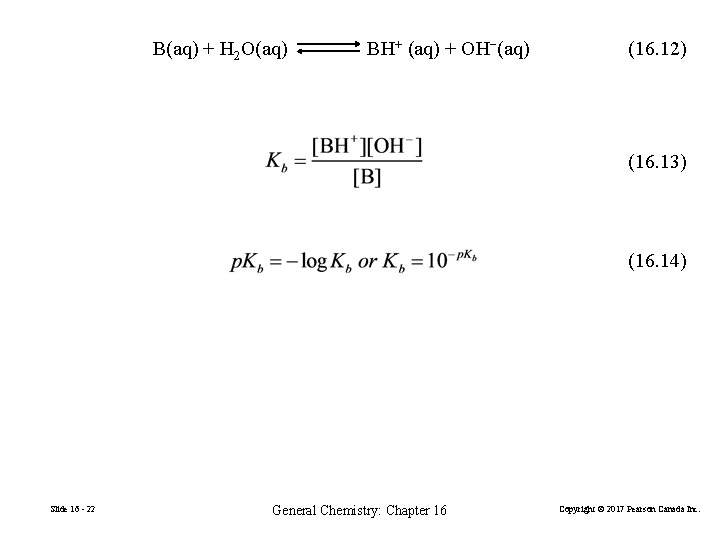
B(aq) + H 2 O(aq) BH+ (aq) + OH−(aq) (16. 12) (16. 13) (16. 14) Slide 16 - 22 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

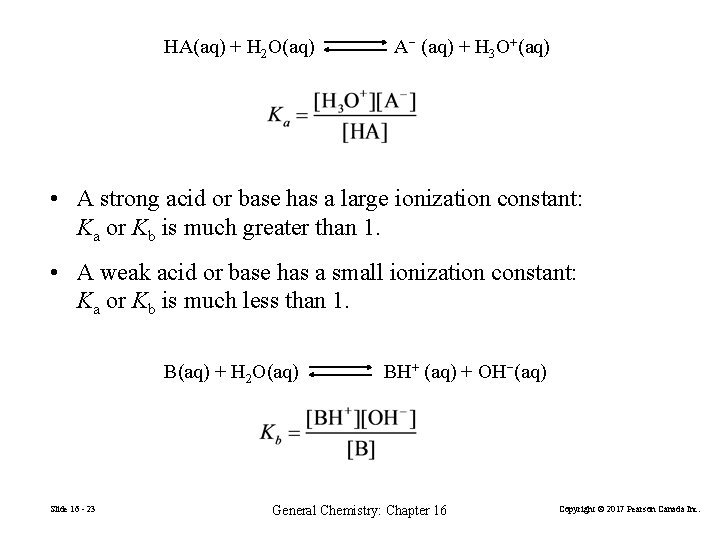
HA(aq) + H 2 O(aq) A− (aq) + H 3 O+(aq) • A strong acid or base has a large ionization constant: Ka or Kb is much greater than 1. • A weak acid or base has a small ionization constant: Ka or Kb is much less than 1. B(aq) + H 2 O(aq) Slide 16 - 23 BH+ (aq) + OH−(aq) General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

Slide 16 - 24 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

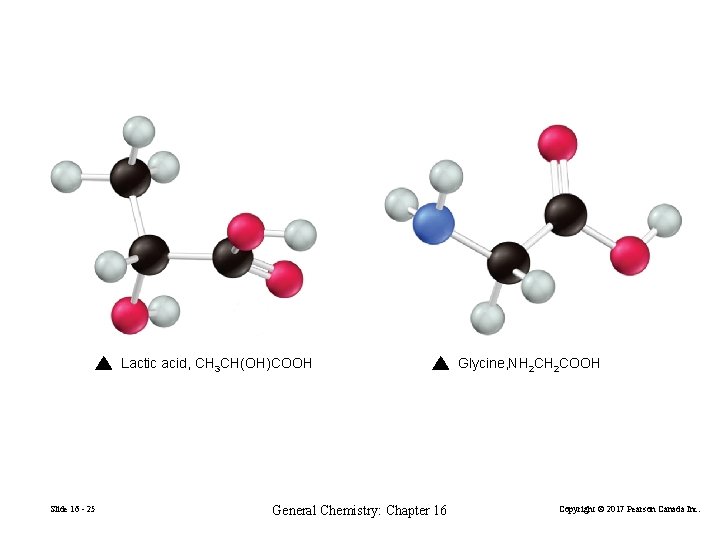
Lactic acid, CH 3 CH(OH)COOH Slide 16 - 25 General Chemistry: Chapter 16 Glycine, NH 2 COOH Copyright © 2017 Pearson Canada Inc.

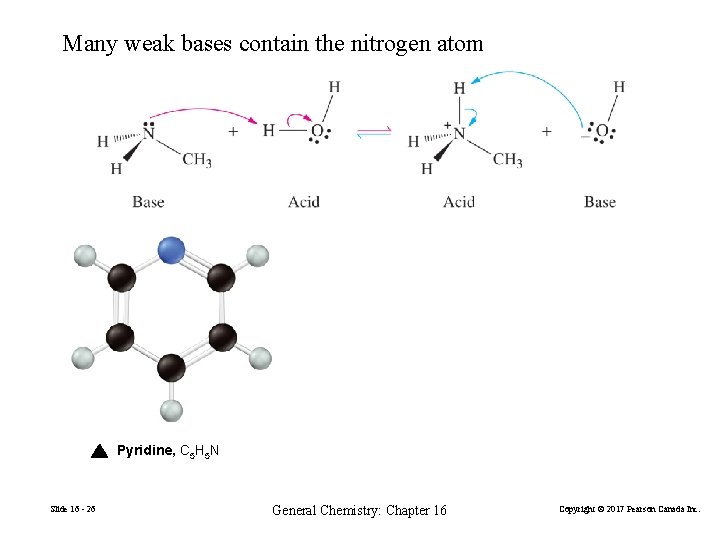
Many weak bases contain the nitrogen atom Pyridine, C 5 H 5 N Slide 16 - 26 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

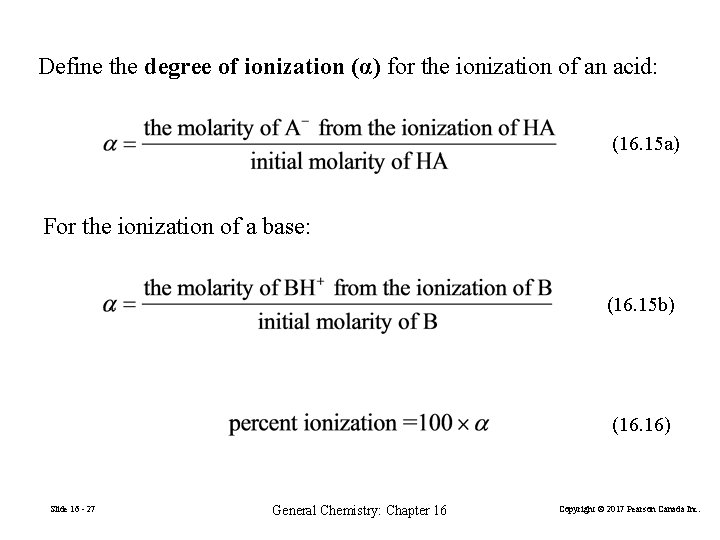
Define the degree of ionization (α) for the ionization of an acid: (16. 15 a) For the ionization of a base: (16. 15 b) (16. 16) Slide 16 - 27 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

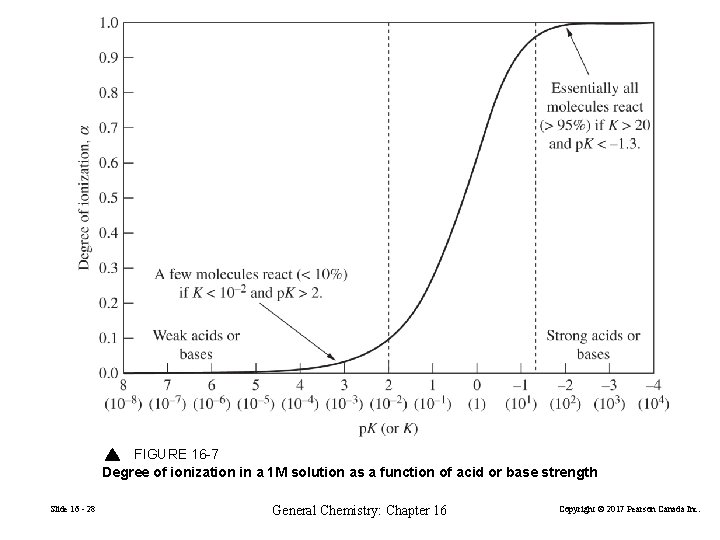
FIGURE 16 -7 Degree of ionization in a 1 M solution as a function of acid or base strength Slide 16 - 28 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

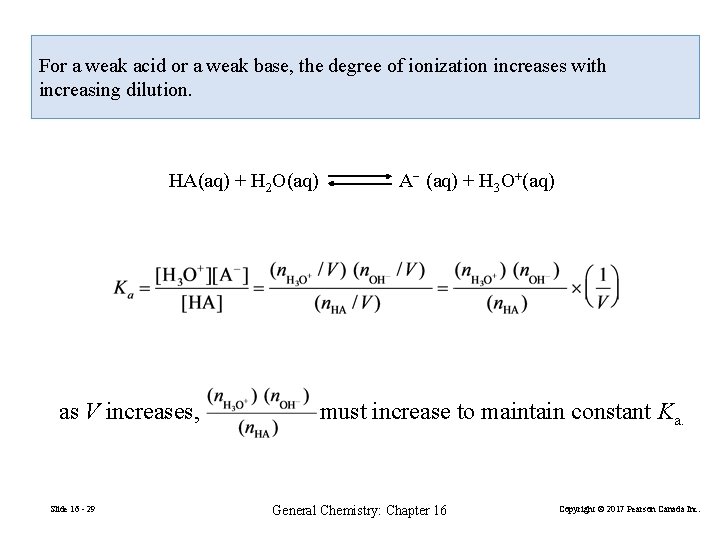
For a weak acid or a weak base, the degree of ionization increases with increasing dilution. HA(aq) + H 2 O(aq) as V increases, Slide 16 - 29 A− (aq) + H 3 O+(aq) must increase to maintain constant Ka. General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

