GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION













- Slides: 25

GENERAL CHEMISTRY PRINCIPLES AND MODERN APPLICATIONS ELEVENTH EDITION PETRUCCI HERRING MADURA Chemical Reactions BISSONNETTE 4 PHILIP DUTTON UNIVERSITY OF WINDSOR DEPARTMENT OF CHEMISTRY AND BIOCHEMISTRY Slide 4 - 1 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

Chemical Compounds Slide 4 - 2 CONTENTS 4 -1 Chemical Reactions and Chemical Equations 4 -2 Chemical Equations and Stoichiometry 4 -3 Chemical Reactions in Solution 4 -4 Determining the Limiting Reactant 4 -5 Other Practical Matters in Reaction Stoichiometry 4 -6 Chemical Reactions and Chemical Equations General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

4 -1 Chemical Reactions and Chemical Equations As reactants are converted to products we observe: Color change Precipitate formation Gas evolution Heat absorption or evolution Chemical evidence may be necessary. Slide 4 - 3 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

FIGURE 4 -1 Precipitation of silver chromate Slide 4 - 4 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

FIGURE 4 -2 Evidence of a chemical reaction Slide 4 - 5 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

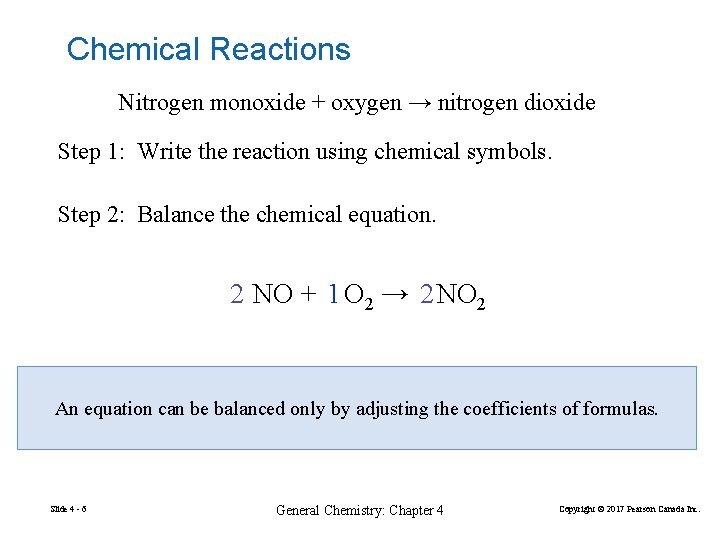
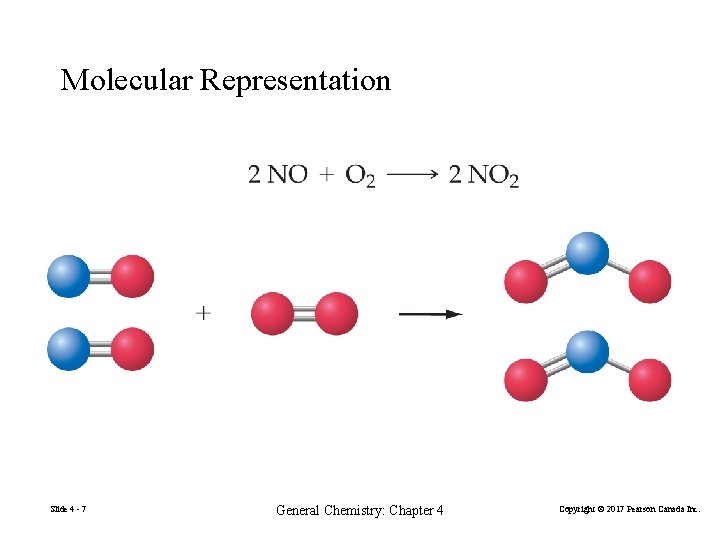
Chemical Reactions Nitrogen monoxide + oxygen → nitrogen dioxide Step 1: Write the reaction using chemical symbols. Step 2: Balance the chemical equation. 2 NO + 1 O 2 → 2 NO 2 An equation can be balanced only by adjusting the coefficients of formulas. Slide 4 - 6 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

Molecular Representation Slide 4 - 7 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

Strategy for Balancing Equations • Balance elements that occur in only one compound on each side first. • Balance free elements last. • Balance unchanged polyatomics (or other groups of atoms) as groups. • Fractional coefficients are acceptable and can be cleared at the end by multiplication. Slide 4 - 8 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