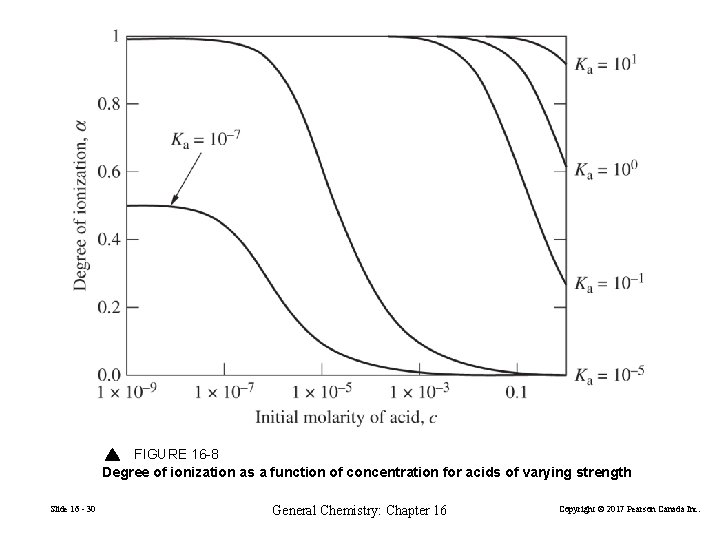
FIGURE 16 -8 Degree of ionization as a function of concentration for acids of varying strength Slide 16 - 30 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

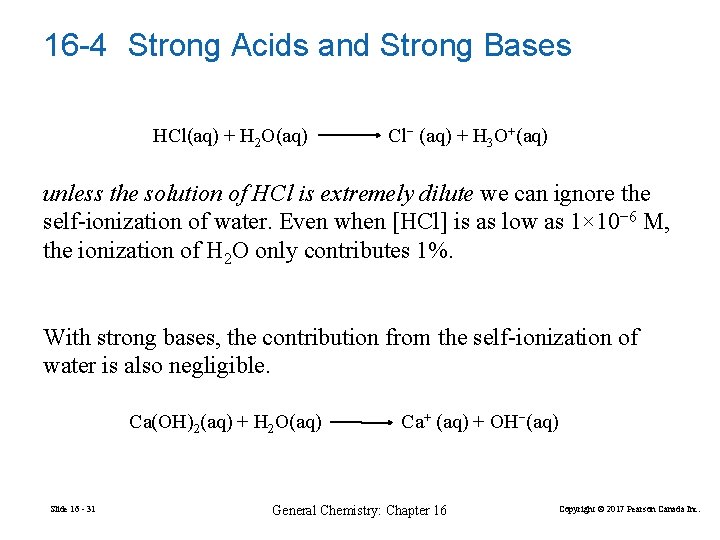
16 -4 Strong Acids and Strong Bases HCl(aq) + H 2 O(aq) Cl− (aq) + H 3 O+(aq) unless the solution of HCl is extremely dilute we can ignore the self-ionization of water. Even when [HCl] is as low as 1× 10− 6 M, the ionization of H 2 O only contributes 1%. With strong bases, the contribution from the self-ionization of water is also negligible. Ca(OH)2(aq) + H 2 O(aq) Slide 16 - 31 Ca+ (aq) + OH−(aq) General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

16 -5 Weak Acids and Weak Bases The key to solving equilibrium problems is to be able to imagine what is going on. Ask yourself: • Which are the principal species in solution? • What are the chemical reactions that produce them? • Can some reactions (for example, the self-ionization of water) be ignored? • Can you make any assumptions that allow you to simplify the equilibrium calculations? • What is a reasonable answer to the problem? For instance, should the final solution be acidic (p. H < 7) or basic (p. H > 7). Slide 16 - 32 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

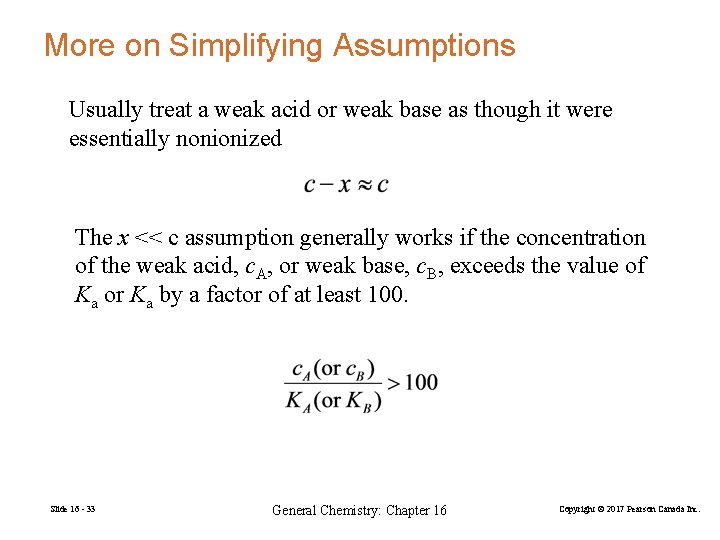
More on Simplifying Assumptions Usually treat a weak acid or weak base as though it were essentially nonionized The x << c assumption generally works if the concentration of the weak acid, c. A, or weak base, c. B, exceeds the value of Ka or Ka by a factor of at least 100. Slide 16 - 33 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

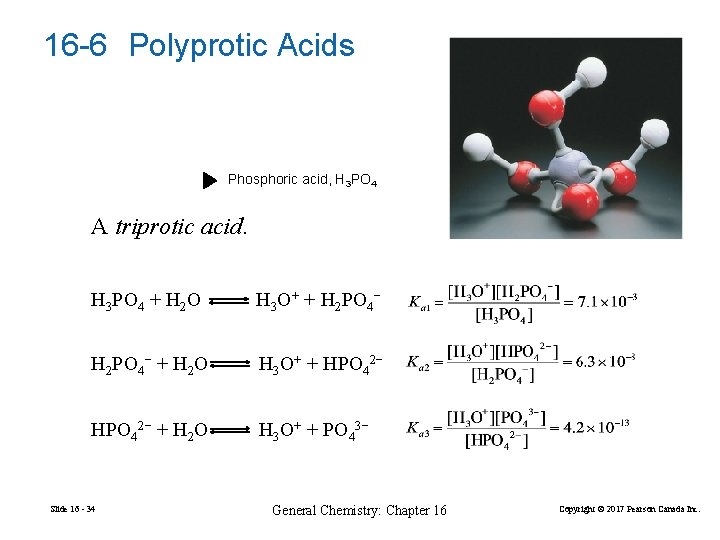
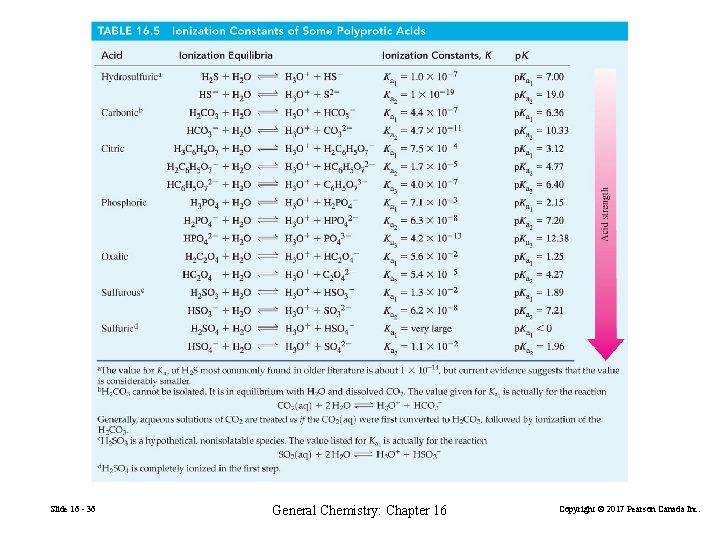
16 -6 Polyprotic Acids Phosphoric acid, H 3 PO 4 A triprotic acid. H 3 PO 4 + H 2 O H 3 O+ + H 2 PO 4− + H 2 O H 3 O+ + HPO 42− + H 2 O H 3 O+ + PO 43− Slide 16 - 34 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

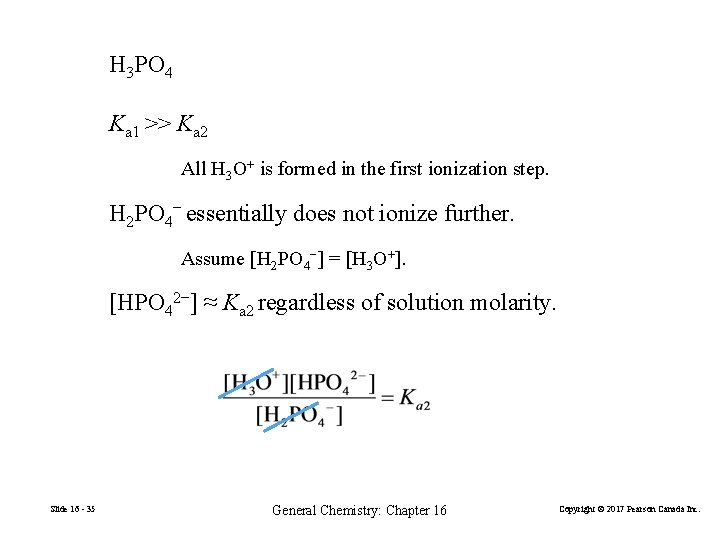
H 3 PO 4 Ka 1 >> Ka 2 All H 3 O+ is formed in the first ionization step. H 2 PO 4− essentially does not ionize further. Assume [H 2 PO 4−] = [H 3 O+]. [HPO 42−] ≈ Ka 2 regardless of solution molarity. Slide 16 - 35 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

Slide 16 - 36 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

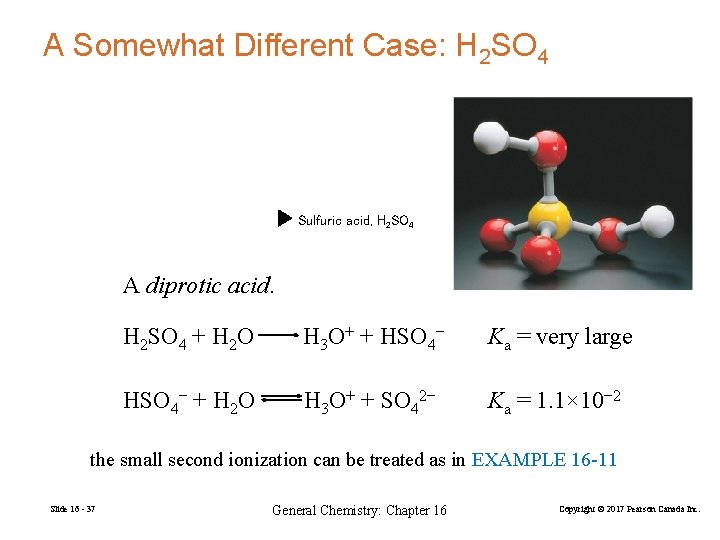
A Somewhat Different Case: H 2 SO 4 Sulfuric acid, H 2 SO 4 A diprotic acid. H 2 SO 4 + H 2 O H 3 O+ + HSO 4− Ka = very large HSO 4− + H 2 O H 3 O+ + SO 42− Ka = 1. 1× 10− 2 the small second ionization can be treated as in EXAMPLE 16 -11 Slide 16 - 37 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

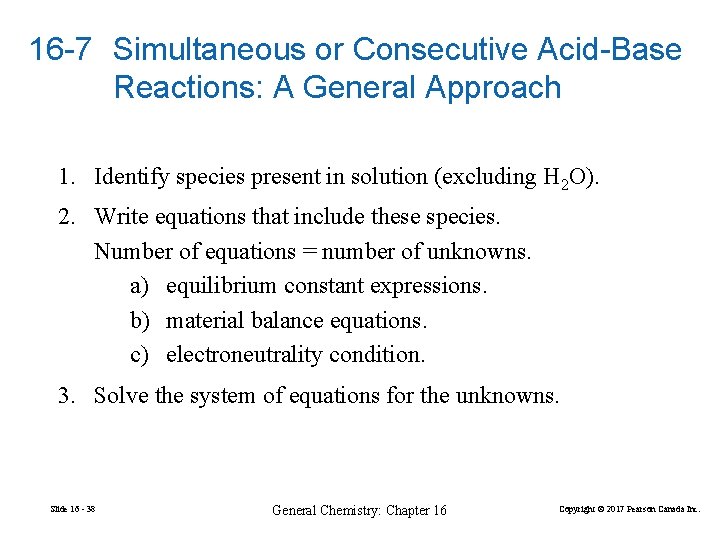
16 -7 Simultaneous or Consecutive Acid-Base Reactions: A General Approach 1. Identify species present in solution (excluding H 2 O). 2. Write equations that include these species. Number of equations = number of unknowns. a) equilibrium constant expressions. b) material balance equations. c) electroneutrality condition. 3. Solve the system of equations for the unknowns. Slide 16 - 38 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

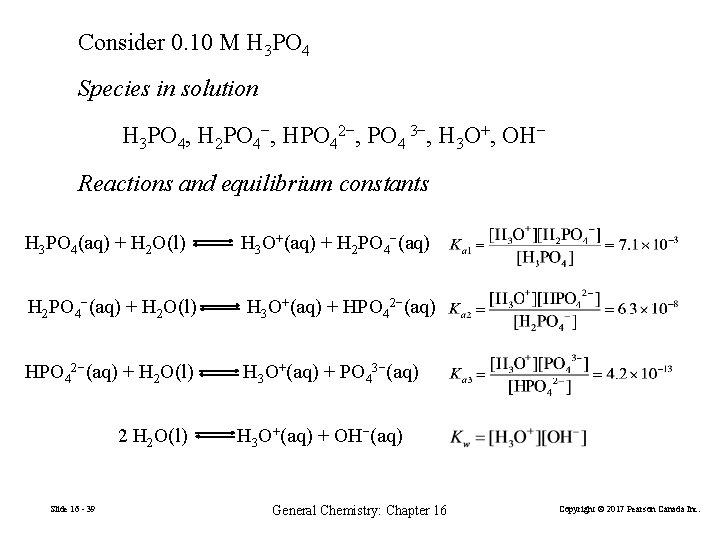
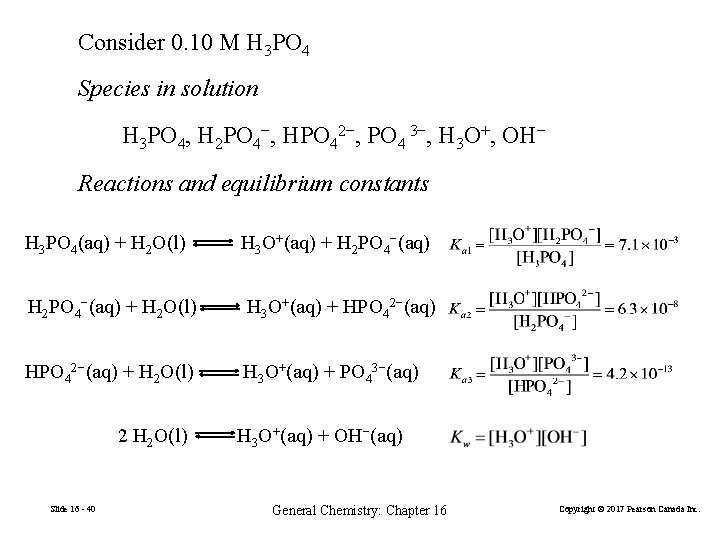
Consider 0. 10 M H 3 PO 4 Species in solution H 3 PO 4, H 2 PO 4−, HPO 42−, PO 4 3−, H 3 O+, OH− Reactions and equilibrium constants H 3 PO 4(aq) + H 2 O(l) H 3 O+(aq) + H 2 PO 4−(aq) + H 2 O(l) H 3 O+(aq) + HPO 42−(aq) + H 2 O(l) H 3 O+(aq) + PO 43−(aq) 2 H 2 O(l) Slide 16 - 39 H 3 O+(aq) + OH−(aq) General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

Consider 0. 10 M H 3 PO 4 Species in solution H 3 PO 4, H 2 PO 4−, HPO 42−, PO 4 3−, H 3 O+, OH− Reactions and equilibrium constants H 3 PO 4(aq) + H 2 O(l) H 3 O+(aq) + H 2 PO 4−(aq) + H 2 O(l) H 3 O+(aq) + HPO 42−(aq) + H 2 O(l) H 3 O+(aq) + PO 43−(aq) 2 H 2 O(l) Slide 16 - 40 H 3 O+(aq) + OH−(aq) General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

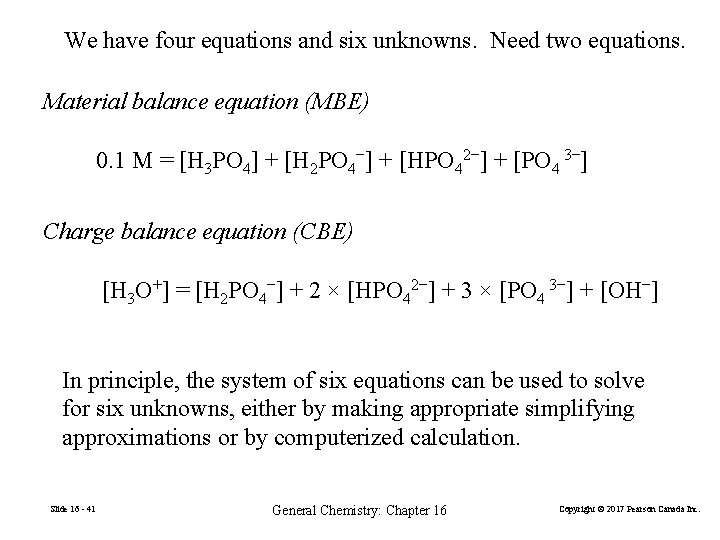
We have four equations and six unknowns. Need two equations. Material balance equation (MBE) 0. 1 M = [H 3 PO 4] + [H 2 PO 4−] + [HPO 42−] + [PO 4 3−] Charge balance equation (CBE) [H 3 O+] = [H 2 PO 4−] + 2 × [HPO 42−] + 3 × [PO 4 3−] + [OH−] In principle, the system of six equations can be used to solve for six unknowns, either by making appropriate simplifying approximations or by computerized calculation. Slide 16 - 41 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