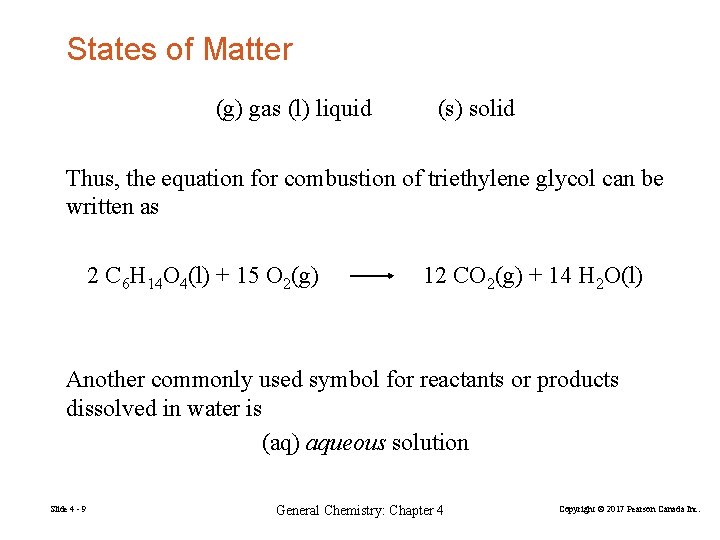
States of Matter (g) gas (l) liquid (s) solid Thus, the equation for combustion of triethylene glycol can be written as 2 C 6 H 14 O 4(l) + 15 O 2(g) 12 CO 2(g) + 14 H 2 O(l) Another commonly used symbol for reactants or products dissolved in water is (aq) aqueous solution Slide 4 - 9 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

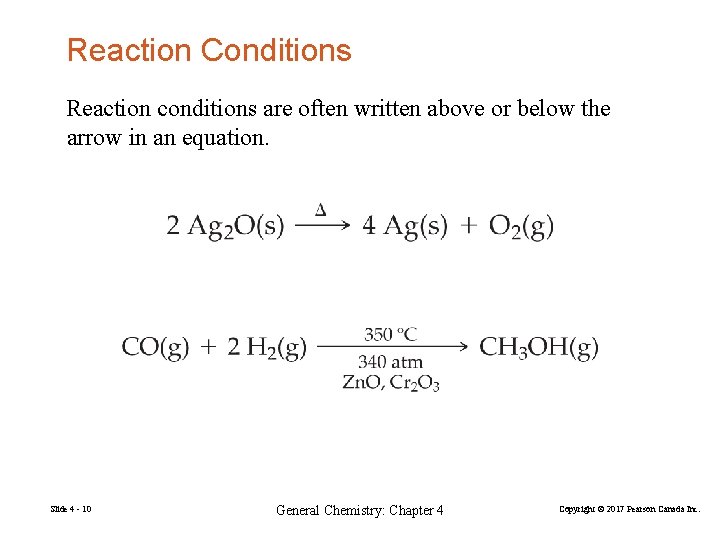
Reaction Conditions Reaction conditions are often written above or below the arrow in an equation. Slide 4 - 10 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

4 -2 Chemical Equations and Stoichiometry includes all the quantitative relationships involving: atomic and formula masses chemical formulas. chemical equations Mole ratio or stoichiometric factor is a central conversion factor. Slide 4 - 11 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

FIGURE 4 -3 A generalized stoichiometry diagram Slide 4 - 12 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

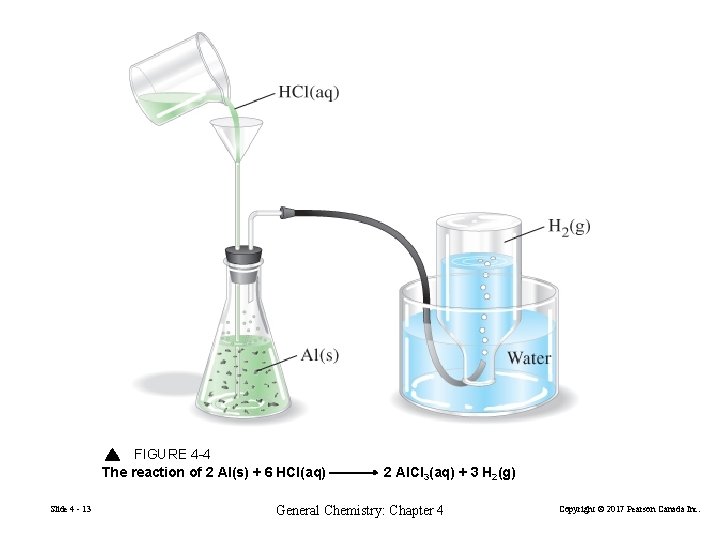
FIGURE 4 -4 The reaction of 2 Al(s) + 6 HCl(aq) Slide 4 - 13 2 Al. Cl 3(aq) + 3 H 2(g) General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

4 -3 Chemical Reactions in Solution Close contact between atoms, ions and molecules necessary for a reaction to occur. Solvent We will usually use aqueous (aq) solution. Solute A material dissolved by the solvent. Slide 4 - 14 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