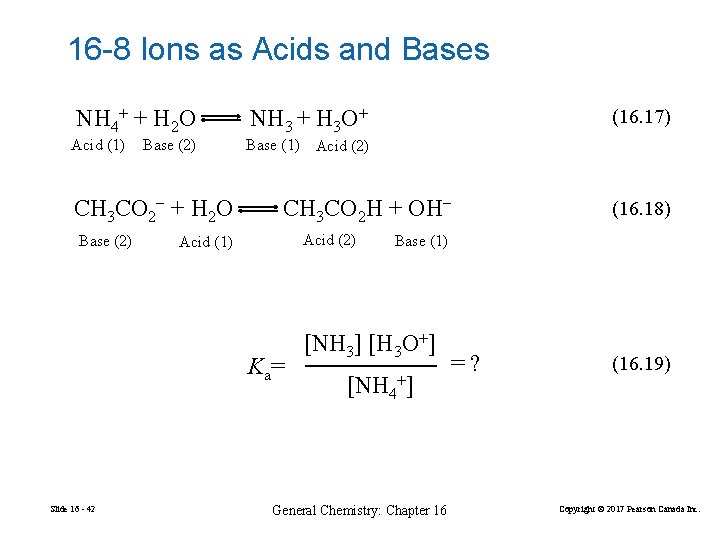
16 -8 Ions as Acids and Bases NH 4+ + H 2 O Acid (1) Base (2) CH 3 CO 2− + H 2 O Base (2) NH 3 + H 3 O+ Base (1) Acid (2) CH 3 CO 2 H + OH− Acid (2) Acid (1) K a= Slide 16 - 42 (16. 17) (16. 18) Base (1) [NH 3] [H 3 O+] [NH 4+] General Chemistry: Chapter 16 =? (16. 19) Copyright © 2017 Pearson Canada Inc.

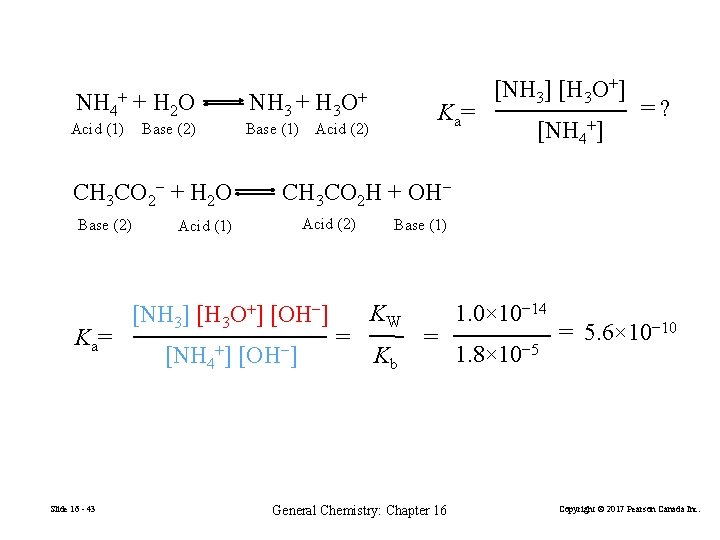
NH 4+ + H 2 O Acid (1) Base (2) CH 3 CO 2− + H 2 O Base (2) K a= Slide 16 - 43 NH 3 + H 3 O+ K a= Base (1) Acid (2) [NH 3] [H 3 O+] [NH 4+] =? CH 3 CO 2 H + OH− Acid (2) Acid (1) [NH 3] [H 3 O+] [OH−-]] [NH 4+] [OH−-]] = Base (1) KW Kb = General Chemistry: Chapter 16 1. 0× 10− 14 1. 8× 10− 5 = 5. 6× 10− 10 Copyright © 2017 Pearson Canada Inc.

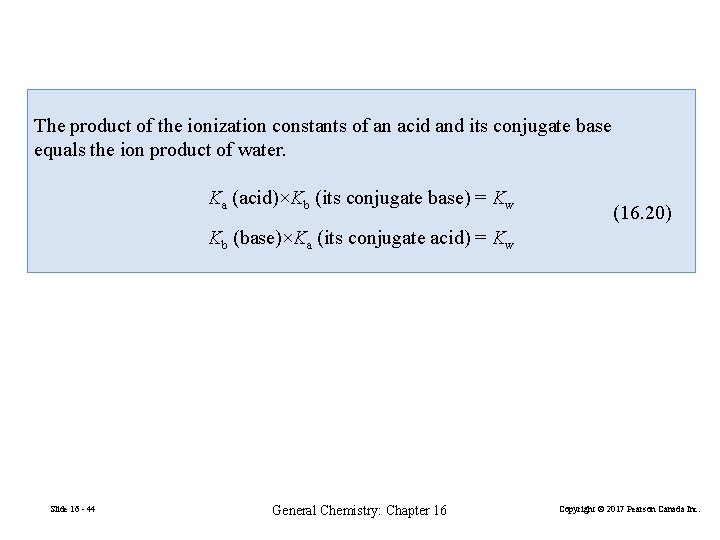
The product of the ionization constants of an acid and its conjugate base equals the ion product of water. Ka (acid)×Kb (its conjugate base) = Kw (16. 20) Kb (base)×Ka (its conjugate acid) = Kw Slide 16 - 44 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

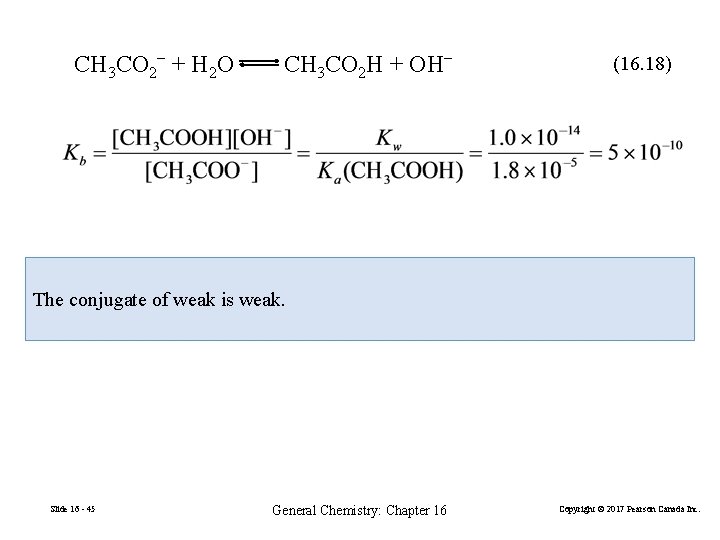
CH 3 CO 2− + H 2 O CH 3 CO 2 H + OH− (16. 18) The conjugate of weak is weak. Slide 16 - 45 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

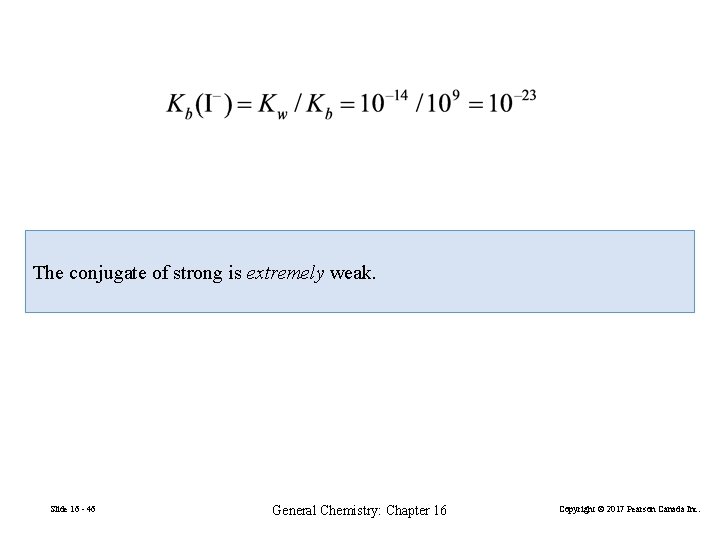
The conjugate of strong is extremely weak. Slide 16 - 46 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

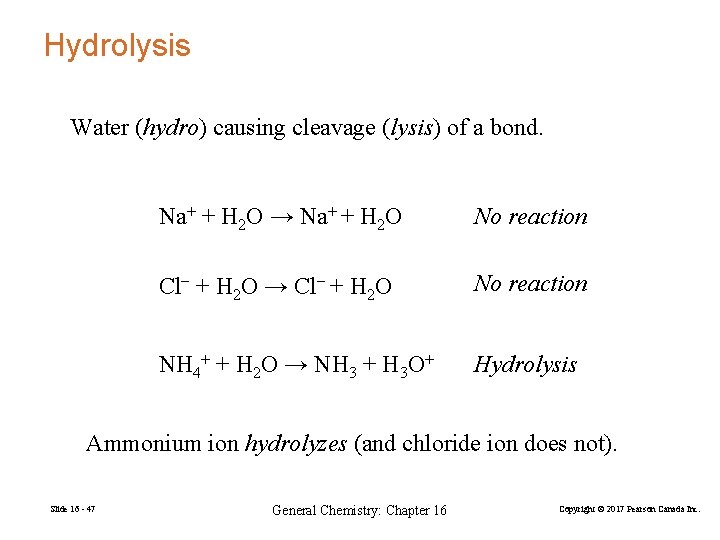
Hydrolysis Water (hydro) causing cleavage (lysis) of a bond. Na+ + H 2 O → Na+ + H 2 O No reaction Cl− + H 2 O → Cl− + H 2 O No reaction NH 4+ + H 2 O → NH 3 + H 3 O+ Hydrolysis Ammonium ion hydrolyzes (and chloride ion does not). Slide 16 - 47 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

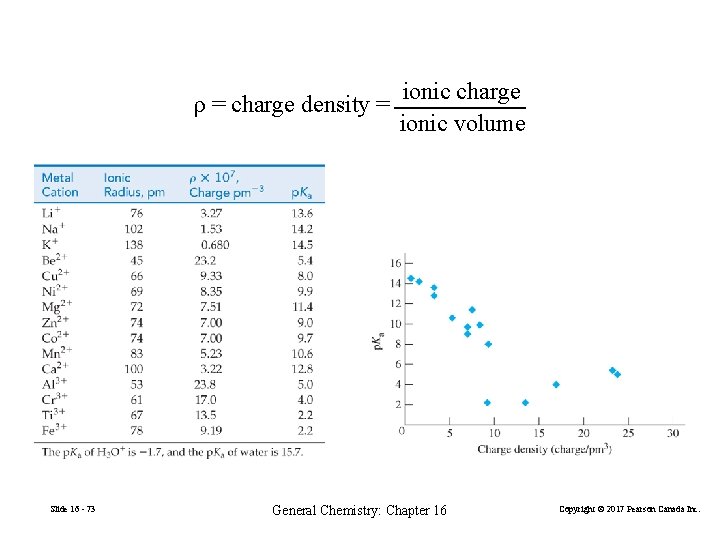
The p. H of Salt Solutions • Metal ions with a +1 or +2 charge usually do not affect the p. H of a solution. Metal ions with higher charges may (for example Al 3+, Fe 3+, Cr 3+, see Slide 16 - 72) • Some polyatomc cations act as acids in water. Important examples are NH 4+ and the protonated forms of amines. • Many anions act as bases in water. It is helpful to think about the acid strength of the conjugate acid, HA. Slide 16 - 48 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

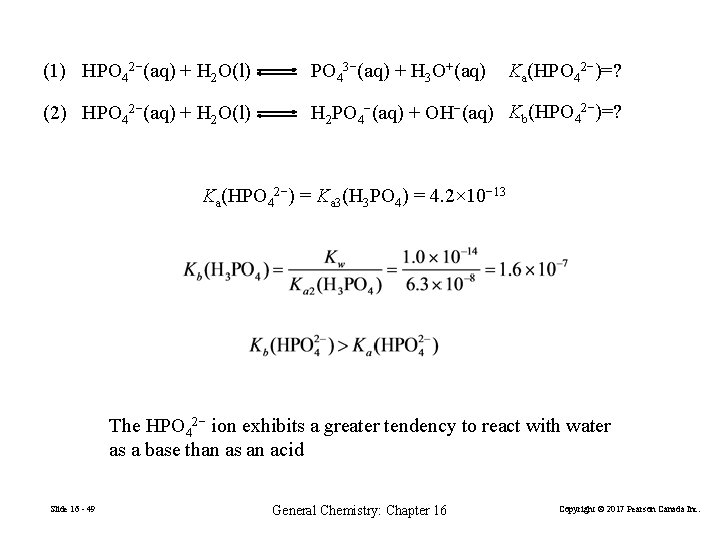
(1) HPO 42−(aq) + H 2 O(l) PO 43−(aq) + H 3 O+(aq) Ka(HPO 42−)=? (2) HPO 42−(aq) + H 2 O(l) H 2 PO 4−(aq) + OH−(aq) Kb(HPO 42−)=? Ka(HPO 42−) = Ka 3(H 3 PO 4) = 4. 2× 10− 13 The HPO 42− ion exhibits a greater tendency to react with water as a base than as an acid Slide 16 - 49 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

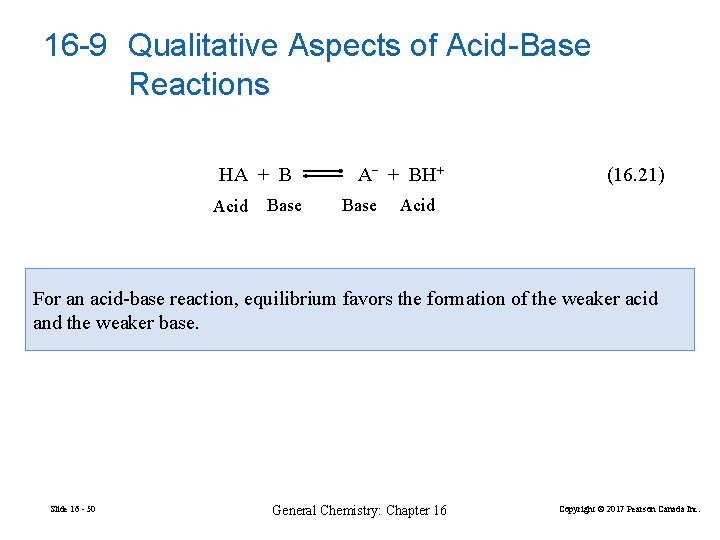
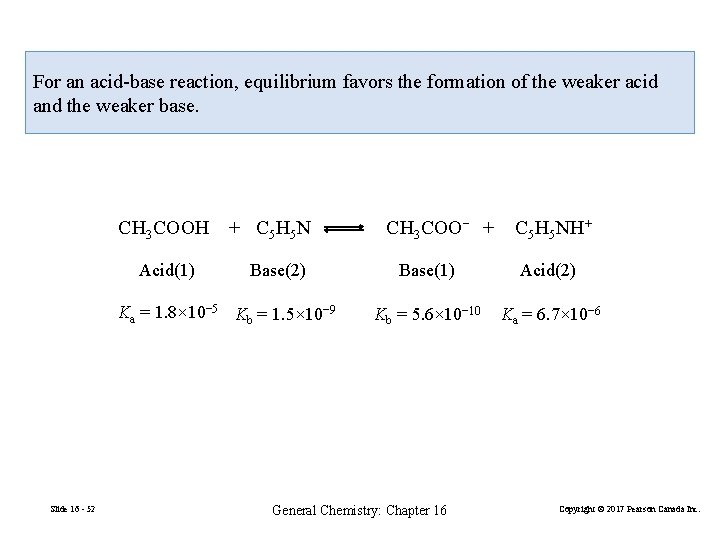
16 -9 Qualitative Aspects of Acid-Base Reactions HA + B Acid Base A− + BH+ Base (16. 21) Acid For an acid-base reaction, equilibrium favors the formation of the weaker acid and the weaker base. Slide 16 - 50 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

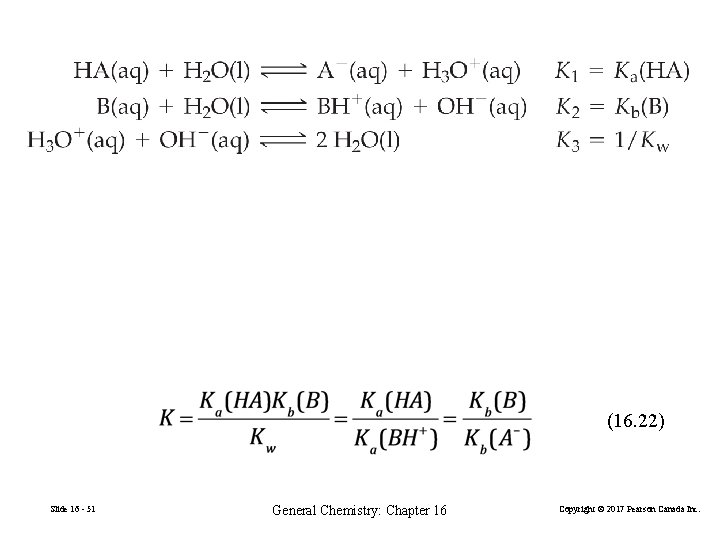
(16. 22) Slide 16 - 51 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

For an acid-base reaction, equilibrium favors the formation of the weaker acid and the weaker base. CH 3 COOH Acid(1) + C 5 H 5 N Base(2) Ka = 1. 8× 10− 5 Kb = 1. 5× 10− 9 Slide 16 - 52 CH 3 COO− + C 5 H 5 NH+ Base(1) Acid(2) Kb = 5. 6× 10− 10 Ka = 6. 7× 10− 6 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

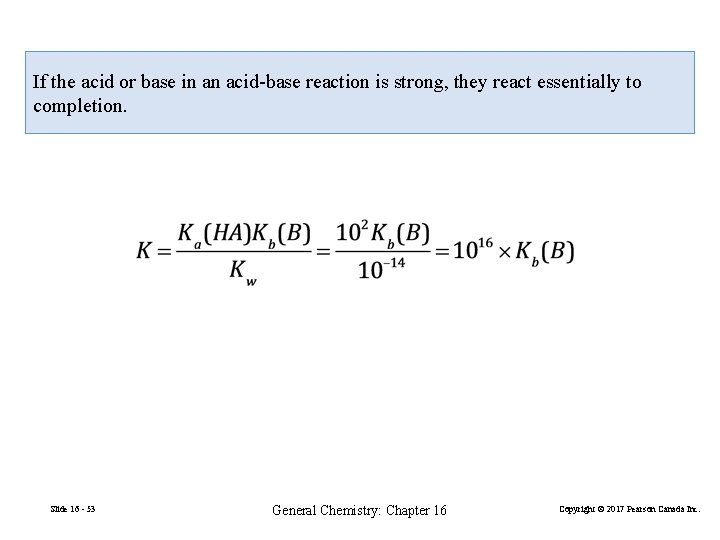
If the acid or base in an acid-base reaction is strong, they react essentially to completion. Slide 16 - 53 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