Molarity (M) = Amount of solute (mol solute) Volume of solution (L) c = n V If 0. 440 mol of urea is dissolved in enough water to make 1. 000 L of solution the concentration is: curea = 0. 440 mol urea = 0. 440 M CO(NH 2)2 1. 000 L Slide 4 - 15 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

FIGURE 4 -5 Preparation of 0. 250 M K 2 Cr 2 O 4 —Example 4 -8 illustrated Slide 4 - 16 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

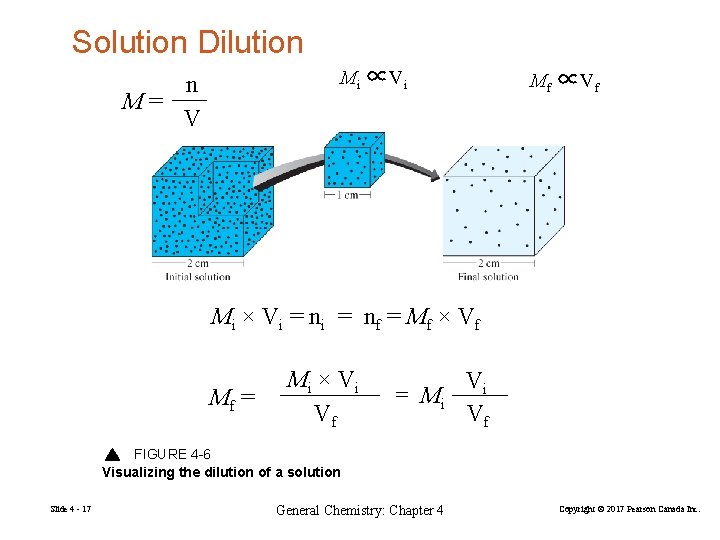
Solution Dilution Mi ∝Vi n M= V Mf ∝Vf Mi × V i = n f = Mf × V f Mf = Mi × V i Vf = Mi Vi Vf FIGURE 4 -6 Visualizing the dilution of a solution Slide 4 - 17 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

FIGURE 4 -7 Preparing a solution by dilution—Example 4 -9 illustrated Slide 4 - 18 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

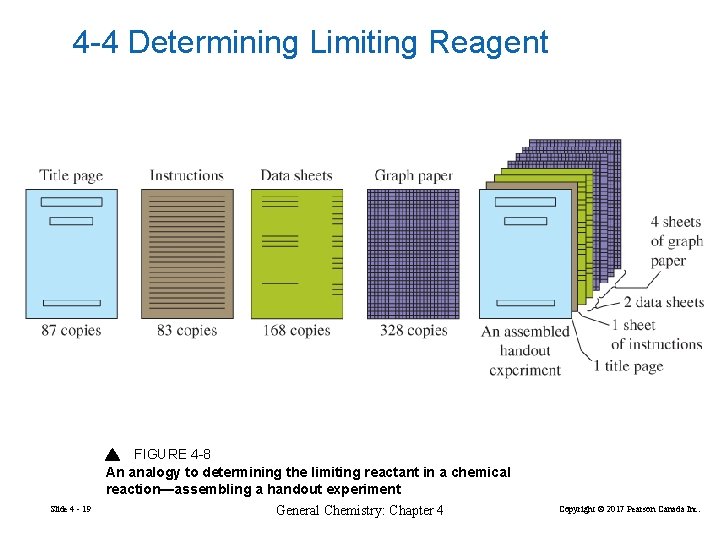
4 -4 Determining Limiting Reagent FIGURE 4 -8 An analogy to determining the limiting reactant in a chemical reaction—assembling a handout experiment Slide 4 - 19 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

4 -5 Other Practical Matters in Reaction Stoichiometry Theoretical, Actual and Percent Yield Theoretical yield is the expected yield from a reactant. Actual yield is the amount of product actually produced. Actual yield Percent yield = ∝ 100% Theoretical yield Slide 4 - 20 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

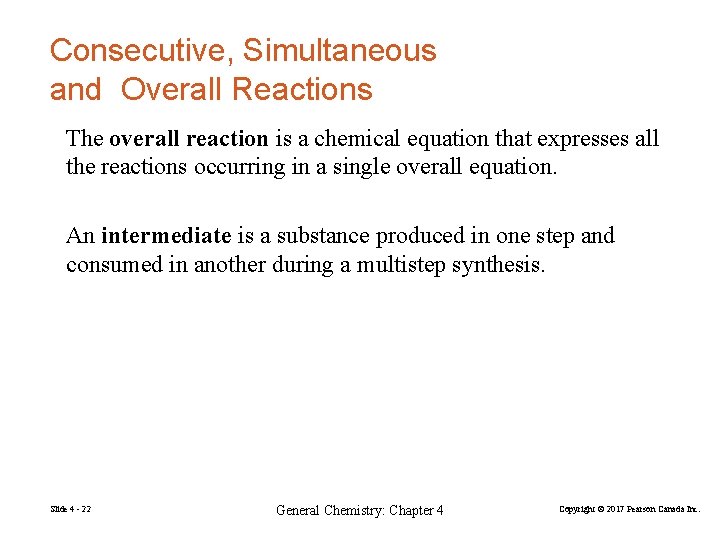
Consecutive, Simultaneous and Overall Reactions Multistep synthesis is often unavoidable. Consecutive reactions are carried out one after another in sequence. In simultaneous reactions, two or more substances react independently of one another in separate reactions occurring at the same time. Slide 4 - 21 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