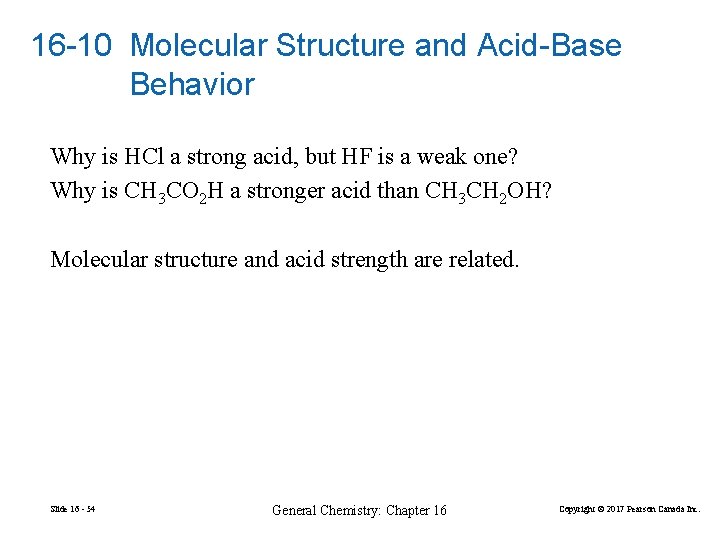
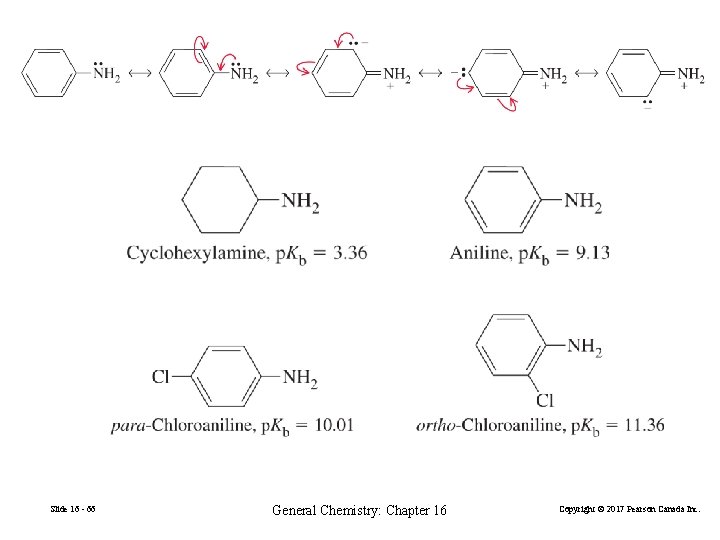
16 -10 Molecular Structure and Acid-Base Behavior Why is HCl a strong acid, but HF is a weak one? Why is CH 3 CO 2 H a stronger acid than CH 3 CH 2 OH? Molecular structure and acid strength are related. Slide 16 - 54 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

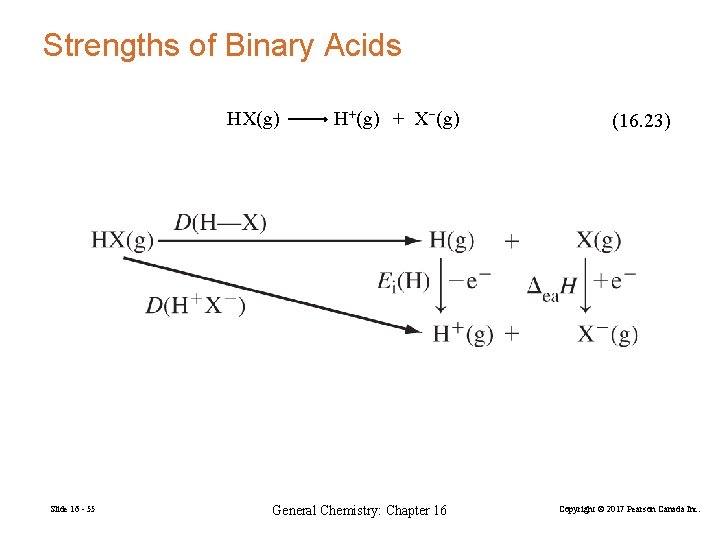
Strengths of Binary Acids HX(g) Slide 16 - 55 H+(g) + X−(g) General Chemistry: Chapter 16 (16. 23) Copyright © 2017 Pearson Canada Inc.

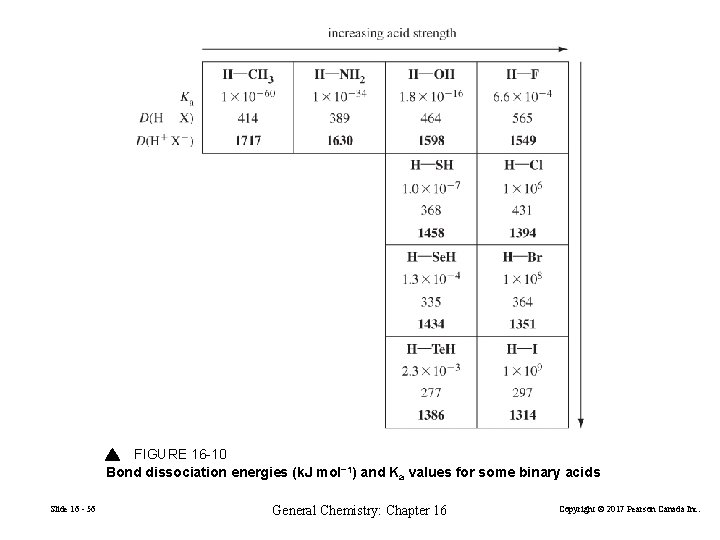
FIGURE 16 -10 Bond dissociation energies (k. J mol− 1) and Ka values for some binary acids Slide 16 - 56 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

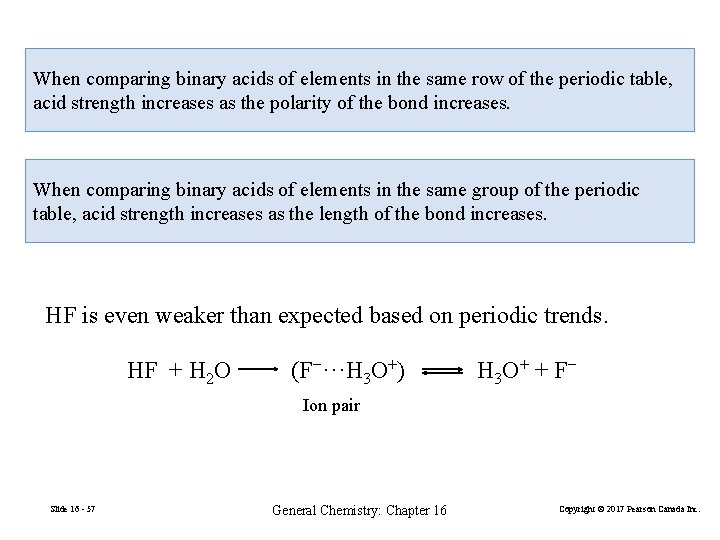
When comparing binary acids of elements in the same row of the periodic table, acid strength increases as the polarity of the bond increases. When comparing binary acids of elements in the same group of the periodic table, acid strength increases as the length of the bond increases. HF is even weaker than expected based on periodic trends. HF + H 2 O (F−···H 3 O+) H 3 O + + F − Ion pair Slide 16 - 57 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

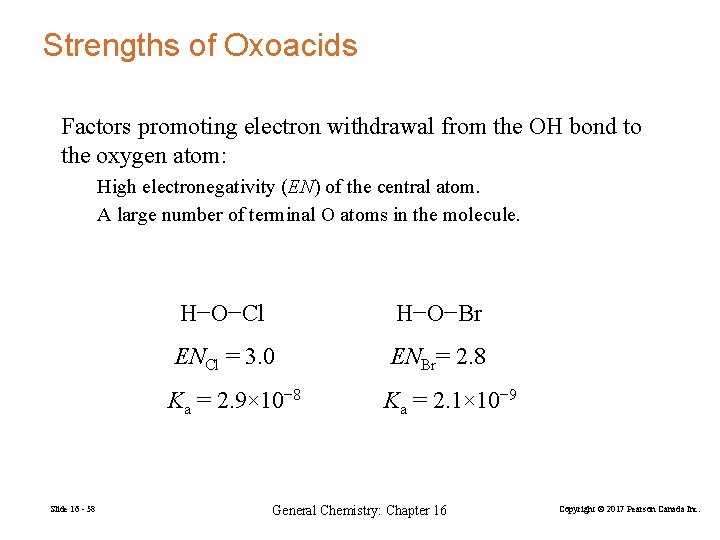
Strengths of Oxoacids Factors promoting electron withdrawal from the OH bond to the oxygen atom: High electronegativity (EN) of the central atom. A large number of terminal O atoms in the molecule. Slide 16 - 58 H−O−Cl H−O−Br ENCl = 3. 0 ENBr= 2. 8 Ka = 2. 9× 10− 8 Ka = 2. 1× 10− 9 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

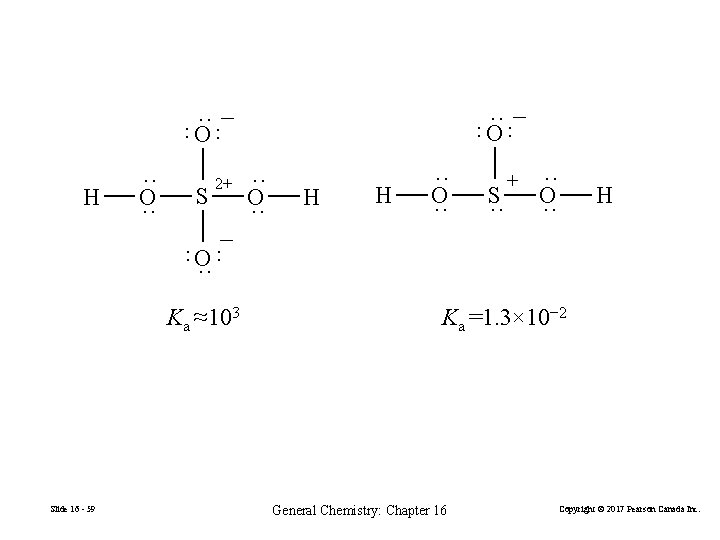
·· − O ·· ·· ·· − O H ·· O ·· S ·· O ·· H H ·· O ·· S ·· + ·· O ·· H − ·· ·· O ·· 2+ Ka ≈103 Slide 16 - 59 Ka =1. 3× 10− 2 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

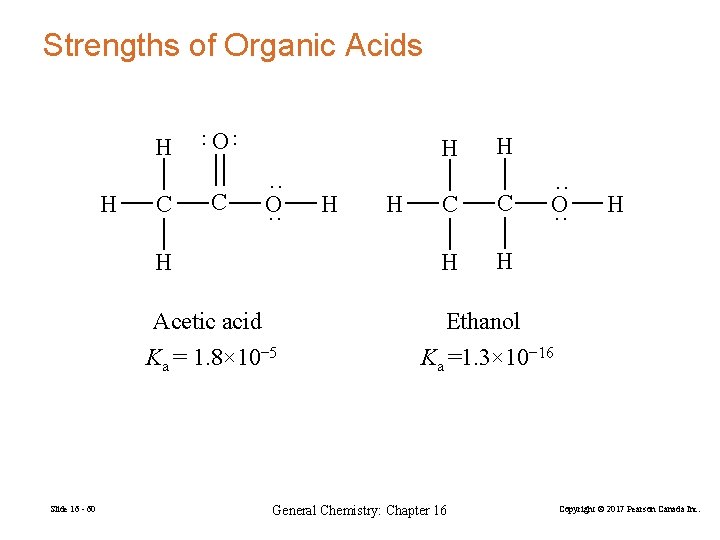
Strengths of Organic Acids H C O ·· ·· H C H ·· O ·· H Acetic acid Ka = 1. 8× 10− 5 Slide 16 - 60 H H H C C H H ·· O ·· H Ethanol Ka =1. 3× 10− 16 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

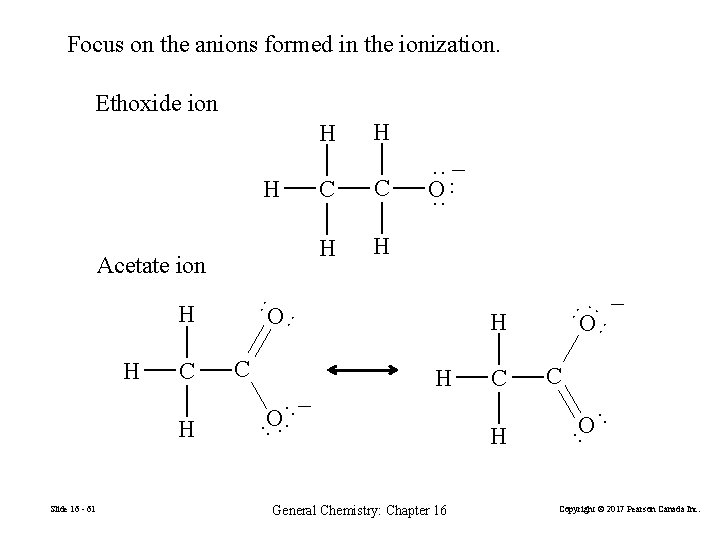
Focus on the anions formed in the ionization. Ethoxide ion H C C H O ·· General Chemistry: Chapter 16 H C ·· O ·− · C O − ·· H ·· O ·· Slide 16 - 61 H ·· H C ·· Acetate ion C ·· − O ·· ·· H H ·· H Copyright © 2017 Pearson Canada Inc.

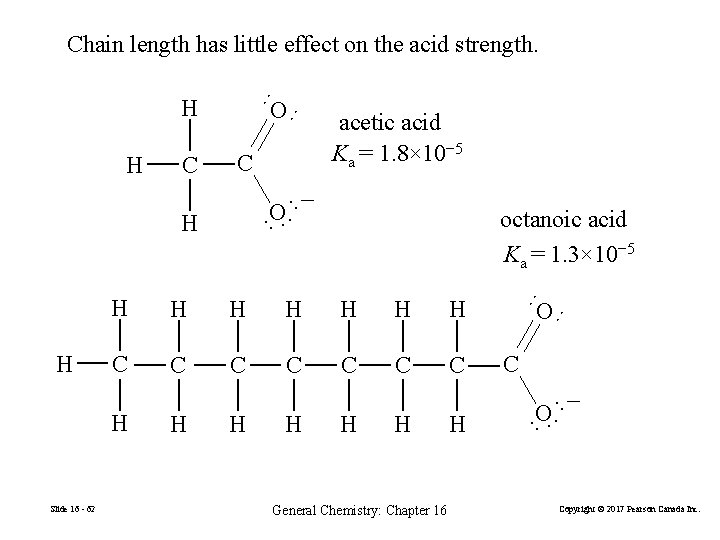
Chain length has little effect on the acid strength. ·· C ·· O ·− · H H H H C C C C H H General Chemistry: Chapter 16 H C O ·− · ·· H O ·· H Slide 16 - 62 octanoic acid Ka = 1. 3× 10− 5 ·· H H acetic acid Ka = 1. 8× 10− 5 ·· C O ·· H Copyright © 2017 Pearson Canada Inc.

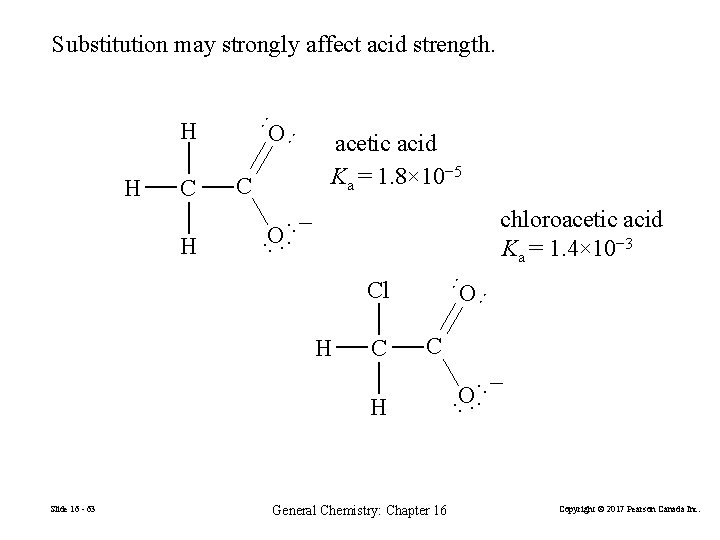
chloroacetic acid Ka = 1. 4× 10− 3 ·· O ·− · ·· H C Cl H C O ·− · ·· Slide 16 - 63 C ·· H O ·· C acetic acid Ka = 1. 8× 10− 5 ·· H O ·· H ·· Substitution may strongly affect acid strength. General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

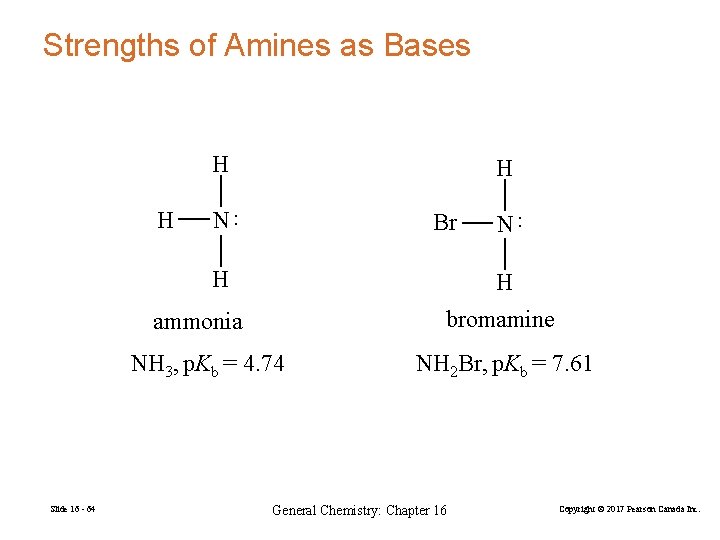
Strengths of Amines as Bases H Br H H bromamine ammonia NH 3, p. Kb = 4. 74 Slide 16 - 64 N ·· H H NH 2 Br, p. Kb = 7. 61 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

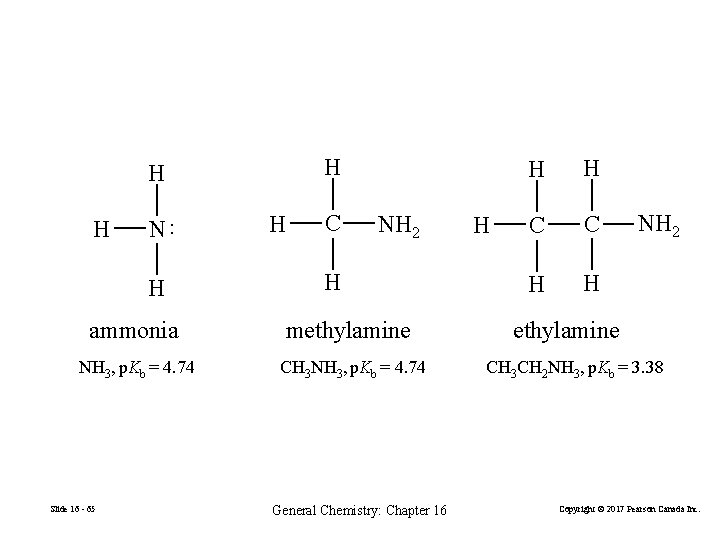
H H N ·· H H H C NH 2 H ammonia methylamine NH 3, p. Kb = 4. 74 CH 3 NH 3, p. Kb = 4. 74 Slide 16 - 65 General Chemistry: Chapter 16 H H H C C H H NH 2 ethylamine CH 3 CH 2 NH 3, p. Kb = 3. 38 Copyright © 2017 Pearson Canada Inc.