Consecutive, Simultaneous and Overall Reactions The overall reaction is a chemical equation that expresses all the reactions occurring in a single overall equation. An intermediate is a substance produced in one step and consumed in another during a multistep synthesis. Slide 4 - 22 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

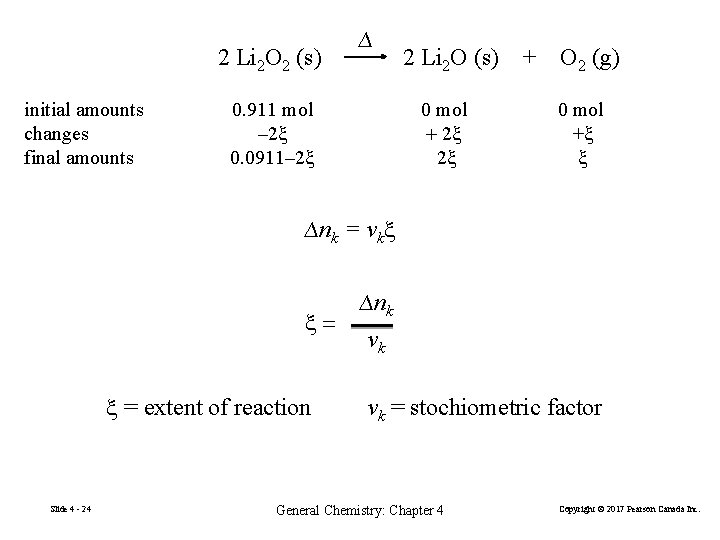
4 -6 The Extent of Reaction 2 Li 2 O 2 (s) initial amounts changes final amounts Slide 4 - 23 ∆ 2 Li 2 O (s) 0. 0911 mol 0 mol – 2× 0. 0350 mol +2× 0. 0350 mol 0. 0911 – 2× 0. 0350 mol +2× 0. 0350 mol 0. 911 mol – 2 x 0. 0911– 2 x 0 mol + 2 x 2 x General Chemistry: Chapter 4 + O 2 (g) 0 mol +1× 0. 0350 mol +0. 0350 mol +x x Copyright © 2017 Pearson Canada Inc.

2 Li 2 O 2 (s) initial amounts changes final amounts ∆ 0. 911 mol – 2 x 0. 0911– 2 x 2 Li 2 O (s) 0 mol + 2 x 2 x + O 2 (g) 0 mol +x x ∆nk = νkx x= x = extent of reaction Slide 4 - 24 ∆nk νk νk = stochiometric factor General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.

End of Chapter Slide 4 - 25 General Chemistry: Chapter 4 Copyright © 2017 Pearson Canada Inc.
 Management eleventh edition
Management eleventh edition Management stephen p robbins 11th edition
Management stephen p robbins 11th edition Management eleventh edition
Management eleventh edition Management eleventh edition
Management eleventh edition General chemistry 11th edition
General chemistry 11th edition Chadha committee
Chadha committee Eleventh 5 year plan
Eleventh 5 year plan Eleventh plan
Eleventh plan For his eleventh birthday elvis presley
For his eleventh birthday elvis presley Introduction to genetic analysis tenth edition
Introduction to genetic analysis tenth edition Fluid mechanics fundamentals and applications
Fluid mechanics fundamentals and applications Plastic scintillators: chemistry and applications
Plastic scintillators: chemistry and applications Modern systems analysis and design 7th edition
Modern systems analysis and design 7th edition Discrete math susanna epp
Discrete math susanna epp Mis chapter 6
Mis chapter 6 Using mis (10th edition)
Using mis (10th edition) Terahertz spectroscopy principles and applications
Terahertz spectroscopy principles and applications Sport management principles and applications
Sport management principles and applications Principles and applications of electrical engineering
Principles and applications of electrical engineering Pearson engineering
Pearson engineering Learning principles and applications
Learning principles and applications 25 m/s
25 m/s Applications of nuclear chemistry
Applications of nuclear chemistry Modern labor economics 12th edition solution
Modern labor economics 12th edition solution Vertical economics
Vertical economics Modern real estate practice in pennsylvania
Modern real estate practice in pennsylvania