Slide 16 - 66 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

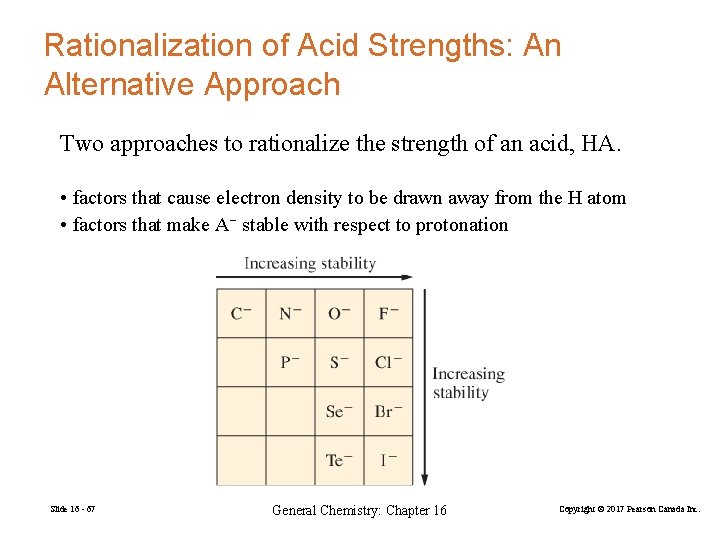
Rationalization of Acid Strengths: An Alternative Approach Two approaches to rationalize the strength of an acid, HA. • factors that cause electron density to be drawn away from the H atom • factors that make A− stable with respect to protonation Slide 16 - 67 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

• the more electronegative the atom is, the better it is able to bear a negative charge. (CH 3 O− is more stable than NH 2−) • the larger the atom, the greater its ability to bear a negative charge. (HS− is more stable than HO−) • the stability of the anion increases as the number of electron-withdrawing groups increases. • the stability of the anion increases as the number of atoms sharing the charge increases Slide 16 - 68 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

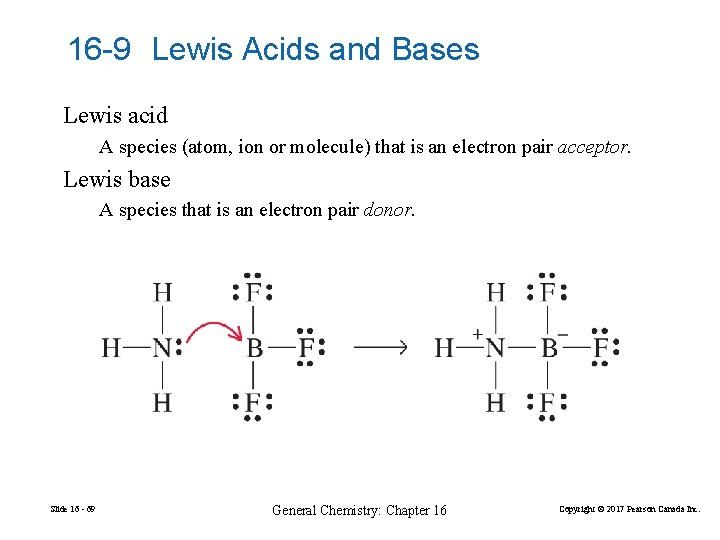
16 -9 Lewis Acids and Bases Lewis acid A species (atom, ion or molecule) that is an electron pair acceptor. Lewis base A species that is an electron pair donor. Slide 16 - 69 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

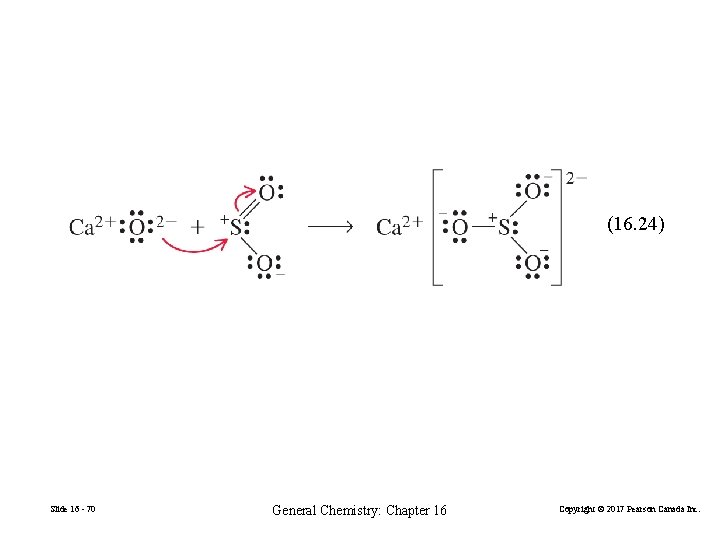
(16. 24) Slide 16 - 70 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.
![Complex ions FIGURE 16 11 The Lewis structure of AlH 2 O63 and a Complex ions FIGURE 16 -11 The Lewis structure of [Al(H 2 O)6]3+ and a](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-71.jpg)
Complex ions FIGURE 16 -11 The Lewis structure of [Al(H 2 O)6]3+ and a ball-and-stick representation Slide 16 - 71 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.
![FIGURE 16 12 Hydrolysis of AlH 2 O63 to produce H 3 O Slide FIGURE 16 -12 Hydrolysis of [Al(H 2 O)6]3+ to produce H 3 O+ Slide](https://slidetodoc.com/presentation_image_h/19a26e280059ada9f75f607f70961de4/image-72.jpg)
FIGURE 16 -12 Hydrolysis of [Al(H 2 O)6]3+ to produce H 3 O+ Slide 16 - 72 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

ionic charge ρ = charge density = ionic volume Slide 16 - 73 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 16 - 74 General Chemistry: Chapter 16 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition Management eleventh edition stephen p robbins
Management eleventh edition stephen p robbins General chemistry
General chemistry Chadha committee
Chadha committee Eleventh 5 year plan
Eleventh 5 year plan 11th five year plan
11th five year plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Introduction to genetic analysis tenth edition
Introduction to genetic analysis tenth edition No slip condition
No slip condition Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition A computer programming team has 13 members
A computer programming team has 13 members Mis chapter 6
Mis chapter 6 Report
Report Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Principles and applications of electrical engineering
Principles and applications of electrical engineering Learning principles and applications
Learning principles and applications Applications of nuclear chemistry
Applications of nuclear chemistry Irradiated food
Irradiated food Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Modern labor economics 12th edition pdf
Modern labor economics 12th edition pdf Modern real estate practice in pennsylvania
Modern real estate practice in pennsylvania Modern database management 12th edition ppt
Modern database management 12th edition ppt Modern database management 10th edition
Modern database management 10th edition Modern operating systems 3rd edition
Modern operating systems 3rd edition Modern operating systems tanenbaum 5th edition
Modern operating systems tanenbaum 5th edition Modern database management 8th edition
Modern database management 8th edition University physics with modern physics fifteenth edition
University physics with modern physics fifteenth edition Modern database management solutions
Modern database management solutions Modern labor economics 12th edition
Modern labor economics 12th edition Computer security principles and practice
Computer security principles and practice Computer security principles and practice 4th edition
Computer security principles and practice 4th edition Expert systems: principles and programming, fourth edition
Expert systems: principles and programming, fourth edition Rearranged most stable carbocation is
Rearranged most stable carbocation is Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Klein organic chemistry 2nd edition
Klein organic chemistry 2nd edition Introductory chemistry 4th edition
Introductory chemistry 4th edition Prefix multipliers
Prefix multipliers Introductory chemistry 5th edition nivaldo j. tro
Introductory chemistry 5th edition nivaldo j. tro The central science 14th edition
The central science 14th edition Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Zumdahl chemistry, 9th edition notes
Zumdahl chemistry, 9th edition notes Organic chemistry third edition david klein
Organic chemistry third edition david klein Lesson 81 drop in molecular views
Lesson 81 drop in molecular views Chemistry by raymond chang 10th edition
Chemistry by raymond chang 10th edition Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Halohydrin
Halohydrin Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Klein
Klein Smart materials names
Smart materials names Chapter 9 review stoichiometry modern chemistry answers
Chapter 9 review stoichiometry modern chemistry answers Modern chemistry chapter 7
Modern chemistry chapter 7 Modern chemistry chapter 15
Modern chemistry chapter 15 Chapter 14 review acids and bases section 1
Chapter 14 review acids and bases section 1 Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Modern chemistry chapter 12 review answers
Modern chemistry chapter 12 review answers Modern chemistry chapter 4
Modern chemistry chapter 4 Modern chemistry solutions
Modern chemistry solutions Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Principles of network applications
Principles of network applications Principles of network applications
Principles of network applications Principles of information security 5th edition pdf
Principles of information security 5th edition pdf Principles of electronic communication systems 3rd edition
Principles of electronic communication systems 3rd edition Principles of economics oxford fajar
Principles of economics oxford fajar Accounting principles second canadian edition
Accounting principles second canadian edition Accounting principles second canadian edition
Accounting principles second canadian edition Accounting principles second canadian edition
Accounting principles second canadian edition Management robbins coulter 14th edition chapter 1
Management robbins coulter 14th edition chapter 1 Involuntary inactive real estate license florida
Involuntary inactive real estate license florida